The Impact of Cornelian Cherry (Cornus mas L.) on Cardiometabolic Risk Factors: A Meta-Analysis of Randomised Controlled Trials
Abstract
1. Introduction
2. Methods
2.1. Literature Search
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Quality Assessment
2.5. Data Synthesis and Statistical Analysis
3. Results
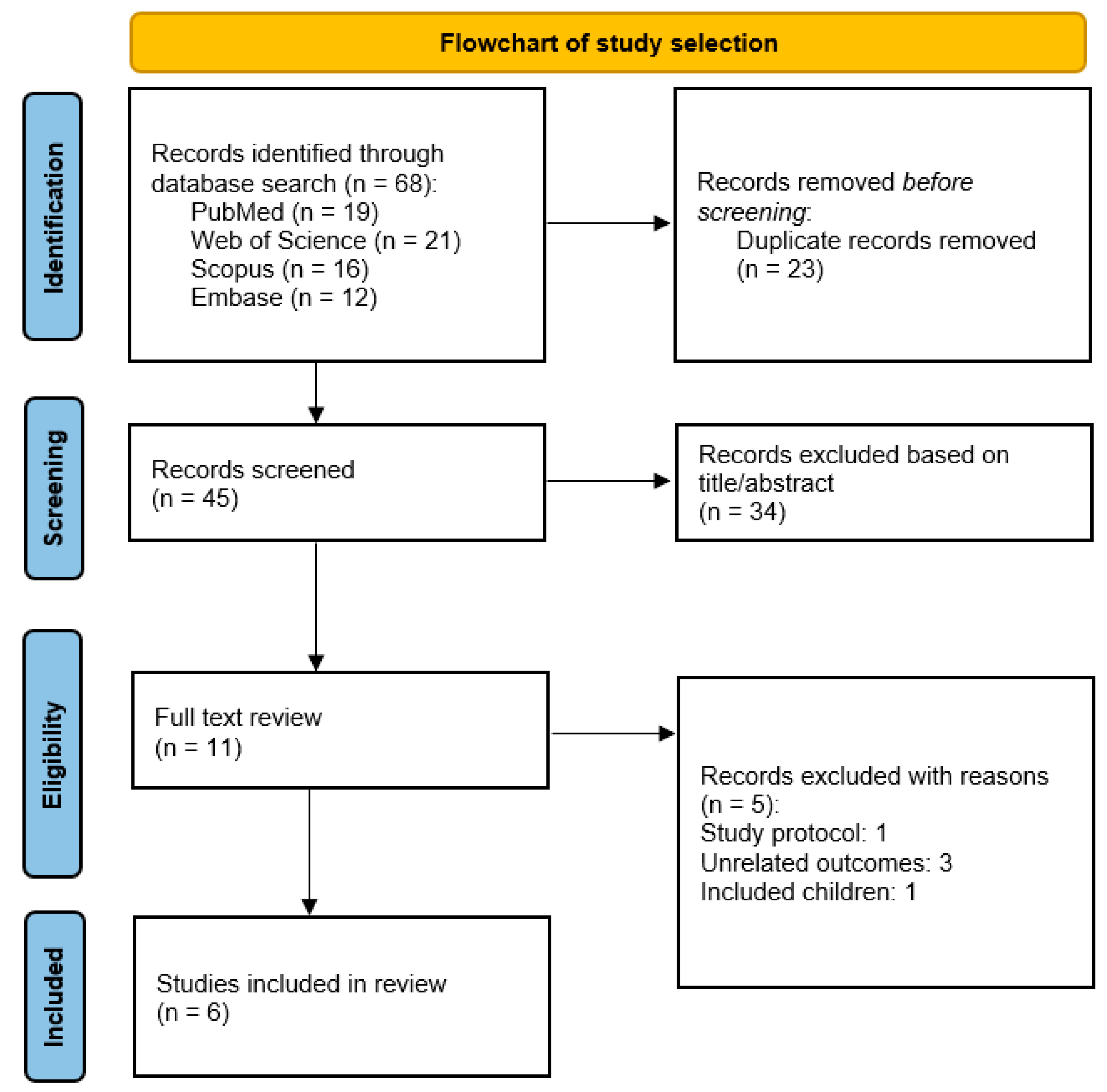
3.1. Study Selection
3.2. Study Characteristics
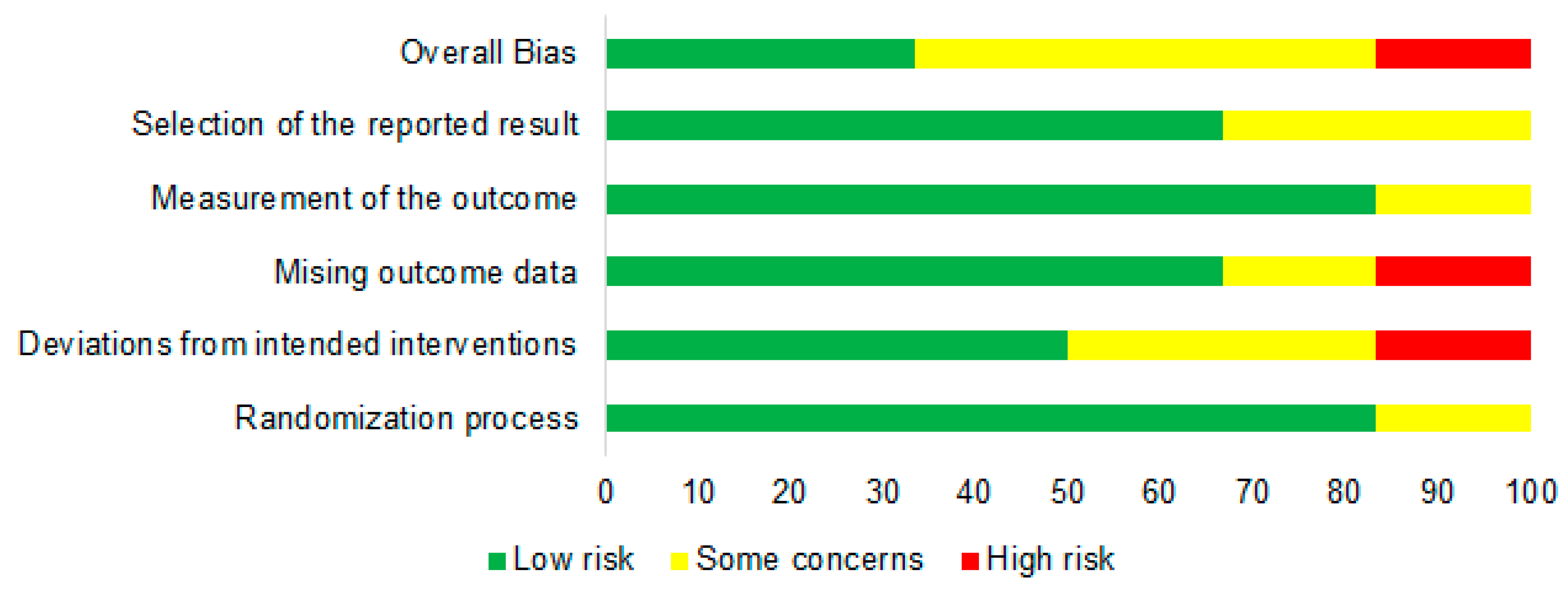
3.3. Risk of Bias Assessment
3.4. Meta-Analysis Results
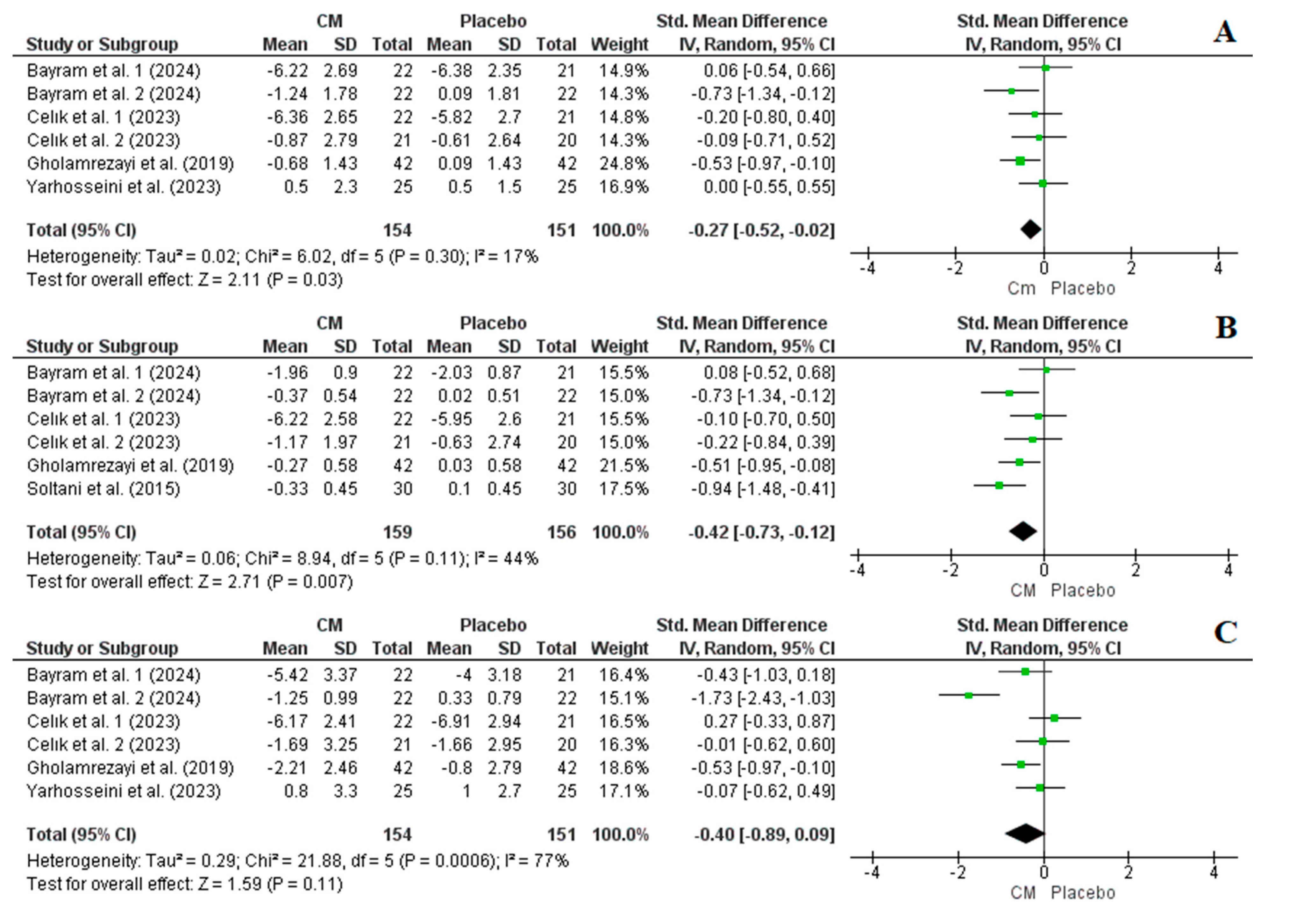
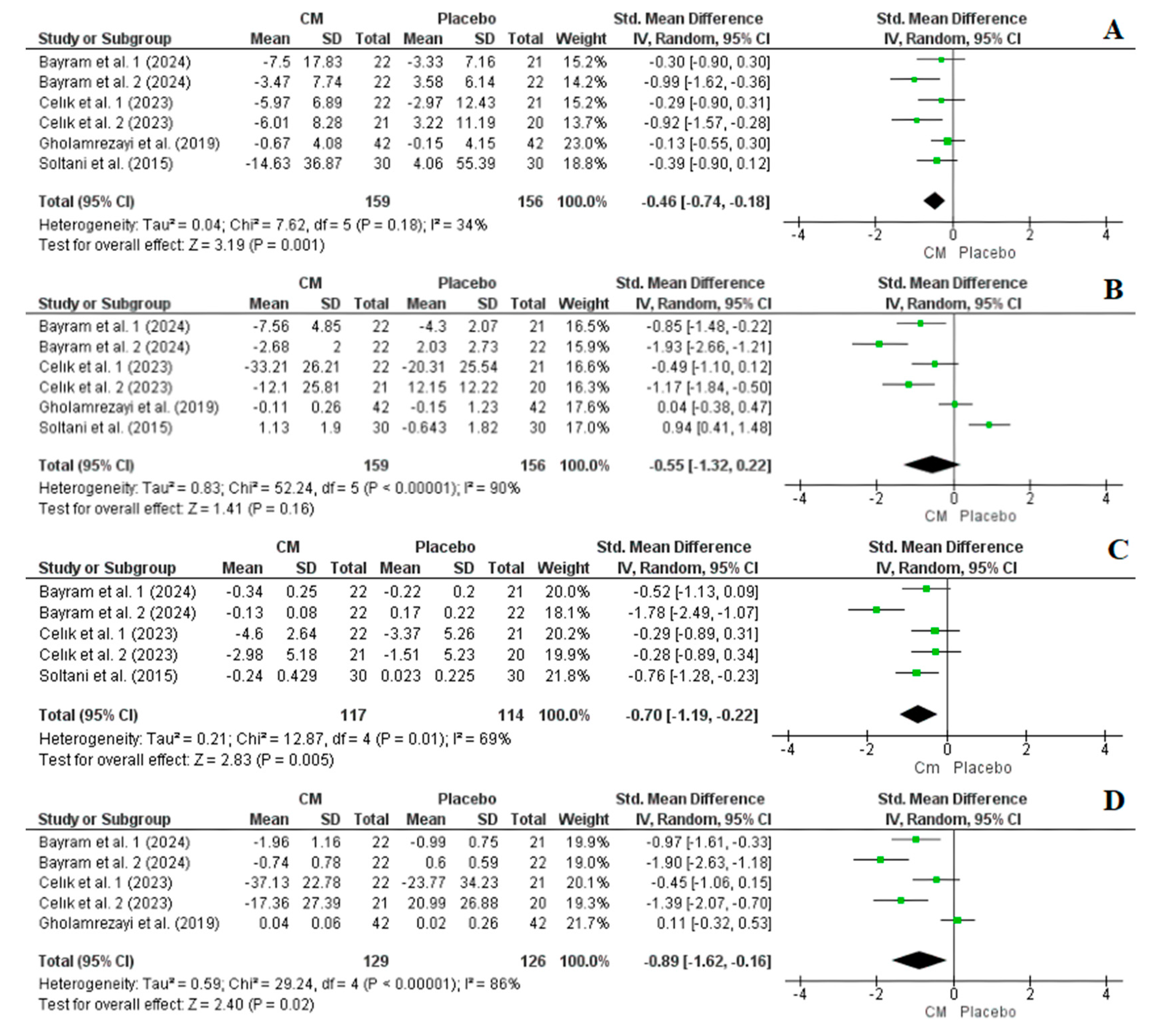
3.4.1. Effect of Cornelian Cherry Supplementation on BW, BMI, and WC
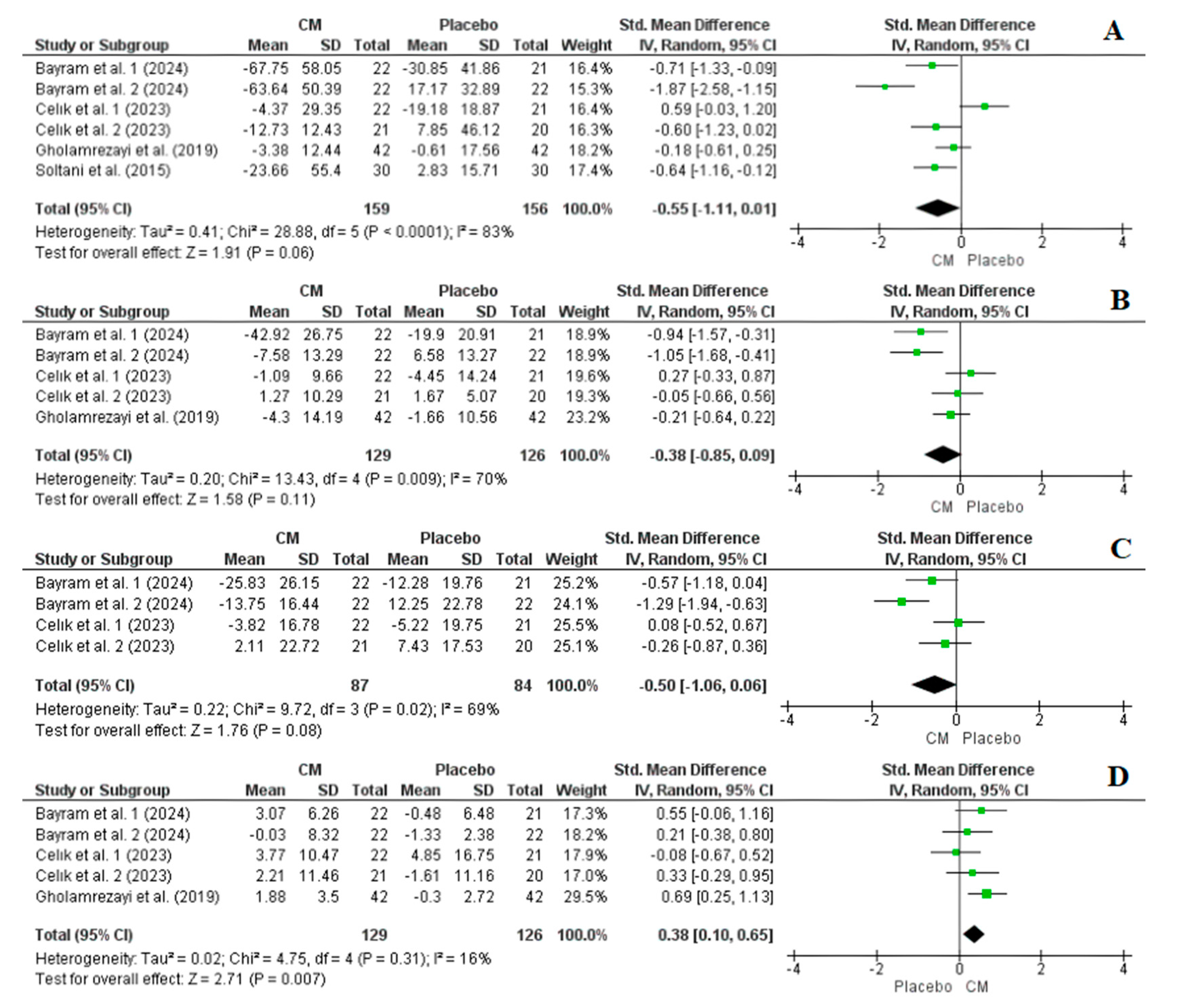
3.4.2. Effect of Cornelian Cherry Supplementation on TG, TC, LDL-C, and HDL-C Levels
3.4.3. Effect of Cornelian Cherry Supplementation on FBG, Insulin, HbA1c, and HOMA-IR
3.4.4. Effect of Cornelian Cherry Supplementation on AST and ALT
3.4.5. Effect of Cornelian Cherry Supplementation on SBP and DBP
3.4.6. Publication Bias
4. Discussion
4.1. The Effect of Cornelian Cherry Supplementation on Anthropometric Measurements
4.2. The Effect of Cornelian Cherry Supplementation on Total Blood Lipid Levels
4.3. The Effect of Cornelian Cherry Supplementation on Glycaemic Parameters
4.4. The Effect of Cornelian Cherry Supplementation on Liver Parameters
4.5. The Effect of Cornelian Cherry Supplementation on Blood Pressure
4.6. Weight-Loss-Dependent and Independent Effects of Cornelian Cherry Supplementation
4.7. Clinical Relevance of the Findings
5. Limitations and Future Perspectives
5.1. Methodological Limitations
5.2. Study-Related Limitations
5.3. Geographic and Supplement Quality Limitations
5.4. Future Research Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qorbani, M.; Mahdavi-Gorabi, A.; Khatibi, N.; Ejtahed, H.S.; Khazdouz, M.; Djalalinia, S.; Sahebkar, A.; Esmaeili-Abdar, M.; Hasani, M. Dietary diversity score and cardio-metabolic risk factors: An updated systematic review and meta-analysis. Eat. Weight Disord. 2022, 27, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N.; Forrest, E.; Preiss, D. Non-alcoholic fatty liver disease. BMJ 2014, 349, g4596. [Google Scholar] [CrossRef] [PubMed]
- Lan, Q.; Zhang, Y.; Lin, F.; Meng, Q.; Buys, N.J.; Fan, H.; Sun, J. Association between serum aminotransferases and risk of new-onset cardiometabolic disease in a healthy chinese population: A cohort study. Front. Public Health 2022, 10, 902393. [Google Scholar] [CrossRef] [PubMed]
- Porter, S.A.; Pedley, A.; Massaro, J.M.; Vasan, R.S.; Hoffmann, U.; Fox, C.S. Aminotransferase levels are associated with cardiometabolic risk above and beyond visceral fat and insulin resistance: The Framingham Heart Study. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.J.; Ozemek, C.; Carbone, S.; Katzmarzyk, P.T.; Blair, S.N. Sedentary behavior, Exercise, and Cardiovascular Health. Circ. Res. 2019, 124, 799–815. [Google Scholar] [CrossRef] [PubMed]
- WHO. Noncommunicable Diseases; WHO: Geneva, Switzerland, 2023. [Google Scholar]
- Shu, J.; Jin, W. Prioritizing non-communicable diseases in the post-pandemic era based on a comprehensive analysis of the GBD 2019 from 1990 to 2019. Sci. Rep. 2023, 13, 13325. [Google Scholar] [CrossRef] [PubMed]
- Rippe, J.M. Lifestyle strategies for risk factor reduction, prevention, and treatment of cardiovascular disease. Am. J. Lifestyle Med. 2019, 13, 204. [Google Scholar] [CrossRef]
- Kaminsky, L.A.; German, C.; Imboden, M.; Ozemek, C.; Peterman, J.E.; Brubaker, P.H. The importance of healthy lifestyle behaviors in the prevention of cardiovascular disease. Prog. Cardiovasc. Dis. 2022, 70, 8–15. [Google Scholar] [CrossRef]
- Laddu, D.; Ma, J.; Kaar, J.; Ozemek, C.; Durant, R.W.; Campbell, T.; Welsh, J.; Turrise, S. Health behavior change programs in primary care and community practices for cardiovascular disease prevention and risk factor management among midlife and older adults: A scientific statement from the American Heart Association. Circulation 2021, 144, E533–E549. [Google Scholar] [CrossRef]
- WHO. Increasing Fruit and Vegetable Consumption to Reduce the Risk of Noncommunicable Diseases. Available online: https://www.who.int/tools/elena/interventions/fruit-vegetables-ncds (accessed on 8 May 2024).
- Dinda, B.; Kyriakopoulos, A.M.; Dinda, S.; Zoumpourlis, V.; Thomaidis, N.S.; Velegraki, A.; Markopoulos, C.; Dinda, M. Cornus mas L. (cornelian cherry), an important European and Asian traditional food and medicine: Ethnomedicine, phytochemistry and pharmacology for its commercial utilization in drug industry. J. Ethnopharmacol. 2016, 193, 670–690. [Google Scholar] [CrossRef]
- Bayram, H.M.; Arda Ozturkcan, S. Bioactive components and biological properties of cornelian cherry (Cornus mas L.): A comprehensive review. J. Funct. Foods 2020, 75, 104252. [Google Scholar] [CrossRef]
- Mesgari Abbasi, M.; Hassanalilou, T.; Khordadmehr, M.; Mohammadzadeh Vardin, A.; Behroozi Kohlan, A.; Khalili, L. Effects of Cornus mas fruit hydro-methanolic extract on liver antioxidants and histopathologic changes induced by cisplatin in rats. Indian J. Clin. Biochem. 2020, 35, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Rafieian-Kopaei, M.; Adelnia, A.; Kazemi, S.; Shamsi, F. Comparing the effects of lovastatin and Cornus mas fruit on fibrinogen level in hypercholesterolemic rabbits. ARYA Atheroscler. 2010, 6, 1–5. [Google Scholar] [PubMed]
- Lotfi, A.; Aghdam Shahryar, H.; Rasoolian, H. Effects of cornelian cherry (Cornus mas L.) fruit on plasma lipids, cortisol, T3 and T4 levels in hamsters. J. Anim. Plant Sci. 2014, 24, 459–462. [Google Scholar]
- Rasoulian, H.; Shahryar, H.A.; Abbaspour, R.; Lotfi, H. Effects of dietary inclusion of cornelian cherry (Cornus mas L.) fruit on body weight, insulin level and glycemic status of hamsters. Pak. J. Biol. Sci. 2012, 15, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, K.; Alizadeh Sani, M.; Nattagh-Eshtivani, E.; Yaribash, S.; Rahmani, J.; Shokrollahi Yancheshmeh, B.; Julian McClements, D. A systematic review and meta-analysis of the impact of cornelian cherry consumption on blood lipid profiles. Food Sci. Nutr. 2021, 9, 4629–4638. [Google Scholar] [CrossRef] [PubMed]
- EFSA Food Supplements. Available online: https://www.efsa.europa.eu/en/topics/topic/food-supplements (accessed on 8 May 2024).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Jang, H.H.; Hwang, I.G.; Lee, Y.M. Effects of anthocyanin supplementation on blood lipid levels: A systematic review and meta-analysis. Front. Nutr. 2023, 10, 1207751. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Cochrane RoB 2: A Revised Cochrane Risk-of-Bias Tool for Randomized Trials. Available online: https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials (accessed on 15 April 2024).
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Khan, S. Meta-analysis of standardized mean difference. In Meta-Analysis: Methods for Health and Experimental Studies; Springer: Singapore, 2020; pp. 159–194. ISBN 978-981-15-5032-4. [Google Scholar]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. Br. Med. J. 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Soltani, R.; Gorji, A.; Asgary, S.; Sarrafzadegan, N.; Siavash, M. Evaluation of the effects of Cornus mas L. fruit extract on glycemic control and insulin level in type 2 diabetic adult patients: A randomized double-blind placebo-controlled clinical trial. Evid. Based Complement. Altern. Med. 2015, 2015, 740954. [Google Scholar] [CrossRef] [PubMed]
- Bayram, H.M.; Iliaz, R.; Gunes, F.E. Effects of Cornus mas L. on anthropometric and biochemical parameters among metabolic associated fatty liver disease patients: Randomized clinical trial. J. Ethnopharmacol. 2024, 318, 117068. [Google Scholar] [CrossRef] [PubMed]
- Gholamrezayi, A.; Aryaeian, N.; Rimaz, S.; Abolghasemi, J.; Fallah, S.; Moradi, N.; Taghizadeh, M. The effect of Cornus mas fruit extract consumption on lipid profile, glycemic indices, and leptin in postmenopausal women—A randomized clinical trial. Phyther. Res. 2019, 33, 2979–2988. [Google Scholar] [CrossRef] [PubMed]
- Sangsefidi, Z.S.; Yarhosseini, F.; Hosseinzadeh, M.; Ranjbar, A.; Akhondi-Meybodi, M.; Fallahzadeh, H.; Mozaffari-Khosravi, H. The effect of (Cornus mas L.) fruit extract on liver function among patients with nonalcoholic fatty liver: A double-blind randomized clinical trial. Phyther. Res. 2021, 35, 5259–5268. [Google Scholar] [CrossRef]
- Yarhosseini, F.; Sangouni, A.A.; Sangsefidi, Z.S.; Hosseinzadeh, M.; Akhondi-Meybodi, M.; Ranjbar, A.; Fallahzadeh, H.; Mozaffari-Khosravi, H. Effect of Cornus mas L. fruit extract on blood pressure, anthropometric and body composition indices in patients with non-alcoholic fatty liver disease: A double-blind randomized controlled trial. Clin. Nutr. ESPEN 2023, 56, 18–24. [Google Scholar] [CrossRef]
- Celık, Z.M.; Sargin, M.; Tamer, H.G.; Gunes, F.E. The effect of lyophilized dried cornelian cherry (Cornus mas L.) intake on anthropometric and biochemical parameters in women with insulin resistance: A randomized controlled trial. Food Sci. Nutr. 2023, 11, 8060–8071. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Mezhal, F.; Oulhaj, A.; Abdulle, A.; AlJunaibi, A.; Alnaeemi, A.; Ahmad, A.; Leinberger-Jabari, A.; Al Dhaheri, A.S.; AlZaabi, E.; Al-Maskari, F.; et al. High prevalence of cardiometabolic risk factors amongst young adults in the United Arab Emirates: The UAE Healthy Future Study. BMC Cardiovasc. Disord. 2023, 23, 137. [Google Scholar] [CrossRef]
- Wang, H.H.; Lee, D.K.; Liu, M.; Portincasa, P.; Wang, D.Q.H. Novel insights into the pathogenesis and management of the metabolic syndrome. Pediatr. Gastroenterol. Hepatol. Nutr. 2020, 23, 189. [Google Scholar] [CrossRef] [PubMed]
- Selinger, E.; Neuenschwander, M.; Koller, A.; Gojda, J.; Kühn, T.; Schwingshackl, L.; Barbaresko, J.; Schlesinger, S. Evidence of a vegan diet for health benefits and risks—An umbrella review of meta-analyses of observational and clinical studies. Crit. Rev. Food Sci. Nutr. 2023, 63, 9926–9936. [Google Scholar] [CrossRef] [PubMed]
- Rees, K.; Takeda, A.; Martin, N.; Ellis, L.; Wijesekara, D.; Vepa, A.; Das, A.; Hartley, L.; Stranges, S. Mediterranean-style diet for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2019, 3, CD009825. [Google Scholar] [CrossRef]
- Rees, K.; Al-Khudairy, L.; Takeda, A.; Stranges, S. Vegan dietary pattern for the primary and secondary prevention of cardiovascular diseases. Cochrane Database Syst. Rev. 2021, 2021, CD013501. [Google Scholar] [CrossRef]
- Klein, S.; Allison, D.B.; Heymsfield, S.B.; Kelley, D.E.; Leibel, R.L.; Nonas, C.; Kahn, R. Waist circumference and cardiometabolic risk: A consensus statement from Shaping America’s Health: Association for Weight Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Am. J. Clin. Nutr. 2007, 85, 1197–1202. [Google Scholar] [PubMed]
- Park, S.; Choi, M.; Lee, M. Effects of anthocyanin supplementation on reduction of obesity criteria: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2021, 13, 2121. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasam, B.; Olson, L.K.; Schutzki, R.E.; Tai, M.H.; Nair, M.G. Amelioration of obesity and glucose intolerance in high-fat-fed C57BL/6 mice by anthocyanins and ursolic acid in cornelian cherry (Cornus mas). J. Agric. Food Chem. 2006, 54, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Dzydzan, O.; Bila, I.; Kucharska, A.Z.; Brodyak, I.; Sybirna, N. Antidiabetic effects of extracts of red and yellow fruits of cornelian cherries (Cornus mas L.) on rats with streptozotocin-induced diabetes mellitus. Food Funct. 2019, 10, 6459–6472. [Google Scholar] [CrossRef]
- Park, E.; Kim, J.; Yeo, S.; Kim, G.; Ko, E.H.; Lee, S.W.; Li, W.Y.; Choi, C.W.; Jeong, S.Y. Antiadipogenic effects of loganic acid in 3T3-L1 preadipocytes and ovariectomized mice. Molecules 2018, 23, 1663. [Google Scholar] [CrossRef] [PubMed]
- Yarhosseini, F.; Darand, M.; Sangsefidi, Z.S.; Mozaffari-Khosravi, H.; Hosseinzadeh, M. Does anthocyanins consumption affect weight and body composition? A systematic review and meta-analysis of randomized controlled trials. Obes. Sci. Pract. 2023, 9, 42–58. [Google Scholar] [CrossRef] [PubMed]
- Sangouni, A.A.; Sangsefidi, Z.S.; Yarhosseini, F.; Hosseinzadeh, M.; Akhondi-Meybodi, M.; Ranjbar, A.; Madadizadeh, F.; Mozaffari-Khosravi, H. Effect of Cornus mas L. fruit extract on lipid accumulation product and cardiovascular indices in patients with non-alcoholic fatty liver disease: A double-blind randomized controlled trial. Clin. Nutr. ESPEN 2022, 47, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Kelishadi, R.; Rafieian-Kopaei, M.; Najafi, S.; Najafi, M.; Sahebkar, A. Investigation of the lipid-modifying and antiinflammatory effects of Cornus mas L. supplementation on dyslipidemic children and adolescents. Pediatr. Cardiol. 2013, 34, 1729–1735. [Google Scholar] [CrossRef] [PubMed]
- Daneshzad, E.; Shab-Bidar, S.; Mohammadpour, Z.; Djafarian, K. Effect of anthocyanin supplementation on cardio-metabolic biomarkers: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2019, 38, 1153–1165. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and human health: The role of bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Stein, R.; Ferrari, F.; Scolari, F. Genetics, dyslipidemia, and cardiovascular disease: New insights. Curr. Cardiol. Rep. 2019, 21, 68. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Xia, M.; Ma, J.; Hao, Y.T.; Liu, J.; Mou, H.Y.; Cao, L.; Ling, W.H. Anthocyanin supplementation improves serum LDL- and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am. J. Clin. Nutr. 2009, 90, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xia, M.; Yan, X.; Li, D.; Wang, L.; Xu, Y.; Jin, T.; Ling, W. Gut microbiota metabolism of anthocyanin promotes reverse cholesterol transport in mice via repressing miRNA-10b. Circ. Res. 2012, 111, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Sozański, T.; Kucharska, A.Z.; Szumny, A.; Magdalan, J.; Bielska, K.; Merwid-Ląd, A.; Woźniak, A.; Dzimira, S.; Piórecki, N.; Trocha, M. The protective effect of the Cornus mas fruits (cornelian cherry) on hypertriglyceridemia and atherosclerosis through PPARα activation in hypercholesterolemic rabbits. Phytomedicine 2014, 21, 1774–1784. [Google Scholar] [CrossRef]
- Sozański, T.; Kucharska, A.Z.; Rapak, A.; Szumny, D.; Trocha, M.; Merwid-Ląd, A.; Dzimira, S.; Piasecki, T.; Piórecki, N.; Magdalan, J.; et al. Iridoid–loganic acid versus anthocyanins from the Cornus mas fruits (cornelian cherry): Common and different effects on diet-induced atherosclerosis, PPARs expression and inflammation. Atherosclerosis 2016, 254, 151–160. [Google Scholar] [CrossRef]
- Zhou, B.; Sheffer, K.E.; Bennett, J.E.; Gregg, E.W.; Danaei, G.; Singleton, R.K.; Shaw, J.E.; Mishra, A.; Lhoste, V.P.F.; Carrillo-Larco, R.M.; et al. Global variation in diabetes diagnosis and prevalence based on fasting glucose and hemoglobin A1c. Nat. Med. 2023, 29, 2885–2901. [Google Scholar] [CrossRef]
- Ruijgrok, C.; Dekker, J.M.; Beulens, J.W.; Brouwer, I.A.; Coupé, V.M.H.; Heymans, M.W.; Sijtsma, F.P.C.; Mela, D.J.; Zock, P.L.; Olthof, M.R.; et al. Size and shape of the associations of glucose, HbA1c, insulin and HOMA-IR with incident type 2 diabetes: The Hoorn Study. Diabetologia 2018, 61, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Rafieian-Kopaei, M.; Shamsi, F.; Najafi, S.; Sahebkar, A. Biochemical and histopathological study of the anti-hyperglycemic and anti-hyperlipidemic effects of cornelian cherry (Cornus mas L.) in alloxan-induced diabetic rats. J. Complement. Integr. Med. 2014, 11, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Capcarova, M.; Kalafova, A.; Schwarzova, M.; Schneidgenova, M.; Svik, K.; Prnova, M.S.; Slovak, L.; Kovacik, A.; Lory, V.; Zorad, S.; et al. Cornelian cherry fruit improves glycaemia and manifestations of diabetes in obese Zucker diabetic fatty rats. Res. Vet. Sci. 2019, 126, 118–123. [Google Scholar] [CrossRef]
- Małodobra-Mazur, M.; Cierzniak, A.; Ryba, M.; Sozański, T.; Piórecki, N.; Kucharska, A.Z. Cornus mas L. increases glucose uptake and the expression of PPARG in insulin-resistant adipocytes. Nutrients 2022, 14, 2307. [Google Scholar] [CrossRef] [PubMed]
- Blagojević, B.; Agić, D.; Serra, A.T.; Matić, S.; Matovina, M.; Bijelić, S.; Popović, B.M. An in vitro and in silico evaluation of bioactive potential of cornelian cherry (Cornus mas L.) extracts rich in polyphenols and iridoids. Food Chem. 2021, 335, 127619. [Google Scholar] [CrossRef]
- Mao, T.; Akshit, F.N.U.; Mohan, M.S. Effects of anthocyanin supplementation in diet on glycemic and related cardiovascular biomarkers in patients with type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Front. Nutr. 2023, 10, 1199815. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Ramírez, B.A.; Catalán, Ú.; Llauradó, E.; Valls, R.M.; Salamanca, P.; Rubió, L.; Yuste, S.; Solà, R. The health benefits of anthocyanins: An umbrella review of systematic reviews and meta-analyses of observational studies and controlled clinical trials. Nutr. Rev. 2022, 80, 1515–1530. [Google Scholar] [CrossRef]
- Gong, L.; Feng, D.; Wang, T.; Ren, Y.; Liu, Y.; Wang, J. Inhibitors of α-amylase and α-glucosidase: Potential linkage for whole cereal foods on prevention of hyperglycemia. Food Sci. Nutr. 2020, 8, 6320–6337. [Google Scholar] [CrossRef]
- Zou, T.B.; Feng, D.; Song, G.; Li, H.W.; Tang, H.W.; Ling, W.H. The role of sodium-dependent glucose transporter 1 and glucose transporter 2 in the absorption of cyanidin-3-O-β-glucoside in caco-2 cells. Nutrients 2014, 6, 4165–4177. [Google Scholar] [CrossRef]
- Yan, F.; Zhang, J.; Zhang, L.; Zheng, X. Mulberry anthocyanin extract regulates glucose metabolism by promotion of glycogen synthesis and reduction of gluconeogenesis in human HepG2 cells. Food Funct. 2016, 7, 425–433. [Google Scholar] [CrossRef]
- Monsalve, F.A.; Pyarasani, R.D.; Delgado-Lopez, F.; Moore-Carrasco, R. Peroxisome proliferator-activated receptor targets for the treatment of metabolic diseases. Mediat. Inflamm. 2013, 2013, 549627. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, A.M.; González-Ortiz, M.; Martínez-Abundis, E.; Acuña Ortega, N. Effect of ursolic acid on metabolic syndrome, insulin sensitivity, and inflammation. J. Med. Food 2017, 20, 882–886. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.J.G.; Frederico, M.J.S.; Cazarolli, L.H.; Mendes, C.P.; Bretanha, L.C.; Schmidt, É.C.; Bouzon, Z.L.; De Medeiros Pinto, V.A.; Da Fonte Ramos, C.; Pizzolatti, M.G.; et al. The mechanism of action of ursolic acid as insulin secretagogue and insulinomimetic is mediated by cross-talk between calcium and kinases to regulate glucose balance. Biochim. Biophys. Acta—Gen. Subj. 2015, 1850, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Szczepaniak, O.; Cielecka-Piontek, J.; Kobus-Cisowska, J. Hypoglycaemic, antioxidative and phytochemical evaluation of Cornus mas varieties. Eur. Food Res. Technol. 2021, 247, 183–191. [Google Scholar] [CrossRef]
- Dzydzan, O.; Brodyak, I.; Sokół-łętowska, A.; Kucharska, A.Z.; Sybirna, N. Loganic acid, an iridoid glycoside extracted from Cornus mas L. fruits, reduces of carbonyl/oxidative stress biomarkers in plasma and restores antioxidant balance in leukocytes of rats with streptozotocin-induced diabetes mellitus. Life 2020, 10, 349. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.F.; Kai, J.; Guha, I.N.; Qureshi, N. The value of aspartate aminotransferase and alanine aminotransferase in cardiovascular disease risk assessment. Open Heart 2015, 2, e000272. [Google Scholar] [CrossRef]
- Kohsari, M.; Moradinazar, M.; Rahimi, Z.; Pasdar, Y.; Shakiba, E. Liver enzymes and their association with some cardiometabolic diseases: Evidence from a large Kurdish cohort. Biomed Res. Int. 2021, 2021, 5584452. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.; Gao, J.; Liu, H.; Zhang, C.; Huang, J.; Fan, J.; Wei, J. Preventive effect and mechanism of anthocyanins from Aronia melanocarpa Elliot on hepatic fibrosis through TGF-β/Smad signaling pathway. Cell Biochem. Biophys. 2022, 80, 737–745. [Google Scholar] [CrossRef]
- Sheikha, M.A.; Soheir, N.A.; Syrageldin, F.M. Synthesis, characterization and protection effect of black rice anthocyanins nano-composite against hepatotoxicity induced by methotrexate in rats. Braz. J. Biol. 2024, 84, e248726. [Google Scholar] [CrossRef]
- Zhou, F.; She, W.; He, L.; Zhu, J.; Gu, L. The effect of anthocyanins supplementation on liver enzymes among patients with metabolic disorders: A systematic review and meta-analysis of randomized clinical trials. Phytother. Res. 2022, 36, 53–61. [Google Scholar] [CrossRef]
- Sangsefidi, Z.S.; Mozaffari-Khosravi, H.; Sarkhosh-Khorasani, S.; Hosseinzadeh, M. The effect of anthocyanins supplementation on liver enzymes: A systematic review and meta-analysis of randomized clinical trials. Food Sci. Nutr. 2021, 9, 3954–3970. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, F.D.; Whelton, P.K. High blood pressure and cardiovascular disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Vitale, M.; Micek, A.; Ray, S.; Martini, D.; Del Rio, D.; Riccardi, G.; Galvano, F.; Grosso, G. Dietary polyphenol intake, blood pressure, and hypertension: A systematic review and meta-analysis of observational studies. Antioxidants 2019, 8, 152. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.R.; Gochenaur, K. Direct vasoactive and vasoprotective properties of anthocyanin-rich extracts. J. Appl. Physiol. 2006, 100, 1164–1170. [Google Scholar] [CrossRef]
- Parichatikanond, W.; Pinthong, D.; Mangmool, S. Blockade of the renin-angiotensin system with delphinidin, cyanin, and quercetin. Planta Med. 2012, 78, 1626–1632. [Google Scholar] [CrossRef]
- Vendrame, S.; Klimis-Zacas, D. Potential factors influencing the effects of anthocyanins on blood pressure regulation in humans: A review. Nutrients 2019, 11, 1431. [Google Scholar] [CrossRef] [PubMed]
- Dattilo, A.M.; Kris-Etherton, P.M. Effects of weight reduction on blood lipids and lipoproteins: A meta-analysis. Am. J. Clin. Nutr. 1992, 56, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Harder, H.; Dinesen, B.; Astrup, A. The effect of a rapid weight loss on lipid profile and glycemic control in obese type 2 diabetic patients. Int. J. Obes. 2004, 28, 180–182. [Google Scholar] [CrossRef]
- Zhou, K.; Wolski, K.; Malin, S.K.; Aminian, A.; Schauer, P.R.; Bhatt, D.L.; Kashyap, S.R. Impact of weight loss trajectory following randomization to bariatric surgery on long-term diabetes glycemic and cardiometabolic parameters. Endocr. Pract. 2019, 25, 572–579. [Google Scholar] [CrossRef]
- Shinde, S.; Thieu, V.T.; Kwan, A.Y.M.; Houghton, K.; Meyers, J.; Schapiro, D. Impact of weight change on glycemic control and metabolic parameters in T2D: A retrospective US study based on real-world data. Diabetes Ther. 2024, 15, 409–426. [Google Scholar] [CrossRef]


| Criteria | Description |
|---|---|
| Population | Adult participants (aged ≥ 18 years), excluding pregnant individuals, whether healthy or otherwise |
| Intervention | Supplementation with cornelian cherry fruits/powder/extract |
| Comparison | Placebo |
| Study | Evaluation of the effects of cornelian cherry on cardiometabolic risk factors, including BW, BMI, WC, TG, TC, LDL-C, HDL-C, SBP, DBP, FBG, insulin, HbA1c, HOMA-IR, AST, and ALT |
| Outcome | Randomised controlled trials with either a crossover or parallel trial design, lasting at least ≥2 weeks |
| Study (Year), Country | Study Design | Participants | Intervention/d | Control | Type of Intervention | Duration | Total Sample (Intervention/Placebo) Sex Distribution (M/F) | Measured Outcomes |
|---|---|---|---|---|---|---|---|---|
| Soltani et al. (2015), Iran [28] | Parallel, double-blinded, randomised clinical trial | T2DM | 500 mg CM extract (150 mg anthocyanins) | Placebo | Extract | 6 wk | 60 (30/30) (39/21) | ↔ BMI, ↔ FBG, ↓ Insulin, ↓ HbA1c, ↓ TG, ↔ ALT, ↔ AST |
| Gholamrezayi et al. (2019), Iran [30] | Parallel, double-blinded, randomised clinical trial | Postmenopausal women | 900 mg CM extract | Placebo | Extract | 8 wk | 84 (42/42) (0/84) | ↓ BW, ↓ BMI, ↓ WC, ↔ FBG, ↔ Insulin, ↔ HOMA-IR, ↔ TG, ↔ LDL-C, ↑ HDL-C, ↔ TC |
| Sangsefidi et al. (2021), Iran [31] | Parallel, double-blinded, randomised clinical trial | MAFLD | 20 mL CM extract (2800 mg dried extract, 32 mg anthocyanins) | Placebo | Extract | 12 wk | 50 (25/25) (23/27) | ↔ ALT, ↔ AST |
| Celık et al. (2023), Turkey [33] | Parallel, randomised controlled trial | Insulin resistance | 20 g lyophilised dried CM powder | Placebo | Powder | 12 wk | 84 (43/41) (0/84) | ↔ BW, ↔ BMI, ↔ WC, ↓ FBG, ↓ HbA1c, ↓ Insulin, ↓ HOMA-IR, ↓ TC, ↔ LDL-C, ↔ HDL-C, ↓ TG |
| Yarhosseini et al. (2023), Iran [32] | Parallel, double-blinded, randomised clinical trial | MAFLD | 20 mL CM extract (2800 mg dried extract, 32 mg anthocyanins) | Placebo | Extract | 12 wk | 50 (25/25) (23/27) | ↔ BW, ↔ WC, ↓ SBP, ↓ DBP |
| Bayram et al. (2024), Turkey [29] | Parallel, single-blinded, randomised controlled trial | MAFLD | 30 g lyophilised dried CM powder | Placebo | Powder | 8 wk | 87 (44/43) (40/47) | ↓ BW, ↓ BMI, ↓ WC, ↓ FBG, ↓ Insulin, ↓ HOMA-IR, ↓ HbA1c, ↓ AST, ↓ ALT, ↓ TG, HDL-C, ↓ LDL-C, ↓ TC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frumuzachi, O.; Kieserling, H.; Rohn, S.; Mocan, A.; Crișan, G. The Impact of Cornelian Cherry (Cornus mas L.) on Cardiometabolic Risk Factors: A Meta-Analysis of Randomised Controlled Trials. Nutrients 2024, 16, 2173. https://doi.org/10.3390/nu16132173
Frumuzachi O, Kieserling H, Rohn S, Mocan A, Crișan G. The Impact of Cornelian Cherry (Cornus mas L.) on Cardiometabolic Risk Factors: A Meta-Analysis of Randomised Controlled Trials. Nutrients. 2024; 16(13):2173. https://doi.org/10.3390/nu16132173
Chicago/Turabian StyleFrumuzachi, Oleg, Helena Kieserling, Sascha Rohn, Andrei Mocan, and Gianina Crișan. 2024. "The Impact of Cornelian Cherry (Cornus mas L.) on Cardiometabolic Risk Factors: A Meta-Analysis of Randomised Controlled Trials" Nutrients 16, no. 13: 2173. https://doi.org/10.3390/nu16132173
APA StyleFrumuzachi, O., Kieserling, H., Rohn, S., Mocan, A., & Crișan, G. (2024). The Impact of Cornelian Cherry (Cornus mas L.) on Cardiometabolic Risk Factors: A Meta-Analysis of Randomised Controlled Trials. Nutrients, 16(13), 2173. https://doi.org/10.3390/nu16132173











