Abstract
The causal association of circulating metabolites with dementia remains uncertain. We assessed the causal association of circulating metabolites with dementia utilizing Mendelian randomization (MR) methods. We performed univariable MR analysis to evaluate the associations of 486 metabolites with dementia, Alzheimer’s disease (AD), and vascular dementia (VaD) risk. For secondary validation, we replicated the analyses using an additional dataset with 123 metabolites. We observed 118 metabolites relevant to the risk of dementia, 59 of which were lipids, supporting the crucial role of lipids in dementia pathogenesis. After Bonferroni adjustment, we identified nine traits of HDL particles as potential causal mediators of dementia. Regarding dementia subtypes, protective effects were observed for epiandrosterone sulfate on AD (OR = 0.60, 95% CI: 0.48–0.75) and glycoproteins on VaD (OR = 0.89, 95% CI: 0.83–0.95). Bayesian model averaging MR (MR-BMA) analysis was further conducted to prioritize the predominant metabolites for dementia risk, which highlighted the mean diameter of HDL particles and the concentration of very large HDL particles as the predominant protective factors against dementia. Moreover, pathway analysis identified 17 significant and 2 shared metabolic pathways. These findings provide support for the identification of promising predictive biomarkers and therapeutic targets for dementia.
1. Introduction
Dementia, in particular Alzheimer’s disease (AD) and vascular dementia (VaD), is the major cause of disability among older individuals globally, resulting in serious physical, financial and social consequences [1]. The prevalence of dementia was estimated to exceed 50 million individuals globally in 2019, with projections indicating more than a twofold increase by 2050 [2]. Due to the absence of well-established disease-modifying therapies, it is an urgent public health priority to explore the potential biomarkers for early identification and prevention of dementia [3].
Metabolomics thoroughly quantifies small molecular metabolites in specific tissues or biofluids, reflecting genetic, environmental, and pathological changes associated with disease progression [4]. Recently, converging evidence has indicated that metabolic dysfunction may be a major hallmark and cause of dementia [5]. Previous observational studies have revealed various circulating metabolites, such as amino acids, fatty acids, and lipids, related to dementia [6,7], but further studies have provided contradictory findings [8,9]. The inconsistencies may arise due to variations in sample characteristics and study designs. Additionally, even with rigorous adjustments for potential confounders, observational studies are still vulnerable to residual unmeasured confounding factors. Therefore, evidence solely from observational studies is insufficient to clarify the causal relationships of circulating metabolites with dementia risk for early identification and prevention.
Mendelian randomization (MR) is an emerging epidemiological method utilizing genetic instrumental variables (IVs) to examine the causal relationship of exposures with outcomes, thus overcoming the inevitable defects of conventional observational studies including reverse causation and confounding [10]. Recent technological advances in mass spectrometry and genotyping have enabled genome-wide association studies (GWASs) to comprehensively reveal the genetic determinants of hundreds of circulating metabolites [11]. In this context, MR design provides a new strategy for accessing the causal association of circulating metabolites with dementia by integrating genomics and metabolomics. However, no large-scale MR analysis has been conducted to systematically estimate the association between circulating metabolites and dementia.
Therefore, we utilized systematic MR analysis to (1) comprehensively evaluate the causal effects of circulating metabolites on dementia; (2) disentangle the prioritization of the predominant metabolites for dementia risk; and (3) identify potential metabolic pathways to enhance the comprehension of the underlying mechanism of dementia.
2. Materials and Methods
2.1. Study Design
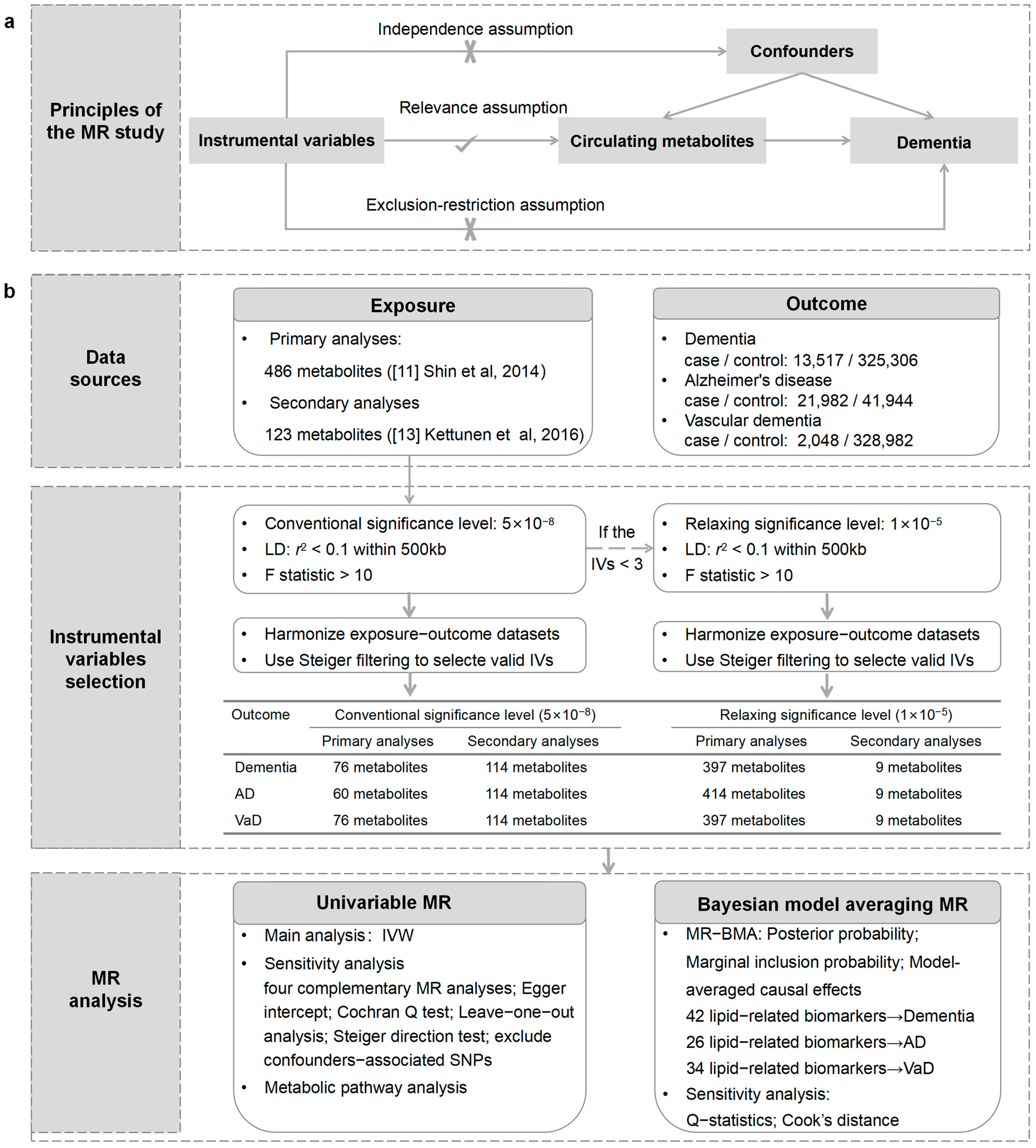
An outline of our study design is presented in Figure 1. Initially, we performed univariable MR analysis to evaluate the causal relationships between circulating metabolites and the risk of outcomes. Subsequently, we performed Bayesian model averaging MR (MR-BMA) analysis to prioritize the metabolites that contribute to the risk of outcomes. Furthermore, we conducted a metabolic pathway analysis to explore the underlying mechanism of dementia. Our analysis followed the Strengthening the Reporting of Observational Studies in Epidemiology using Mendelian Randomization (STROBE-MR) guidelines [12].
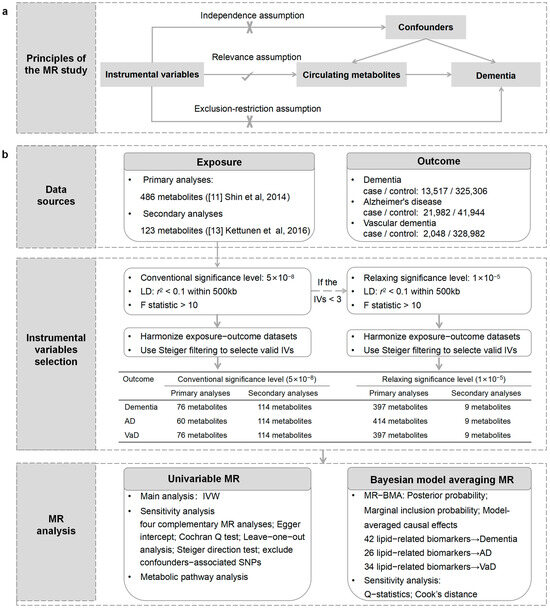
Figure 1.
An overview of the study design. (a) The principles of the MR study. (b) Flowchart describing the sequence of analytical steps in this research. LD: linkage disequilibrium; IVs: instrumental variables; IVW: inverse-variance weighting; WM: weighted median.
2.2. GWAS Data Sources for Circulating Metabolites and Dementia
The summary-level datasets for circulating metabolites were obtained from two large-scale comprehensive metabolite GWASs. Shin et al. investigated 486 circulating metabolites in 7824 European adults with approximately 2.1 million SNPs from 2 cohorts [11]. Of the 486 metabolites, 309 known metabolites could be further assigned to eight broad metabolic groups (amino acids, carbohydrates, cofactors and vitamins, energy, lipids, nucleotides, peptides, and xenobiotic metabolism). Moreover, Kettunen et al. investigated 123 circulating metabolites, including lipoprotein lipids and lipid subclasses, fatty acids, amino acids, and glycolysis precursors, in 24,925 European individuals with approximately 12 million SNPs from 14 cohorts [13]. The lipoprotein subclass-specific lipids have supplemented the corresponding deficiencies of previous GWAS summary statistics.
The GWAS datasets for dementia (13,517 cases and 325,306 controls) and VaD (2048 cases and 328,982 controls) were obtained from the FinnGen consortium [14]. Summarized data for AD were obtained from the International Genomics of Alzheimer’s Project (IGAP), including 21,982 cases and 41,944 controls from 46 case–control studies [15]. Detailed information on GWAS datasets is summarized in Table S1. Furthermore, we accessed the potential bias attributable to participant overlap utilizing a web-based tool (https://sb452.shinyapps.io/overlap/, accessed on 2 July 2023) and estimated type 1 error rates < 0.05 [16].
2.3. Selection of IVs
To obtain unbiased estimates of the causal effects, MR analysis should adhere to three assumptions: relevance, independence, and exclusion restriction. First, we extracted SNPs at the conventional genome-wide significance level (p < 5 × 10−8) with a linkage disequilibrium (LD) threshold of r2 < 0.1 within 500 kilobases’ (kb) distance. If the number of SNPs for metabolites was fewer than 3, we adopted a relaxed genome-wide threshold (p < 1 × 10−5) in reference to previous studies [17,18]. Steiger filtering was used to remove SNPs that were correlated with outcomes stronger than exposures [19]. We then measured the explained genetic variation (R2) utilizing the MR Steiger directionality test. The F statistic was computed by the formula F = R2(N − K − 1)/K(1 − R2), where N is the sample size of the GWAS for each metabolite and K is the number of IVs [20]. Metabolites with an F statistic of less than 10 were excluded to avoid the potential weak instrument bias [21]. Finally, the metabolites with more than 2 SNPs were included in the MR analysis [22]. Detailed information on IVs is presented in Table S2.
2.4. Univariable MR
Inverse-variance weighting (IVW) was adopted to assess the causal effects of metabolites on outcomes (Table S3). To render the conclusions more reliable, we conducted several sensitivity analyses for the identified significant metabolites (Table S4). First, five MR models were utilized as complementary methods to evaluate the consistency and robustness of the results, including MR-Egger, weighted median, penalized weighted median, maximum likelihood methods, and MR pleiotropy residual sum and outlier (MR–PRESSO) [23,24]. Then, the Cochran Q test and MR-Egger intercept test were carried out to establish the existence of heterogeneity and horizontal pleiotropy, respectively. The MR-PRESSO global test was further applied to detect horizontal pleiotropy and possible outliers. Moreover, we performed the MR Steiger directionality test to validate whether the observed causalities were biased owing to reverse causation [19]. Finally, we also employed the PhenoScanner function in the MendelianRandomization package to exclude SNPs that were associated with education, body mass index, blood pressure, smoking and drinking at the threshold of p < 1 × 10−5 because these factors are known to be primary contributors to mortality and are also significant risk factors for outcomes [25]. The IVW was repeated after dropping the above SNPs to ensure robustness.
A reliable and robust causal association between circulating metabolites and dementia was determined in compliance with the following criteria: (1) the IVW method demonstrated a significant difference (p < 0.05); (2) consistent direction and magnitude among the five complementary MR methods; (3) no heterogeneity or pleiotropy was detected by Cochran’s Q test, MR-Egger intercept test, or MR-PRESSO global test (p > 0.05); (4) the MR-Steiger directionality test indicated that the effect direction from metabolites to dementia was true; and (5) the MR estimates remained significant after excluding SNPs associated with potential confounders.
2.5. MR-BMA Analysis
MR-BMA, an innovative expansion of multivariable MR using the Bayesian framework, shows the advantage of selecting and prioritizing highly correlated candidate risk factors in high-dimensional datasets [26]. MR-BMA analysis was further conducted in the subcategory showing significant enrichment of metabolic traits. We pooled SNPs that were associated with all selected lipid-related traits (p < 5 × 10−9) and strictly clumped them with an LD threshold of r2 < 0.001 within 10,000 kb (Table S5). Posterior probability (PP) was calculated for each specific model to prioritize the best model. After the initial analysis, we calculated Cochran’s Q statistics and Cook’s distance for the models with PP > 0.02 to screen potential outliers. Subsequently, MR-BMA analysis was repeated after excluding the outliers (Table S6). The marginal inclusion probability (MIP), representing the sum of the PP, was used to prioritize the candidate risk factors. The model-averaged causal effects (MACE) demonstrated the average causal effect of each lipid-related trait on the outcomes. Empirical p values for the MIP of metabolites were computed with 1000 permutations [27].
2.6. Statistical Analysis
All MR estimates are presented as odds ratios (ORs) with 95% confidence intervals (CIs) of outcomes. We adopted a conservative Bonferroni-adjusted threshold of p < 1.03 × 10−4 (0.05/486) for primary analyses and p < 4.07 × 10−4 (0.05/123) for secondary analyses to determine a statistically significant causal relationship. The metabolites with a two-sided p < 0.05 but above the Bonferroni-adjusted threshold were considered potential risk predictors for dementia. All analyses were conducted using R software (version 4.3.1) with the R packages TwoSampleMR, MendelianRandomization, and MR-PRESSO. The R-code for MR-BMA was accessible on GitHub (https://github.com/verena-zuber/demo_AMD, accessed on 15 March 2023).
2.7. Metabolic Pathway Analysis
The metabolites identified by IVW (p < 0.05) were imported into the pathway analysis module in MetaboAnalyst 5.0 (https://www.metaboanalyst.ca/, accessed on 5 May 2023). Metabolic pathway analysis was conducted utilizing a hypergeometric test. We tested human metabolic pathways from two metabolite set libraries: the Kyoto Encyclopedia of Genes and Genomes (KEGG) and the Small Molecule Pathway Database (SMPDB).
3. Results
3.1. Strength of the IVs
The analysis included a total of 472 metabolites for dementia and VaD, 473 metabolites for AD analysis, and all 123 metabolites were retained for validation. The F statistics of metabolites ranged from 20 to 504, indicating a considerable strength of the genetic instruments employed.
3.2. Univariable MR Analyses
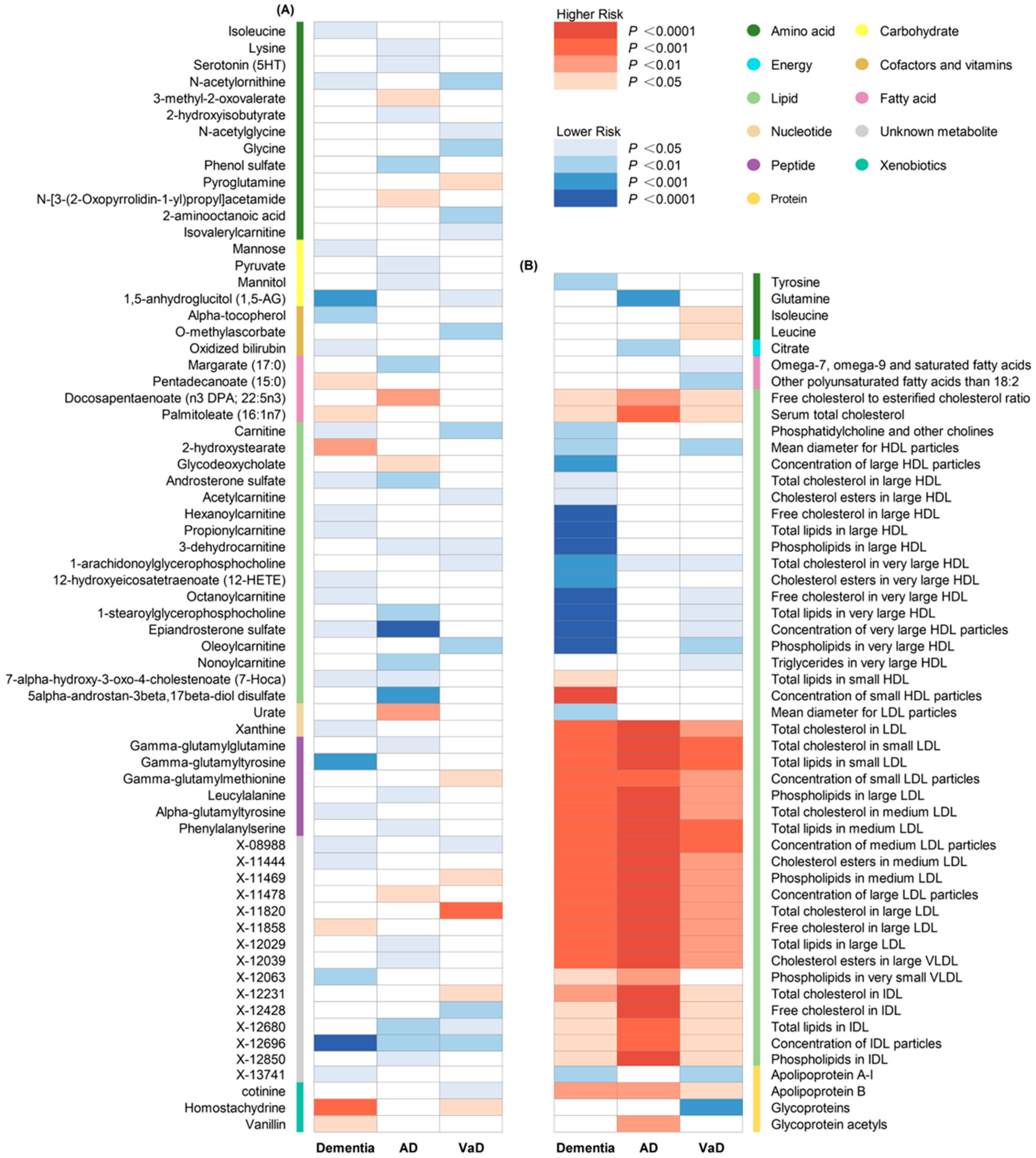
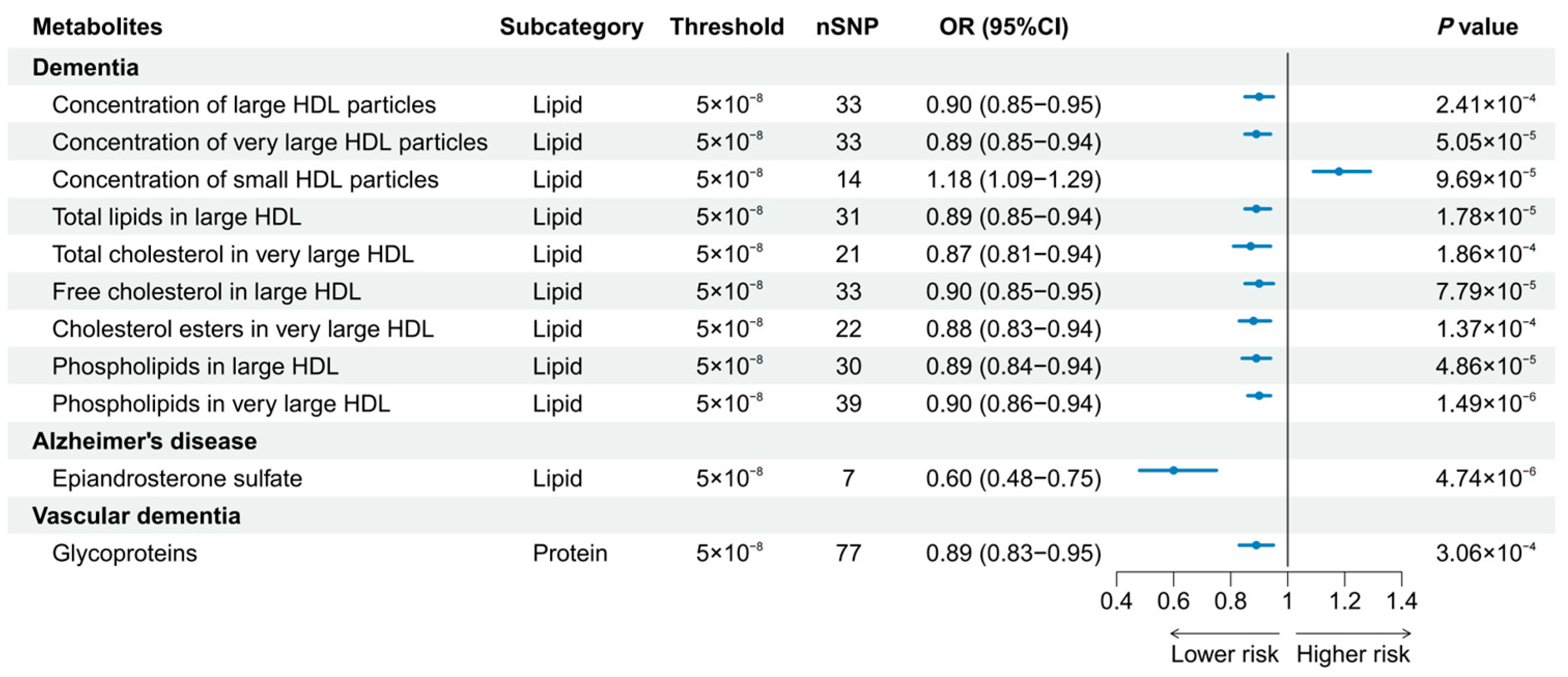
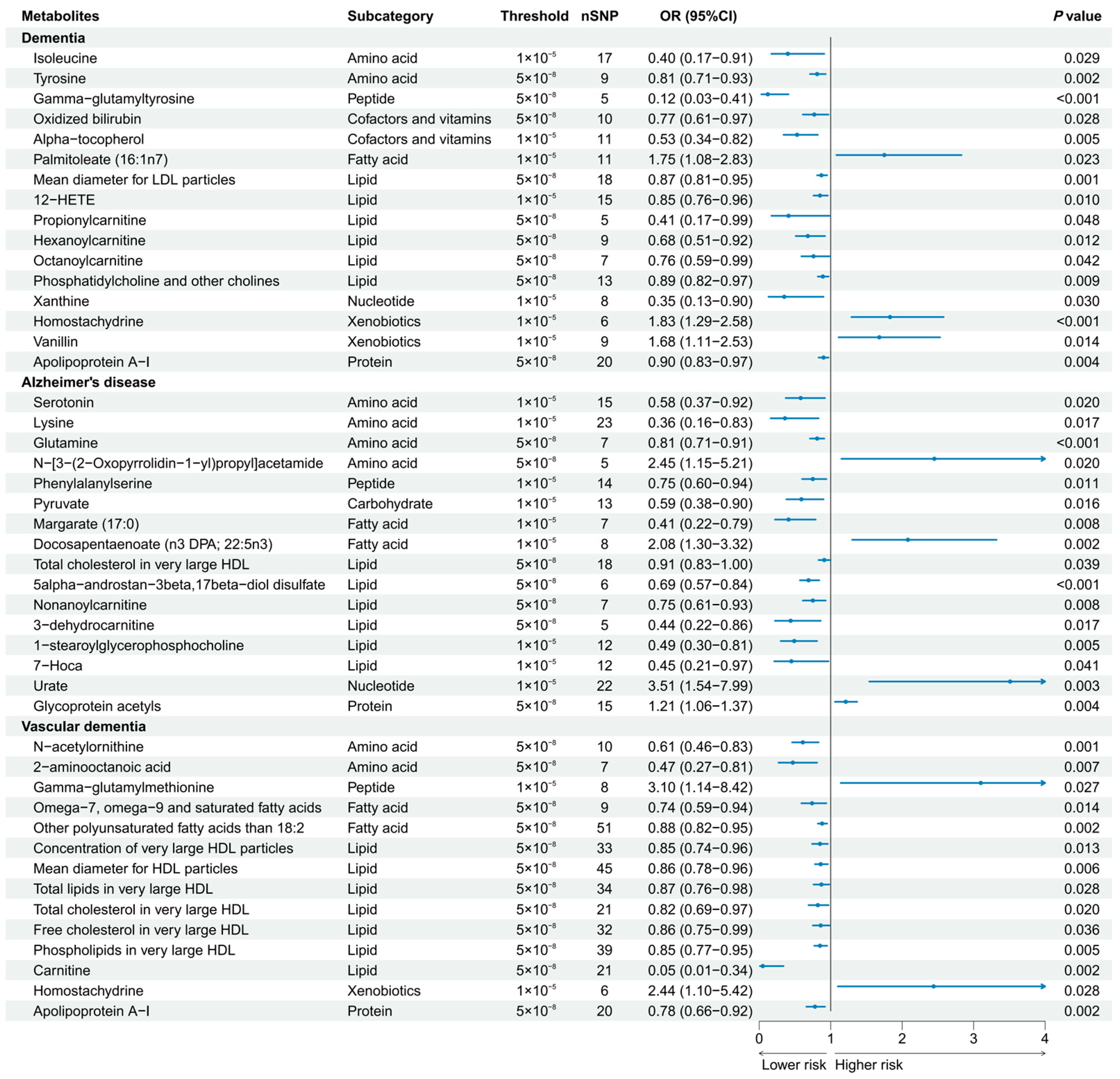
A total of 186 causal features, corresponding to 118 unique metabolites, were preliminarily identified using IVW (Figure 2 and Table S3). After the Bonferroni adjustment, we detected 44 statistically significant causal features. A total of 68 causal features demonstrated consistent associations in the sensitivity analyses (Table S4), with 11 of them reaching the Bonferroni-adjusted threshold (Figure 3). Specifically, the genetically determined concentrations of large HDL particles (OR = 0.90, 95% CI: 0.85–0.95, p = 2.41 × 10−4), very large HDL particles (OR = 0.89, 95% CI: 0.85–0.94, p = 5.05 × 10−5), and small HDL particles (OR = 1.18, 95% CI: 1.09–1.29, p = 9.69 × 10−5) were causally associated with dementia susceptibility. The subfractions of large HDL particles (total lipids, free cholesterol, and phospholipids) and very large HDL particles (total cholesterol, cholesterol esters, and phospholipids) were associated with decreased risks of dementia. Regarding the subtypes of dementia, epiandrosterone sulfate (OR = 0.60, 95% CI: 0.48–0.75, p = 4.74 × 10−6) and glycoproteins (OR = 0.89, 95% CI: 0.83–0.95, p = 3.06 × 10−4) were associated with decreased risk of AD and VaD, respectively. Additionally, we identified 46 potential risk predictors that remained robust in the sensitivity analyses, particularly amino acids and acylcarnitines (Figure 4). With regard to the potentially shared molecules, 3-dehydrocarnitine was simultaneously proven to be related to a lower risk of AD and VaD.
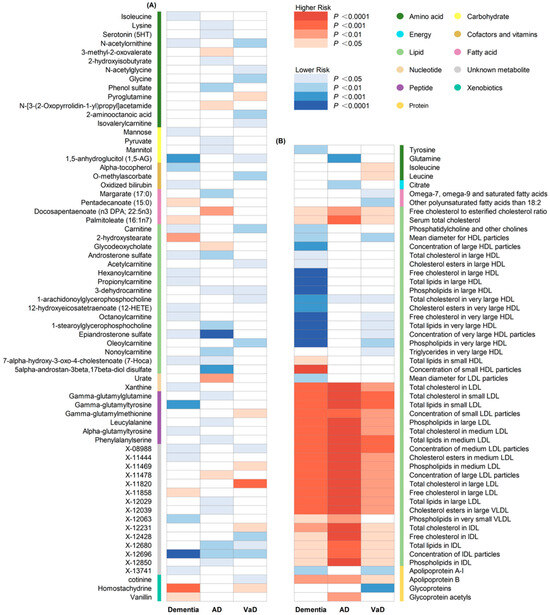
Figure 2.
Heatmap illustrating the causal estimates of circulating metabolites on dementia, AD and VaD. (A) Heatmap showing the univariable MR analysis result of 486 circulating metabolites in the primary analyses; (B) heatmap showing the univariable MR analysis result of 123 circulating metabolites in the secondary analyses. AD: Alzheimer’s disease; VaD: vascular dementia; IVW: inverse-variance weighting.
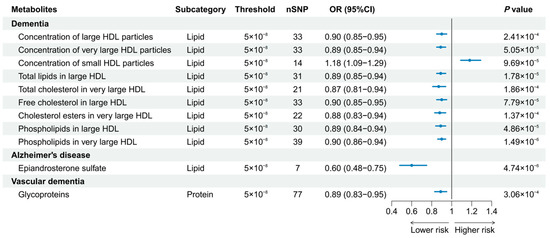
Figure 3.
Forest plot of 11 causal features reaching the Bonferroni-adjusted threshold.
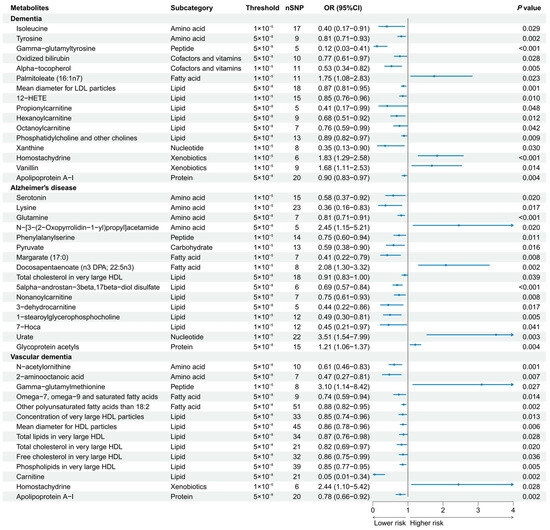
Figure 4.
Forest plot of 46 potential risk predictors (p < 0.05) that remained robust in sensitivity analyses. 12-HETE, 12-hydroxyeicosatetraenoate; 7-Hoca, 7-alpha-hydroxy-3-oxo-4-cholestenoate.
3.3. MR-BMA Analyses
We performed an MR–BMA analysis with lipid-related traits among 123 metabolites identified by the IVW (Table 1 and Table 2). For dementia, the top-ranked model included only the mean diameter for HDL particles, followed by the concentration of very large HDL particles, and these two metabolite traits were identified as the dominant risk factors for the risk of dementia with the highest MIP. For AD, the top-ranked model included only total cholesterol in very large HDL particles, and it was also the metabolite trait with the strongest overall evidence. For VaD, the top-ranked model included triglycerides in very large HDL particles, followed by omega-7, omega-9, and saturated fatty acids, and the latter metabolite trait of fatty acids had the highest MIP rank.

Table 1.
Prioritization of the top 5 lipid-related traits for dementia, AD, and VaD.

Table 2.
Models of lipid-related traits for dementia, AD, and VaD.
3.4. Metabolic Pathway Analysis
As shown in Table 3, a total of 17 significant metabolic pathways (hypergeometric test, p < 0.05) were identified in the metabolic pathway analysis. Two shared metabolic pathways were implicated in the development of dementia, AD and VaD, including the “aminoacyl-tRNA biosynthesis” pathway and the “valine, leucine and isoleucine biosynthesis” pathway.

Table 3.
Significant metabolic pathways (hypergeometric test, p < 0.05) involved in dementia, AD, and VaD.
4. Discussion
This comprehensive MR study identified 118 metabolites relevant to the risk of dementia, 59 of which were lipids, supporting the crucial role of lipids in dementia pathogenesis. Our study revealed causal relationships of nine traits of HDL particles on dementia, epiandrosterone sulfate on AD, and glycoproteins on VaD. Our MR-BMA results indicated that the mean diameter of HDL particles and concentration of very large HDL particles had a predominant influence on the risk of dementia. Moreover, we detected 17 significant metabolic pathways involved in dementia, of which 2 were shared metabolic pathways among different subtypes of dementia.
Observational studies have documented the protective role of HDL cholesterol (HDL-C) on the risk of dementia [28]. In contrast, several studies have indicated that elevated levels of HDL-C are related to a higher risk of dementia [29,30]. The inconsistency observed in these findings can be attributed to variations in sample size, study designs, unmeasured confounders, and reverse causation, emphasizing the importance of employing the MR method to disentangle such scenarios. Moreover, HDL particles display remarkable heterogeneity in terms of structure, density, size, and composition, providing them with diverse functionalities that are essential to numerous biological processes [31]. Previous MR studies also show inconclusive evidence [32,33], possibly because they did not adequately consider the heterogeneity and complexity of HDL particles. Our comprehensive MR study clarified this controversial issue by conducting a meticulous analysis of specific subfractions and found protective effects of subfractions of large and very large HDL particles against dementia. Moreover, our study identified the predominant role of the mean diameter of HDL particles and the concentration of very large HDL particles in dementia. The following may explain the mechanisms underlying the involvement of HDL particles in dementia pathophysiology. Recent studies have proposed that HDL particle size serves as a critical determinant of HDL cholesterol efflux capacity [34] and may play a central role in mitigating cognitive decline. Furthermore, the lipid constituents encapsulated within HDL, such as phospholipids, cholesteryl esters, and free cholesterol, have been acknowledged as potent modulators of HDL functionality encompassing cholesterol efflux, vasodilation, antioxidative, and anti-inflammatory properties [35]. These multifaceted functions may contribute significantly to the pathophysiology of dementia, indicating their potential therapeutic relevance. Our study reaffirmed the potential health benefits of HDL in protecting against dementia [36] and emphasized the role of the mean diameter and concentration of HDL particles.
Regarding dementia subtypes, our study found protective effects of epiandrosterone sulfate and glycoproteins against AD and VaD, respectively. Epiandrosterone sulfate is classified as a sulfated steroid that reflects the metabolism of androgens [37]. Similarly, prior research has proposed the protective role of epiandrosterone sulfate against AD, which may improve plaque formation, enhance cognitive performance and increase longevity [38,39]. Glycoproteins, markers of inflammation, consist of protein molecules covalently linked to carbohydrate chains or glycans [40]. The increased expression of P-glycoprotein is a temporary physiological compensatory response in blood–brain barrier impairment to discharge undesirable substances [41]. A recent observational study proposed a glycoprotein to be a valid diagnostic biomarker for VaD [42]. Our study provides further compelling evidence, confirming the potential diagnostic value and therapeutic targets associated with glycoproteins in VaD.
Our study identified various potential risk predictors for dementia, with particular emphasis on amino acids and acylcarnitines, which were corroborated by previous observational studies [43]. Amino acids play multifaceted roles as neuromodulators, neurotransmitters and regulators of energy metabolism. Our study is consistent with existing evidence indicating the protective effect of essential amino acids against dementia [44]. Acylcarnitines represent derivatives of carnitine during the fatty acid transport to mitochondria for subsequent β-oxidation and participate in energy metabolism and neuroprotection [45]. Several studies have reported the beneficial impact of acylcarnitines on dementia, recognizing them as predictive diagnostic biomarkers for dementia [43].
The metabolic pathway analysis identified two shared pathways involved in dementia, AD, and VaD. The “aminoacyl-tRNA biosynthesis” pathway plays a vital role in protein synthesis and immune regulation [46], and may be related to the critical hypotheses explaining the pathophysiology of dementia. For the “valine, leucine and isoleucine biosynthesis” pathways, known as branched-chain amino acid (BCAA) pathways, previous studies have recognized abnormal metabolism of BCAA as the characteristic alteration in the development of dementia [47]. Furthermore, recent studies have indicated that fatty acid metabolism appears to be an essential determinant in the onset of dementia [48]. Our study further suggested that the “oxidation of branched-chain fatty acids” pathway was associated with VaD, consistent with the MR-BMA results highlighting the predominant role of fatty acids in VaD.
This study represents a comprehensive and systematic examination of the causal associations between circulating metabolites and dementia. Our study possesses several notable strengths. First, by utilizing genomic and metabolomic data, we have provided novel insights into the potential mediators of dementia and its subtypes through this systematic MR analysis. Second, by leveraging multiple large-scale GWASs, we were able to establish a robust causal inference with high statistical power. Additionally, we employed MR-BMA analysis, which facilitated the prioritization of the predominant metabolites associated with the risk of dementia in high-dimensional datasets. However, our research has several limitations deserving attention. First, we conducted a GWAS of circulating metabolites, which may not directly reflect the abnormal brain function associated with dementia. Future studies should focus on cerebrospinal fluid or brain tissue to provide more precise insights into the association of metabolites with dementia. Second, our study was exclusively from European populations, limiting extrapolation to other populations. It is crucial for future research to access the validity and applicability of our findings across different ethnicities. Third, the included GWAS datasets were heterogeneous in terms of population, sample and metabolic detection technology, which may contribute to the differences in metabolites identified by the primary and secondary analyses. We did not perform a meta-analysis owing to the limited overlap of metabolites. Furthermore, the MR method employed in our study assumes a lifetime exposure, which may not accurately represent reality. This assumption restricts our ability to identify potential nonlinear correlations between circulating metabolites and dementia. Exploring alternative analytical approaches that account for nonlinear associations could provide valuable insights into the complex relationship of metabolites with dementia.
5. Conclusions
In this systematic MR analysis, we identified nine traits of HDL particles as potential causal mediators of dementia, among which the mean diameter of HDL particles and the concentration of very large HDL particles played predominant roles in reducing the risk of dementia. Regarding dementia subtypes, epiandrosterone sulfate and glycoproteins were associated with decreased risk of AD and VaD, respectively.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/nu16172879/s1. Table S1. Detailed information of summary statistics for circulating metabolites, dementia, Alzheimer’s disease and vascular dementia; Table S2. Instrumental variables of circulating metabolites and their associations with dementia, Alzheimer’s disease and vascular dementia; Table S3. Mendelian randomization results for circulating metabolites on risk of dementia, Alzheimer’s disease and vascular dementia; Table S4. Mendelian randomization results and sensitivity analysis for 186 causal features identified by inverse-variance weighting; Table S5. Detailed information on all SNPs in MR-BMA analyses; Table S6. The results of single-metabolite causal rankings in accordance with their marginal posterior probability (MIP).
Author Contributions
H.-M.L. and C.-S.Q. designed the study and drafted the manuscript. Z.-H.L. directed the study. L.-Y.D., X.-L.T., D.-Q.L. and Z.-Y.X. reviewed the manuscript. S.-M.L., H.-X.H., L.K. and B.-Y.Z. contributed to the visualization. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (82204115), the Guangdong Basic and Applied Basic Research Foundation (2021A1515110230), the Science and Technology Program of Guangzhou (2024A04J4571), and the Youth S&T Talent Support Programme of Guangdong Provincial Association for Science and Technology (SKXRC202408).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data used are accessible at https://gwas.mrcieu.ac.uk/ (accessed on 3 April 2023), www.finngen.fi/en (accessed on 20 April 2023), and http://www.metabolomix.com/the-metabolomics-gwas-server-gwas-eu/ (accessed on 2 April 2023).
Acknowledgments
The authors thank the participants and investigators of the FinnGen study and all GWASs used in this study.
Conflicts of Interest
The authors declare no competing interests.
References
- Grande, G.; Qiu, C.; Fratiglioni, L. Prevention of dementia in an ageing world: Evidence and biological rationale. Ageing Res. Rev. 2020, 64, 101045. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef] [PubMed]
- Lefevre-Arbogast, S.; Wagner, M.; Proust-Lima, C.; Samieri, C. Nutrition and Metabolic Profiles in the Natural History of Dementia: Recent Insights from Systems Biology and Life Course Epidemiology. Curr. Nutr. Rep. 2019, 8, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Lin, Y.; Li, X.; Driver, J.A.; Liang, L. Shared genetic architecture between metabolic traits and Alzheimer’s disease: A large-scale genome-wide cross-trait analysis. Hum. Genet. 2019, 138, 271–285. [Google Scholar] [CrossRef]
- de la Monte, S.M.; Tong, M. Brain metabolic dysfunction at the core of Alzheimer’s disease. Biochem. Pharmacol. 2014, 88, 548–559. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, W.; Wang, Y.; Wang, W.; Liao, H.; Zhang, X.; Kiburg, K.V.; Shang, X.; Bulloch, G.; Huang, Y.; et al. Plasma metabolomic profiles of dementia: A prospective study of 110,655 participants in the UK Biobank. BMC Med. 2022, 20, 252. [Google Scholar] [CrossRef] [PubMed]
- Proitsi, P.; Kuh, D.; Wong, A.; Maddock, J.; Bendayan, R.; Wulaningsih, W.; Hardy, R.; Richards, M. Lifetime cognition and late midlife blood metabolites: Findings from a British birth cohort. Transl. Psychiatry 2018, 8, 203. [Google Scholar] [CrossRef]
- van der Lee, S.J.; Teunissen, C.E.; Pool, R.; Shipley, M.J.; Teumer, A.; Chouraki, V.; Melo van Lent, D.; Tynkkynen, J.; Fischer, K.; Hernesniemi, J.; et al. Circulating metabolites and general cognitive ability and dementia: Evidence from 11 cohort studies. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2018, 14, 707–722. [Google Scholar] [CrossRef]
- Tynkkynen, J.; Chouraki, V.; van der Lee, S.J.; Hernesniemi, J.; Yang, Q.; Li, S.; Beiser, A.; Larson, M.G.; Sääksjärvi, K.; Shipley, M.J.; et al. Association of branched-chain amino acids and other circulating metabolites with risk of incident dementia and Alzheimer’s disease: A prospective study in eight cohorts. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2018, 14, 723–733. [Google Scholar] [CrossRef]
- Sanderson, E.; Richardson, T.G.; Morris, T.T.; Tilling, K.; Davey Smith, G. Estimation of causal effects of a time-varying exposure at multiple time points through multivariable mendelian randomization. PLoS Genet. 2022, 18, e1010290. [Google Scholar] [CrossRef]
- Shin, S.Y.; Fauman, E.B.; Petersen, A.K.; Krumsiek, J.; Santos, R.; Huang, J.; Arnold, M.; Erte, I.; Forgetta, V.; Yang, T.P.; et al. An atlas of genetic influences on human blood metabolites. Nat. Genet. 2014, 46, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.R.; Yarmolinsky, J.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Higgins, J.P.T.; Timpson, N.J.; Dimou, N.; et al. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. Jama 2021, 326, 1614–1621. [Google Scholar] [CrossRef] [PubMed]
- Kettunen, J.; Demirkan, A.; Wurtz, P.; Draisma, H.H.; Haller, T.; Rawal, R.; Vaarhorst, A.; Kangas, A.J.; Lyytikainen, L.P.; Pirinen, M.; et al. Genome-wide study for circulating metabolites identifies 62 loci and reveals novel systemic effects of LPA. Nat. Commun. 2016, 7, 11122. [Google Scholar] [CrossRef]
- Kurki, M.I.; Karjalainen, J.; Palta, P.; Sipilä, T.P.; Kristiansson, K.; Donner, K.M.; Reeve, M.P.; Laivuori, H.; Aavikko, M.; Kaunisto, M.A.; et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature 2023, 613, 508–518. [Google Scholar] [CrossRef]
- Kunkle, B.W.; Grenier-Boley, B.; Sims, R.; Bis, J.C.; Damotte, V.; Naj, A.C.; Boland, A.; Vronskaya, M.; van der Lee, S.J.; Amlie-Wolf, A.; et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Abeta, tau, immunity and lipid processing. Nat. Genet. 2019, 51, 414–430. [Google Scholar] [CrossRef]
- Burgess, S.; Davies, N.M.; Thompson, S.G. Bias due to participant overlap in two-sample Mendelian randomization. Genet. Epidemiol. 2016, 40, 597–608. [Google Scholar] [CrossRef]
- Sanna, S.; van Zuydam, N.R.; Mahajan, A.; Kurilshikov, A.; Vich Vila, A.; Vosa, U.; Mujagic, Z.; Masclee, A.A.M.; Jonkers, D.; Oosting, M.; et al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat. Genet. 2019, 51, 600–605. [Google Scholar] [CrossRef]
- Yang, J.; Yan, B.; Zhao, B.; Fan, Y.; He, X.; Yang, L.; Ma, Q.; Zheng, J.; Wang, W.; Bai, L.; et al. Assessing the Causal Effects of Human Serum Metabolites on 5 Major Psychiatric Disorders. Schizophr. Bull. 2020, 46, 804–813. [Google Scholar] [CrossRef]
- Hemani, G.; Tilling, K.; Davey Smith, G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. 2017, 13, e1007081. [Google Scholar]
- Pierce, B.L.; Ahsan, H.; Vanderweele, T.J. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int. J. Epidemiol. 2011, 40, 740–752. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G. Avoiding bias from weak instruments in Mendelian randomization studies. Int. J. Epidemiol. 2011, 40, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Li, X.; Wu, S.; Tian, Y.; Zhang, Y.; Wei, Z.; Jin, Z.; Li, X.; Chen, X.; Chen, W.X. Assessing the causal association between human blood metabolites and the risk of epilepsy. J. Transl. Med. 2022, 20, 437. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Huang, Y.; Hu, J.; Shao, Z. Mendelian randomization study of inflammatory bowel disease and bone mineral density. BMC Med. 2020, 18, 312. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Shen, X.; Pan, W. Constrained maximum likelihood-based Mendelian randomization robust to both correlated and uncorrelated pleiotropic effects. Am. J. Hum. Genet. 2021, 108, 1251–1269. [Google Scholar] [CrossRef]
- Yeung, C.H.C.; Au Yeung, S.L.; Kwok, M.K.; Zhao, J.V.; Schooling, C.M. The influence of growth and sex hormones on risk of alzheimer’s disease: A mendelian randomization study. Eur. J. Epidemiol. 2023, 38, 745–755. [Google Scholar] [CrossRef]
- Zuber, V.; Colijn, J.M.; Klaver, C.; Burgess, S. Selecting likely causal risk factors from high-throughput experiments using multivariable Mendelian randomization. Nat. Commun. 2020, 11, 29. [Google Scholar] [CrossRef]
- Levin, M.G.; Zuber, V.; Walker, V.M.; Klarin, D.; Lynch, J.; Malik, R.; Aday, A.W.; Bottolo, L.; Pradhan, A.D.; Dichgans, M.; et al. Prioritizing the Role of Major Lipoproteins and Subfractions as Risk Factors for Peripheral Artery Disease. Circulation 2021, 144, 353–364. [Google Scholar] [CrossRef]
- Hottman, D.A.; Chernick, D.; Cheng, S.; Wang, Z.; Li, L. HDL and cognition in neurodegenerative disorders. Neurobiol. Dis. 2014, 72 Pt A, 22–36. [Google Scholar] [CrossRef]
- Kjeldsen, E.W.; Thomassen, J.Q.; Juul Rasmussen, I.; Nordestgaard, B.G.; Tybjærg-Hansen, A.; Frikke-Schmidt, R. Plasma high-density lipoprotein cholesterol and risk of dementia: Observational and genetic studies. Cardiovasc. Res. 2022, 118, 1330–1343. [Google Scholar] [CrossRef]
- Sáiz-Vazquez, O.; Puente-Martínez, A.; Ubillos-Landa, S.; Pacheco-Bonrostro, J.; Santabárbara, J. Cholesterol and Alzheimer’s Disease Risk: A Meta-Meta-Analysis. Brain Sci. 2020, 10, 386. [Google Scholar] [CrossRef]
- Li, R.; Chen, J.X.; Lu, Q.; Geng, T.T.; Xia, P.F.; Wang, Y.; Chen, L.K.; Shan, Z.L.; Pan, A.; Liu, G. Associations of lipoprotein subclasses with risk of all-cause and cardiovascular disease mortality in individuals with type 2 diabetes: A prospective cohort study. Diabetes Obes. Metab. 2023, 25, 3259–3267. [Google Scholar] [CrossRef] [PubMed]
- Proitsi, P.; Lupton, M.K.; Velayudhan, L.; Newhouse, S.; Fogh, I.; Tsolaki, M.; Daniilidou, M.; Pritchard, M.; Kloszewska, I.; Soininen, H.; et al. Genetic predisposition to increased blood cholesterol and triglyceride lipid levels and risk of Alzheimer disease: A Mendelian randomization analysis. PLoS Med. 2014, 11, e1001713. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, S.D.; Mukherjee, S.; Sharp, S.J.; Proitsi, P.; Lotta, L.A.; Day, F.; Perry, J.R.; Boehme, K.L.; Walter, S.; Kauwe, J.S.; et al. Associations between Potentially Modifiable Risk Factors and Alzheimer Disease: A Mendelian Randomization Study. PLoS Med. 2015, 12, e1001841. [Google Scholar] [CrossRef] [PubMed]
- Fazio, S.; Pamir, N. HDL Particle Size and Functional Heterogeneity. Circ. Res. 2016, 119, 704–707. [Google Scholar] [CrossRef] [PubMed]
- Kontush, A.; Lhomme, M.; Chapman, M.J. Unraveling the complexities of the HDL lipidome. J. Lipid Res. 2013, 54, 2950–2963. [Google Scholar] [CrossRef]
- Turri, M.; Marchi, C.; Adorni, M.P.; Calabresi, L.; Zimetti, F. Emerging role of HDL in brain cholesterol metabolism and neurodegenerative disorders. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2022, 1867, 159123. [Google Scholar] [CrossRef]
- Moayyeri, A.; Cheung, C.L.; Tan, K.C.; Morris, J.A.; Cerani, A.; Mohney, R.P.; Richards, J.B.; Hammond, C.; Spector, T.D.; Menni, C. Metabolomic Pathways to Osteoporosis in Middle-Aged Women: A Genome-Metabolome-Wide Mendelian Randomization Study. J. Bone Min. Res. 2018, 33, 643–650. [Google Scholar] [CrossRef]
- Sun, L.; Guo, D.; Jia, Y.; Shi, M.; Yang, P.; Wang, Y.; Liu, F.; Chen, G.C.; Zhang, Y.; Zhu, Z. Association between Human Blood Metabolome and the Risk of Alzheimer’s Disease. Ann. Neurol. 2022, 92, 756–767. [Google Scholar] [CrossRef]
- Pérez-Jiménez, M.M.; Monje-Moreno, J.M.; Brokate-Llanos, A.M.; Venegas-Calerón, M.; Sánchez-García, A.; Sansigre, P.; Valladares, A.; Esteban-García, S.; Suárez-Pereira, I.; Vitorica, J.; et al. Steroid hormones sulfatase inactivation extends lifespan and ameliorates age-related diseases. Nat. Commun. 2021, 12, 49. [Google Scholar] [CrossRef]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol. 2019, 15, 346–366. [Google Scholar] [CrossRef]
- Haran, J.P.; Bhattarai, S.K.; Foley, S.E.; Dutta, P.; Ward, D.V.; Bucci, V.; McCormick, B.A. Alzheimer’s Disease Microbiome Is Associated with Dysregulation of the Anti-Inflammatory P-Glycoprotein Pathway. mBio 2019, 10, e00632-19. [Google Scholar] [CrossRef]
- Llorens, F.; Hermann, P.; Villar-Piqué, A.; Diaz-Lucena, D.; Nägga, K.; Hansson, O.; Santana, I.; Schmitz, M.; Schmidt, C.; Varges, D.; et al. Cerebrospinal fluid lipocalin 2 as a novel biomarker for the differential diagnosis of vascular dementia. Nat. Commun. 2020, 11, 619. [Google Scholar] [CrossRef]
- Horgusluoglu, E.; Neff, R.; Song, W.M.; Wang, M.; Wang, Q.; Arnold, M.; Krumsiek, J.; Galindo-Prieto, B.; Ming, C.; Nho, K.; et al. Integrative metabolomics-genomics approach reveals key metabolic pathways and regulators of Alzheimer’s disease. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2022, 18, 1260–1278. [Google Scholar] [CrossRef] [PubMed]
- Glenn, J.M.; Madero, E.N.; Bott, N.T. Dietary Protein and Amino Acid Intake: Links to the Maintenance of Cognitive Health. Nutrients 2019, 11, 1315. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.E.; Evans, A.M. Carnitine and acylcarnitines: Pharmacokinetic, pharmacological and clinical aspects. Clin. Pharmacokinet. 2012, 51, 553–572. [Google Scholar] [CrossRef] [PubMed]
- Nie, A.; Sun, B.; Fu, Z.; Yu, D. Roles of aminoacyl-tRNA synthetases in immune regulation and immune diseases. Cell Death Dis. 2019, 10, 901. [Google Scholar] [CrossRef]
- Polis, B.; Samson, A.O. Role of the metabolism of branched-chain amino acids in the development of Alzheimer’s disease and other metabolic disorders. Neural Regen. Res. 2020, 15, 1460–1470. [Google Scholar] [CrossRef]
- González-Domínguez, R.; Castellano-Escuder, P.; Lefèvre-Arbogast, S.; Low, D.Y.; Du Preez, A.; Ruigrok, S.R.; Lee, H.; Helmer, C.; Pallàs, M.; Urpi-Sarda, M.; et al. Apolipoprotein E and sex modulate fatty acid metabolism in a prospective observational study of cognitive decline. Alzheimers Res. Ther. 2022, 14, 1. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).