Nutritional Strategies for Chronic Craniofacial Pain and Temporomandibular Disorders: Current Clinical and Preclinical Insights
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Quality of Evidence Assessments
3. Results
3.1. Characteristics
3.1.1. Clinical Articles
3.1.2. Preclinical Articles
3.2. Risk of Bias Assessment
3.2.1. Clinical Articles
3.2.2. Preclinical Articles
3.3. Effect of Interventions—Outcomes
3.3.1. Clinical Articles
3.3.2. Preclinical Articles
4. Discussion
4.1. Characteristics of Dietary Habits and Nutritional Intakes in Painful TMDs
4.2. Nutraceutical, Nutrition, and Painful TMDs
4.2.1. Vitamins
- Vitamin D
- 2.
- Vitamin B complex and C
4.2.2. Minerals
- Magnesium (Mg)
- 2.
- Zinc (Zn)
- 3.
- Strontium
- 4.
- Sulfur
4.2.3. Polyunsaturated Fatty Acids
4.2.4. Polyphenols
4.2.5. Isoprenoids
4.2.6. Carotenoids
4.2.7. Lectin
4.2.8. Polysaccharide
4.2.9. Glucosamine
4.2.10. Palmitoylethanolamide
5. Limitations
6. Future Directions: An Alternative Diet—Rice-Fermented Food
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shrivastava, M.; Ye, L. Neuroimaging and Artificial Intelligence for Assessment of Chronic Painful Temporomandibular Disorders-a Comprehensive Review. Int. J. Oral. Sci. 2023, 15, 58. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, E.; Ohrbach, R.; Truelove, E.; Look, J.; Anderson, G.; Goulet, J.-P.; List, T.; Svensson, P.; Gonzalez, Y.; Lobbezoo, F.; et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J. Oral. Facial Pain. Headache 2014, 28, 6–27. [Google Scholar] [CrossRef] [PubMed]
- Minervini, G.; Franco, R.; Crimi, S.; Di Blasio, M.; D’Amico, C.; Ronsivalle, V.; Cervino, G.; Bianchi, A.; Cicciù, M. Pharmacological Therapy in the Management of Temporomandibular Disorders and Orofacial Pain: A Systematic Review and Meta-Analysis. BMC Oral. Health 2024, 24, 78. [Google Scholar] [CrossRef] [PubMed]
- Busse, J.W.; Casassus, R.; Carrasco-Labra, A.; Durham, J.; Mock, D.; Zakrzewska, J.M.; Palmer, C.; Samer, C.F.; Coen, M.; Guevremont, B.; et al. Management of Chronic Pain Associated with Temporomandibular Disorders: A Clinical Practice Guideline. BMJ 2023, 383, e076227. [Google Scholar] [CrossRef]
- Qaseem, A.; Wilt, T.J.; McLean, R.M.; Forciea, M.A.; Clinical Guidelines Committee of the American College of Physicians; Denberg, T.D.; Barry, M.J.; Boyd, C.; Chow, R.D.; Fitterman, N.; et al. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline from the American College of Physicians. Ann. Intern. Med. 2017, 166, 514–530. [Google Scholar] [CrossRef]
- Rodgers-Melnick, S.; Trager, R.; Love, T.; Dusek, J. Engagement in Integrative and Nonpharmacologic Pain Management Modalities Among Adults with Chronic Pain: Analysis of the 2019 National Health Interview Survey. J. Pain Res. 2024, 17, 253–264. [Google Scholar] [CrossRef]
- Duca, L.M.; Helmick, C.G.; Barbour, K.E.; Nahin, R.L.; Von Korff, M.; Murphy, L.B.; Theis, K.; Guglielmo, D.; Dahlhamer, J.; Porter, L.; et al. A Review of Potential National Chronic Pain Surveillance Systems in the United States. J. Pain 2022, 23, 1492–1509. [Google Scholar] [CrossRef]
- Umberger, W. Complementary and Integrative Approaches to Pain and Patient Preference. Pain Manag. Nurs. 2019, 20, 1–2. [Google Scholar] [CrossRef]
- Corp, N.; Jordan, J.L.; Croft, P.R. Justifications for Using Complementary and Alternative Medicine Reported by Persons with Musculoskeletal Conditions: A Narrative Literature Synthesis. PLoS ONE 2018, 13, e0200879. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Wan, L.; Jamison, R.N. Using Integrative Medicine in Pain Management: An Evaluation of Current Evidence. Anesth. Analg. 2017, 125, 2081–2093. [Google Scholar] [CrossRef]
- Clarke, T.C.; Nahin, R.L.; Barnes, P.M.; Stussman, B.J. Use of Complementary Health Approaches for Musculoskeletal Pain Disorders among Adults: United States, 2012. Natl. Health Stat. Rep. 2016, 98, 1–12. [Google Scholar]
- Agarwal, V. Patient Communication of Chronic Pain in the Complementary and Alternative Medicine Therapeutic Relationship. J. Patient Exp. 2020, 7, 238–244. [Google Scholar] [CrossRef]
- Key, M.N.; Szabo-Reed, A.N. Impact of Diet and Exercise Interventions on Cognition and Brain Health in Older Adults: A Narrative Review. Nutrients 2023, 15, 2495. [Google Scholar] [CrossRef] [PubMed]
- Taekman, J.M.; Bonakdar, R. Integrative Pain Management Must Include Diet Considerations. Anesth. Analg. 2018, 127, 305. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Zhang, L.; Sun, Y.; Yan, J.; Jiang, H. Causal Associations between Dietary Habits and Chronic Pain: A Two-Sample Mendelian Randomization Study. Nutrients 2023, 15, 3709. [Google Scholar] [CrossRef] [PubMed]
- Pasdar, Y.; Hamzeh, B.; Karimi, S.; Moradi, S.; Cheshmeh, S.; Shamsi, M.B.; Najafi, F. Major Dietary Patterns in Relation to Chronic Low Back Pain; a Cross-Sectional Study from RaNCD Cohort. Nutr. J. 2022, 21, 28. [Google Scholar] [CrossRef]
- Mosalmanzadeh, N.; Jandari, S.; Soleimani, D.; Shadmand Foumani Moghadam, M.R.; Khorramrouz, F.; Araste, A.; Molavi, S.F.; Fakhlaie, R.; Jokar, M.; Rezvani, R. Major Dietary Patterns and Food Groups in Relation to Rheumatoid Arthritis in Newly Diagnosed Patients. Food Sci. Nutr. 2020, 8, 6477–6486. [Google Scholar] [CrossRef]
- Philippou, E.; Nikiphorou, E. Are We Really What We Eat? Nutrition and Its Role in the Onset of Rheumatoid Arthritis. Autoimmun. Rev. 2018, 17, 1074–1077. [Google Scholar] [CrossRef]
- Brown, R.B. Non-Specific Low Back Pain, Dietary Salt Intake, and Posterior Lumbar Subcutaneous Edema. Int. J. Environ. Res. Public Health 2022, 19, 9158. [Google Scholar] [CrossRef]
- Takhrifa, N.; Taik, F.Z.; Berrichi, I.; Adnine, A.; Abourazzak, F.E. Diets and Joint Symptoms: A Survey of Moroccan Patients with Chronic Inflammatory Rheumatic Disease. Cureus 2024, 16, e53868. [Google Scholar] [CrossRef]
- Ruiz-Cabello, P.; Soriano-Maldonado, A.; Delgado-Fernandez, M.; Alvarez-Gallardo, I.C.; Segura-Jimenez, V.; Estevez-Lopez, F.; Camiletti-Moirón, D.; Aparicio, V.A. Association of Dietary Habits with Psychosocial Outcomes in Women with Fibromyalgia: The al-Ándalus Project. J. Acad. Nutr. Diet. 2017, 117, 422–432.e1. [Google Scholar] [CrossRef]
- Gerdle, B.; Dahlqvist Leinhard, O.; Lund, E.; Bengtsson, A.; Lundberg, P.; Ghafouri, B.; Forsgren, M.F. Fibromyalgia: Associations between Fat Infiltration, Physical Capacity, and Clinical Variables. J. Pain Res. 2022, 15, 2517–2535. [Google Scholar] [CrossRef]
- Min, Y.; Heo, Y.; Feng, F.; Kim, D.; Kim, M.; Yang, J.; Kim, H.J.; Jee, Y.; Ghosh, M.; Kang, I.; et al. High-Sucrose Diet Accelerates Arthritis Progression in a Collagen-Induced Rheumatoid Arthritis Model. Mol. Nutr. Food Res. 2023, 67, e2300244. [Google Scholar] [CrossRef] [PubMed]
- Gogga, P.; Mika, A.; Janczy, A.; Sztendel, A.; Sledzinski, T.; Małgorzewicz, S. Profiles of Serum Fatty Acids in Healthy Women on Different Types of Vegetarian Diets. Nutrients 2024, 16, 516. [Google Scholar] [CrossRef]
- Bassolino, L.; Petroni, K.; Polito, A.; Marinelli, A.; Azzini, E.; Ferrari, M.; Ficco, D.B.M.; Mazzucotelli, E.; Tondelli, A.; Fricano, A.; et al. Does Plant Breeding for Antioxidant-Rich Foods Have an Impact on Human Health? Antioxidants 2022, 11, 794. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.J.; Mangels, A.R.; Fresán, U.; Marsh, K.; Miles, F.L.; Saunders, A.V.; Haddad, E.H.; Heskey, C.E.; Johnston, P.; Larson-Meyer, E.; et al. The Safe and Effective Use of Plant-Based Diets with Guidelines for Health Professionals. Nutrients 2021, 13, 4144. [Google Scholar] [CrossRef]
- Casale, R.; Symeonidou, Z.; Ferfeli, S.; Micheli, F.; Scarsella, P.; Paladini, A. Food for Special Medical Purposes and Nutraceuticals for Pain: A Narrative Review. Pain Ther. 2021, 10, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Mesa, N.C.; Alves, I.A.; Vilela, F.M.P.; E Silva, D.M.; Forero, L.A.P.; Novoa, D.M.A.; de Carvalho da Costa, J. Fruits as Nutraceuticals: A Review of the Main Fruits Included in Nutraceutical Patents. Food Res. Int. 2023, 170, 113013. [Google Scholar] [CrossRef] [PubMed]
- Gianò, M.; Franco, C.; Castrezzati, S.; Rezzani, R. Involvement of Oxidative Stress and Nutrition in the Anatomy of Orofacial Pain. Int. J. Mol. Sci. 2023, 24, 13128. [Google Scholar] [CrossRef]
- Mesquita, M.L.M.; Magalhães, A.K.P.G.; Nascimento, M.V.; Pascoal, S.C.D.; Pontes, K.M.d.F.; Bonjardim, L.R.; Conti, P.C.R.; Pinto Fiamengui, L.M.S. Nutrition and Chronic Musculoskeletal Pain: A Narrative Review and Directions for Temporomandibular Disorder Research and Management. J. Oral. Rehabil. 2024, 51, 1925–1931. [Google Scholar] [CrossRef]
- Nasri-Heir, C.; Epstein, J.B.; Touger-Decker, R.; Benoliel, R. What Should We Tell Patients with Painful Temporomandibular Disorders about What to Eat? J. Am. Dent. Assoc. 2016, 147, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Durham, J.; Touger-Decker, R.; Nixdorf, D.R.; Rigassio-Radler, D.; Moynihan, P. Oro-Facial Pain and Nutrition: A Forgotten Relationship? J. Oral. Rehabil. 2015, 42, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Hasegawa, M.; Piriyaprasath, K.; Kakihara, Y.; Saeki, M.; Yamamura, K. Preclinical Models of Deep Craniofacial Nociception and Temporomandibular Disorder Pain. Jpn. Dent. Sci. Rev. 2021, 57, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Shimazu, Y. Modulatory Mechanism Underlying How Dietary Constituents Attenuate Orofacial Pain. J. Oral. Sci. 2020, 62, 140–143. [Google Scholar] [CrossRef]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA-a Scale for the Quality Assessment of Narrative Review Articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s Risk of Bias Tool for Animal Studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, R.; Gill, S. Effectiveness of Vitamin D along with Splint Therapy in the Vit D Deficient Patients with Temporomandibular Disorder-A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Indian Prosthodont. Soc. 2022, 22, 65–73. [Google Scholar] [CrossRef]
- Reis, L.N.C.; Falabella, M.E.V.; Carvalho, F.R.D.; Camargos, G.L. The Effectiveness of Laserpuncture Associated with Vitamin B12 in the Treatment of Temporomandibular Disorders and Orofacial Pain. Res. Soc. Dev. 2023, 12, e17812541593. [Google Scholar] [CrossRef]
- Cömert Kılıç, S. Does Glucosamine, Chondroitin Sulfate, and Methylsulfonylmethane Supplementation Improve the Outcome of Temporomandibular Joint Osteoarthritis Management with Arthrocentesis plus Intraarticular Hyaluronic Acid Injection. A Randomized Clinical Trial. J. Cranio-Maxillofac. Surg. 2021, 49, 711–718. [Google Scholar] [CrossRef]
- Damlar, I.; Esen, E.; Tatli, U. Effects of Glucosamine-Chondroitin Combination on Synovial Fluid IL-1β, IL-6, TNF-α and PGE2 Levels in Internal Derangements of Temporomandibular Joint. Med. Oral. Patol. Oral. Cir. Bucal 2015, 20, e278–e283. [Google Scholar] [CrossRef]
- Nguyen, P.; Mohamed, S.E.; Gardiner, D.; Salinas, T. A Randomized Double-Blind Clinical Trial of the Effect of Chondroitin Sulfate and Glucosamine Hydrochloride on Temporomandibular Joint Disorders: A Pilot Study. Cranio 2001, 19, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Cen, X.; Liu, Y.; Wang, S.; Yang, X.; Shi, Z.; Liang, X. Glucosamine Oral Administration as an Adjunct to Hyaluronic Acid Injection in Treating Temporomandibular Joint Osteoarthritis. Oral. Dis. 2018, 24, 404–411. [Google Scholar] [CrossRef]
- Yang, W.; Liu, W.; Miao, C.; Sun, H.; Li, L.; Li, C. Oral Glucosamine Hydrochloride Combined with Hyaluronate Sodium Intra-Articular Injection for Temporomandibular Joint Osteoarthritis: A Double-Blind Randomized Controlled Trial. J. Oral. Maxillofac. Surg. 2018, 76, 2066–2073. [Google Scholar] [CrossRef] [PubMed]
- Refahee, S.M.; Mahrous, A.I.; Shabaan, A.A. Clinical Efficacy of Magnesium Sulfate Injection in the Treatment of Masseter Muscle Trigger Points: A Randomized Clinical Study. BMC Oral. Health 2022, 22, 408. [Google Scholar] [CrossRef] [PubMed]
- Thie, N.M.; Prasad, N.G.; Major, P.W. Evaluation of Glucosamine Sulfate Compared to Ibuprofen for the Treatment of Temporomandibular Joint Osteoarthritis: A Randomized Double Blind Controlled 3 Month Clinical Trial. J. Rheumatol. 2001, 28, 1347–1355. [Google Scholar] [PubMed]
- Haghighat, A.; Behnia, A.; Kaviani, N.; Khorami, B. Evaluation of Glucosamine Sulfate and Ibuprofen Effects in Patients with Temporomandibular Joint Osteoarthritis Symptom. J. Res. Pharm. Pract. 2013, 2, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Cahlin, B.J.; Dahlström, L. No Effect of Glucosamine Sulfate on Osteoarthritis in the Temporomandibular Joints—A Randomized, Controlled, Short-Term Study. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2011, 112, 760–766. [Google Scholar] [CrossRef]
- Marini, I.; Bartolucci, M.L.; Bortolotti, F.; Gatto, M.R.; Bonetti, G.A. Palmitoylethanolamide versus a Nonsteroidal Anti-Inflammatory Drug in the Treatment of Temporomandibular Joint Inflammatory Pain. J. Orofac. Pain. 2012, 26, 99–104. [Google Scholar]
- Marana, R.R.; Benedicto Dos Santos, V.A.; Groppo, F.C.; Ferreira, L.E.N.; Sánchez, J.B.; Barbin, T.; Figueroba, S.R. Omega 3 Polyunsaturated Fatty Acids: Potential Anti-Inflammatory Effect in a Model of Ovariectomy and Temporomandibular Joint Arthritis Induction in Rats. Arch. Oral. Biol. 2022, 134, 105340. [Google Scholar] [CrossRef]
- Mittal, N.; Joshi, R.; Hota, D.; Chakrabarti, A. Evaluation of Antihyperalgesic Effect of Curcumin on Formalin-Induced Orofacial Pain in Rat. Phytother. Res. 2009, 23, 507–512. [Google Scholar] [CrossRef]
- Sashide, Y.; Toyota, R.; Takeda, M. Local Administration of the Phytochemical, Quercetin, Attenuates the Hyperexcitability of Rat Nociceptive Primary Sensory Neurons Following Inflammation Comparable to Lidocaine. J. Pain 2024, 25, 755–765. [Google Scholar] [CrossRef]
- Toyota, R.; Ito, H.; Sashide, Y.; Takeda, M. Suppression of the Excitability of Rat Nociceptive Primary Sensory Neurons Following Local Administration of the Phytochemical, Quercetin. J. Pain 2023, 24, 540–549. [Google Scholar] [CrossRef]
- Liu, Z.; Shan, Z.; Yang, H.; Xing, Y.; Guo, W.; Cheng, J.; Jiang, Y.; Cai, S.; Wu, C.; Liu, J.A.; et al. Quercetin, Main Active Ingredient of Moutan Cortex, Alleviates Chronic Orofacial Pain via Block of Voltage-Gated Sodium Channel. Anesth. Analg. 2024, 138, 1324–1336. [Google Scholar] [CrossRef] [PubMed]
- Itou, H.; Toyota, R.; Takeda, M. Phytochemical Quercetin Alleviates Hyperexcitability of Trigeminal Nociceptive Neurons Associated with Inflammatory Hyperalgesia Comparable to NSAIDs. Mol. Pain 2022, 18, 17448069221108971. [Google Scholar] [CrossRef] [PubMed]
- Uchino, M.; Sashide, Y.; Takeda, M. Suppression of the Excitability of Rat Nociceptive Secondary Sensory Neurons Following Local Administration of the Phytochemical, (-)-Epigallocatechin-3-Gallate. Brain Res. 2023, 1813, 148426. [Google Scholar] [CrossRef] [PubMed]
- Shimazu, Y.; Shibuya, E.; Takehana, S.; Sekiguchi, K.; Oshima, K.; Kamata, H.; Karibe, H.; Takeda, M. Local Administration of Resveratrol Inhibits Excitability of Nociceptive Wide-Dynamic Range Neurons in Rat Trigeminal Spinal Nucleus Caudalis. Brain Res. Bull. 2016, 124, 262–268. [Google Scholar] [CrossRef]
- Takehana, S.; Kubota, Y.; Uotsu, N.; Yui, K.; Iwata, K.; Shimazu, Y.; Takeda, M. The Dietary Constituent Resveratrol Suppresses Nociceptive Neurotransmission via the NMDA Receptor. Mol. Pain 2017, 13, 1–9. [Google Scholar] [CrossRef]
- Erfanparast, A.; Escort, M.; Tamaddonfard, E.; Maroufi, S.; Kazemi-Shojaei, S.; Dabbaghi, M.; Taati, M. Systemic and Local Peripheral Injections of Vitamin B12 Suppressed Orofacial Nociception Induced by Formalin in Rats. Drug Res. 2014, 64, 85–90. [Google Scholar] [CrossRef]
- Erfanparast, A.; Tamaddonfard, E.; Nemati, S. Effects of Intra-Hippocampal Microinjection of Vitamin B12 on the Orofacial Pain and Memory Impairments Induced by Scopolamine and Orofacial Pain in Rats. Physiol. Behav. 2017, 170, 68–77. [Google Scholar] [CrossRef]
- Cavalcante, A.L.C.; Siqueira, R.M.P.; Araujo, J.C.B.; Gondim, D.V.; Ribeiro, R.A.; Quetz, J.S.; Havt, A.; Lima, A.A.M.; Vale, M.L. Role of NMDA Receptors in the Trigeminal Pathway, and the Modulatory Effect of Magnesium in a Model of Rat Temporomandibular Joint Arthritis. Eur. J. Oral. Sci. 2013, 121, 573–583. [Google Scholar] [CrossRef]
- Srebro, D.P.; Vučković, S.M.; Dožić, I.S.; Dožić, B.S.; Savić Vujović, K.R.; Milovanović, A.P.; Karadžić, B.V.; Prostran, M.Š. Magnesium Sulfate Reduces Formalin-Induced Orofacial Pain in Rats with Normal Magnesium Serum Levels. Pharmacol. Rep. 2018, 70, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Srebro, D.; Dožić, B.; Vučković, S.; Savić Vujović, K.; Medić Brkić, B.; Dožić, I.; Srebro, M. The Interactions of Magnesium Sulfate and Cromoglycate in a Rat Model of Orofacial Pain; The Role of Magnesium on Mast Cell Degranulation in Neuroinflammation. Int. J. Mol. Sci. 2023, 24, 6241. [Google Scholar] [CrossRef] [PubMed]
- Alves, S.M.; Abreu, S.C.; Lemos, J.C.; Gomes, F.I.F.; Alves, S.M.; do Val, D.R.; Freitas, R.S.; Pereira, K.M.A.; de Paulo Teixeira Pinto, V.; de Castro Brito, G.A.; et al. Anti-Inflammatory and Anti-Nociceptive Effects of Strontium Ranelate on the Zymosan-Induced Temporomandibular Joint Inflammatory Hypernociception in Rats Depend on TNF-α Inhibition. Pharmacol. Rep. 2017, 69, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Ceotto, B.H.; Figueroba, S.R.; Ferreira, L.E.N.; Amorim, K.S.; Sánchez, J.B.; Gercina, A.C.; Dos Santos, V.A.B.; Groppo, F.C. The Effect of Association of Aspirin and Omega 3 in Rat Temporomandibular Joint with Induced Arthritis. Ann. Anat. 2022, 239, 151812. [Google Scholar] [CrossRef] [PubMed]
- Barbin, T.; Groppo, F.C.; Toledo, F.C.; Costa, Y.M.; Clemente-Napimoga, J.T.; Figueroba, S.R. The Effect of Omega-3 in Temporomandibular Joint Synovial Tissues of Rats with Induced Arthritis: Pilot Study. Int. J. Oral. Maxillofac. Surg. 2020, 49, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Nakazaki, S.; Tadokoro, K.; Takehana, S.; Syoji, Y.; Shimazu, Y.; Takeda, M. Docosahexaenoic Acid Attenuates Inflammation-Induced Hyperexcitability of Trigeminal Spinal Nucleus Caudalis Neurons Associated with Hyperalgesia in Rats. Eur. J. Oral. Sci. 2018, 126, 458–465. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, S.; Shu, H.; Crawford, J.; Xing, Y.; Tao, F. Resveratrol Alleviates Temporomandibular Joint Inflammatory Pain by Recovering Disturbed Gut Microbiota. Brain Behav. Immun. 2020, 87, 455–464. [Google Scholar] [CrossRef]
- Luca, A.; Alexa, T.; Dondaş, A.; Andron, G.; Bădescu, M.; Alexa, I.D.; Bohotin, C. Pain Modulation by Curcumin and Ascorbic Acid in Mice. Med.-Surg. J. 2014, 118, 346–351. [Google Scholar]
- Wu, Y.; Qin, D.; Yang, H.; Fu, H. Evidence for the Participation of Acid-Sensing Ion Channels (ASICs) in the Antinociceptive Effect of Curcumin in a Formalin-Induced Orofacial Inflammatory Model. Cell. Mol. Neurobiol. 2017, 37, 635–642. [Google Scholar] [CrossRef]
- Yeon, K.Y.; Kim, S.A.; Kim, Y.H.; Lee, M.K.; Ahn, D.K.; Kim, H.J.; Kim, J.S.; Jung, S.J.; Oh, S.B. Curcumin Produces an Antihyperalgesic Effect via Antagonism of TRPV1. J. Dent. Res. 2010, 89, 170–174. [Google Scholar] [CrossRef]
- Pereira, E.W.M.; Heimfarth, L.; Santos, T.K.; Passos, F.R.S.; Siqueira-Lima, P.; Scotti, L.; Scotti, M.T.; Almeida, J.R.G.d.S.; Campos, A.R.; Coutinho, H.D.M.; et al. Limonene, a Citrus Monoterpene, Non-Complexed and Complexed with Hydroxypropyl-β-Cyclodextrin Attenuates Acute and Chronic Orofacial Nociception in Rodents: Evidence for Involvement of the PKA and PKC Pathway. Phytomedicine 2022, 96, 153893. [Google Scholar] [CrossRef] [PubMed]
- Santos, B.M.; Santos, W.S.; Solon, I.G.; Garcia, F.S.; Emilio-Silva, M.T.; Jesus, A.A.; Hiruma-Lima, C.A.; Nascimento, G.C.; Cárnio, E.C.; Branco, L.G.S. Orofacial Anti-Hypernociceptive Effect of Citral in Acute and Persistent Inflammatory Models in Rats. Arch. Oral. Biol. 2023, 152, 105734. [Google Scholar] [CrossRef] [PubMed]
- Alves Rodrigues Santos, S.A.; de Barros Mamede Vidal Damasceno, M.; Alves Magalhães, F.E.; Sessle, B.J.; Amaro de Oliveira, B.; Alves Batista, F.L.; Vieira-Neto, A.E.; Rolim Campos, A. Transient Receptor Potential Channel Involvement in Antinociceptive Effect of Citral in Orofacial Acute and Chronic Pain Models. EXCLI J. 2022, 21, 869–887. [Google Scholar] [CrossRef] [PubMed]
- Tamaddonfard, E.; Tamaddonfard, S.; Pourbaba, S. Effects of Intra-Fourth Ventricle Injection of Crocin on Capsaicin-Induced Orofacial Pain in Rats. Avicenna J. Phytomedicine 2015, 5, 450–457. [Google Scholar]
- Shimazu, Y.; Kobayashi, A.; Endo, S.; Takemura, J.; Takeda, M. Effect of Lutein on the Acute Inflammation-Induced c-Fos Expression of Rat Trigeminal Spinal Nucleus Caudalis and C1 Dorsal Horn Neurons. Eur. J. Oral. Sci. 2019, 127, 379–385. [Google Scholar] [CrossRef]
- Syoji, Y.; Kobayashi, R.; Miyamura, N.; Hirohara, T.; Kubota, Y.; Uotsu, N.; Yui, K.; Shimazu, Y.; Takeda, M. Suppression of Hyperexcitability of Trigeminal Nociceptive Neurons Associated with Inflammatory Hyperalgesia Following Systemic Administration of Lutein via Inhibition of Cyclooxygenase-2 Cascade Signaling. J Inflamm 2018, 15, 24. [Google Scholar] [CrossRef]
- Rivanor, R.L.d.C.; Do Val, D.R.; Ribeiro, N.A.; Silveira, F.D.; de Assis, E.L.; Franco, Á.X.; Vieira, L.V.; de Queiroz, I.N.L.; Chaves, H.V.; Bezerra, M.M.; et al. A Lectin Fraction from Green Seaweed Caulerpa Cupressoides Inhibits Inflammatory Nociception in the Temporomandibular Joint of Rats Dependent from Peripheral Mechanisms. Int. J. Biol. Macromol. 2018, 115, 331–340. [Google Scholar] [CrossRef]
- da Conceição Rivanor, R.L.; Chaves, H.V.; do Val, D.R.; de Freitas, A.R.; Lemos, J.C.; Rodrigues, J.A.G.; Pereira, K.M.A.; de Araújo, I.W.F.; Bezerra, M.M.; Benevides, N.M.B. A Lectin from the Green Seaweed Caulerpa Cupressoides Reduces Mechanical Hyper-Nociception and Inflammation in the Rat Temporomandibular Joint during Zymosan-Induced Arthritis. Int. Immunopharmacol. 2014, 21, 34–43. [Google Scholar] [CrossRef]
- de Oliveira Leite, G.; Santos, S.A.A.R.; Dos Santos Silva, R.R.; Teixeira, C.S.; Campos, A.R. Parkia Platycephala Lectin (PPL) Inhibits Orofacial Nociception Responses via TRPV1 Modulation. Molecules 2022, 27, 7506. [Google Scholar] [CrossRef]
- Alves, S.M.; Freitas, R.S.; do Val, D.R.; Vieira, L.V.; de Assis, E.L.; Gomes, F.I.F.; Gadelha, C.A.d.A.; Gadelha, T.S.; de Lacerda, J.T.J.G.; Clemente-Napimoga, J.T.; et al. The Efficacy of a Lectin from Abelmoschus Esculentus Depends on Central Opioid Receptor Activation to Reduce Temporomandibular Joint Hypernociception in Rats. Biomed. Pharmacother. 2018, 101, 478–484. [Google Scholar] [CrossRef]
- Freitas, R.S.; do Val, D.R.; Fernandes, M.E.F.; Gomes, F.I.F.; de Lacerda, J.T.J.G.; SantiGadelha, T.; de Almeida Gadelha, C.A.; de Paulo Teixeira Pinto, V.; Cristino-Filho, G.; Pereira, K.M.A.; et al. Lectin from Abelmoschus Esculentus Reduces Zymosan-Induced Temporomandibular Joint Inflammatory Hypernociception in Rats via Heme Oxygenase-1 Pathway Integrity and Tnf-α and Il-1β Suppression. Int. Immunopharmacol. 2016, 38, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Damasceno, M.B.M.V.; de Melo Júnior, J.d.M.A.; Santos, S.A.A.R.; Melo, L.T.M.; Leite, L.H.I.; Vieira-Neto, A.E.; Moreira, R.d.A.; Monteiro-Moreira, A.C.d.O.; Campos, A.R. Frutalin Reduces Acute and Neuropathic Nociceptive Behaviours in Rodent Models of Orofacial Pain. Chem. Biol. Interact. 2016, 256, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Araújo, I.W.F.; Chaves, H.V.; Pachêco, J.M.; Val, D.R.; Vieira, L.V.; Santos, R.; Freitas, R.S.; Rivanor, R.L.; Monteiro, V.S.; Clemente-Napimoga, J.T.; et al. Role of Central Opioid on the Antinociceptive Effect of Sulfated Polysaccharide from the Red Seaweed Solieria Filiformis in Induced Temporomandibular Joint Pain. Int. Immunopharmacol. 2017, 44, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.Á.P.B.; de Oliveira, B.A.; Santos, S.A.A.R.; Batista, F.L.A.; Andrade, F.R.N.; Neto, E.J.R.; de Melo Júnior, J.d.M.A.; Silva Mendes, F.R.d.; Barroso, L.K.V.; Canuto, K.M.; et al. Orofacial Antinociceptive Effect of Sulphated Polysaccharide from the Marine Algae Hypnea Pseudomusciformis in Rodents. Inflammopharmacology 2019, 27, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.A.G.; Chaves, H.V.; Alves, K.D.S.; Filgueira, A.A.; Bezerra, M.M.; Benevides, N.M.B. Structural Features and Assessment of Zymosan-Induced Arthritis in Rat Temporomandibular Joint Model Using Sulfated Polysaccharide. Acta Sci. Biol. Sci. 2014, 36, 127. [Google Scholar] [CrossRef]
- Bartolucci, M.L.; Marini, I.; Bortolotti, F.; Impellizzeri, D.; Di Paola, R.; Bruschetta, G.; Crupi, R.; Portelli, M.; Militi, A.; Oteri, G.; et al. Micronized Palmitoylethanolamide Reduces Joint Pain and Glial Cell Activation. Inflamm. Res. 2018, 67, 891–901. [Google Scholar] [CrossRef]
- Licina, E.; Radojicic, A.; Jeremic, M.; Tomic, A.; Mijajlovic, M. Non-Pharmacological Treatment of Primary Headaches-A Focused Review. Brain Sci. 2023, 13, 1432. [Google Scholar] [CrossRef]
- Maddox, E.K.; Massoni, S.C.; Hoffart, C.M.; Takata, Y. Dietary Effects on Pain Symptoms in Patients with Fibromyalgia Syndrome: Systematic Review and Future Directions. Nutrients 2023, 15, 716. [Google Scholar] [CrossRef]
- Chiarioni, G.; Popa, S.L.; Ismaiel, A.; Pop, C.; Dumitrascu, D.I.; Brata, V.D.; Duse, T.A.; Incze, V.; Surdea-Blaga, T. The Effect of Polyphenols, Minerals, Fibers, and Fruits on Irritable Bowel Syndrome: A Systematic Review. Nutrients 2023, 15, 4070. [Google Scholar] [CrossRef]
- Gunes-Bayir, A.; Mendes, B.; Dadak, A. The Integral Role of Diets Including Natural Products to Manage Rheumatoid Arthritis: A Narrative Review. Curr. Issues Mol. Biol. 2023, 45, 5373–5388. [Google Scholar] [CrossRef]
- Elma, Ö.; Yilmaz, S.T.; Deliens, T.; Clarys, P.; Nijs, J.; Coppieters, I.; Polli, A.; Malfliet, A. Chronic Musculoskeletal Pain and Nutrition: Where Are We and Where Are We Heading? PM&R 2020, 12, 1268–1278. [Google Scholar] [CrossRef]
- Hansen, K.A.; McKernan, L.C.; Carter, S.D.; Allen, C.; Wolever, R.Q. A Replicable and Sustainable Whole Person Care Model for Chronic Pain. J. Altern. Complement. Med. 2019, 25, S86–S94. [Google Scholar] [CrossRef] [PubMed]
- Olfert, M.D.; Wattick, R.A. Vegetarian Diets and the Risk of Diabetes. Curr. Diabetes Rep. 2018, 18, 101. [Google Scholar] [CrossRef]
- Dybvik, J.S.; Svendsen, M.; Aune, D. Vegetarian and Vegan Diets and the Risk of Cardiovascular Disease, Ischemic Heart Disease and Stroke: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Eur. J. Nutr. 2023, 62, 51–69. [Google Scholar] [CrossRef]
- Shen, Y.-C.; Chang, C.-E.; Lin, M.-N.; Lin, C.-L. Vegetarian Diet Is Associated with Lower Risk of Depression in Taiwan. Nutrients 2021, 13, 1059. [Google Scholar] [CrossRef]
- Nadal-Nicolás, Y.; Miralles-Amorós, L.; Martínez-Olcina, M.; Sánchez-Ortega, M.; Mora, J.; Martínez-Rodríguez, A. Vegetarian and Vegan Diet in Fibromyalgia: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 4955. [Google Scholar] [CrossRef]
- Forsyth, C.; Kouvari, M.; D’Cunha, N.M.; Georgousopoulou, E.N.; Panagiotakos, D.B.; Mellor, D.D.; Kellett, J.; Naumovski, N. The Effects of the Mediterranean Diet on Rheumatoid Arthritis Prevention and Treatment: A Systematic Review of Human Prospective Studies. Rheumatol. Int. 2018, 38, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Kurapatti, M.; Carreira, D. Diet Composition’s Effect on Chronic Musculoskeletal Pain: A Narrative Review. Pain Physician 2023, 26, 527–534. [Google Scholar] [PubMed]
- Mendonça, C.R.; Noll, M.; Castro, M.C.R.; Silveira, E.A. Effects of Nutritional Interventions in the Control of Musculoskeletal Pain: An Integrative Review. Nutrients 2020, 12, 3075. [Google Scholar] [CrossRef]
- Karimi, E.; Tirani, S.A.; Azimi, E.S.; Askari, G.; As’habi, A.; Arab, A. Is There an Association between a Plant-Based Eating Pattern and Clinical Findings of a Migraine Headache? Front. Nutr. 2023, 10, 1117740. [Google Scholar] [CrossRef]
- Arab, A.; Khorvash, F.; Karimi, E.; Hadi, A.; Askari, G. Associations between Adherence to Mediterranean Dietary Pattern and Frequency, Duration, and Severity of Migraine Headache: A Cross-Sectional Study. Nutr. Neurosci. 2023, 26, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sessle, B.J. Fifty Years of Development of Neuroscientific Insights into Oro-Facial Pain and Its Control. J. Oral. Rehabil. 2023, 50, 860–876. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.C.; Bowes, C.C.; Penlington, C.; Durham, J. Temporomandibular Disorders and Dietary Changes: A Cross-Sectional Survey. J. Oral. Rehabil. 2021, 48, 873–879. [Google Scholar] [CrossRef]
- Órla, G.; Béchet, S.; Walshe, M. Modified Diet Use in Adults with Temporomandibular Disorders Related to Rheumatoid Arthritis: A Systematic Review. Mediterr. J. Rheumatol. 2020, 31, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, A.; Morimoto, T.; Hu, J.W.; Tsuboi, Y.; Tashiro, A.; Noguchi, K.; Nakagawa, H.; Iwata, K. Hard-Food Mastication Suppresses Complete Freund’s Adjuvant-Induced Nociception. Neuroscience 2003, 120, 1081–1092. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.J.; Stephan, M.; Kielstein, H.; Rahne, H.; Nugraha, B.; Gutenbrunner, C.; Ro, J.Y.; Svensson, P. Functions of the Temporomandibular System in Extracranial Chronic Pain Conditions: Modulatory Effects on Nocifensive Behavior in an Animal Model. J. Manip. Physiol. Ther. 2014, 37, 485–493. [Google Scholar] [CrossRef]
- Sigurðsson, K.; Andersen, B.V.; Bendixen, K.H.; Baad-Hansen, L. Are Orofacial Pain and Xerostomia Associated with Differences in Diet, Sensory Perception, Appetite and Enjoyment of Eating?—An Explorative Study. J. Oral. Rehabil. 2024, 51, 703–711. [Google Scholar] [CrossRef]
- Aktaş, A.; Ilgaz, F.; Serel Arslan, S. Dietary Intakes of Individuals with Temporomandibular Disorders: A Comparative Study. J. Oral. Rehabil. 2023, 50, 655–663. [Google Scholar] [CrossRef]
- Greene, C.S.; Lerman, M.D.; Sutcher, H.D.; Laskin, D.M. The TMJ Pain-Dysfunction Syndrome: Heterogeneity of the Patient Population. J. Am. Dent. Assoc. 1969, 79, 1168–1172. [Google Scholar] [CrossRef]
- Irving, J.; Wood, G.D.; Hackett, A.F. Does Temporomandibular Disorder Pain Dysfunction Syndrome Affect Dietary Intake? Dent. Update 1999, 26, 405–407. [Google Scholar] [CrossRef]
- Raphael, K.G.; Marbach, J.J.; Touger-Decker, R. Dietary Fiber Intake in Patients with Myofascial Face Pain. J. Orofac. Pain 2002, 16, 39–47. [Google Scholar]
- Kalra, E.K. Nutraceutical—Definition and Introduction. AAPS Pharm. Sci. 2003, 5, E25. [Google Scholar] [CrossRef]
- Colletti, A.; Cicero, A.F.G. Nutraceutical Approach to Chronic Osteoarthritis: From Molecular Research to Clinical Evidence. Int. J. Mol. Sci. 2021, 22, 12920. [Google Scholar] [CrossRef]
- Abdelrahman, K.M.; Hackshaw, K.V. Nutritional Supplements for the Treatment of Neuropathic Pain. Biomedicines 2021, 9, 674. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Bauer, B.A.; Wu, Q.; Xiong, D.; Wahner-Roedler, D.L.; Chon, T.Y.; Ganesh, R. Impact of Herbs and Dietary Supplements in Patients with Fibromyalgia: A Protocol for a Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicine 2020, 99, e20257. [Google Scholar] [CrossRef] [PubMed]
- Radziszewska, M.; Smarkusz-Zarzecka, J.; Ostrowska, L. Nutrition, Physical Activity and Supplementation in Irritable Bowel Syndrome. Nutrients 2023, 15, 3662. [Google Scholar] [CrossRef]
- Durham, P.L.; Antonopoulos, S.R. Benefit of Dietary Supplementation of Nutraceuticals as an Integrative Approach for Management of Migraine: Evidence From Preclinical and Clinical Studies. Curr. Pain. Headache Rep. 2024, 28, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Im, Y.-G.; Han, M.-Y.; Baek, H.-S. Association of Serum Vitamin D Level with Temporomandibular Disorder Incidence: A Retrospective, Multi-Center Cohort Study Using Six Hospital Databases. Nutrients 2023, 15, 2860. [Google Scholar] [CrossRef]
- Ekici, Ö.; Çelik, S. The Relationship of Biochemical Factors Related to Calcium Metabolism with Temporomandibular Disorders. J. Stomatol. Oral. Maxillofac. Surg. 2023, 124, 101315. [Google Scholar] [CrossRef]
- Nemati, M.; Tabrizi, R.; Rasooli, F.; Ghafari, S. Is the Prevalence of Vitamin D Deficiency in Patients with Temporomandibular Disorder Higher than Healthy Control Group? J. Maxillofac. Oral. Surg. 2022, 21, 1205–1208. [Google Scholar] [CrossRef]
- Yildiz, S.; Tumer, M.K.; Yigit, S.; Nursal, A.F.; Rustemoglu, A.; Balel, Y. Relation of Vitamin D and BsmI Variant with Temporomandibular Diseases in the Turkish Population. Br. J. Oral. Maxillofac. Surg. 2021, 59, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Kui, A.; Buduru, S.; Labunet, A.; Balhuc, S.; Negucioiu, M. Vitamin D and Temporomandibular Disorders: What Do We Know So Far? Nutrients 2021, 13, 1286. [Google Scholar] [CrossRef]
- Staniszewski, K.; Lygre, H.; Berge, T.; Rosén, A. Serum Analysis in Patients with Temporomandibular Disorders: A Controlled Cross-Sectional Study in Norway. Pain Res. Manag. 2019, 2019, 1360725. [Google Scholar] [CrossRef] [PubMed]
- Mehra, P.; Wolford, L.M. Serum Nutrient Deficiencies in the Patient with Complex Temporomandibular Joint Problems. Bayl. Univ. Med. Cent. Proc. 2008, 21, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Kucuk, A.; Baykara, R.A.; Tuzcu, A.; Omma, A.; Cure, M.C.; Cure, E.; Acet, G.K.; Dogan, E. Could Ferritin, Vitamin B12, and Vitamin D Play a Role in the Etiopathogenesis of Fibromyalgia Syndrome? Rom. J. Intern. Med. 2021, 59, 384–393. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Madani, A.; Shamsian, S.A.; Layegh, P.; Abrisham, S.M.; Ravaghi, A.; Tayarani Najjaran, N. Are Certain Factors Involved in Calcium Metabolism Associated with Temporomandibular Disorders? Cranio 2021, 39, 202–208. [Google Scholar] [CrossRef]
- Demir, C.Y.; Ersoz, M.E. Biochemical Changes Associated with Temporomandibular Disorders. J. Int. Med. Res. 2019, 47, 765–771. [Google Scholar] [CrossRef]
- Tekeli, S.Ö.; Köse, Ö.; Yapar, D.; Tekeli, F.Y.; Asoğlu, M.M.; Kartal, E.M. Relationship between Serum Vitamin D Levels and the Prevalence of Knee Osteoarthritis: A Retrospective Study on 3424 Subjects. Technol. Health Care 2024, 1–10. [Google Scholar] [CrossRef]
- Li, H.-M.; Liu, Y.; Zhang, R.-J.; Ding, J.-Y.; Shen, C.-L. Vitamin D Receptor Gene Polymorphisms and Osteoarthritis: A Meta-Analysis. Rheumatology 2021, 60, 538–548. [Google Scholar] [CrossRef]
- Yilmaz, A.D.; Yazicioglu, D.; Tüzüner Öncül, A.M.; Yilmaz, E.; Ereş, G. Vitamin D Receptor Gene Polymorphisms (Apa1 and Taq1) in Temporomandibular Joint Internal Derangement/Osteoarthritis in a Group of Turkish Patients. Mol. Biol. Rep. 2018, 45, 1839–1848. [Google Scholar] [CrossRef] [PubMed]
- Al-Saoodi, H.; Kolahdooz, F.; Andersen, J.R.; Jalili, M. Effect of Vitamin D on Inflammatory and Clinical Outcomes in Patients with Rheumatoid Arthritis: A Systematic Review and Dose-Response Meta-Analysis of Randomized Controlled Trials. Nutr. Rev. 2024, 82, 600–611. [Google Scholar] [CrossRef]
- Sanghi, D.; Mishra, A.; Sharma, A.C.; Singh, A.; Natu, S.M.; Agarwal, S.; Srivastava, R.N. Does Vitamin D Improve Osteoarthritis of the Knee: A Randomized Controlled Pilot Trial. Clin. Orthop. Relat. Res. 2013, 471, 3556–3562. [Google Scholar] [CrossRef]
- Lombardo, M.; Feraco, A.; Ottaviani, M.; Rizzo, G.; Camajani, E.; Caprio, M.; Armani, A. The Efficacy of Vitamin D Supplementation in the Treatment of Fibromyalgia Syndrome and Chronic Musculoskeletal Pain. Nutrients 2022, 14, 3010. [Google Scholar] [CrossRef]
- Rahman, A.; Waterhouse, M.; Baxter, C.; Romero, B.D.; McLeod, D.S.A.; Armstrong, B.K.; Ebeling, P.R.; English, D.R.; Hartel, G.; Kimlin, M.G.; et al. The Effect of Vitamin D Supplementation on Pain: An Analysis of Data from the D-Health Randomised Controlled Trial. Br. J. Nutr. 2023, 130, 633–640. [Google Scholar] [CrossRef]
- Okumus, M.; Ceceli, E.; Tuncay, F.; Kocaoglu, S.; Palulu, N.; Yorgancioglu, Z.R. The Relationship between Serum Trace Elements, Vitamin B12, Folic Acid and Clinical Parameters in Patients with Myofascial Pain Syndrome. J. Back. Musculoskelet. Rehabil. 2010, 23, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Yu, X.; Wu, L.; Zheng, H.; Zhong, X.; Xie, Y.; Wu, W. Vitamin B6 and Folate Intake Are Associated with Lower Risk of Severe Headache or Migraine in Adults: An Analysis Based on NHANES 1999–2004. Nutr. Res. 2024, 121, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Quintana, S.; Russo, M.; Torelli, P. Nutraceuticals and Migraine: Further Strategy for the Treatment of Specific Conditions. Neurol. Sci. 2022, 43, 6565–6567. [Google Scholar] [CrossRef]
- Plantone, D.; Pardini, M.; Rinaldi, G. Riboflavin in Neurological Diseases: A Narrative Review. Clin. Drug Investig. 2021, 41, 513–527. [Google Scholar] [CrossRef]
- Gholizadeh, N.; Sheykhbahaei, N. Micronutrients Status as a Contributing Factor in Secondary Burning Mouth Syndrome: A Review of the Literature. Health Sci. Rep. 2024, 7, e1906. [Google Scholar] [CrossRef]
- Giat, E.; Yom-Tov, E. Evidence From Web-Based Dietary Search Patterns to the Role of B12 Deficiency in Non-Specific Chronic Pain: A Large-Scale Observational Study. J. Med. Internet Res. 2018, 20, e4. [Google Scholar] [CrossRef] [PubMed]
- Frediani, J.K.; Lal, A.A.; Kim, E.; Leslie, S.L.; Boorman, D.W.; Singh, V. The Role of Diet and Non-Pharmacologic Supplements in the Treatment of Chronic Neuropathic Pain: A Systematic Review. Pain Pract. 2024, 24, 186–210. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Song, X.-J. Vitamins in Neuropathy: Pathophysiological and Therapeutic Roles. Curr. Opin. Neurol. 2023, 36, 388–393. [Google Scholar] [CrossRef]
- Karaganis, S.; Song, X.-J. B Vitamins as a Treatment for Diabetic Pain and Neuropathy. J. Clin. Pharm. Ther. 2021, 46, 1199–1212. [Google Scholar] [CrossRef]
- Krawinkel, M.B.; Strohm, D.; Weissenborn, A.; Watzl, B.; Eichholzer, M.; Bärlocher, K.; Elmadfa, I.; Leschik-Bonnet, E.; Heseker, H. Revised D-A-CH Intake Recommendations for Folate: How Much Is Needed? Eur. J. Clin. Nutr. 2014, 68, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Jayabalan, B.; Low, L.L. Vitamin B Supplementation for Diabetic Peripheral Neuropathy. Singap. Med. J. 2016, 57, 55–59. [Google Scholar] [CrossRef]
- Zhang, M.; Han, W.; Hu, S.; Xu, H. Methylcobalamin: A Potential Vitamin of Pain Killer. Neural Plast. 2013, 2013, 424651. [Google Scholar] [CrossRef]
- Marques, D.P.; Chacur, M.; Martins, D.O. Photobiomodulation and Vitamin B Treatment Alleviate Both Thermal and Mechanical Orofacial Pain in Rats. Photochem. Photobiol. Sci. 2023, 22, 2315–2327. [Google Scholar] [CrossRef]
- Carr, A.C.; McCall, C. The Role of Vitamin C in the Treatment of Pain: New Insights. J. Transl. Med. 2017, 15, 77. [Google Scholar] [CrossRef]
- Teixeira, A.; Carrié, A.S.; Généreau, T.; Herson, S.; Cherin, P. Vitamin C Deficiency in Elderly Hospitalized Patients. Am. J. Med. 2001, 111, 502. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Schemann, K.; Min, S.S.; Sullivan, D.R.; Fuller, S.J. Serum Vitamin C Status of People in New South Wales: Retrospective Analysis of Findings at a Public Referral Hospital. Med. J. Aust. 2023, 219, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Warzocha, J.; Gadomska-Krasny, J.; Mrowiec, J. Etiologic Factors of Temporomandibular Disorders: A Systematic Review of Literature Containing Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) and Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD) from 2018 to 2022. Healthcare 2024, 12, 575. [Google Scholar] [CrossRef] [PubMed]
- Powers, C.D.; Sternberg, M.R.; Patel, S.B.; Pfeiffer, C.M.; Storandt, R.J.; Schleicher, R.L. Vitamin C Status of US Adults Assessed as Part of the National Health and Nutrition Examination Survey Remained Unchanged between 2003–2006 and 2017–2018. J. Appl. Lab. Med. 2023, 8, 272–284. [Google Scholar] [CrossRef]
- Crook, J.; Horgas, A.; Yoon, S.-J.; Grundmann, O.; Johnson-Mallard, V. Insufficient Vitamin C Levels among Adults in the United States: Results from the NHANES Surveys, 2003–2006. Nutrients 2021, 13, 3910. [Google Scholar] [CrossRef]
- Shrivastava, M.; Battaglino, R.; Ye, L. A Comprehensive Review on Biomarkers Associated with Painful Temporomandibular Disorders. Int. J. Oral. Sci. 2021, 13, 23. [Google Scholar] [CrossRef]
- Omidpanah, N.; Ebrahimi, S.; Raygani, A.V.; Mozafari, H.; Rezaei, M. Total Antioxidant Capacity, Catalase Activity and Salivary Oxidative Parameters in Patients with Temporomandibular Disorders. Front. Dent. 2020, 17, 16. [Google Scholar] [CrossRef]
- Fatima, M.; Farhat, K.; Ali, S.; Noor, M.; Usman, C.M.; Gilani, F.F. Evaluation Of Anti-Inflammatory Efficacy Of Ascorbic Acid After Third Molar Surgery. J. Ayub Med. Coll. Abbottabad 2023, 35, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, A.E.; Sarlo, G.L.; Holton, K.F. The Role of Magnesium in Neurological Disorders. Nutrients 2018, 10, 730. [Google Scholar] [CrossRef]
- Gröber, U.; Schmidt, J.; Kisters, K. Magnesium in Prevention and Therapy. Nutrients 2015, 7, 8199–8226. [Google Scholar] [CrossRef]
- von Luckner, A.; Riederer, F. Magnesium in Migraine Prophylaxis-Is There an Evidence-Based Rationale? A Systematic Review. Headache 2018, 58, 199–209. [Google Scholar] [CrossRef]
- Bayram, S.; Şahin, K.; Anarat, F.B.; Chousein, C.M.; Kocazeybek, E.; Altan, M.; Akgül, T. The Effect of Oral Magnesium Supplementation on Acute Non-Specific Low Back Pain: Prospective Randomized Clinical Trial. Am. J. Emerg. Med. 2021, 47, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.-H.; Wu, Z.-S.; Huang, S.-Y.; Chou, T.-L.; Cheng, H.-J.; Lo, Y.-H.; Jean, Y.-H.; Sung, C.-S. Local Magnesium Sulfate Administration Ameliorates Nociception, Peripheral Inflammation, and Spinal Sensitization in a Rat Model of Incisional Pain. Neuroscience 2024, 547, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-J.; Li, Y.-L.; Fang, Z.-H.; Liao, H.-L.; Zhang, Y.-Y.; Lin, J.; Liu, F.; Shen, J.-F. NMDARs Mediate Peripheral and Central Sensitization Contributing to Chronic Orofacial Pain. Front. Cell. Neurosci. 2022, 16, 999509. [Google Scholar] [CrossRef]
- Okamoto, K.; Bereiter, D.F.; Thompson, R.; Tashiro, A.; Bereiter, D.A. Estradiol Replacement Modifies C-Fos Expression at the Spinomedullary Junction Evoked by Temporomandibular Joint Stimulation in Ovariectomized Female Rats. Neuroscience 2008, 156, 729–736. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jankovskis, V.; Selga, G. Vitamin B and Zinc Supplements and Capsaicin Oral Rinse Treatment Options for Burning Mouth Syndrome. Medicina 2021, 57, 391. [Google Scholar] [CrossRef]
- Morr Verenzuela, C.S.; Davis, M.D.P.; Bruce, A.J.; Torgerson, R.R. Burning Mouth Syndrome: Results of Screening Tests for Vitamin and Mineral Deficiencies, Thyroid Hormone, and Glucose Levels-Experience at Mayo Clinic over a Decade. Int. J. Dermatol. 2017, 56, 952–956. [Google Scholar] [CrossRef]
- Bahramian, A.; Rahbaran, M.; Bahramian, M.; Bohlouli, S.; Katebi, K. Effect of Zinc Supplementation as an Adjuvant to Corticosteroid Treatment in Patients with Oral Lichen Planus: A Systematic Review. J. Adv. Periodontol. Implant. Dent. 2023, 15, 128–133. [Google Scholar] [CrossRef]
- Barros-Neto, J.A.; Souza-Machado, A.; Kraychete, D.C.; Jesus, R.P.d.; Cortes, M.L.; Lima, M.D.S.; Freitas, M.C.; Santos, T.M. de M.; Viana, G.F. de S.; Menezes-Filho, J.A. Selenium and Zinc Status in Chronic Myofascial Pain: Serum and Erythrocyte Concentrations and Food Intake. PLoS ONE 2016, 11, e0164302. [Google Scholar] [CrossRef]
- Yokota, S.; Ishizu, H.; Miyazaki, T.; Takahashi, D.; Iwasaki, N.; Shimizu, T. Osteoporosis, Osteoarthritis, and Subchondral Insufficiency Fracture: Recent Insights. Biomedicines 2024, 12, 843. [Google Scholar] [CrossRef]
- Liao, B.; Ding, M.; Wang, Y.; Xu, H.; Shangguan, L. Strontium Ion Attenuates Osteoarthritis through Inhibiting Senescence and Enhancing Autophagy in Fibroblast-like Synoviocytes. Mol. Biol. Rep. 2023, 50, 1437–1446. [Google Scholar] [CrossRef]
- Saito, A.I.; Inoue, T.; Kinoshita, M.; Kosaka, T.; Mitsuhashi, T. Strontium-89 Chloride Delivery for Painful Bone Metastases in Patients with a History of Prior Irradiation. Ir. J. Med. Sci. 2023, 192, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Nimni, M.E.; Han, B.; Cordoba, F. Are We Getting Enough Sulfur in Our Diet? Nutr. Metab. 2007, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, N.; Sakoda, A.; Kataoka, T.; Sun, L.; Tanaka, H.; Ohtsu, I.; Yamaoka, K. Changes in Sulfur Metabolism in Mouse Brains Following Radon Inhalation. Int. J. Environ. Res. Public Health 2022, 19, 10750. [Google Scholar] [CrossRef]
- Horváth, K.; Noker, P.E.; Somfai-Relle, S.; Glávits, R.; Financsek, I.; Schauss, A.G. Toxicity of Methylsulfonylmethane in Rats. Food Chem. Toxicol. 2002, 40, 1459–1462. [Google Scholar] [CrossRef]
- Toguchi, A.; Noguchi, N.; Kanno, T.; Yamada, A. Methylsulfonylmethane Improves Knee Quality of Life in Participants with Mild Knee Pain: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2023, 15, 2995. [Google Scholar] [CrossRef] [PubMed]
- Tennent, D.J.; Hylden, C.M.; Kocher, B.K.; Aden, J.K.; Johnson, A.E. A Randomized Controlled Trial Evaluating Methylsulfonylmethane versus Placebo to Prevent Knee Pain in Military Initial Entry Trainees. US Army Med. Dep. J. 2017, 21–25. [Google Scholar]
- Chang, J.P.-C.; Tseng, P.-T.; Zeng, B.-S.; Chang, C.-H.; Su, H.; Chou, P.-H.; Su, K.-P. Safety of Supplementation of Omega-3 Polyunsaturated Fatty Acids: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2023, 14, 1326–1336. [Google Scholar] [CrossRef]
- Zhang, C.; Hou, B.; Xu, Y.; Zeng, S.; Luo, X.; Zhang, B. Association between Eicosapentaenoic Acid Consumption and the Risk of Depressive Symptoms in US Adults: Analyses from NHANES 2005–2018. J. Affect. Disord. 2024, 354, 62–67. [Google Scholar] [CrossRef]
- Norouziasl, R.; Zeraattalab-Motlagh, S.; Jayedi, A.; Shab-Bidar, S. Efficacy and Safety of N-3 Fatty Acids Supplementation on Depression: A Systematic Review and Dose-Response Meta-Analysis of Randomised Controlled Trials. Br. J. Nutr. 2024, 131, 658–671. [Google Scholar] [CrossRef]
- Chilton, F.H.; Dutta, R.; Reynolds, L.M.; Sergeant, S.; Mathias, R.A.; Seeds, M.C. Precision Nutrition and Omega-3 Polyunsaturated Fatty Acids: A Case for Personalized Supplementation Approaches for the Prevention and Management of Human Diseases. Nutrients 2017, 9, 1165. [Google Scholar] [CrossRef]
- Loef, M.; Schoones, J.W.; Kloppenburg, M.; Ioan-Facsinay, A. Fatty Acids and Osteoarthritis: Different Types, Different Effects. Jt. Bone Spine 2019, 86, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Sanders, A.E.; Weatherspoon, E.D.; Ehrmann, B.M.; Soma, P.S.; Shaikh, S.R.; Preisser, J.S.; Ohrbach, R.; Fillingim, R.B.; Slade, G.D. Circulating Polyunsaturated Fatty Acids and Pain Intensity in Five Chronic Pain Conditions. J. Pain 2023, 24, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Yi, Z.; Yin, E.; Lu, R.; You, H.; Yuan, X. Effect of Omega-3 Polyunsaturated Fatty Acids Supplementation for Patients with Osteoarthritis: A Meta-Analysis. J. Orthop. Surg. Res. 2023, 18, 381. [Google Scholar] [CrossRef]
- Fares, S.; Omar, M.; Laurence, A.; Abu-Baker, S.; Shaza, A.; Fadi, H.; Jonathan, M.; Georges, K.; Koushik, S.; Elie, B.S.; et al. Over-the-Counter Anti-Inflammatory Supplements for Adjunctive Rheumatoid Arthritis Therapy: A Comprehensive Narrative Review. Aging Dis. 2024, 16, 1–15. [Google Scholar] [CrossRef]
- Marchetti, M.; Gualtieri, P.; De Lorenzo, A.; Trombetta, D.; Smeriglio, A.; Ingegneri, M.; Cianci, R.; Frank, G.; Schifano, G.; Bigioni, G.; et al. Dietary ω-3 Intake for the Treatment of Morning Headache: A Randomized Controlled Trial. Front. Neurol. 2022, 13, 987958. [Google Scholar] [CrossRef]
- Zhou, S.; Zhu, G.; Xu, Y.; Gao, R.; Li, H.; Han, G.; Su, W.; Wang, R. Mendelian Randomization Study on the Putative Causal Effects of Omega-3 Fatty Acids on Low Back Pain. Front. Nutr. 2022, 9, 819635. [Google Scholar] [CrossRef]
- Dai, Y.; Chen, Y.; Gu, R.; Zhang, C.; Jiang, R. Causal Association of Polyunsaturated Fatty Acids with Chronic Pain: A Two-Sample Mendelian Randomization Study. Front. Nutr. 2023, 10, 1265928. [Google Scholar] [CrossRef]
- Liao, H.-Y.; Yen, C.-M.; Hsiao, I.-H.; Hsu, H.-C.; Lin, Y.-W. Eicosapentaenoic Acid Modulates Transient Receptor Potential V1 Expression in Specific Brain Areas in a Mouse Fibromyalgia Pain Model. Int. J. Mol. Sci. 2024, 25, 2901. [Google Scholar] [CrossRef]
- Nakamoto, K.; Nishinaka, T.; Mankura, M.; Fujita-Hamabe, W.; Tokuyama, S. Antinociceptive Effects of Docosahexaenoic Acid against Various Pain Stimuli in Mice. Biol. Pharm. Bull. 2010, 33, 1070–1072. [Google Scholar] [CrossRef]
- Chávez-Castillo, M.; Ortega, Á.; Cudris-Torres, L.; Duran, P.; Rojas, M.; Manzano, A.; Garrido, B.; Salazar, J.; Silva, A.; Rojas-Gomez, D.M.; et al. Specialized Pro-Resolving Lipid Mediators: The Future of Chronic Pain Therapy? Int. J. Mol. Sci. 2021, 22, 10370. [Google Scholar] [CrossRef]
- Ikeda, A.; Muroki, A.; Suzuki, C.; Shimazu, Y.; Takeda, M. Resolvin D1 Suppresses Inflammation-Induced Hyperexcitability of Nociceptive Trigeminal Neurons Associated with Mechanical Hyperalgesia. Brain Res. Bull. 2020, 154, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Tokuyama, S.; Nakamoto, K. Unsaturated Fatty Acids and Pain. Biol. Pharm. Bull. 2011, 34, 1174–1178. [Google Scholar] [CrossRef]
- Ramsden, C.E.; Faurot, K.R.; Zamora, D.; Palsson, O.S.; MacIntosh, B.A.; Gaylord, S.; Taha, A.Y.; Rapoport, S.I.; Hibbeln, J.R.; Davis, J.M.; et al. Targeted Alterations in Dietary N-3 and n-6 Fatty Acids Improve Life Functioning and Reduce Psychological Distress among Patients with Chronic Headache: A Secondary Analysis of a Randomized Trial. Pain 2015, 156, 587–596. [Google Scholar] [CrossRef]
- Sibille, K.T.; King, C.; Garrett, T.J.; Glover, T.L.; Zhang, H.; Chen, H.; Reddy, D.; Goodin, B.R.; Sotolongo, A.; Petrov, M.E.; et al. Omega-6: Omega-3 PUFA Ratio, Pain, Functioning, and Distress in Adults with Knee Pain. Clin. J. Pain 2018, 34, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Boyd, J.T.; LoCoco, P.M.; Furr, A.R.; Bendele, M.R.; Tram, M.; Li, Q.; Chang, F.-M.; Colley, M.E.; Samenuk, G.M.; Arris, D.A.; et al. Elevated Dietary ω-6 Polyunsaturated Fatty Acids Induce Reversible Peripheral Nerve Dysfunction That Exacerbates Comorbid Pain Conditions. Nat. Metab. 2021, 3, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Sanders, A.E.; Weatherspoon, E.D.; Ehrmann, B.M.; Soma, P.S.; Shaikh, S.R.; Preisser, J.S.; Ohrbach, R.; Fillingim, R.B.; Slade, G.D. Ratio of Omega-6/Omega-3 Polyunsaturated Fatty Acids Associated with Somatic and Depressive Symptoms in People with Painful Temporomandibular Disorder and Irritable Bowel Syndrome. J. Pain 2022, 23, 1737–1748. [Google Scholar] [CrossRef]
- Sanders, A.E.; Weatherspoon, E.D.; Ehrmann, B.M.; Soma, P.S.; Shaikh, S.R.; Preisser, J.S.; Ohrbach, R.; Fillingim, R.B.; Slade, G.D. Circulating Omega-6 and Omega-3 Polyunsaturated Fatty Acids in Painful Temporomandibular Disorder and Low Back Pain. J. Pain 2022, 23, 1724–1736. [Google Scholar] [CrossRef]
- Larrieu, T.; Hilal, M.L.; Fourrier, C.; De Smedt-Peyrusse, V.; Sans, N.; Capuron, L.; Layé, S. Nutritional Omega-3 Modulates Neuronal Morphology in the Prefrontal Cortex along with Depression-Related Behaviour through Corticosterone Secretion. Transl. Psychiatry 2014, 4, e437. [Google Scholar] [CrossRef]
- Suneson, K.; Söderberg Veibäck, G.; Lindahl, J.; Tjernberg, J.; Ståhl, D.; Ventorp, S.; Ängeby, F.; Lundblad, K.; Wolkowitz, O.M.; Lindqvist, D. Omega-3 Fatty Acids for Inflamed Depression—A Match/Mismatch Study. Brain Behav. Immun. 2024, 118, 192–201. [Google Scholar] [CrossRef]
- Zhou, L.; Xiong, J.-Y.; Chai, Y.-Q.; Huang, L.; Tang, Z.-Y.; Zhang, X.-F.; Liu, B.; Zhang, J.-T. Possible Antidepressant Mechanisms of Omega-3 Polyunsaturated Fatty Acids Acting on the Central Nervous System. Front. Psychiatry 2022, 13, 933704. [Google Scholar] [CrossRef]
- Mu, G.; Ren, C.; Zhang, Y.; Lu, B.; Feng, J.; Wu, D.; Xu, X.; Ou, C. Amelioration of Central Neurodegeneration by Docosahexaenoic Acid in Trigeminal Neuralgia Rats through the Regulation of Central Neuroinflammation. Int. Immunopharmacol. 2023, 114, 109544. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-W.; Chou, A.I.W.; Su, H.; Su, K.-P. Transient Receptor Potential V1 (TRPV1) Modulates the Therapeutic Effects for Comorbidity of Pain and Depression: The Common Molecular Implication for Electroacupuncture and Omega-3 Polyunsaturated Fatty Acids. Brain Behav. Immun. 2020, 89, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Pavlou, I.A.; Spandidos, D.A.; Zoumpourlis, V.; Adamaki, M. Nutrient Insufficiencies and Deficiencies Involved in the Pathogenesis of Bruxism (Review). Exp. Ther. Med. 2023, 26, 563. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Mizushima, N.; Virgin, H.W. Autophagy in Immunity and Inflammation. Nature 2011, 469, 323–335. [Google Scholar] [CrossRef]
- Inoue, T.; Tanaka, M.; Masuda, S.; Ohue-Kitano, R.; Yamakage, H.; Muranaka, K.; Wada, H.; Kusakabe, T.; Shimatsu, A.; Hasegawa, K.; et al. Omega-3 Polyunsaturated Fatty Acids Suppress the Inflammatory Responses of Lipopolysaccharide-Stimulated Mouse Microglia by Activating SIRT1 Pathways. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 552–560. [Google Scholar] [CrossRef]
- Du, C.; Smith, A.; Avalos, M.; South, S.; Crabtree, K.; Wang, W.; Kwon, Y.-H.; Vijayagopal, P.; Juma, S. Blueberries Improve Pain, Gait Performance, and Inflammation in Individuals with Symptomatic Knee Osteoarthritis. Nutrients 2019, 11, 290. [Google Scholar] [CrossRef]
- Farid, R.; Rezaieyazdi, Z.; Mirfeizi, Z.; Hatef, M.R.; Mirheidari, M.; Mansouri, H.; Esmaelli, H.; Bentley, G.; Lu, Y.; Foo, Y.; et al. Oral Intake of Purple Passion Fruit Peel Extract Reduces Pain and Stiffness and Improves Physical Function in Adult Patients with Knee Osteoarthritis. Nutr. Res. 2010, 30, 601–606. [Google Scholar] [CrossRef]
- Schell, J.; Scofield, R.H.; Barrett, J.R.; Kurien, B.T.; Betts, N.; Lyons, T.J.; Zhao, Y.D.; Basu, A. Strawberries Improve Pain and Inflammation in Obese Adults with Radiographic Evidence of Knee Osteoarthritis. Nutrients 2017, 9, 949. [Google Scholar] [CrossRef]
- Kadayifci, F.Z.; Bradley, M.J.; Onat, A.M.; Shi, H.N.; Zheng, S. Review of Nutritional Approaches to Fibromyalgia. Nutr. Rev. 2022, 80, 2260–2274. [Google Scholar] [CrossRef]
- Mansouri, M.; Sharifi, F.; Varmaghani, M.; Shokri, A.; Rahdar, H.; Keshtkar, A.; Sadeghi, O. Fruit and Vegetable Consumption in Relation to Primary Headaches: The MEPHASOUS Study. Eat. Weight. Disord. 2021, 26, 1617–1626. [Google Scholar] [CrossRef]
- Costa de Miranda, R.; Paiva, E.S.; Suter Correia Cadena, S.M.; Brandt, A.P.; Vilela, R.M. Polyphenol-Rich Foods Alleviate Pain and Ameliorate Quality of Life in Fibromyalgic Women. Int. J. Vitam. Nutr. Res. 2017, 87, 66–74. [Google Scholar] [CrossRef]
- Moccia, S.; Nucci, L.; Spagnuolo, C.; d’Apuzzo, F.; Piancino, M.G.; Minervini, G. Polyphenols as Potential Agents in the Management of Temporomandibular Disorders. Appl. Sci. 2020, 10, 5305. [Google Scholar] [CrossRef]
- Catunda, I.S.; Vasconcelos, B.C.d.E.; Andrade, E.S.d.S.; Costa, D.F.N. Clinical Effects of an Avocado-Soybean Unsaponifiable Extract on Arthralgia and Osteoarthritis of the Temporomandibular Joint: Preliminary Study. Int. J. Oral. Maxillofac. Surg. 2016, 45, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Braz, M.A.; Freitas Portella, F.; Seehaber, K.A.; Bavaresco, C.S.; Rivaldo, E.G. Association between Oxidative Stress and Temporomandibular Joint Dysfunction: A Narrative Review. J. Oral. Rehabil. 2020, 47, 536–546. [Google Scholar] [CrossRef]
- Vrbanović, E.; Alajbeg, I.Z.; Vuletić, L.; Lapić, I.; Rogić, D.; Andabak Rogulj, A.; Illeš, D.; Knezović Zlatarić, D.; Badel, T.; Alajbeg, I. Salivary Oxidant/Antioxidant Status in Chronic Temporomandibular Disorders Is Dependent on Source and Intensity of Pain—A Pilot Study. Front. Physiol. 2018, 9, 1405. [Google Scholar] [CrossRef] [PubMed]
- Cady, R.J.; Hirst, J.J.; Durham, P.L. Dietary Grape Seed Polyphenols Repress Neuron and Glia Activation in Trigeminal Ganglion and Trigeminal Nucleus Caudalis. Mol. Pain 2010, 6, 91. [Google Scholar] [CrossRef]
- Magni, G.; Marinelli, A.; Riccio, D.; Lecca, D.; Tonelli, C.; Abbracchio, M.P.; Petroni, K.; Ceruti, S. Purple Corn Extract as Anti-Allodynic Treatment for Trigeminal Pain: Role of Microglia. Front. Cell Neurosci. 2018, 12, 378. [Google Scholar] [CrossRef]
- Bowden, L.N.; Rohrs, E.L.; Omoto, K.; Durham, P.L.; Holliday, L.S.; Morris, A.D.; Allen, K.D.; Caudle, R.M.; Neubert, J.K. Effects of Cocoa-Enriched Diet on Orofacial Pain in a Murine Model. Orthod. Craniofacial Res. 2017, 20 (Suppl. S1), 157–161. [Google Scholar] [CrossRef] [PubMed]
- Cady, R.J.; Denson, J.E.; Durham, P.L. Inclusion of Cocoa as a Dietary Supplement Represses Expression of Inflammatory Proteins in Spinal Trigeminal Nucleus in Response to Chronic Trigeminal Nerve Stimulation. Mol. Nutr. Food Res. 2013, 57, 996–1006. [Google Scholar] [CrossRef]
- Hajati, A.; Brondani, M.; Angerstig, L.; Klein, V.; Liljeblad, L.; Al-Moraissi, E.A.; Louca Jounger, S.; Brondani, B.; Christidis, N. Chocolate Intake and Muscle Pain Sensation: A Randomized Experimental Study. PLoS ONE 2023, 18, e0284769. [Google Scholar] [CrossRef]
- Abbey, M.J.; Patil, V.V.; Vause, C.V.; Durham, P.L. Repression of Calcitonin Gene-Related Peptide Expression in Trigeminal Neurons by a Theobroma Cacao Extract. J. Ethnopharmacol. 2008, 115, 238–248. [Google Scholar] [CrossRef]
- Toledano-Martos, R.; Bagó-Mas, A.; Deulofeu, M.; Homs, J.; Fiol, N.; Verdú, E.; Boadas-Vaello, P. Natural Polyphenolic Coffee Extract Administration Relieves Chronic Nociplastic Pain in a Reserpine-Induced Fibromyalgia-like Female Mouse Model. Brain Behav. 2024, 14, e3386. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Takehana, S.; Sekiguchi, K.; Kubota, Y.; Shimazu, Y. Modulatory Mechanism of Nociceptive Neuronal Activity by Dietary Constituent Resveratrol. Int. J. Mol. Sci. 2016, 17, 1702. [Google Scholar] [CrossRef] [PubMed]
- Tajik, H.; Tamaddonfard, E.; Hamzeh-Gooshchi, N. Interaction between Curcumin and Opioid System in the Formalin Test of Rats. Pak. J. Biol. Sci. 2007, 10, 2583–2586. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, L.; Ma, Z.; Wu, Z.; Jin, M.; An, L.; Xue, F. Curcumin Improves Chronic Pain Induced Depression through Regulating Serum Metabolomics in a Rat Model of Trigeminal Neuralgia. J. Pain Res. 2020, 13, 3479–3492. [Google Scholar] [CrossRef]
- Jiang, C.; Luo, P.; Li, X.; Liu, P.; Li, Y.; Xu, J. Nrf2/ARE Is a Key Pathway for Curcumin-Mediated Protection of TMJ Chondrocytes from Oxidative Stress and Inflammation. Cell Stress Chaperones 2020, 25, 395–406. [Google Scholar] [CrossRef]
- Sirše, M. Effect of Dietary Polyphenols on Osteoarthritis-Molecular Mechanisms. Life 2022, 12, 436. [Google Scholar] [CrossRef]
- Kumar, A.; P, N.; Kumar, M.; Jose, A.; Tomer, V.; Oz, E.; Proestos, C.; Zeng, M.; Elobeid, T.; K, S.; et al. Major Phytochemicals: Recent Advances in Health Benefits and Extraction Method. Molecules 2023, 28, 887. [Google Scholar] [CrossRef]
- Araújo-Filho, H.G.; Pereira, E.W.M.; Rezende, M.M.; Menezes, P.P.; Araújo, A.A.S.; Barreto, R.S.S.; Martins, A.O.B.P.B.; Albuquerque, T.R.; Silva, B.A.F.; Alcantara, I.S.; et al. D-Limonene Exhibits Superior Antihyperalgesic Effects in a β-Cyclodextrin-Complexed Form in Chronic Musculoskeletal Pain Reducing Fos Protein Expression on Spinal Cord in Mice. Neuroscience 2017, 358, 158–169. [Google Scholar] [CrossRef]
- Araújo-Filho, H.G.; Pereira, E.W.M.; Heimfarth, L.; Souza Monteiro, B.; Santos Passos, F.R.; Siqueira-Lima, P.; Gandhi, S.R.; Viana Dos Santos, M.R.; Guedes da Silva Almeida, J.R.; Picot, L.; et al. Limonene, a Food Additive, and Its Active Metabolite Perillyl Alcohol Improve Regeneration and Attenuate Neuropathic Pain after Peripheral Nerve Injury: Evidence for IL-1β, TNF-α, GAP, NGF and ERK Involvement. Int. Immunopharmacol. 2020, 86, 106766. [Google Scholar] [CrossRef]
- Nishijima, C.M.; Ganev, E.G.; Mazzardo-Martins, L.; Martins, D.F.; Rocha, L.R.M.; Santos, A.R.S.; Hiruma-Lima, C.A. Citral: A Monoterpene with Prophylactic and Therapeutic Anti-Nociceptive Effects in Experimental Models of Acute and Chronic Pain. Eur. J. Pharmacol. 2014, 736, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.C.; Almeida, J.R.G.S.; Quintans, J.S.S.; Gopalsamy, R.G.; Shanmugam, S.; Serafini, M.R.; Oliveira, M.R.C.; Silva, B.A.F.; Martins, A.O.B.P.B.; Castro, F.F.; et al. Enhancement of Orofacial Antinociceptive Effect of Carvacrol, a Monoterpene Present in Oregano and Thyme Oils, by β-Cyclodextrin Inclusion Complex in Mice. Biomed. Pharmacother. 2016, 84, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Regules, A.E.; Martínez-Thomas, J.A.; Schürenkämper-Carrillo, K.; de Parrodi, C.A.; López-Mena, E.R.; Mejía-Méndez, J.L.; Lozada-Ramírez, J.D. Recent Advances in the Therapeutic Potential of Carotenoids in Preventing and Managing Metabolic Disorders. Plants 2024, 13, 1584. [Google Scholar] [CrossRef]
- Dos Santos, O.V.; do Rosário, R.C.; Teixeira-Costa, B.E. Sources of Carotenoids in Amazonian Fruits. Molecules 2024, 29, 2190. [Google Scholar] [CrossRef]
- Rezaei, F.; Saebipour, M.R.; Ghaemi, K.; Hassanzadeh-Taheri, M.M.; Foadoddini, M.; Hosseini, M. Intra-Cerebroventricular Administration of Crocin Attenuates Sleep Deprivation-Induced Hyperalgesia in Rats. Basic. Clin. Neurosci. 2020, 11, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Elkholy, N.S.; Mohammed, H.S.; Shafaa, M.W. Assessment of the Therapeutic Potential of Lutein and Beta-Carotene Nanodispersions in a Rat Model of Fibromyalgia. Sci. Rep. 2023, 13, 19712. [Google Scholar] [CrossRef]
- Wu, M.; Cai, J.; Yu, Y.; Hu, S.; Wang, Y.; Wu, M. Therapeutic Agents for the Treatment of Temporomandibular Joint Disorders: Progress and Perspective. Front. Pharmacol. 2020, 11, 596099. [Google Scholar] [CrossRef]
- Oliveira, J.P.; Nampo, F.K.; Souza, M.T.S.; Cercato, L.M.; Camargo, E.A. The Effect of Natural Products in Animal Models of Temporomandibular Disorders. J. Appl. Oral. Sci. 2020, 28, e20200272. [Google Scholar] [CrossRef]
- Gao, J.; Liang, Y.; Liu, P. Along the Microbiota-Gut-Brain Axis: Use of Plant Polysaccharides to Improve Mental Disorders. Int. J. Biol. Macromol. 2024, 265, 130903. [Google Scholar] [CrossRef]
- Jang, H.J.; Lee, N.-K.; Paik, H.-D. A Narrative Review on the Advance of Probiotics to Metabiotics. J. Microbiol. Biotechnol. 2024, 34, 487–494. [Google Scholar] [CrossRef]
- Sivakumar, S.; Sivakumar, G.; Sundramoorthy, A.K. Effects of Glucosamine in the Temporomandibular Joint Osteoarthritis: A Review. Curr. Rheumatol. Rev. 2024, 20, 373–378. [Google Scholar] [CrossRef]
- Ruiz-Romero, V.; Toledano-Serrabona, J.; Gay-Escoda, C. Efficacy of the Use of Chondroitin Sulphate and Glucosamine for the Treatment of Temporomandibular Joint Dysfunction: A Systematic Review and Meta-Analysis. Cranio 2022, 1–10. [Google Scholar] [CrossRef]
- Rabade, A.; Viswanatha, G.L.; Nandakumar, K.; Kishore, A. Evaluation of Efficacy and Safety of Glucosamine Sulfate, Chondroitin Sulfate, and Their Combination Regimen in the Management of Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Inflammopharmacology 2024, 32, 1759–1775. [Google Scholar] [CrossRef]
- Derwich, M.; Górski, B.; Amm, E.; Pawłowska, E. Oral Glucosamine in the Treatment of Temporomandibular Joint Osteoarthritis: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 4925. [Google Scholar] [CrossRef]
- Figueroba, S.R.; Moreira, J.C.; Amorim, K.S.; Cunha, L.D.L.L.; Morais, T.M.L.; Ferreira, L.E.N.; Groppo, F.C. Effect of Glucosamine Sulphate on the Temporomandibular Joint of Ovariectomised Rats. Br. J. Oral. Maxillofac. Surg. 2021, 59, 202–208. [Google Scholar] [CrossRef]
- Hawkins, J.L.; Durham, P.L. Enriched Chicken Bone Broth as a Dietary Supplement Reduces Nociception and Sensitization Associated with Prolonged Jaw Opening. J. Oral. Facial Pain Headache 2018, 32, 208–215. [Google Scholar] [CrossRef]
- Santonocito, S.; Donzella, M.; Venezia, P.; Nicolosi, G.; Mauceri, R.; Isola, G. Orofacial Pain Management: An Overview of the Potential Benefits of Palmitoylethanolamide and Other Natural Agents. Pharmaceutics 2023, 15, 1193. [Google Scholar] [CrossRef] [PubMed]
- Peritore, A.F.; Siracusa, R.; Crupi, R.; Cuzzocrea, S. Therapeutic Efficacy of Palmitoylethanolamide and Its New Formulations in Synergy with Different Antioxidant Molecules Present in Diets. Nutrients 2019, 11, 2175. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, V.; Schievano, C.; Martini, A.; Polati, L.; Del Balzo, G.; Simari, S.; Milan, B.; Finco, G.; Varrassi, G.; Polati, E. Extended Treatment with Micron-Size Oral Palmitoylethanolamide (PEA) in Chronic Pain: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 1653. [Google Scholar] [CrossRef]
- Shekhar, A.; Srivastava, A.; Verma, N.; Verma, A.; Chaturvedi, T.P. The Comparative Efficacy of Palmitoylethanolamide (PEA) with the Combination of Pregabalin and Nortriptyline on Post-Extraction Trigeminal Neuropathy by Using Magnetic Resonance (MR) Neurography: A Randomized Clinical Trial. Cureus 2024, 16, e54843. [Google Scholar] [CrossRef] [PubMed]
- Piriyaprasath, K.; Hasegawa, M.; Kakihara, Y.; Iwamoto, Y.; Kamimura, R.; Saito, I.; Fujii, N.; Yamamura, K.; Okamoto, K. Effects of Stress Contagion on Anxiogenic- and Orofacial Inflammatory Pain-like Behaviors with Brain Activation in Mice. Eur. J. Oral. Sci. 2023, 131, e12942. [Google Scholar] [CrossRef]
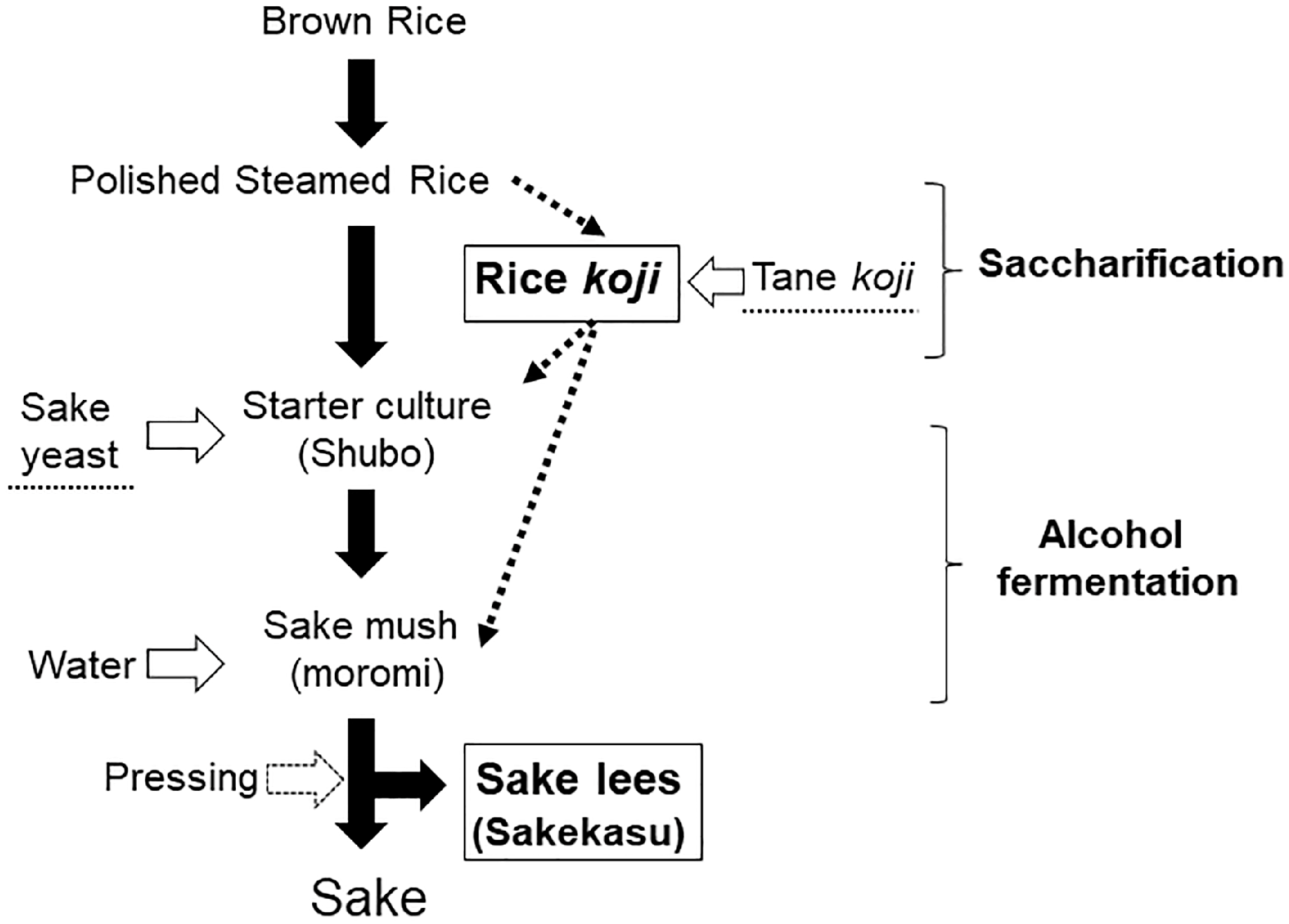
- Allwood, J.G.; Wakeling, L.T.; Bean, D.C. Fermentation and the Microbial Community of Japanese Koji and Miso: A Review. J. Food Sci. 2021, 86, 2194–2207. [Google Scholar] [CrossRef] [PubMed]
- Kurahashi, A. Ingredients, Functionality, and Safety of the Japanese Traditional Sweet Drink Amazake. J. Fungi 2021, 7, 469. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.E.; Lee, S.; Singh, D.; Jang, E.S.; Shin, H.W.; Moon, B.S.; Lee, C.H. Time-Resolved Comparative Metabolomes for Koji Fermentation with Brown-, White-, and Giant Embryo-Rice. Food Chem. 2017, 231, 258–266. [Google Scholar] [CrossRef]
- Tsutsui, N.; Yamamoto, Y.; Iwami, K. Protein-Nutritive Assessment of Sake Lees Obtained by Brewing from Liquefied Rice. J. Nutr. Sci. Vitaminol. 1998, 44, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Izu, H.; Yamashita, S.; Arima, H.; Fujii, T. Nutritional Characterization of Sake Cake (Sake-Kasu) after Heat-Drying and Freeze-Drying. Biosci. Biotechnol. Biochem. 2019, 83, 1477–1483. [Google Scholar] [CrossRef]
- Paul, A.K.; Lim, C.L.; Apu, M.A.I.; Dolma, K.G.; Gupta, M.; de Lourdes Pereira, M.; Wilairatana, P.; Rahmatullah, M.; Wiart, C.; Nissapatorn, V. Are Fermented Foods Effective against Inflammatory Diseases? Int. J. Environ. Res. Public Health 2023, 20, 2481. [Google Scholar] [CrossRef]
- Zhang, K.; Wu, W.; Yan, Q. Research Advances on Sake Rice, Koji, and Sake Yeast: A Review. Food Sci. Nutr. 2020, 8, 2995–3003. [Google Scholar] [CrossRef]
- Hor, P.K.; Pal, S.; Mondal, J.; Halder, S.K.; Ghosh, K.; Santra, S.; Ray, M.; Goswami, D.; Chakrabarti, S.; Singh, S.; et al. Antiobesity, Antihyperglycemic, and Antidepressive Potentiality of Rice Fermented Food through Modulation of Intestinal Microbiota. Front. Microbiol. 2022, 13, 794503. [Google Scholar] [CrossRef]
- Piriyaprasath, K.; Kakihara, Y.; Kurahashi, A.; Taiyoji, M.; Kodaira, K.; Aihara, K.; Hasegawa, M.; Yamamura, K.; Okamoto, K. Preventive Roles of Rice-Koji Extracts and Ergothioneine on Anxiety- and Pain-like Responses under Psychophysical Stress Conditions in Male Mice. Nutrients 2023, 15, 3989. [Google Scholar] [CrossRef]
- Yun, J.-W.; Kim, S.-H.; Kim, Y.-S.; You, J.-R.; Cho, E.-Y.; Yoon, J.-H.; Kwon, E.; Lee, S.J.; Kim, S.P.; Seo, J.H.; et al. Absence of Subchronic Oral Toxicity and Genotoxicity of Rice Koji with Aspergillus Terreus. Regul. Toxicol. Pharmacol. 2017, 89, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Nakatani, Y.; Kakihara, Y.; Taiyoji, M.; Saeki, M.; Takagi, R.; Yamamura, K.; Okamoto, K. Daily Administration of Sake Lees (Sake Kasu) Reduced Psychophysical Stress-Induced Hyperalgesia and Fos Responses in the Lumbar Spinal Dorsal Horn Evoked by Noxious Stimulation to the Hindpaw in the Rats. Biosci. Biotechnol. Biochem. 2020, 84, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Kakihara, Y.; Ohkura, N.; Tohma, A.; Washio, A.; Kitamura, C.; Noiri, Y.; Yamamura, K.; Saeki, M. Effects of Rice Fermented Extracts, “Sake Lees”, on the Functional Activity of Odontoblast-like Cells (KN-3 Cells). Odontology 2022, 110, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, Y.; Kakihara, Y.; Shimizu, S.; Kurose, M.; Sato, T.; Kaneoke, M.; Saeki, M.; Takagi, R.; Yamamura, K.; Okamoto, K. Japanese Rice Wine Can Reduce Psychophysical Stress-Induced Depression-like Behaviors and Fos Expression in the Trigeminal Subnucleus Caudalis Evoked by Masseter Muscle Injury in the Rats. Biosci. Biotechnol. Biochem. 2019, 83, 155–165. [Google Scholar] [CrossRef] [PubMed]
| Nutraceuticals | |
|---|---|
| Vitamin Mineral Polyunsaturated fatty acids Polyphenols Isoprenoids Carotenoid Lectin Polysaccharide Glucosamine Palmitoylethanolamide | Vitamin D, Vitamin B, Vitamin C Magnesium, Zinc, Strontium, Sulfur Omega-3 fatty acids, Docosahexaenoic acid Quercetin, (-)-epigallocatechin-3-gallate, Resveratrol, Curcumin Limonene, Citral Crocin, Lutein |
| Category | Keywords |
|---|---|
| a. Clinical | “temporomandibular disorder” OR “TMD” OR “temporomandibular joint” OR “TMJ” OR “masseter muscle” OR “orofacial pain” OR “patient” OR “clinical” |
| b. Preclinical | “craniofacial tissue” OR “animal model” OR “Complete Freund’s Adjuvant” OR “CFA” OR “formalin” OR “mice” OR “rat” OR “nociception” OR “preclinical model” |
| c. Exposure | “antioxidant” OR “diet” OR “dietary” OR “dietary supplement” OR “supplement” OR “fatty acid” OR “fiber” OR “food” OR “mineral” OR “natural product” OR “nutraceutical” OR “nutrition” OR “supplement” OR “phytochemical” OR “polyphenol” OR “vitamin” OR “fruits” OR “vegetable” OR “isoprenoid” OR “carotenoid” OR “lectin” OR “polysaccharide” OR “glucosamine” |
| Authors | Pain Condition | Study Duration | Interventions | Groups | Outcomes |
|---|---|---|---|---|---|
| Vitamins | |||||
| Gupta et al., 2022, India [37] | Axis I group II TMDs with vitamin D levels < 30 ng/mL. | 3 months | Vitamin D tablets 60,000 IU once a week for eight weeks. | 1. Splint alone 2. Splint + Vitamin D supplement | In TMD patients with vitamin D deficiency, a significant difference was seen in VAS score and maximum mouth opening between the splint with vitamin D supplementation and the splint with a placebo drug. |
| Reis et al., 2023, Brazil [38] | Chronic myofascial pain and arthralgia. | 1 month | Methylcobalamin (B12) 1000 μg/day, orally. | 1. Laser and B12 placebo 2. Effective laser + B12 placebo 3. Effective laser + B12 | Vitamin B12 facilitates the inhibitory effects of laserpuncture in treating painful TMDs. |
| Minerals | |||||
| Refahee et al., 2022, Egypt [44] | Myofascial pain and trigger points in the masseter muscle. | 6 months | Magnesium sulfate (MS, 0.41 mMol/mL) 2 mL, trigger point injection. | 1. Saline 2. Magnesium sulfate | MS reduced the facial pain scores, and the maximum mouth opening distance was higher up to 3 months in the MS than in the saline group. |
| Kiliç, 2021, Turkey [39] | Temporomandibular joint osteoarthritis (TMJ OA). | 12 months | GCM supplementation, containing 750 mg GH, 600 mg chondroitin sulfate, and 350 mg MSM at 2 × 1 dosage daily for 3 months. | 1. arthrocentesis plus intraarticular hyaluronic acid (HA) injection only 2. arthrocentesis plus intraarticular HA injection followed by 3 months of GCM | GCM supplementation after arthro-centesis plus intraarticular hyaluronic acid injection produced no additional clinical benefits or improvements for patients with TMJ-OA compared with arthrocentesis plus intraarticular hyaluronic acid injection alone. |
| Glucosamine | |||||
| Thie et al., 2001, Canada [45] | Degenerative joint Disease of TMJ. | 4 months | Glucosamine sulfate (GS) 500 mg tid for 90 days, orally. | 1. Ibuprofen 2. GS | GS decreased TMJ pain compared with ibuprofen administrations. |
| Damlar et al., 2014, Turkey [40] | Internal derangements of TMJ | 2 months | 1500 mg glucosamine and 1200 mg chondroitin sulfate (CS) /day, orally. | 1. Tramadol HCl 2. glucosamine and chondroitin sulfate | A combination of glucosamine and chondroitin sulfate reduced pain compared with the tramadol group. |
| Haghighat et al., 2013, Iran [46] | Painful TMJ, TMJ crepitation or limitation of mouth opening. | 3 months | Glucosamine sulfate (GS) 1500 mg/day, orally. | 1. Ibuprofen 2. GS | GS improved craniofacial pain and mandibular opening distance compared to baseline and showed more post-treatment improvement when compared with ibuprofen. |
| Cahlin et al., 2011, Sweden [47] | TMJ OA | 6 weeks | Glucosamine sulfate (GS) 1200 mg/day, orally. | 1. Placebo drug 2. GS | GS showed less beneficial effects compared with the placebo group in reducing pain associated with TMJ osteoarthritis. |
| Nguyen et al., 2016, USA [41] | Capsulitis, disk displacement, disk dislocation, or painful osteoarthritis of TMJ | 12 weeks | 1500 mg of glucosamine hydrochloride (GH) and 1200 mg of chondroitin sulfate (CS)/day, orally. | 1. Placebo drug 2. GH + CS | GH-CS reduced craniofacial pain compared with the placebo group. |
| Cen et al., 2017 [42] | TMJ OA | 1 year | Two tablets of 240 mg glucosamine hydrochloride (GH) tid for 3 months, orally | 1. Placebo + HA injection 2. GH + HA injection | GH with HA injection decreased craniofacial pain at 1 month and one year follow-up compared to baseline. One year later, pain score was reduced, and IL-6 and IL-1β levels were lower in group GH + HA than in group placebo + HA. |
| Yang et al., 2018, China [43] | TMJ OA | 1 year | Glucosamine hydrochloride (GH) 1.44 g/day for 3 months, orally. | 1. Placebo + hyaluronate sodium injection 2. GH + hyaluronate sodium injection | GH and hyaluronate sodium injection improved maximal mouth opening distance and facial pain intensity compared to the placebo + hyaluronate sodium injection group in the long-term follow-up. |
| Palmitoylethanolamide | |||||
| Marini et al., 2012, Italy [48] | TMJ OA or arthralgia | 2 weeks | Palmitoylethanolamide (PEA) 300 mg in the morning and 600 mg in the evening for 7 days and then 300 mg twice a day for 7 more days, orally. | 1. Ibuprofen 2. PEA | PEA improved pain related to maximum mouth opening compared with the ibuprofen-treated group. |
| Authors | Model | Interventions | Groups | Outcomes |
|---|---|---|---|---|
| Vitamins | ||||
| Erfanparast et al., 2014 [58] | Male rats, formalin test | Vitamin B12, 1, 2, and 4 mg/kg, i.p. or peripheral (2.5, 5 and 10 µg/rat) injections. | 1. Saline 2. Diclofenac 3. Vitamin B12 4. Vitamin B12 + Diclofenac | Vitamin B12 reduced facial pain-like behavior. Co-treatments with vitamin B12 and diclofenac facilitated antinociceptive effects. |
| Erfanparast et al., 2017 [59] | Male rats, formalin test | Vitamin B12, 0.5 µL intra-hippocampal injection. | 1. Saline 2. Naloxone 3. Vitamin B12 4. Vitamin B12 +Naloxone | Vitamin B12 reduced facial pain-like behaviors associated with laserpuncture intervention. |
| Minerals | ||||
| Cavalcante et al, 2013 [60] | Male rats, carrageenan-induced TMJ inflammation | Magnesium chloride (MgCl2), 10, 30, and 90 mg kg/day, orally. | 1. Naive (no carrageenan) 2. Carrageenan + Vehicle 3. Carrageenan + MgCl2 | MgCl2 reduced facial pain-like behaviors evoked by mechanical threshold. |
| Srebro et al., 2018 [61] | Male rats, formalin test | Magnesium sulfate (MS), 0.005–45 mg/kg, s.c. | 1. Naïve (no formalin) 2. Formalin + Vehicle 3. Formalin + MS | MS reduced facial pain-like behaviors. |
| Srebro et al., 2023 [62] | Male rats, formalin test | Magnesium sulfate (MS), 5, 15 mg/kg, s.c. | 1. Saline 2. Cromoglycate 3. MS 4. MS + Cromoglycate | MS reduced formalin-induced facial pain-like behaviors. |
| Alves et al., 2017 [63] | Male rats, zymosan-induced TMJ inflammation | Strontium, 0.5, 5, or 50 mg/kg, orally. | 1. Naïve (no zymosan) 2. Zymosan 3. Zymosan + Indomethacin 4. Zymosan + Strontium ranelate | Strontium reduced the facial pain-like behaviors evoked by mechanical threshold. |
| Fatty acids | ||||
| Marana et al, 2022 [49] | Female rats, type II bovine collagen (CII) +CFA-induced rheumatoid arthritis (RA) | Omega-3 FAs, 300 mg/kg/day, orally. | 1. Naïve (non-RA) 2. ovariectomized rats (OVX) 3. OVX + RA 4. OVX + Omega-3 FAs | Omega-3 FAs reduced TMJ damage and increased proinflammatory cytokine levels. |
| Ceotto et al., 2022 [64] | Male rats, CII +CFA-induced RA | Omega-3 FAs, 85 mg/kg/day, orally. | 1. Naïve (non-RA) 2. RA 3. RA + Omega-3 FAs 4. RA + Omega-3 FAs +Aspirin | Omega-3 FAs reduced TMJ damage identified and increased various cytokines. |
| Barbin et al., 2020 [65] | Rats, Sex: not described, CFA-induced facial inflammation | Omega-3 FAs, 300 mg/kg/day, orally. | 1. Naïve (no CFA) 2. CFA + Saline 3. CFA + dexamethasone 4. CFA + Omega-3 FAs | Omega-3 FAs reduced TMJ damage identified by histo-morphometric analysis. Differences in the level of IL-1 beta, TNF-alpha, and IL-10 between control and CFA groups were found. |
| Nakazaki et al., 2018 [66] | Male rats, CFA-induced TMJ inflammation) | Docosahexaenoic acid (DHA), 328 mg/kg, i.p. | 1. Naïve (no CFA) 2. CFA + Saline 3. CFA + DHA | DHA reduced mechanical behavioral sensitivities and neural activities in the trigeminal subnucleus caudalis. |
| Polyphenols | ||||
| Sashide et al., 2024 [51] | Male rats, CFA-induced facial inflammation | Quercetin, 1–10 mM, local injection. | 1. Before quercetin injection 2. After quercetin injection | Quercetin reduced facial stimulation-evoked neural activities in the trigeminal ganglion (TG). |
| Toyota et al., 2023 [52] | Male rats, naïve | Quercetin, 1, 10 mM, local injection. | 1. Before quercetin injection 2. After quercetin injection | Quercetin reduced facial stimulation-evoked nociceptive neural activities in the TG. |
| Liu et al., 2024 [53] | Male rats, LPS into the TG | Quercetin, 10 mM, trigeminal ganglion (TG) injection. | 1. Naïve (no LPS) 2. LPS + Saline 3. LPS + Quercetin | Quercetin reduced mechanical hyperalgesia-like behaviors in rats with the intra-TG injection of LPS. |
| Itou et al., 2022 [54] | Male rats, CFA-induced whisker pad inflammation | Quercetin, 50 mg/kg, i.p. | 1. Naïve (no CFA) 2. CFA + Saline 3. CFA + Diclofenac 4. CFA + Quercetin | Quercetin reduced mechanical hyperalgesia-like behaviors and neural activities in the trigeminal subnucleus caudalis. |
| Uchino et al., 2023 [55] | Male rats, naïve | (-)-epigallocatechin-3-gallate (EGCG), 1, 10 mM, local injection. | 1. Before EGCG injection 2. After EGCG injection | EGCG reduced neural activities in the trigeminal subnucleus caudalis region evoked by mechanical stimulation to the facial skin. |
| Shimazu et al., 2016 [56] | Male rats, naïve | Resveratrol (RSV), 1–10 mM, local injection. | 1. Before RSV injection 2. After RSV injection | Resveratrol suppresses the excitability of the trigeminal subnucleus caudalis neurons. |
| Ma et al., 2020 [67] | Male rats, CFA-induced facial inflammation | Resveratrol (RSV), 40 mg/kg or 80 mg/kg, i.p. | 1. Naïve (no CFA) + Dimethyl sulfoxide (DMSO) 2. Naïve (no CFA) + RSV 3. CFA + DSMO 4. CFA + RSV | Resveratrol increased the CFA-decreased mechanical withdrawal threshold. |
| Takehana et al., 2017 [57] | Male rats, naïve | Resveratrol, 2 mg/kg, i.v. | 1. Before RSV injection 2. After RSV injection | Resveratrol reduced neurotransmission of the trigeminal nucleus caudalis neurons evoked by mechanical stimulation. |
| Mittal et al., 2009 [50] | Male and female rats, formalin test | Curcumin, 25, 50, 100, 200, 400, and 600 mg/kg, i.p. | 1. DMSO 2. Curcumin 3. Diclofenac + Curcumin | Curcumin reduced facial pain-like behaviors. |
| Luca et al., 2014 [68] | Male mice, formalin test | Curcumin, 120 mg/kg, orally. | 1. Vehicle 2. Curcumin | Curcumin reduced facial pain-like behaviors. |
| Wu et al., 2017 [69] | Male rats, formalin test | Curcumin, 50 mg/kg, i.p. | 1. Naïve (no formalin) 2. Formalin 3. Formalin + Amiloride 4. Formalin + Curcumin | Curcumin reduced facial pain-like behaviors. |
| Yeon et al., 2010 [70] | Male rats, capsaicin test | Curcumin, 5, 25, or 50 mg/kg, i.p. | 1. Naïve (no capsaicin) 2. Naïve (no capsaicin) + Curcumin 3. Capsaicin + Vehicle 4. Capsaicin + Curcumin | Curcumin reduced capsaicin-induced thermal hyperalgesia-like behaviors in rats. |
| Isoprenoids | ||||
| Pereira et al., 2022 [71] | Male rats, formalin test | Limonene (LIM), 50 mg/kg, orally. | 1. Naïve (no formalin) 2. Formalin 3. Formalin + Morphine 4. Formalin + LIM 5. Formalin + LIM/HPβCD | Limonene reduced TMJ pain-like behaviors. |
| Santos et al., 2023 [72] | Male rats, formalin test or CFA-induced TMJ inflammation | Citral, 100 and 300 mg/kg, orally. | 1. Formalin + Tween80 2. Formalin + Citral 1. Naïve (no CFA) + Tween80 2. CFA + Tween80 3. CFA + Citral | Citral reduced formalin-induced orofacial nociceptive behavior and mechanical hyper-nociception in CFA-induced TMJ inflammation. |
| Santos et al., 2022 [73] | Male mice and rats, formalin, mustard oil, cinnamaldehyde, menthol, and capsaicin tests | Citral, 0.1, 0.3, or 1.0 mg/kg, orally. | 1. Naïve (non-inflamed) 2. Inflamed + vehicle 3. Inflamed + Citral | Citral reduced TMJ- or masseter muscle-evoked pain-like behaviors via TRPV1, TRPM3, and TRPM8 mechanisms. |
| Carotenoids | ||||
| Tamaddonfard et al., 2015 [74] | Male rats, capsaicin test | Crocin, 10 and 40 µg/rat, Intra-fourth ventricle injection. | 1. Capsaicin + Saline 2. Capsaicin + Morphine 3. Capsaicin + Crocin 4. Capsaicin + Morphine + Crocin | Intra-fourth ventricle administration of crocin reduced facial pain-like behavior independent of opioid mechanisms. |
| Shimazu et al., 2019 [75] | Male rats, mustard oil-evoked inflammation | Lutein, 10 mg/kg, i.p. | 1. Naïve (no mustard oil) 2. Mustard oil 3. Mustard oil + Lutein | Lutein reduced facial pain-like behaviors and c-Fos responses in the trigeminal caudalis and upper cervical dorsal horn. |
| Syoji et al., 2018 [76] | Male rats, CFA-induced inflammation | Lutein, 10 mg/kg, i.p. | 1. Naïve (no CFA) 2. CFA 3. CFA + Lutein | Lutein reduced mechanical hyperalgesia-like behaviors via the Cox-2 signaling cascade. |
| Lectins | ||||
| Rivanor et al., 2018 [77] | Male rats, formalin or carrageenan or capsaicin test | Lectin, 0. 1, 1, or 10 mg/kg, i.v. | 1. Naïve (non-inflamed) 2. Inflamed + Saline 3. Inflamed + Lectin | Lectin from green seaweed Caulerpa cupressoides reduced orofacial pain-like behaviors. |
| Rivanor et al., 2014 [78] | Male rats, Zymosan | Lectin, 0.1, 1, or 10 mg/kg, i.v. | 1. Naïve (no zymosan) 2. Zymosan + Saline 3. Zymosan + Indomethacin 4. Zymosan + Lectin | Lectin from the green seaweed Caulerpa cupressoides reduced mechanical hyper-nociception. |
| Leite et al., 2022 [79] | Mice and rats, Sex: not described, capsaicin, formalin test | Lectin, 0.25, 0.5, 1, 10 mL/kg, i.p. | 1. Naïve (non-inflamed) 2. Inflamed + Saline 3. Inflamed + Lectin | Parkia platycephala lectin reduced orofacial pain-like behavior. |
| Alves et al., 2018 [80] | Male rats, formalin test | Lectin, 0.001–0.1 mg/kg, i.v. | 1. Naïve (no formalin) 2. Formalin 3. Formalin + Morphine 4. Formalin + Lectin | Lectin from Abelmoschus esculentus reduced orofacial pain-like behavior. |
| Freitas et al., 2016 [81] | Male rats, zymosan-induced TMJ inflammation | Lectin, 0.01–1 mg/kg, i.v. | 1. Naïve (no zymosan) 2. Zymosan + Saline 3. Zymosan + Indomethacin 4. Zymosan + Lectin | Lectin from Abelmoschus esculentus reduced mechanical hyperalgesia-like behaviors. |
| Damasceno et al., 2016 [82] | Mice and rats, Sex: not described, formalin, capsaicin, glutamate test | Frutalin, 0.25, 0.5, or 1 mg/kg, i.p. | 1. Naïve (non-inflamed) 2. Inflamed + Saline 3. Inflamed + Frutalin | Frutalin reduced facial pain-like behaviors via nitrogenic oxide mechanisms. |
| Polysaccharides | ||||
| Araújo et al., 2017 [83] | Male rats, formalin test | Sulfated polysaccharide (SP), 0.03, 0.3 or 3.0 mg/kg, s.c. | 1. Naïve (no formalin) 2. Formalin 3. Formalin + Sulfated polysaccharide | SP from the red seaweed Solieria filiformis reduced facial pain-like behaviors, inhibited the plasma extravasation and inflammatory cytokines release, and increased the β-endorphin release in the trigeminal caudalis. |
| Souza et al., 2019 [84] | Male mice or rats, formalin test | Sulfated polysaccharide (SP), 5, 10 mg/kg, orally | 1. Naïve (no formalin) 2. Formalin 3. Formalin + Sulfated polysaccharide | SP from the marine algae Hypnea pseudomusciformis reduced orofacial pain-like behaviors. |
| Rodrigues et al., 2014 [85] | Male rats, zymosan-evoked TMJ inflammation | Sulfated polysaccharide (SP), 1, 3 and 9 mg/kg, s.c. | 1. Naïve (no zymosan) 2. Zymosan + Saline 3. Zymosan + Indomethacin 4. Zymosan + Sulfated polysaccharide | SP from the green seaweed Caulerpa cupressoides var. lycopodium reduced mechanical hyperalgesia-like behaviors. |
| Palmitoylethanolamide | ||||
| Bartolucci et al., 2018 [86] | Male rats, CFA-induced facial inflammation | Palmitoylethanolamide (PEA), 10 mg/kg, i.p. | 1. Naïve (no CFA) 2. Naïve (no CFA) + PEA 3. CFA + Vehicle 4. CFA + PEA | PEA reduced facial hyperalgesia-like behaviors after TMJ inflammation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piriyaprasath, K.; Kakihara, Y.; Hasegawa, M.; Iwamoto, Y.; Hasegawa, Y.; Fujii, N.; Yamamura, K.; Okamoto, K. Nutritional Strategies for Chronic Craniofacial Pain and Temporomandibular Disorders: Current Clinical and Preclinical Insights. Nutrients 2024, 16, 2868. https://doi.org/10.3390/nu16172868
Piriyaprasath K, Kakihara Y, Hasegawa M, Iwamoto Y, Hasegawa Y, Fujii N, Yamamura K, Okamoto K. Nutritional Strategies for Chronic Craniofacial Pain and Temporomandibular Disorders: Current Clinical and Preclinical Insights. Nutrients. 2024; 16(17):2868. https://doi.org/10.3390/nu16172868
Chicago/Turabian StylePiriyaprasath, Kajita, Yoshito Kakihara, Mana Hasegawa, Yuya Iwamoto, Yoko Hasegawa, Noritaka Fujii, Kensuke Yamamura, and Keiichiro Okamoto. 2024. "Nutritional Strategies for Chronic Craniofacial Pain and Temporomandibular Disorders: Current Clinical and Preclinical Insights" Nutrients 16, no. 17: 2868. https://doi.org/10.3390/nu16172868
APA StylePiriyaprasath, K., Kakihara, Y., Hasegawa, M., Iwamoto, Y., Hasegawa, Y., Fujii, N., Yamamura, K., & Okamoto, K. (2024). Nutritional Strategies for Chronic Craniofacial Pain and Temporomandibular Disorders: Current Clinical and Preclinical Insights. Nutrients, 16(17), 2868. https://doi.org/10.3390/nu16172868





