Gastroprotective Effect of Isoferulic Acid Derived from Foxtail Millet Bran against Ethanol-Induced Gastric Mucosal Injury by Enhancing GALNT2 Enzyme Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
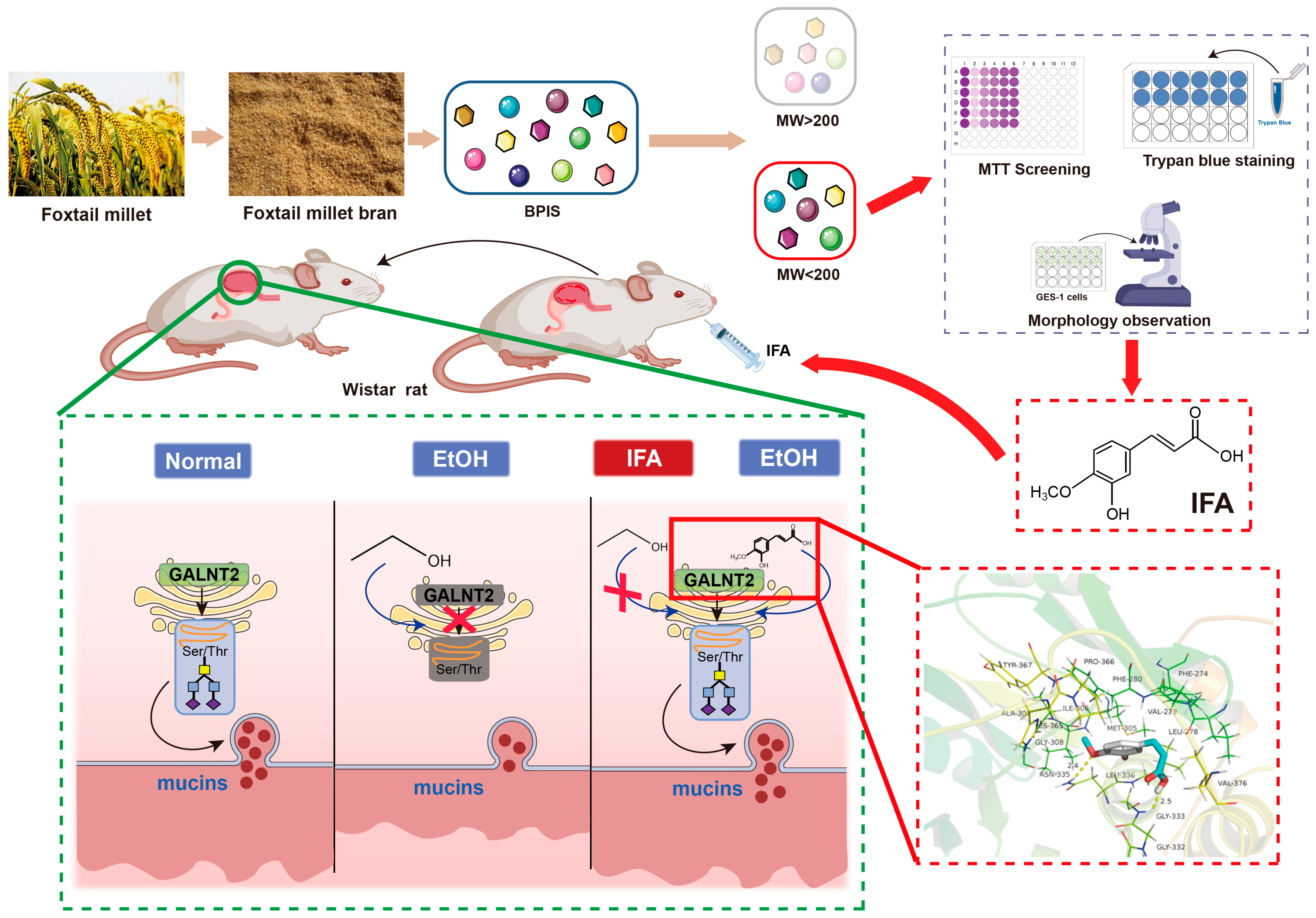
2.2. BPIS Extraction and Component Separation
2.3. Cell Experiment
2.3.1. Cell Line and Cell Culture
2.3.2. Protective Effects of Different Components of BPIS on Ethanol-Induced Damaged Cells
2.4. Animal Experiment
2.4.1. Animal Experiment Design
2.4.2. Macroscopic Assessment of the Gastric Mucosal Injury and Guth Injury Index Score
2.4.3. H&E Staining and Pathological Index Score
2.4.4. Determination of Aminohexose and Gastric Wall-Binding Mucus
2.4.5. RNA Extraction, Reverse Transcription, and Real-Time PCR Analysis
2.4.6. Vicia villosa Lectin Immunoprecipitation
2.4.7. Deglycosylation of Mucins
2.5. GALNT–Transferase Activity Assays
2.6. Molecular Docking of IFA to GALNTs
2.7. Determination of the Interaction between IFA and GALNT2
2.8. Statistical Analysis
3. Results
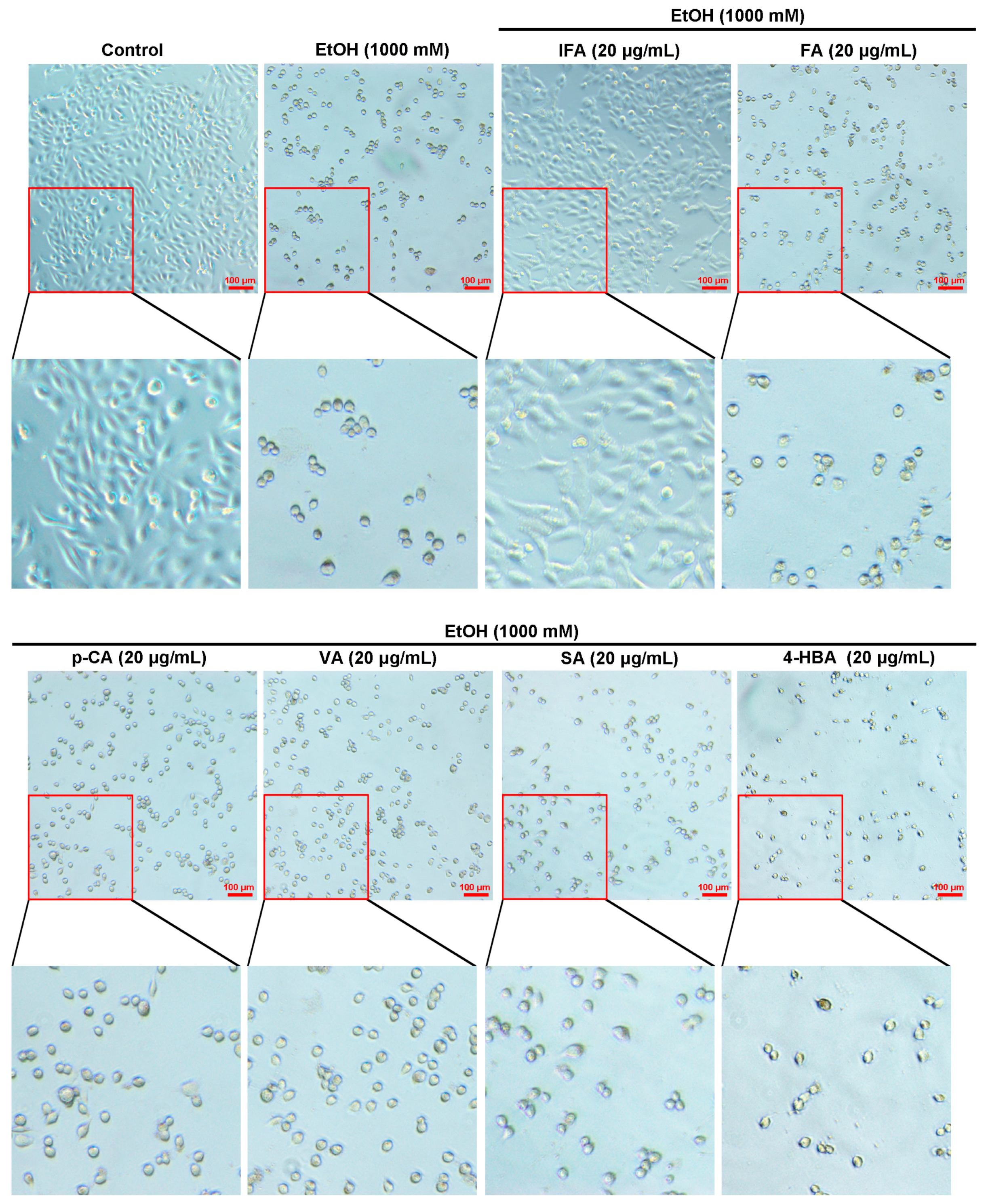
3.1. BPIS (MW < 200 D) Significantly Alleviates Alcohol-Induced Gastric Epithelial Cell Injury
3.2. IFA Is the Main Component of BPIS (MW < 200 D) in Inhibiting Alcoholic Gastric Mucosa Injury
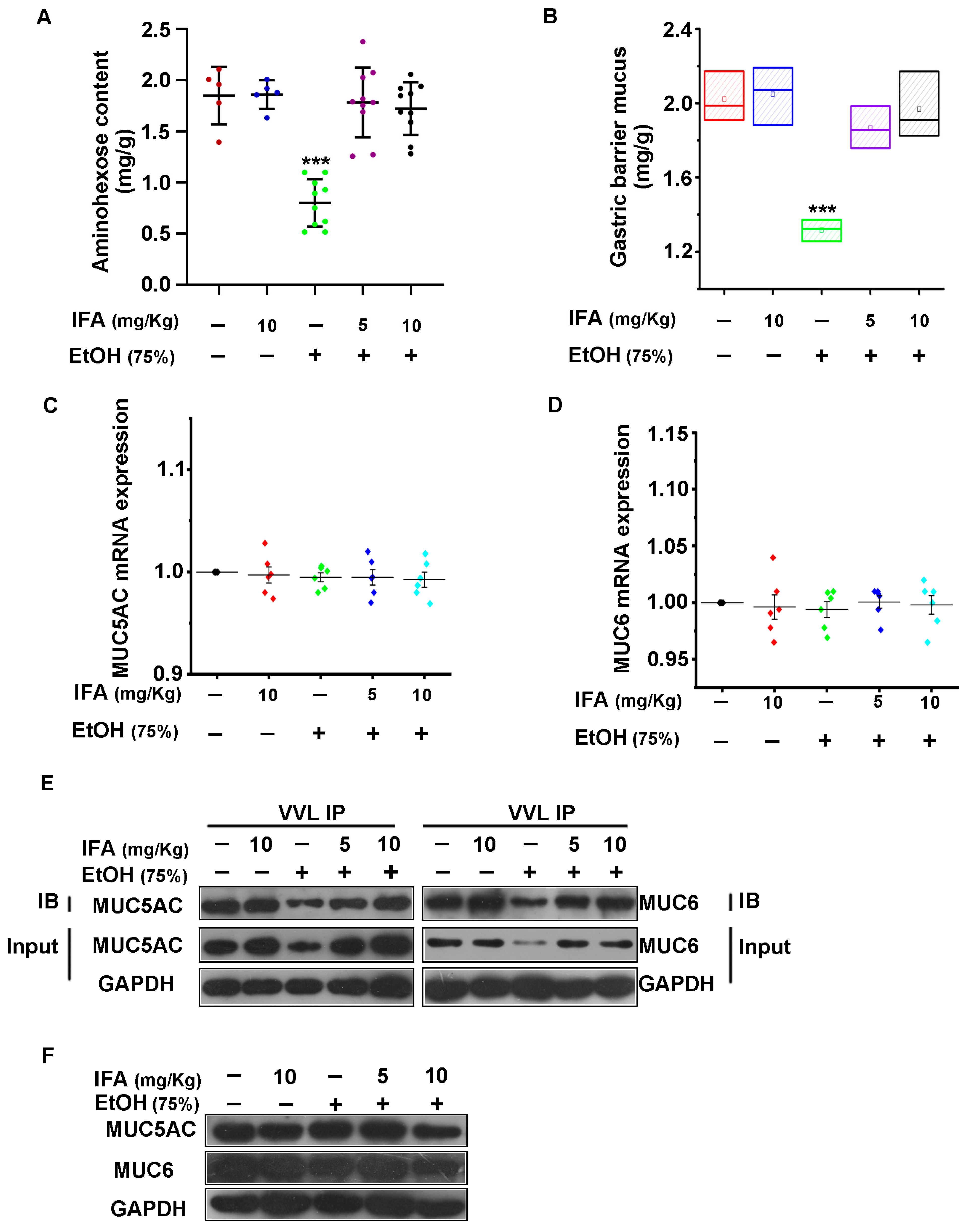
3.3. IFA Improves Alcoholic Gastric Mucosa Injury in Wistar Rats
3.4. IFA Promotes the Expression of Mucin in the Gastric Mucosa of Wistar Rats
3.5. IFA Facilitates Mucin Synthesis by Increasing the Activity of Glycosyltransferase GALNT2
3.6. IFA Alleviates the Inhibitory Effect of Alcohol on GALNT2 Activity by Interacting with GALNT2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Y.; Hu, J.; Long, X.; Pan, Y.; Mu, J.; Park, K.Y.; Zhao, X. Lactobacillus plantarum ZS62 Alleviates Alcohol-Induced Gastric Injury in Mice via an Anti-Oxidative Mechanism. Drug Des. Devel Ther. 2021, 15, 1667–1676. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sui, D.; Fu, W.; Sun, L.; Li, Y.; Yu, P.; Yu, X.; Zhou, Y.; Xu, H. Protective effects of polysaccharides from Panax ginseng on acute gastric ulcers induced by ethanol in rats. Food Funct. 2021, 12, 2741–2749. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.; Synnerstad, I.; Holm, L. Acid transport through channels in the mucous layer of rat stomach. Gastroenterology 2000, 119, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Chun, S.; Ohk, S.O.; Kim, S.; Kim, J.; Lee, S.; Kim, H.; Kim, S. Amelioration of alcohol-induced gastric mucosa damage by oral administration of food-polydeoxyribonucleotides. Mol. Med. Rep. 2021, 24, 790. [Google Scholar] [CrossRef] [PubMed]
- Yeo, D.; Hwang, S.J.; Song, Y.S.; Lee, H.J. Humulene Inhibits Acute Gastric Mucosal Injury by Enhancing Mucosal Integrity. Antioxidants 2021, 10, 761. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, J.; Wang, Y.; Gupta, R.; Li, Y.; Haridass, P.; Subramani, D.B.; Reidel, B.; Morton, L.; Ridley, C.; O’Neal, W.K.; et al. Assembly and organization of the N-terminal region of mucin MUC5AC: Indications for structural and functional distinction from MUC5B. Proc. Natl. Acad. Sci. USA 2021, 118, e2104490118. [Google Scholar] [CrossRef] [PubMed]
- Battista, S.; Ambrosio, M.R.; Limarzi, F.; Gallo, G.; Saragoni, L. Molecular Alterations in Gastric Preneoplastic Lesions and Early Gastric Cancer. Int. J. Mol. Sci. 2021, 22, 6652. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, M.A.; Swanson, B.J. Mucins in cancer: Protection and control of the cell surface. Nat. Rev. Cancer 2004, 4, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Fu, J.; Bergstrom, K.; Shan, X.; McDaniel, J.M.; McGee, S.; Bai, X.; Chen, W.; Xia, L. Core 1-derived mucin-type O-glycosylation protects against spontaneous gastritis and gastric cancer. J. Exp. Med. 2020, 217, e20182325. [Google Scholar] [CrossRef] [PubMed]
- Melhem, H.; Regan-Komito, D.; Niess, J.H. Mucins Dynamics in Physiological and Pathological Conditions. Int. J. Mol. Sci. 2021, 22, 13642. [Google Scholar] [CrossRef]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef] [PubMed]
- Bennett, E.P.; Mandel, U.; Clausen, H.; Gerken, T.A.; Fritz, T.A.; Tabak, L.A. Control of mucin-type O-glycosylation: A classification of the polypeptide GalNAc-transferase gene family. Glycobiology 2012, 22, 736–756. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Bi, X.; Wu, R.; Belfield, E.J.; Harberd, N.P.; Christensen, B.T.; Charles, M.; Bogaard, A. The potential of stable carbon and nitrogen isotope analysis of foxtail and broomcorn millets for investigating ancient farming systems. Front. Plant Sci. 2022, 13, 1018312. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; Dekanski, D.; Ristić, S.; Radonjić, N.V.; Petronijević, N.D.; Giampieri, F.; Astolfi, P.; González-Paramás, A.M.; Santos-Buelga, C.; Tulipani, S.; et al. Strawberry polyphenols attenuate ethanol-induced gastric lesions in rats by activation of antioxidant enzymes and attenuation of MDA increase. PLoS ONE 2011, 6, e25878. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Sun, F.; Ren, C.; Zhai, L.; Xiong, R.; Yang, Y.; Yang, W.; Yi, R.; Li, C.; Zhao, X. Hunan insect tea polyphenols provide protection against gastric injury induced by HCl/ethanol through an antioxidant mechanism in mice. Food Funct. 2021, 12, 747–760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, D.Y.; Yuan, Y.; Chen, J.; Yi, S.; Chen, B.; Zhao, X. Liubao Insect tea polyphenols prevent HCl/ethanol induced gastric damage through its antioxidant ability in mice. RSC Adv. 2020, 10, 4984–4995. [Google Scholar] [CrossRef] [PubMed]
- Ghani, I.; An, Y.; Qiao, Q.; He, S.; Li, Z. Polyphenols from Foxtail Millet Improve Non-Alcoholic Fatty Liver Disease by Regulating Intestinal Microbiome in Mice. Foods 2024, 13, 1683. [Google Scholar] [CrossRef] [PubMed]
- La, X.; Liu, Y.; Zhang, L.; Li, H.; Li, Z. Protective Effect of Foxtail Millet Bran-Derived Polyphenols on Alcohol-Induced Gastric Mucosal Injury. Food Sci. 2022, 43, 64–71. [Google Scholar]
- Shi, J. The preparation of foxtail millet bran-derived bound polyphenol and molecular mechanism of anti-colorectal cancer effects. Ph.D. Thesis, Shanxi University, Taiyuan, China, 2018; pp. 23–25. [Google Scholar]
- La, X.; Zhang, L.; Li, Z.; Yang, P.; Wang, Y. Berberine-induced autophagic cell death by elevating GRP78 levels in cancer cells. Oncotarget 2017, 8, 20909–20924. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Feng, L.; Gong, X. Study and clinical application of the method for determination of hexaminose in gastric mucosa. Shaanxi Med. Lab. 2001, 4, 22–24. [Google Scholar]
- Corne, S.J.; Morrissey, S.M.; Woods, R.J. Proceedings: A method for the quantitative estimation of gastric barrier mucus. J. Physiol. 1974, 242, 116–117. [Google Scholar]
- Tian, Y.; Denda-Nagai, K.; Tsukui, T.; Ishii-Schrade, K.B.; Okada, K.; Nishizono, Y.; Matsuzaki, K.; Hafley, M.; Bresalier, R.S.; Irimura, T. Mucin 21 confers resistance to apoptosis in an O-glycosylation-dependent manner. Cell Death Discov. 2022, 8, 194. [Google Scholar] [CrossRef] [PubMed]
- Zlocowski, N.; Sendra, V.G.; Lorenz, V.; Villarreal, M.A.; Jorge, A.; Núñez, Y.; Bennett, E.P.; Clausen, H.; Nores, G.A.; Irazoqui, F.J. Catalytic and glycan-binding abilities of ppGalNAc-T2 are regulated by acetylation. Biochem. Biophys. Res. Commun. 2011, 410, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, L.; La, X.; Li, Z.; Li, H.; Guo, S. Salvianolic acid A inhibits tumor-associated angiogenesis by blocking GRP78 secretion. Naunyn Schmiedebergs Arch. Pharmacol. 2019, 392, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Duan, Z.; Li, P.; Wang, S.; Guo, L.; Xia, G.; Xie, H. Protective Effect of Polyphenols Purified from Mallotus oblongfolius on Ethanol-Induced Gastric Mucosal Injury by Regulating Nrf2 and MAPKs Pathways. Antioxidants 2022, 11, 2452. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wu, Y.; Zhu, Y.; Yao, S.; Zhu, Y. ANKRD22 is a novel therapeutic target for gastric mucosal injury. Biomed. Pharmacother. 2022, 147, 112649. [Google Scholar] [CrossRef] [PubMed]
- Al-Sayed, E.; Michel, H.E.; Khattab, M.A.; El-Shazly, M.; Singab, A.N. Protective Role of Casuarinin from Melaleuca leucadendra against Ethanol-Induced Gastric Ulcer in Rats. Planta Med. 2020, 86, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Yang, Q.; Tian, T.; Chang, Y.; Li, Y.; Duan, L.R.; Li, H.; Wang, S.W. Gastroprotective effect of gallic acid against ethanol-induced gastric ulcer in rats: Involvement of the Nrf2/HO-1 signaling and anti-apoptosis role. Biomed. Pharmacother. 2020, 126, 110075. [Google Scholar] [CrossRef] [PubMed]
- Abreu Miranda, M.; Lemos, M.; Alves Cowart, K.; Rodenburg, D.; McChesney, J.D.; Radwan, M.M.; Furtado, N.A.; Kenupp Bastos, J. Gastroprotective activity of the hydroethanolic extract and isolated compounds from the leaves of Solanum cernuum Vell. J. Ethnopharmacol. 2015, 172, 421–429. [Google Scholar] [CrossRef]
- Schjoldager, K.T.; Clausen, H. Site-specific protein O-glycosylation modulates proprotein processing—Deciphering specific functions of the large polypeptide GalNAc-transferase gene family. Biochim. Biophys. Acta 2012, 1820, 2079–2094. [Google Scholar] [CrossRef]



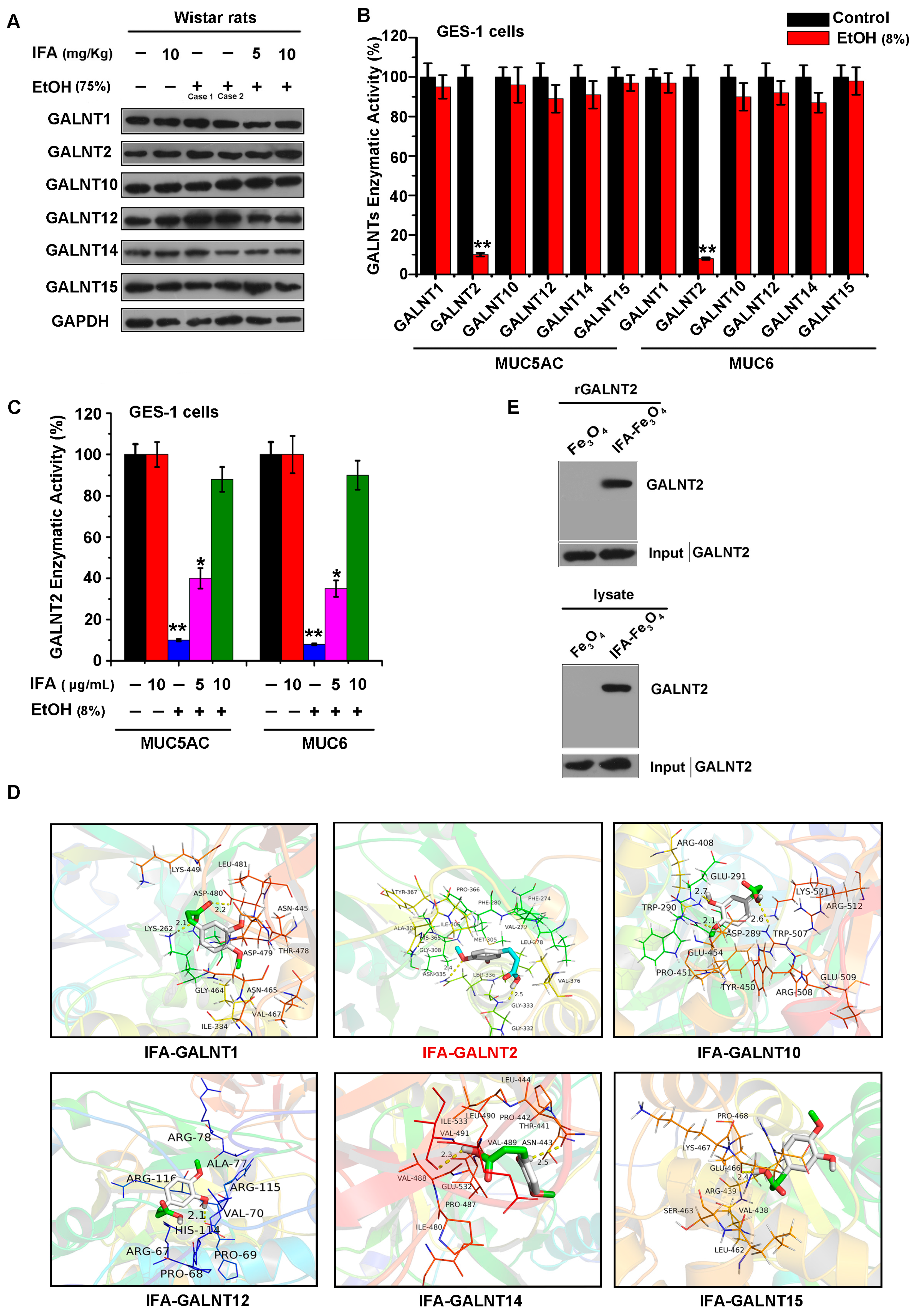
| Protein Name | NCBI Gene Number | UniProtKB/Swiss-Prot Number | Residue Involved in H-Bonding | H-Bond Length (A) | Binding Energy (kcal/mol) |
|---|---|---|---|---|---|
| GALNT1 | 2589 | Q10472 | LYS262; ASP480 | 2.1; 2.2 | −5.7 |
| GALNT2 | 2590 | Q10471 | GLY333; ASN335 | 2.5; 2.4 | −6.2 |
| GALNT10 | 55568 | Q86SR1 | TRP290; ARG408; ARG508 | 2.1; 2.7; 2.6 | −5.3 |
| GALNT12 | 79695 | Q8IXK2 | HIS114 | 2.1 | −5.2 |
| GALNT14 | 79623 | Q96FL9 | ASN443; ILE533 | 2.5; 2.3 | −4.9 |
| GALNT15 | 117248 | Q8N3T1 | GLU466 | 2.4 | −5.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La, X.; He, X.; Liang, J.; Zhang, Z.; Li, H.; Liu, Y.; Liu, T.; Li, Z.; Wu, C. Gastroprotective Effect of Isoferulic Acid Derived from Foxtail Millet Bran against Ethanol-Induced Gastric Mucosal Injury by Enhancing GALNT2 Enzyme Activity. Nutrients 2024, 16, 2148. https://doi.org/10.3390/nu16132148
La X, He X, Liang J, Zhang Z, Li H, Liu Y, Liu T, Li Z, Wu C. Gastroprotective Effect of Isoferulic Acid Derived from Foxtail Millet Bran against Ethanol-Induced Gastric Mucosal Injury by Enhancing GALNT2 Enzyme Activity. Nutrients. 2024; 16(13):2148. https://doi.org/10.3390/nu16132148
Chicago/Turabian StyleLa, Xiaoqin, Xiaoting He, Jingyi Liang, Zhaoyan Zhang, Hanqing Li, Yizhi Liu, Ting Liu, Zhuoyu Li, and Changxin Wu. 2024. "Gastroprotective Effect of Isoferulic Acid Derived from Foxtail Millet Bran against Ethanol-Induced Gastric Mucosal Injury by Enhancing GALNT2 Enzyme Activity" Nutrients 16, no. 13: 2148. https://doi.org/10.3390/nu16132148
APA StyleLa, X., He, X., Liang, J., Zhang, Z., Li, H., Liu, Y., Liu, T., Li, Z., & Wu, C. (2024). Gastroprotective Effect of Isoferulic Acid Derived from Foxtail Millet Bran against Ethanol-Induced Gastric Mucosal Injury by Enhancing GALNT2 Enzyme Activity. Nutrients, 16(13), 2148. https://doi.org/10.3390/nu16132148





