Effects of Creatine Supplementation on the Myostatin Pathway and Myosin Heavy Chain Isoforms in Different Skeletal Muscles of Resistance-Trained Rats
Abstract
1. Introduction
2. Materials and Methods
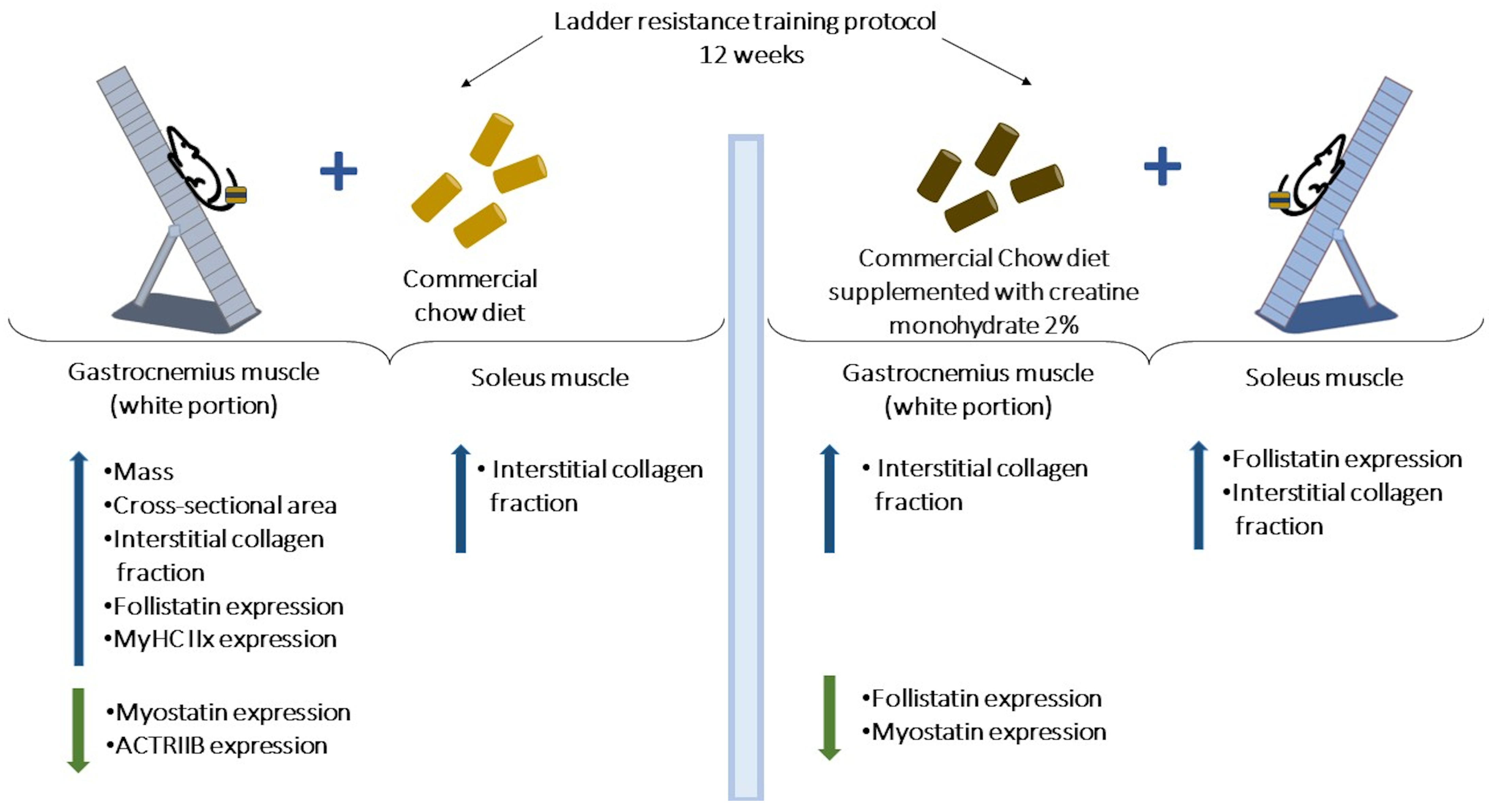
2.1. Animal and Experimental Design
2.2. Climbing Exercise Familiarization
2.3. Initial Maximal Carrying Capacity
2.4. Resistance Training Protocol
2.5. Final Maximal Carrying Capacity
2.6. General Characteristics, Tissue Collection, and Serum Biochemical Analysis
2.7. Skeletal Muscle Morphology
2.8. Western Blotting
2.9. Myosin Heavy Chain Isoforms
2.10. Statistical Analysis
3. Results
3.1. Maximal Carrying Capacity
3.2. Nutritional and Serum Biochemical Data
3.3. Morphological Characterization
3.4. Myosin Heavy Chain Isoforms Distribution
3.5. Protein Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Butts, J.; Jacobs, B.; Silvis, M. Creatine Use in Sports. Sport. Health Multidiscip. Approach 2017, 10, 31–34. [Google Scholar] [CrossRef]
- Terjung, R.L.; Clarkson, P.; Eichner, E.R.; Greenhaff, P.L.; Hespel, P.J.; Israel, R.G.; Kraemer, W.J.; Meyer, R.A.; Spriet, L.L.; Tarnopolsky, M.A.; et al. Physiological and Health Effects of Oral Creatine Supplementation. Med. Sci. Sports Exerc. 2000, 32, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Wax, B.; Kerksick, C.M.; Jagim, A.R.; Mayo, J.J.; Lyons, B.C.; Kreider, R.B. Creatine for Exercise and Sports Performance, with Recovery Considerations for Healthy Populations. Nutrients 2021, 13, 1915. [Google Scholar] [CrossRef] [PubMed]
- Pinder, M.A.; Myrie, S.B. Creatine Supplementation and Skeletal Muscle Metabolism for Building Muscle Mass-Review of the Potential Mechanisms of Action. Curr. Protein Pept. Sci. 2017, 18, 1273–1287. [Google Scholar] [CrossRef]
- Verdijk, L.B.; Gleeson, B.G.; Jonkers, R.A.M.; Meijer, K.; Savelberg, H.H.C.M.; Dendale, P.; van Loon, L.J. Skeletal Muscle Hypertrophy Following Resistance Training Is Accompanied by a Fiber Type-Specific Increase in Satellite Cell Content in Elderly Men. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2009, 64, 332–339. [Google Scholar] [CrossRef]
- Mitchell, C.J.; Churchward-Venne, T.A.; Bellamy, L.; Parise, G.; Baker, S.K.; Phillips, S.M. Muscular and Systemic Correlates of Resistance Training-Induced Muscle Hypertrophy. PLoS ONE 2013, 8, e78636. [Google Scholar] [CrossRef]
- Chilibeck, P.D.; Kaviani, M.; Candow, D.G.; Zello, G.A. Effect of creatine supplementation during resistance training on lean tissue mass and muscular strength in older adults: A meta-analysis. Open Access J. Sport. Med. 2017, 8, 213–226. [Google Scholar] [CrossRef]
- Bonilla, D.A.; Kreider, R.B.; Petro, J.L.; Romance, R.; García-Sillero, M.; Benítez-Porres, J.; Vargas-Molina, S. Creatine Enhances the Effects of Cluster-Set Resistance Training on Lower-Limb Body Composition and Strength in Resistance-Trained Men: A Pilot Study. Nutrients 2021, 13, 2303. [Google Scholar] [CrossRef]
- Nouri, H.; Sheikholeslami-Vatani, D.; Moloudi, M.R. Changes in UPR-PERK pathway and muscle hypertrophy following resistance training and creatine supplementation in rats. J. Physiol. Biochem. 2021, 77, 331–339. [Google Scholar] [CrossRef]
- Pinto, C.L.; Botelho, P.B.; Carneiro, J.A.; Mota, J.F. Impact of creatine supplementation in combination with resistance training on lean mass in the elderly. J. Cachex-Sarcopenia Muscle 2016, 7, 413–421. [Google Scholar] [CrossRef]
- Aguiar, A.F.; Januário, R.S.B.; Junior, R.P.; Gerage, A.; Pina, F.L.C.; Nascimento, M.A.D.; Padovani, C.R.; Cyrino, E. Long-term creatine supplementation improves muscular performance during resistance training in older women. Eur. J. Appl. Physiol. 2012, 113, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, D.S.; Rosene, J. Effects of oral creatine and resistance training on myosin heavy chain expression. Med. Sci. Sport. Exerc. 2001, 33, 1674–1681. [Google Scholar] [CrossRef] [PubMed]
- Taes, Y.E.; Speeckaert, M.; Bauwens, E.; De Buyzere, M.R.; Libbrecht, J.; Lameire, N.H.; Delanghe, J.R. Effect of Dietary Creatine on Skeletal Muscle Myosin Heavy Chain Isoform Expression in an Animal Model of Uremia. Nephron Exp. Nephrol. 2004, 96, e103–e110. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.; Gordon, T.; Syrotuik, D.; Shu, Y.; Tyreman, N.; MacLean, I.; Kenwell, Z.; Putman, C.T. Effects of long-term creatine feeding and running on isometric functional measures and myosin heavy chain content of rat skeletal muscles. Pflug. Arch. Eur. J. Physiol. 2006, 452, 744–755. [Google Scholar] [CrossRef]
- Gallo, M.; MacLean, I.; Tyreman, N.; Martins, K.J.B.; Syrotuik, D.; Gordon, T.; Putman, C.T. Adaptive responses to creatine loading and exercise in fast-twitch rat skeletal muscle. Am. J. Physiol. Integr. Comp. Physiol. 2008, 294, R1319–R1328. [Google Scholar] [CrossRef]
- Aguiar, A.F.; Aguiar, D.H.; Felisberto, A.D.; Carani, F.R.; Milanezi, R.C.; Padovani, C.R.; Dal-Pai-Silva, M. Effects of Creatine Supplementation During Resistance Training on Myosin Heavy Chain (MHC) Expression in Rat Skeletal Muscle Fibers. J. Strength Cond. Res. 2010, 24, 88–96. [Google Scholar] [CrossRef]
- Staron, R.S.; Kraemer, W.J.; Hikida, R.S.; Fry, A.C.; Murray, J.D.; Campos, G.E.R. Fiber type composition of four hindlimb muscles of adult Fisher 344 rats. Histochem. 1999, 111, 117–123. [Google Scholar] [CrossRef]
- Bloemberg, D.; Quadrilatero, J. Rapid Determination of Myosin Heavy Chain Expression in Rat, Mouse, and Human Skeletal Muscle Using Multicolor Immunofluorescence Analysis. PLoS ONE 2012, 7, e35273. [Google Scholar] [CrossRef]
- Wigmore, P.M.; Evans, D.J. Molecular and cellular mechanisms involved in the generation of fiber diversity during myogenesis. Int. Rev. Cytol. 2002, 216, 175–232. [Google Scholar] [CrossRef]
- Schiaffino, S.; Reggiani, C. Fiber Types in Mammalian Skeletal Muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef]
- Elkina, Y.; Von Haehling, S.; Anker, S.D.; Springer, J. The role of myostatin in muscle wasting: An overview. J. Cachexia Sarcopenia Muscle 2011, 2, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-M.; Zhao, Y.-P.; Zhao, Y.; Deng, S.-L.; Yu, K. Regulation of Myostatin on the Growth and Development of Skeletal Muscle. Front. Cell Dev. Biol. 2021, 9, 785712. [Google Scholar] [CrossRef] [PubMed]
- Artaza, J.N.; Bhasin, S.; Mallidis, C.; Taylor, W.; Ma, K.; Gonzalez-Cadavid, N.F. Endogenous expression and localization of myostatin and its relation to myosin heavy chain distribution in C2C12 skeletal muscle cells. J. Cell. Physiol. 2002, 190, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Hennebry, A.; Berry, C.; Siriett, V.; O’Callaghan, P.; Chau, L.; Watson, T.; Sharma, M.; Kambadur, R. Myostatin regulates fiber-type composition of skeletal muscle by regulating MEF2 and MyoD gene expression. Am. J. Physiol.-Cell Physiol. 2009, 296, C525–C534. [Google Scholar] [CrossRef]
- Wang, M.; Yu, H.; Kim, Y.S.; Bidwell, C.A.; Kuang, S. Myostatin facilitates slow and inhibits fast myosin heavy chain expression during myogenic differentiation. Biochem. Biophys. Res. Commun. 2012, 426, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.-X.; Xuan, M.-F.; Jin, L.; Guo, Q.; Luo, Z.-B.; Wang, J.-X.; Luo, Q.-R.; Zhang, G.-L.; Cui, C.-D.; Cui, Z.-Y.; et al. Fiber-type distribution and expression of myosin heavy chain isoforms in newborn heterozygous myostatin-knockout pigs. Biotechnol. Lett. 2017, 39, 1811–1819. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Møller, L.L.V.; De Groote, E.; Bojsen-Møller, K.N.; Davey, J.; Henríquez-Olguin, C.; Li, Z.; Knudsen, J.R.; Jensen, T.E.; Madsbad, S.; et al. Mechanisms involved in follistatin-induced hypertrophy and increased insulin action in skeletal muscle. J. Cachex-Sarcopenia Muscle 2019, 10, 1241–1257. [Google Scholar] [CrossRef] [PubMed]
- Domin, R.; Dadej, D.; Pytka, M.; Zybek-Kocik, A.; Ruchała, M.; Guzik, P. Effect of Various Exercise Regimens on Selected Exercise-Induced Cytokines in Healthy People. Int. J. Environ. Res. Public Health 2021, 18, 1261. [Google Scholar] [CrossRef]
- Saremi, A.; Gharakhanloo, R.; Sharghi, S.; Gharaati, M.; Larijani, B.; Omidfar, K. Effects of oral creatine and resistance training on serum myostatin and GASP-1. Mol. Cell. Endocrinol. 2010, 317, 25–30. [Google Scholar] [CrossRef]
- Mobley, C.B.; Fox, C.D.; Ferguson, B.S.; Amin, R.H.; Dalbo, V.J.; Baier, S.; Rathmacher, J.A.; Wilson, J.M.; Roberts, M.D. L-leucine, beta-hydroxy-beta-methylbutyric acid (HMB) and creatine monohydrate prevent myostatin-induced Akirin-1/Mighty mRNA down-regulation and myotube atrophy. J. Int. Soc. Sport. Nutr. 2014, 11, 38. [Google Scholar] [CrossRef]
- Young, J.; Bertram, H.; Theil, P.; Petersen, A.-G.; Poulsen, K.; Rasmussen, M.; Malmendal, A.; Nielsen, N.; Vestergaard, M.; Oksbjerg, N. In vitro and in vivo studies of creatine monohydrate supplementation to Duroc and Landrace pigs. Meat Sci. 2007, 76, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, M.; Kong, Y.; Ma, H.; Zou, S. Comparison of the novel compounds creatine and pyruvateon lipid and protein metabolism in broiler chickens. Animal 2011, 5, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Rooney, K.; Bryson, J.; Phuyal, J.; Denyer, G.; Caterson, I.; Thompson, C. Creatine supplementation alters insulin secretion and glucose homeostasis in vivo. Metab. Clin. Exp. 2002, 51, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.-S.; Smith, J.L.; Oppelt, P.J.; Fisher, J.S. Creatine feeding increases GLUT4 expression in rat skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E347–E352. [Google Scholar] [CrossRef]
- Hornberger, T.A., Jr.; Farrar, R.P. Physiological Hypertrophy of the FHL Muscle Following 8 Weeks of Progressive Resistance Exercise in the Rat. Can. J. Appl. Physiol. 2004, 29, 16–31. [Google Scholar] [CrossRef]
- Medeiros, C.S.; Neto, I.V.d.S.; Silva, K.K.S.; Cantuária, A.P.C.; Rezende, T.M.B.; Franco, O.L.; Marqueti, R.d.C.; Freitas-Lima, L.C.; Araujo, R.C.; Yildirim, A.; et al. The Effects of High-Protein Diet and Resistance Training on Glucose Control and Inflammatory Profile of Visceral Adipose Tissue in Rats. Nutrients 2021, 13, 1969. [Google Scholar] [CrossRef]
- Tibana, R.A.; Franco, O.L.; Cunha, G.V.; Sousa, N.M.F.; Neto, I.V.S.; Carvalho, M.M.; Almeida, J.A.; Durigan, J.L.Q.; Marqueti, R.C.; Navalta, J.W.; et al. The Effects of Resistance Training Volume on Skeletal Muscle Proteome. Int. J. Exerc. Sci. 2017, 10, 1051–1066. [Google Scholar]
- De Sousa Neto, I.V.; Tibana, R.A.; da Silva, L.G.O.; de Lira, E.M.; do Prado, G.P.G.; de Almeida, J.A.; Franco, O.L.; Durigan, J.L.Q.; Adesida, A.B.; de Sousa, M.V.; et al. Paternal Resistance Training Modulates Calcaneal Tendon Proteome in the Offspring Exposed to High-Fat Diet. Front. Cell Dev. Biol. 2020, 8, 380. [Google Scholar] [CrossRef]
- De Sousa Neto, I.V.; Durigan, J.L.Q.; Guzzoni, V.; Tibana, R.A.; Prestes, J.; de Araujo, H.S.S.; Marqueti, R.C. Effects of Resistance Training on Matrix Metalloproteinase Activity in Skeletal Muscles and Blood Circulation during Aging. Front. Physiol. 2018, 9, 190. [Google Scholar] [CrossRef]
- Oliveira, S.A., Jr.; Pai-Silva, M.D.; Martinez, P.F.; Lima-Leopoldo, A.P.; Campos, D.H.; Leopoldo, A.S.; Politi, O.M.; Okoshi, M.P.; Okoshi, K.; Roberto, P.C.; et al. Diet-induced obesity causes metabolic, endocrine and cardiac alterations in spontaneously hypertensive rats. Med. Sci. Monit. 2010, 16, 367–373. [Google Scholar]
- Carvalho, M.R.; Mendonça, M.L.M.; Oliveira, J.M.; Romanenghi, R.B.; Morais, C.S.; Ota, G.E.; Lima, A.R.; Oliveira, R.J.; Filiú, W.F.; Okoshi, K.; et al. Influence of high-intensity interval training and intermittent fasting on myocardium apoptosis pathway and cardiac morphology of healthy rats. Life Sci. 2020, 264, 118697. [Google Scholar] [CrossRef] [PubMed]
- Basilio, P.G.; De Oliveira, A.P.C.; De Castro, A.C.F.; De Carvalho, M.R.; Zagatto, A.M.; Martinez, P.F.; Okoshi, M.P.; Okoshi, K.; Ota, G.E.; Dos Reis, F.A.; et al. Intermittent fasting attenuates exercise training-induced cardiac remodeling. Arq. Bras. Cardiol. 2020, 115, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Martinez, P.F.; Bonomo, C.; Guizoni, D.M.; Oliveira-Junior, S.A.; Damatto, R.L.; Cezar, M.D.; Lima, A.R.; Pagan, L.U.; Seiva, F.; Bueno, R.T.; et al. Modulation of MAPK and NF-κB Signaling Pathways by Antioxidant Therapy in Skeletal Muscle of Heart Failure Rats. Cell. Physiol. Biochem. 2016, 39, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Junior, S.A.; Martinez, P.F.; Guizoni, D.M.; Campos, D.H.S.; Fernandes, T.; Oliveira, E.M.; Okoshi, M.P.; Okoshi, K.; Padovani, C.R.; Cicogna, A.C. AT1 Receptor Blockade Attenuates Insulin Resistance and Myocardial Remodeling in Rats with Diet-Induced Obesity. PLoS ONE 2014, 9, e86447. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Martinez, P.F.; Okoshi, K.; Zornoff, L.A.M.; Carvalho, R.F.; Oliveira, S.A., Jr.; Lima, A.R.R.; Campos, D.H.S.; Damatto, R.L.; Padovani, C.R.; Nogueira, C.R.; et al. Chronic heart failure-induced skeletal muscle atrophy, necrosis, and changes in myogenic regulatiory factors. Med. Sci. Monit. 2010, 16, 374–383. [Google Scholar]
- Lima, A.R.R.; Martinez, P.F.; Damatto, R.L.; Cezar, M.D.M.; Guizoni, D.M.; Bonomo, C.; Oliveira-Junior, S.A.; Silva, M.D.-P.; Zornoff, L.A.M.; Okoshi, K.; et al. Heart Failure-Induced Diaphragm Myopathy. Cell. Physiol. Biochem. 2014, 34, 333–345. [Google Scholar] [CrossRef]
- Aguiar, A.F.; De Souza, R.W.A.; Aguiar, D.H.; Aguiar, R.C.M.; Vechetti, I.J., Jr.; Dal-Pai-Silva, M. Creatine does not promote hypertrophy in skeletal muscle in supplemented compared with nonsupplemented rats subjected to a similar workload. Nutr. Res. 2011, 31, 652–657. [Google Scholar] [CrossRef]
- Damatto, R.; Martinez, P.; Lima, A.; Cezar, M.; Campos, D.; Junior, S.O.; Guizoni, D.; Bonomo, C.; Nakatani, B.; Silva, M.D.P.; et al. Heart failure-induced skeletal myopathy in spontaneously hypertensive rats. Int. J. Cardiol. 2013, 167, 698–703. [Google Scholar] [CrossRef]
- Pereira, J.A.S.A.; De Haan, A.; Wessels, A.; Moorman, A.F.; Sargeant, A.J. The mATPase histochemical profile of rat type IIX fibres: Correlation with myosin heavy chain immunolabelling. Histochem. J. 1995, 27, 715–722. [Google Scholar] [CrossRef]
- Qaisar, R.; Bhaskaran, S.; Van Remmen, H. Muscle fiber type diversification during exercise and regeneration. Free. Radic. Biol. Med. 2016, 98, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, W.J.; Ratamess, N.A. Fundamentals of Resistance Training: Progression and Exercise Prescription. Med. Sci. Sports Exerc. 2004, 36, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J.; Grgic, J.; Ogborn, D.; Krieger, J.W. Strength and Hypertrophy Adaptations Between Low- vs. High-Load Resistance Training: A Systematic Review and Meta-analysis. J. Strength Cond. Res. 2017, 31, 3508–3523. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.; Junior, R.M.; Barreira, J.; Schoenfeld, B.J.; Orazem, J.; Barroso, R. Muscle hypertrophy and strength gains after resistance training with different volume-matched loads: A systematic review and meta-analysis. Appl. Physiol. Nutr. Metab. 2022, 47, 357–368. [Google Scholar] [CrossRef]
- Škarabot, J.; Brownstein, C.G.; Casolo, A.; Del Vecchio, A.; Ansdell, P. The knowns and unknowns of neural adaptations to resistance training. Eur. J. Appl. Physiol. 2020, 121, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Stotzer, U.S.; Pisani, G.F.D.; Canevazzi, G.H.R.; Shiguemoto, G.E.; Duarte, A.C.G.D.O.; Perez, S.E.D.A.; Selistre-De-Araújo, H.S. Benefits of resistance training on body composition and glucose clearance are inhibited by long-term low carbohydrate diet in rats. PLoS ONE 2018, 13, e0207951. [Google Scholar] [CrossRef]
- Bae, J.Y. Resistance Exercise Regulates Hepatic Lipolytic Factors as Effective as Aerobic Exercise in Obese Mice. Int. J. Environ. Res. Public Health 2020, 17, 8307. [Google Scholar] [CrossRef]
- Folland, J.P.; Williams, A.G. The adaptations to strength training morphological and neurological contributions to increased strength. Sport. Med. 2007, 37, 145–168. [Google Scholar] [CrossRef]
- Wackerhage, H.; Schoenfeld, B.J.; Hamilton, D.; Lehti, M.; Hulmi, J.J. Stimuli and sensors that initiate skeletal muscle hypertrophy following resistance exercise. J. Appl. Physiol. 2019, 126, 30–43. [Google Scholar] [CrossRef]
- Ribeiro, M.B.T.; Guzzoni, V.; Hord, J.M.; Lopes, G.N.; Marqueti, R.D.C.; de Andrade, R.V.; Selistre-De-Araujo, H.S.; Durigan, J.L.Q. Resistance training regulates gene expression of molecules associated with intramyocellular lipids, glucose signaling and fiber size in old rats. Sci. Rep. 2017, 7, 8593. [Google Scholar] [CrossRef]
- Deschenes, M.R.; Sherman, E.G.; Roby, M.A.; Glass, E.K.; Harris, M.B. Effect of resistance training on neuromuscular junctions of young and aged muscles featuring different recruitment patterns. J. Neurosci. Res. 2014, 93, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Neto, W.K.; Gama, E. Strength training and anabolic steroid do not affect muscle capillarization of middle-aged rats. Rev. Bras. Med. Esporte 2017, 23, 137–141. [Google Scholar] [CrossRef]
- Aagaard, P.; Andersen, J.L.; Dyhre-Poulsen, P.; Leffers, A.-M.; Wagner, A.; Magnusson, S.P.; Halkjaer-Kristensen, J.; Simonsen, E.B. A mechanism for increased contractile strength of human pennate muscle in response to strength training: Changes in muscle architecture. J. Physiol. 2001, 534, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S.; Park, Y.-M.; Lee, S.-R.; Masad, I.S.; Khamoui, A.V.; Jo, E.; Park, B.-S.; Arjmandi, B.H.; Panton, L.B.; Lee, W.J.; et al. β-hydroxy-β-methylbutyrate did not enhance high intensity resistance training-induced improvements in myofiber dimensions and myogenic capacity in aged female rats. Mol. Cells 2012, 34, 439–448. [Google Scholar] [CrossRef]
- Douglas, J.; Pearson, S.; Ross, A.; McGuigan, M. Chronic Adaptations to Eccentric Training: A Systematic Review. Sport. Med. 2016, 47, 917–941. [Google Scholar] [CrossRef]
- Lourenço, Í.; Neto, W.K.; Amorim, L.D.S.P.; Ortiz, V.M.M.; Geraldo, V.L.; Ferreira, G.H.D.S.; Caperuto, C.; Gama, E.F. Muscle hypertrophy and ladder-based resistance training for rodents: A systematic review and meta-analysis. Physiol. Rep. 2020, 8, e14502. [Google Scholar] [CrossRef]
- Braggion, G.F.; Ornelas, E.D.M.; Cury, J.C.S.; de Sousa, J.P.; Nucci, R.A.B.; Fonseca, F.L.A.; Maifrino, L.B.M. Remodeling of the soleus muscle of ovariectomized old female rats submitted to resistance training and different diet intake. Acta Histochem. 2020, 122, 151570. [Google Scholar] [CrossRef]
- Csapo, R.; Gumpenberger, M.; Wessner, B. Skeletal Muscle Extracellular Matrix—What Do We Know About Its Composition, Regulation, and Physiological Roles? A Narrative Review. Front. Physiol. 2020, 11, 253. [Google Scholar] [CrossRef]
- McPherron, A.C.; Lee, S.-J. Suppression of body fat accumulation in myostatin-deficient mice. J. Clin. Investig. 2002, 109, 595–601. [Google Scholar] [CrossRef]
- Hamrick, M.W.; Pennington, C.; Webb, C.N.; Isales, C.M. Resistance to body fat gain in ‘double-muscled’ mice fed a high-fat diet. Int. J. Obes. 2006, 30, 868–870. [Google Scholar] [CrossRef]
- McPherron, A.C.; Lee, S.-J. Double muscling in cattle due to mutations in the myostatin gene. Proc. Natl. Acad. Sci. USA 1997, 94, 12457–12461. [Google Scholar] [CrossRef] [PubMed]
- Grobet, L.; Martin, L.J.R.; Poncelet, D.; Pirottin, D.; Brouwers, B.; Riquet, J.; Schoeberlein, A.; Dunner, S.; Ménissier, F.; Massabanda, J.; et al. A deletion in the bovine myostatin gene causes the double–muscled phenotype in cattle. Nat. Genet. 1997, 17, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Mosher, D.S.; Quignon, P.; Bustamante, C.D.; Sutter, N.B.; Mellersh, C.S.; Parker, H.G.; Ostrander, E. A Mutation in the Myostatin Gene Increases Muscle Mass and Enhances Racing Performance in Heterozygote Dogs. PLoS Genet. 2007, 3, e79. [Google Scholar] [CrossRef] [PubMed]
- Schuelke, M.; Wagner, K.R.; Stolz, L.E.; Hübner, C.; Riebel, T.; Kömen, W.; Braun, T.; Tobin, J.F.; Lee, S.-J. Myostatin Mutation Associated with Gross Muscle Hypertrophy in a Child. N. Engl. J. Med. 2004, 350, 2682–2688. [Google Scholar] [CrossRef]
- Raue, U.; Slivka, D.; Jemiolo, B.; Hollon, C.; Trappe, S. Myogenic gene expression at rest and after a bout of resistance exercise in young (18–30 yr) and old (80–89 yr) women. J. Appl. Physiol. 2006, 101, 53–59. [Google Scholar] [CrossRef]
- Hayashi, S.; Miyake, M.; Watanabe, K.; Aso, H.; Hayashi, S.; Ohwada, S.; Yamaguchi, T. Myostatin preferentially down-regulates the expression of fast 2x myosin heavy chain in cattle. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2008, 84, 354–362. [Google Scholar] [CrossRef]
- Santos, A.; Neves, M.; Gualano, B.; Laurentino, G.; Lancha, A.; Ugrinowitsch, C.; Lima, F.; Aoki, M.S. Blood flow restricted resistance training attenuates myostatin gene expression in a patient with inclusion body myositis. Biol. Sport 2014, 31, 121–124. [Google Scholar] [CrossRef]
- Tang, L.; Luo, K.; Liu, C.; Wang, X.; Zhang, D.; Chi, A.; Zhang, J.; Sun, L. Decrease in myostatin by ladder-climbing training is associated with insulin resistance in diet-induced obese rats. Chin. Med. J. 2014, 127, 2342–2349. [Google Scholar]
- Negaresh, R.; Ranjbar, R.; Baker, J.S.; Habibi, A.; Mokhtarzade, M.; Gharibvand, M.M.; Fokin, A. Skeletal muscle hypertrophy, insulin-like growth factor 1, myostatin and follistatin in healthy and sarcopenic elderly men: The effect of whole-body resistance training. Int. J. Prev. Med. 2019, 10, 29. [Google Scholar] [CrossRef]
- Deldicque, L.; Atherton, P.; Patel, R.; Theisen, D.; Nielens, H.; Rennie, M.J.; Francaux, M. Effects of resistance exercise with and without creatine supplementation on gene expression and cell signaling in human skeletal muscle. J. Appl. Physiol. 2008, 104, 371–378. [Google Scholar] [CrossRef]
- Volek, J.S.; Kraemer, W.J.; Bush, J.A.; Boetes, M.; Incledon, T.; Clark, K.L.; Lynch, J.M. Creatine Supplementation Enhances Muscular Performance during High-Intensity Resistance Exercise. J. Am. Diet. Assoc. 1997, 97, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.B.; Brabham, B.; Buford, T.W.; Shelmadine, B.D.; McPheeters, M.; Hudson, G.M.; Stathis, C.; Greenwood, M.; Kreider, R.; Willoughby, D.S. Creatine supplementation post-exercise does not enhance training-induced adaptations in middle to older aged males. Eur. J. Appl. Physiol. 2014, 114, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Jiao, H.; Wang, X.; Li, H.; Zhou, Y.; Zhao, J.; Lin, H. The regulating pathway of creatine on muscular protein metabolism depends on the energy state. Am. J. Physiol. Physiol. 2022, 322, C1022–C1035. [Google Scholar] [CrossRef] [PubMed]
- Mazzetti, S.; Douglass, M.; Yocum, A.; Harber, M. Effect of Explosive versus Slow Contractions and Exercise Intensity on Energy Expenditure. Med. Sci. Sport. Exerc. 2007, 39, 1291–1301. [Google Scholar] [CrossRef] [PubMed]



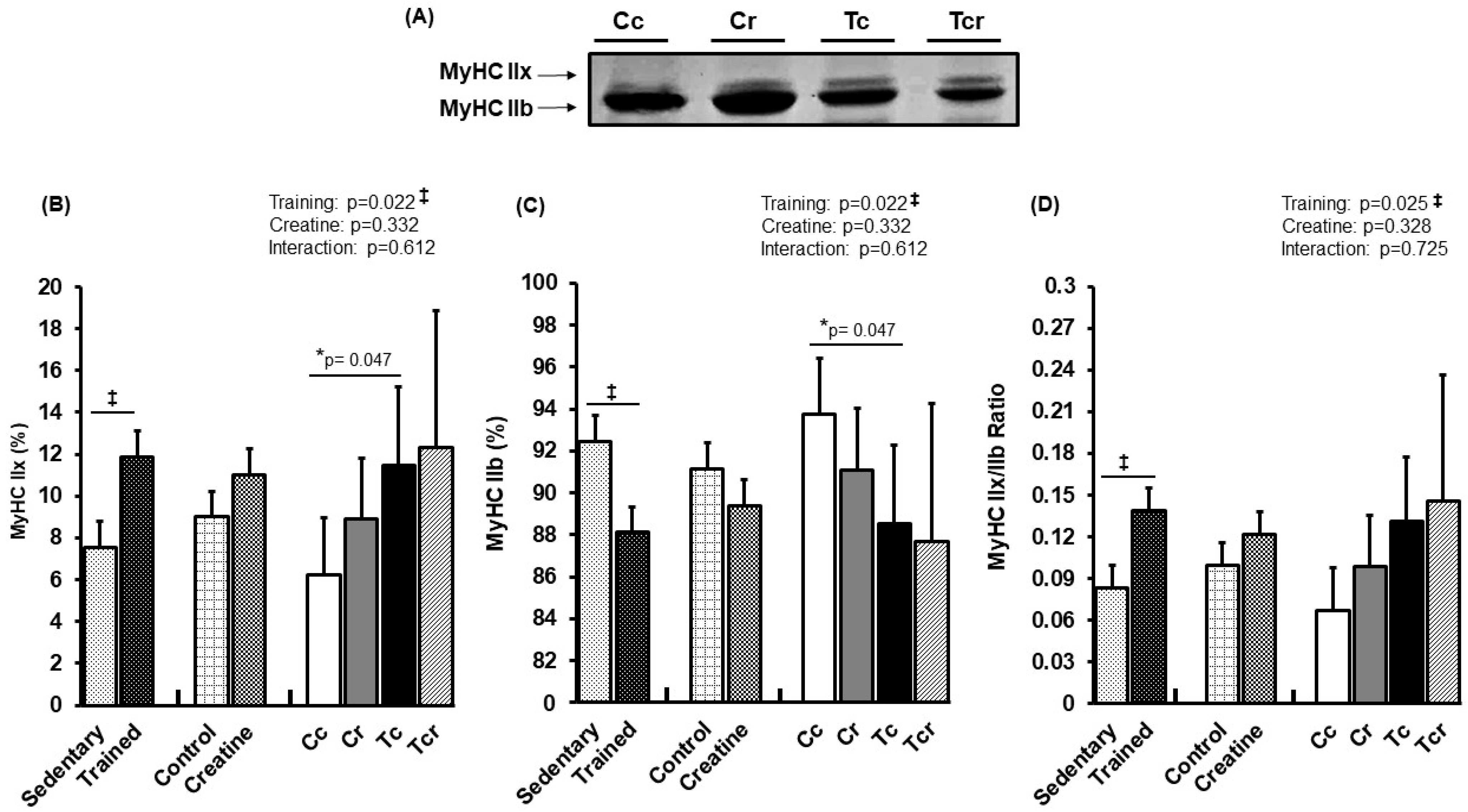
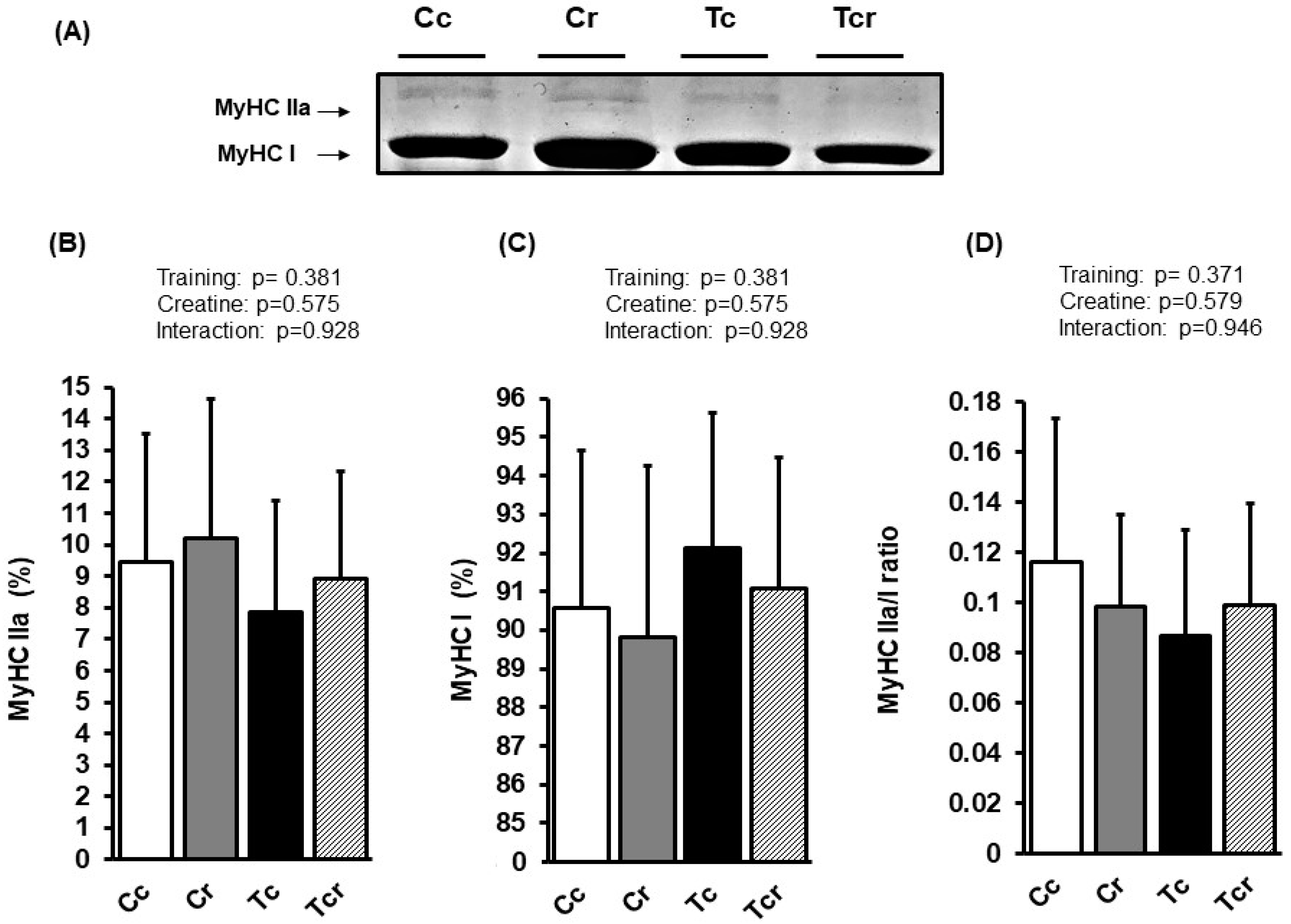

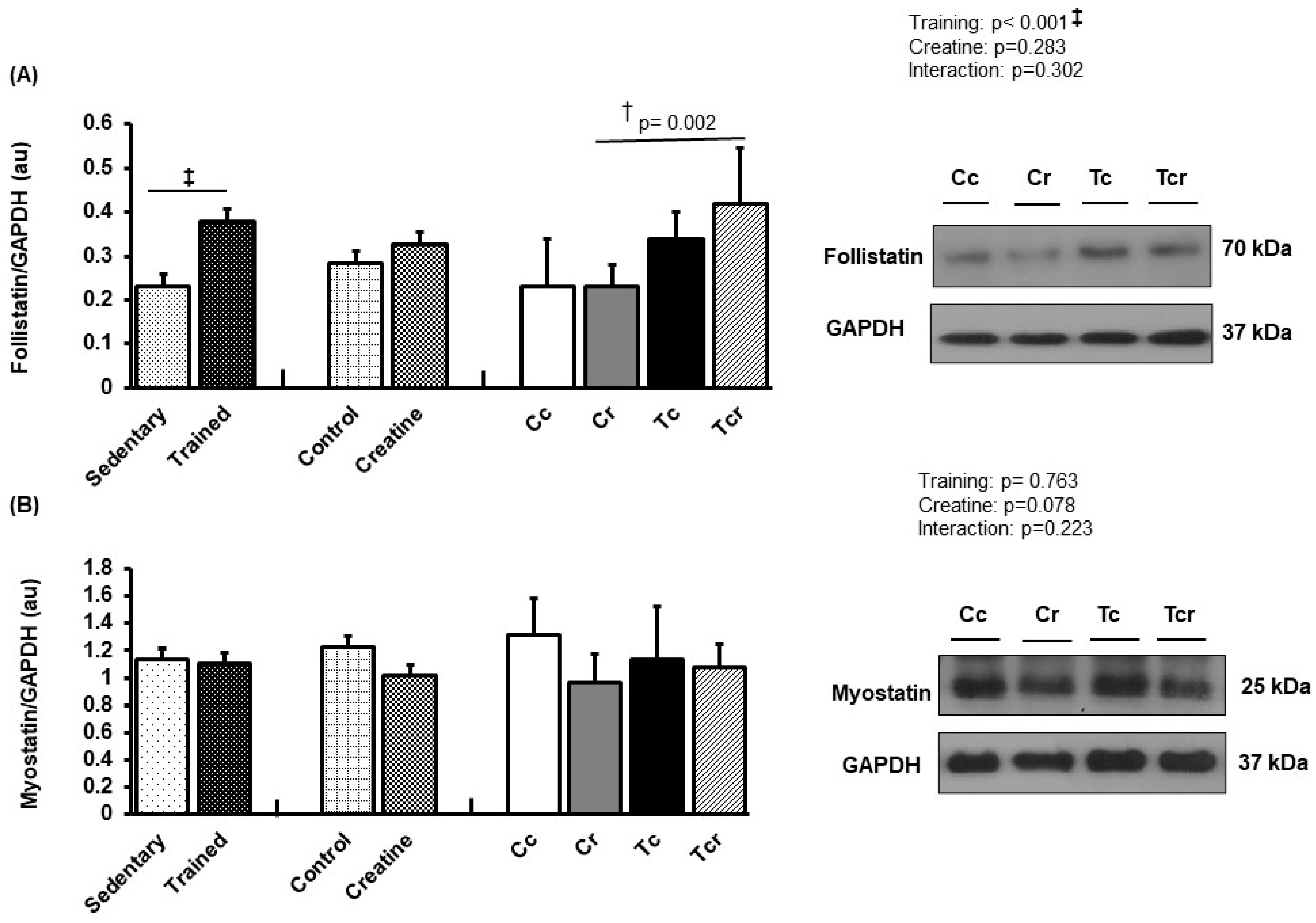
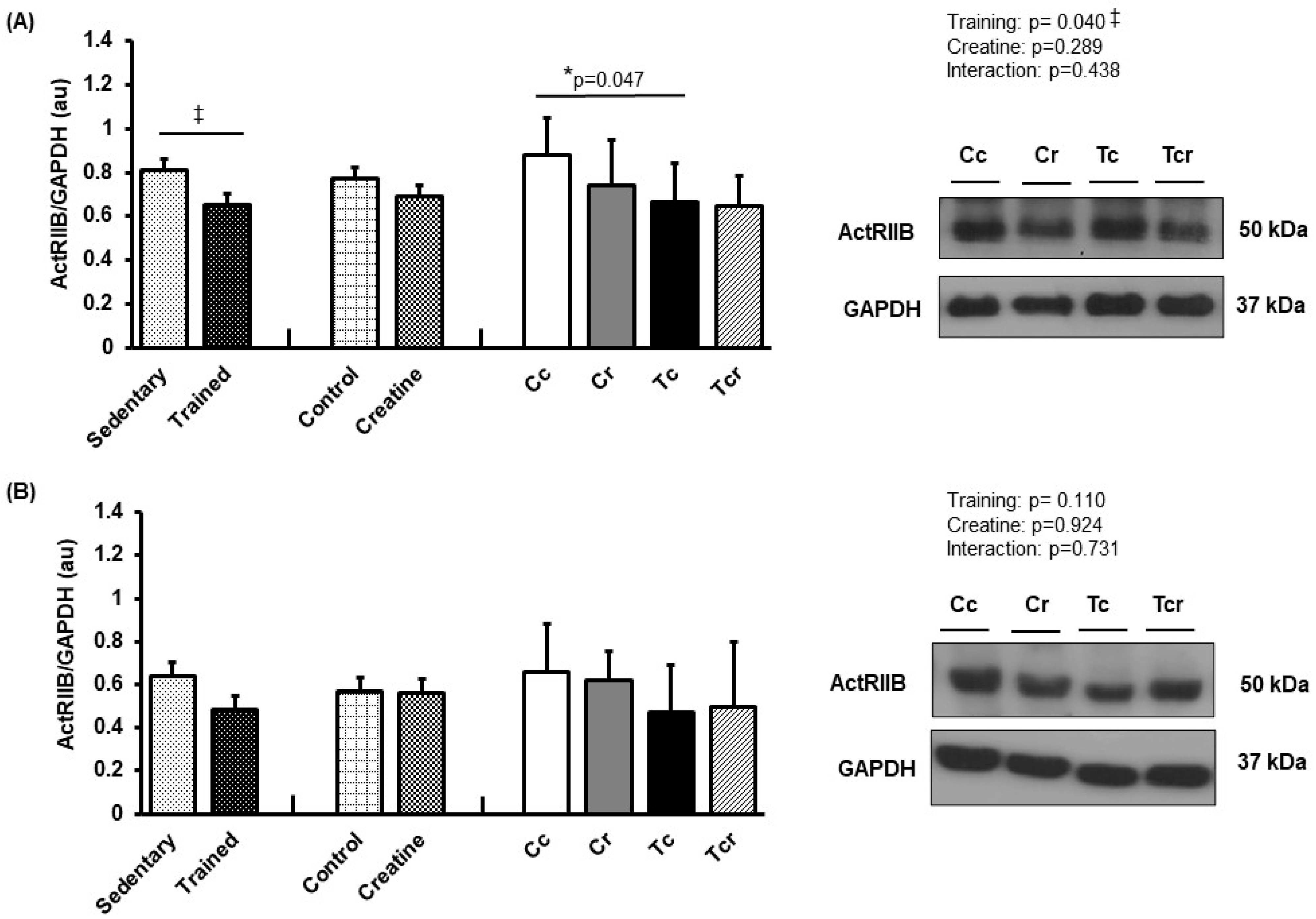
| Variables | Groups | Factors (p-Value) | ||||||
|---|---|---|---|---|---|---|---|---|
| Cc | Cr | Tc | Tcr | Training | Creatine | Interaction | ||
| Nutritional | Initial body weight (g) | 240 ± 22 | 242 ± 25 | 237 ± 20 | 251 ± 29 | 0.740 | 0.409 | 0.547 |
| Final body weight (g) | 392 ± 34 | 407 ± 34 | 350 ± 71 * | 390 ± 72 # | 0.044 ‡ | 0.052 | 0.374 | |
| Food intake (g) | 2132 ± 150 | 2016 ± 82 | 2022 ± 160 | 1983 ± 169 | 0.162 | 0.130 | 0.444 | |
| Creatine intake (g) | - | 40.33 ± 1.64 | - | 39.67 ± 2.32 | 0.551 | - | - | |
| Calorie intake (Kcal) | 7699 ± 544 | 7421 ± 302 | 7301 ± 578 | 7325 ± 444 | 0.186 | 0.489 | 0.414 | |
| Feed efficiency (mg/Kcal) | 1.9 ± 0.1 | 2.2 ± 0.1 * | 1.7 ± 0.2 * | 1.9 ± 0.1 † | <0.001 ‡ | 0.007 § | 0.437 | |
| Biochemical | Glucose (mg/dL) | 134 ± 17 | 131 ± 24 | 133 ± 24 | 144 ± 21 | 0.492 | 0.629 | 0.410 |
| Total cholesterol (mg/dL) | 71.3 ± 9.1 | 77.8 ± 16.6 | 78.6 ± 9.3 | 76.5 ± 6.4 | 0.481 | 0.600 | 0.309 | |
| HDL cholesterol (mg/dL) | 38.7 ± 6.2 | 45.1 ± 8.2 | 42.1 ± 4.3 | 42.4 ± 4.2 | 0.880 | 0.156 | 0.188 | |
| Non-HDL (mg/dL) | 33.9 ± 5.4 | 32.8 ± 11.4 | 36.2 ± 8.5 | 34.1 ± 2.8 | 0.537 | 0.602 | 0.858 | |
| Triglycerides (mg/dL) | 95.8 ± 7.7 | 98.9 ± 6.3 | 91.0 ± 3.4 | 94.2 ± 6.4 | 0.049 ‡ | 0.190 | 0.983 | |
| Albumine (mg/dL) | 2.30 ± 0.15 | 2.30 ± 0.12 | 2.27 ± 0.17 | 2.44 ± 0.11 | 0.291 | 0.119 | 0.119 | |
| Total protein (mg/dL) | 5.16 ± 2.24 | 5.93 ± 0.41 | 6.13 ± 0.54 | 6.46 ± 0.35 | 0.107 | 0.231 | 0.625 | |
| Variables | Groups | Factors (p-Value) | |||||
|---|---|---|---|---|---|---|---|
| Cc | Cr | Tc | Tcr | Training | Creatine | Interaction | |
| Gastrocnemius (mg) | 2255 ± 172 | 2378 ± 106 | 2433 ± 572 | 2531 ± 463 | 0.262 | 0.451 | 0.931 |
| Gastrocnemius/BW (mg/g) | 5.76 ± 0.24 | 5.86 ± 0.34 | 6.92 ± 1.35 * | 6.52 ± 1.30 | 0.020 ‡ | 0.676 | 0.498 |
| Gastrocnemius/tibia (mg/mm) | 52.9 ± 3.8 | 56.6 ± 1.6 | 59.0 ± 13.0 | 59.7 ± 11.0 | 0.181 | 0.512 | 0.646 |
| Soleus (mg) | 141 ± 19 | 146 ± 20 | 125 ± 26 | 153 ± 30 | 0.633 | 0.095 | 0.241 |
| Soleus/BW (mg/g) | 0.36 ± 0.03 | 0.36 ± 0.03 | 0.35 ± 0.05 | 0.39 ± 0.05 | 0.463 | 0.359 | 0.307 |
| Soleus/tibia (mg/mm) | 3.33 ± 0.47 | 3.50 ± 0.46 | 3.05 ± 0.60 | 3.60 ± 0.62 | 0.677 | 0.097 | 0.362 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Carvalho, M.R.; Duarte, E.F.; Mendonça, M.L.M.; de Morais, C.S.; Ota, G.E.; Gaspar-Junior, J.J.; de Oliveira Filiú, W.F.; Damatto, F.C.; Okoshi, M.P.; Okoshi, K.; et al. Effects of Creatine Supplementation on the Myostatin Pathway and Myosin Heavy Chain Isoforms in Different Skeletal Muscles of Resistance-Trained Rats. Nutrients 2023, 15, 2224. https://doi.org/10.3390/nu15092224
de Carvalho MR, Duarte EF, Mendonça MLM, de Morais CS, Ota GE, Gaspar-Junior JJ, de Oliveira Filiú WF, Damatto FC, Okoshi MP, Okoshi K, et al. Effects of Creatine Supplementation on the Myostatin Pathway and Myosin Heavy Chain Isoforms in Different Skeletal Muscles of Resistance-Trained Rats. Nutrients. 2023; 15(9):2224. https://doi.org/10.3390/nu15092224
Chicago/Turabian Stylede Carvalho, Marianna Rabelo, Ellen Fernandes Duarte, Maria Lua Marques Mendonça, Camila Souza de Morais, Gabriel Elias Ota, Jair José Gaspar-Junior, Wander Fernando de Oliveira Filiú, Felipe Cesar Damatto, Marina Politi Okoshi, Katashi Okoshi, and et al. 2023. "Effects of Creatine Supplementation on the Myostatin Pathway and Myosin Heavy Chain Isoforms in Different Skeletal Muscles of Resistance-Trained Rats" Nutrients 15, no. 9: 2224. https://doi.org/10.3390/nu15092224
APA Stylede Carvalho, M. R., Duarte, E. F., Mendonça, M. L. M., de Morais, C. S., Ota, G. E., Gaspar-Junior, J. J., de Oliveira Filiú, W. F., Damatto, F. C., Okoshi, M. P., Okoshi, K., Oliveira, R. J., Martinez, P. F., & de Oliveira-Junior, S. A. (2023). Effects of Creatine Supplementation on the Myostatin Pathway and Myosin Heavy Chain Isoforms in Different Skeletal Muscles of Resistance-Trained Rats. Nutrients, 15(9), 2224. https://doi.org/10.3390/nu15092224








