Evaluation of Primary DNA Damage in Young Healthy Females Based on Their Dietary Preferences
Highlights
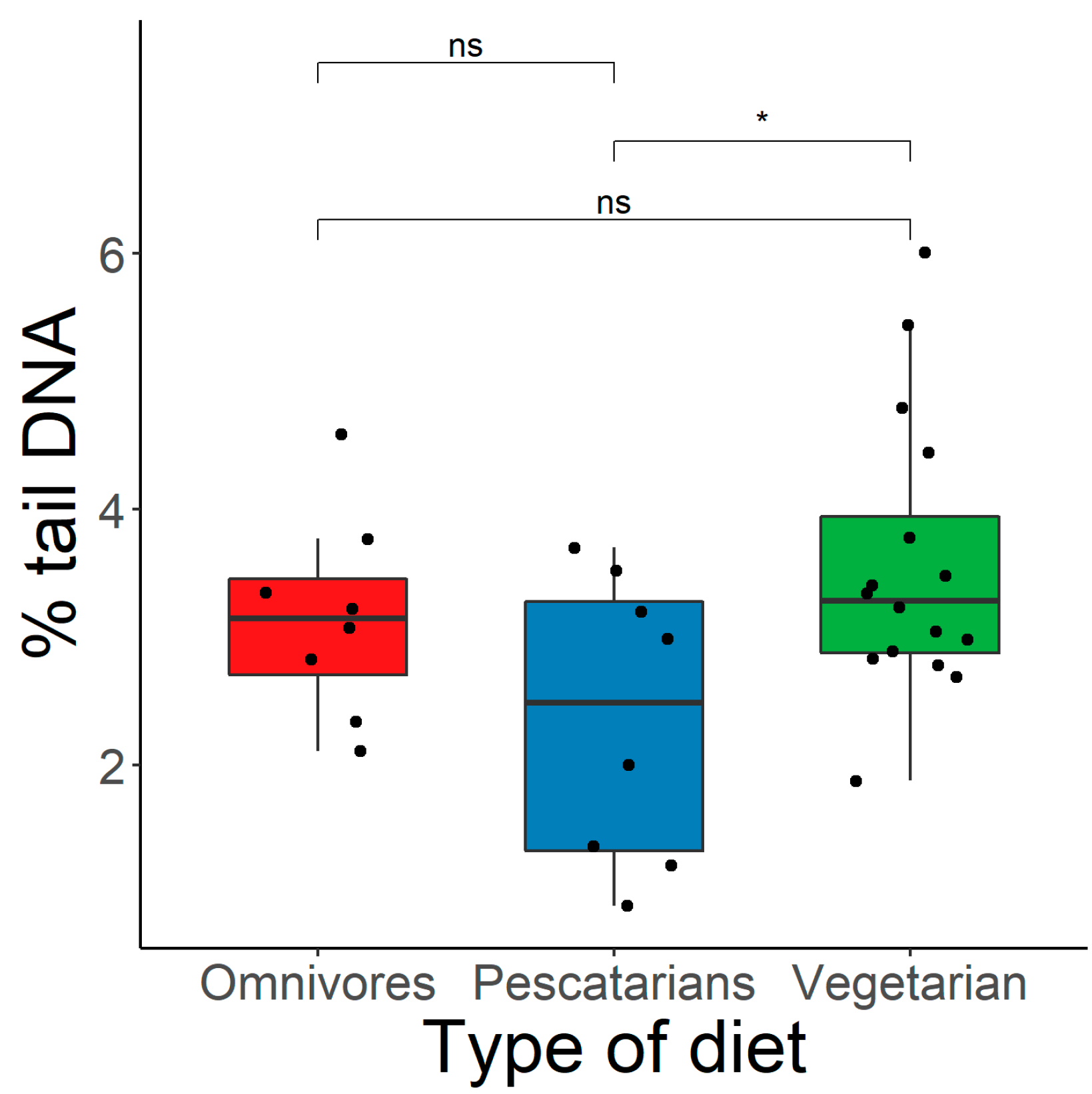
- Vegetarians exhibited significantly higher primary DNA damage compared to non-vegetarians.
- Among the non-vegetarians, the pescatarians had the lowest DNA damage.
- The pescatarian diet was more beneficial for maintaining DNA integrity compared to the vegetarian and omnivorous diets.
- The pescatarian diet may offer advantages in preserving genome stability, potentially reducing the risk of DNA damage-related diseases.
- Further research on dietary preferences and DNA integrity is needed to explore the broader health implications and confirm these findings.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sample and Participant Selection
2.2. Blood Sampling
2.3. Alkaline Comet Assay
2.3.1. Procedure
2.3.2. Image Analysis
2.4. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. Dietary Preferences and Genome Damage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Helleday, T.; Eshtad, S.; Nik-Zainal, S. Mechanisms Underlying Mutational Signatures in Human Cancers. Nat. Rev. Genet. 2014, 15, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Nikfarjam, S.; Singh, K.K. DNA Damage Response Signaling: A Common Link between Cancer and Cardiovascular Diseases. Cancer Med. 2023, 12, 4380–4404. [Google Scholar] [CrossRef] [PubMed]
- Ribezzo, F.; Shiloh, Y.; Schumacher, B. Systemic DNA Damage Responses in Aging and Diseases. Semin. Cancer Biol. 2016, 37–38, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Gajski, G.; Gerić, M.; Jakaša, I.; Peremin, I.; Domijan, A.-M.; Vučić Lovrenčić, M.; Kežić, S.; Bituh, M.; Moraes de Andrade, V. Inflammatory, Oxidative and DNA Damage Status in Vegetarians: Is the Future of Human Diet Green? Crit. Rev. Food Sci. Nutr. 2021. [Google Scholar] [CrossRef]
- Vidaček Škrobot, N.; Nanić, L.; Ravlić, S.; Sopta, M.; Gerić, M.; Gajski, G.; Garaj-Vrhovac, V.; Rubelj, I. Telomeres, Nutrition, and Longevity: Can We Really Navigate Our Aging? J. Gerontol. Ser. A 2018, 73, 39–47. [Google Scholar] [CrossRef]
- Mattioli, R.; Mosca, L.; Sánchez-Lamar, A.; Tempera, I.; Hausmann, R. Natural Bioactive Compounds Acting against Oxidative Stress in Chronic, Degenerative, and Infectious Diseases. Oxid. Med. Cell. Longev. 2018, 2018, 3894381. [Google Scholar] [CrossRef]
- Teodoro, A.J. Bioactive Compounds of Food: Their Role in the Prevention and Treatment of Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 3765986. [Google Scholar] [CrossRef]
- Riso, P.; Del Bo’, C.; Vendrame, S.; Brusamolino, A.; Martini, D.; Bonacina, G.; Porrini, M. Modulation of Plasma Antioxidant Levels, Glutathione S-Transferase Activity and DNA Damage in Smokers Following a Single Portion of Broccoli: A Pilot Study. J. Sci. Food Agric. 2014, 94, 522–528. [Google Scholar] [CrossRef]
- Moser, B.; Szekeres, T.; Bieglmayer, C.; Wagner, K.-H.; Mišík, M.; Kundi, M.; Zakerska, O.; Nersesyan, A.; Kager, N.; Zahrl, J.; et al. Impact of Spinach Consumption on DNA Stability in Peripheral Lymphocytes and on Biochemical Blood Parameters: Results of a Human Intervention Trial. Eur. J. Nutr. 2011, 50, 587–594. [Google Scholar] [CrossRef]
- Fogarty, M.C.; Hughes, C.M.; Burke, G.; Brown, J.C.; Davison, G.W. Acute and Chronic Watercress Supplementation Attenuates Exercise-Induced Peripheral Mononuclear Cell DNA Damage and Lipid Peroxidation. Br. J. Nutr. 2013, 109, 293–301. [Google Scholar] [CrossRef]
- Bakuradze, T.; Lang, R.; Hofmann, T.; Schipp, D.; Galan, J.; Eisenbrand, G.; Richling, E. Coffee Consumption Rapidly Reduces Background DNA Strand Breaks in Healthy Humans: Results of a Short-Term Repeated Uptake Intervention Study. Mol. Nutr. Food Res. 2016, 60, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Gajski, G.; Gerić, M.; Vučić Lovrenčić, M.; Božičević, S.; Rubelj, I.; Nanić, L.; Škrobot Vidaček, N.; Bendix, L.; Peraica, M.; Rašić, D.; et al. Analysis of Health-Related Biomarkers between Vegetarians and Non-Vegetarians: A Multi-Biomarker Approach. J. Funct. Foods 2018, 48, 643–653. [Google Scholar] [CrossRef]
- Vučić Lovrenčić, M.; Gerić, M.; Košuta, I.; Dragičević, M.; Garaj-Vrhovac, V.; Gajski, G. Sex-Specific Effect of Vegetarian Diet on Adiponectin Levels and Insulin Sensitivity in Healthy Non-Obese Individuals. Nutrition 2020, 79–80, 110862. [Google Scholar] [CrossRef] [PubMed]
- Azqueta, A.; Ladeira, C.; Giovannelli, L.; Boutet-Robinet, E.; Bonassi, S.; Neri, M.; Gajski, G.; Duthie, S.; Del Bo’, C.; Riso, P.; et al. Application of the Comet Assay in Human Biomonitoring: An HCOMET Perspective. Mutat. Res. Mutat. Res. 2020, 783, 108288. [Google Scholar] [CrossRef] [PubMed]
- Gerić, M.; Gajski, G.; Oreščanin, V.; Garaj-Vrhovac, V. Seasonal Variations as Predictive Factors of the Comet Assay Parameters: A Retrospective Study. Mutagenesis 2018, 33, 53–60. [Google Scholar] [CrossRef]
- Gajski, G.; Gerić, M.; Živković Semren, T.; Tariba Lovaković, B.; Oreščanin, V.; Pizent, A. Application of the Comet Assay for the Evaluation of DNA Damage from Frozen Human Whole Blood Samples: Implications for Human Biomonitoring. Toxicol. Lett. 2020, 319, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.; Møller, P.; Gajski, G.; Vodenková, S.; Abdulwahed, A.; Anderson, D.; Bankoglu, E.E.; Bonassi, S.; Boutet-Robinet, E.; Brunborg, G.; et al. Measuring DNA Modifications with the Comet Assay: A Compendium of Protocols. Nat. Protoc. 2023, 18, 929–989. [Google Scholar] [CrossRef]
- Azqueta, A.; Collins, A.R. The Essential Comet Assay: A Comprehensive Guide to Measuring DNA Damage and Repair. Arch. Toxicol. 2013, 87, 949–968. [Google Scholar] [CrossRef]
- Bonassi, S.; Ceppi, M.; Møller, P.; Azqueta, A.; Milić, M.; Monica, N.; Brunborg, G.; Godschalk, R.; Koppen, G.; Langie, S.A.S.; et al. DNA Damage in Circulating Leukocytes Measured with the Comet Assay May Predict the Risk of Death. Sci. Rep. 2021, 11, 16793. [Google Scholar] [CrossRef]
- Milić, M.; Ceppi, M.; Bruzzone, M.; Azqueta, A.; Brunborg, G.; Godschalk, R.; Koppen, G.; Langie, S.; Møller, P.; Teixeira, J.P.; et al. The HCOMET Project: International Database Comparison of Results with the Comet Assay in Human Biomonitoring. Baseline Frequency of DNA Damage and Effect of Main Confounders. Mutat. Res. Mutat. Res. 2021, 787, 108371. [Google Scholar] [CrossRef]
- Møller, P.; Azqueta, A.; Boutet-Robinet, E.; Koppen, G.; Bonassi, S.; Milić, M.; Gajski, G.; Costa, S.; Teixeira, J.P.; Costa Pereira, C.; et al. Minimum Information for Reporting on the Comet Assay (MIRCA): Recommendations for Describing Comet Assay Procedures and Results. Nat. Protoc. 2020, 15, 3817–3826. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C.; Koplan, J.P.; Nugent, R.; Dusenbury, C.; Puska, P.; Gaziano, T.A. Prevention of Chronic Disease by Means of Diet and Lifestyle Changes; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2006; ISBN 0821361791. [Google Scholar]
- Kimokoti, R.W.; Millen, B.E. Nutrition for the Prevention of Chronic Diseases. Med. Clin. N. Am. 2016, 100, 1185–1198. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.J. Nutrition Concerns and Health Effects of Vegetarian Diets. Nutr. Clin. Pract. 2010, 25, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.J. Health Effects of Vegan Diets. Am. J. Clin. Nutr. 2009, 89, 1627S–1633S. [Google Scholar] [CrossRef] [PubMed]
- Melina, V.; Craig, W.; Levin, S. Position of the Academy of Nutrition and Dietetics: Vegetarian Diets. J. Acad. Nutr. Diet. 2016, 116, 1970–1980. [Google Scholar] [CrossRef]
- Hargreaves, S.M.; Rosenfeld, D.L.; Moreira, A.V.B.; Zandonadi, R.P. Plant-Based and Vegetarian Diets: An Overview and Definition of These Dietary Patterns. Eur. J. Nutr. 2023, 62, 1109–1121. [Google Scholar] [CrossRef]
- Wulf, H.C.; Iversen, A.S.; Husum, B.; Neibuhr, E. Very Low Sister-Chromatid Exchange Rate in Seventh-Day Adventists. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1986, 162, 131–135. [Google Scholar] [CrossRef]
- Fenech, M. Cytokinesis-Block Micronucleus Cytome Assay. Nat. Protoc. 2007, 2, 1084–1104. [Google Scholar] [CrossRef]
- Gajski, G.; Gerić, M.; Oreščanin, V.; Garaj-Vrhovac, V. Cytokinesis-Block Micronucleus Cytome Assay Parameters in Peripheral Blood Lymphocytes of the General Population: Contribution of Age, Sex, Seasonal Variations and Lifestyle Factors. Ecotoxicol. Environ. Saf. 2018, 148, 561–570. [Google Scholar] [CrossRef]
- Kopjar, N.; Kašuba, V.; Milić, M.; Rozgaj, R.; Želježić, D.; Gajski, G.; Mladinić, M.; Garaj-Vrhovac, V. Normal and Cut-off Values of the Cytokinesis-Block Micronucleus Assay on Peripheral Blood Lymphocytes in the Croatian General Population. Arh. Hig. Rada Toksikol. 2010, 61, 219–234. [Google Scholar] [CrossRef]
- Fenech, M.; Kirsch-Volders, M.; Natarajan, A.T.; Surralles, J.; Crott, J.W.; Parry, J.; Norppa, H.; Eastmond, D.A.; Tucker, J.D.; Thomas, P. Molecular Mechanisms of Micronucleus, Nucleoplasmic Bridge and Nuclear Bud Formation in Mammalian and Human Cells. Mutagenesis 2011, 26, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Fenech, M. Cytokinesis-Block Micronucleus Cytome Assay Evolution into a More Comprehensive Method to Measure Chromosomal Instability. Genes 2020, 11, 1203. [Google Scholar] [CrossRef] [PubMed]
- Kazimírová, A.; Barancoková, M.; Volkovová, K.; Staruchová, M.; Krajcovicová-Kudlácková, M.; Wsólová, L.; Collins, A.R.; Dusinská, M. Does a Vegetarian Diet Influence Genomic Stability? Eur. J. Nutr. 2004, 43, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Krajcovicova-Kudlackova, M.; Valachovicová, M.; Pauková, V.; Dusinská, M. Effects of Diet and Age on Oxidative Damage Products in Healthy Subjects. Physiol. Res. 2008, 57, 647–651. [Google Scholar] [CrossRef]
- Krajcovicova-Kudlackova, M.; Dusinská, M. Oxidative DNA Damage in Relation to Nutrition. Neoplasma 2004, 51, 30–33. [Google Scholar]
- Kotova, N.; Frostne, C.; Abramsson-Zetterberg, L.; Tareke, E.; Bergman, R.; Haghdoost, S.; Paulsson, B.; Törnqvist, M.; Segerbäck, D.; Jenssen, D.; et al. Differences in Micronucleus Frequency and Acrylamide Adduct Levels with Hemoglobin between Vegetarians and Non-Vegetarians. Eur. J. Nutr. 2015, 54, 1181–1190. [Google Scholar] [CrossRef]
- Dhawan, A.; Mathur, N.; Seth, P.K. The Effect of Smoking and Eating Habits on DNA Damage in Indian Population as Measured in the Comet Assay. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2001, 474, 121–128. [Google Scholar] [CrossRef]
- Bajpayee, M.; Dhawan, A.; Parmar, D.; Pandey, A.K.; Mathur, N.; Seth, P.K. Gender-Related Differences in Basal DNA Damage in Lymphocytes of a Healthy Indian Population Using the Alkaline Comet Assay. Mutat. Res. 2002, 520, 83–91. [Google Scholar] [CrossRef]
- Verhagen, H.; Rauma, A.L.; Törrönen, R.; de Vogel, N.; Bruijntjes-Rozier, G.C.; Drevo, M.A.; Bogaards, J.J.; Mykkänen, H.; Dreve, M.; Bogaards, J.J.; et al. Effect of a Vegan Diet on Biomarkers of Chemoprevention in Females. Hum. Exp. Toxicol. 1996, 15, 821–825. [Google Scholar] [CrossRef]
- Davies, H.W.; Kennedy, S.M.; Teschke, K.; Quintana, P.J.E. Cytogenetic Analysis of South Asian Berry Pickers in British Columbia Using the Micronucleus Assay in Peripheral Lymphocytes. Mutat. Res. Toxicol. Environ. Mutagen. 1998, 416, 101–113. [Google Scholar] [CrossRef]
- Fenech, M.; Rinaldi, J. A Comparison of Lymphocyte Micronuclei and Plasma Micronutrients in Vegetarians and Non-Vegetarians. Carcinogenesis 1995, 16, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Khuda-Bukhsh, A.R.; Das, S.; Saha, S.K. Molecular Approaches toward Targeted Cancer Prevention with Some Food Plants and Their Products: Inflammatory and Other Signal Pathways. Nutr. Cancer 2014, 66, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, N.; Bakovic, M.; Paliyath, G. Molecular Mechanisms and Pathways as Targets for Cancer Prevention and Progression with Dietary Compounds. Int. J. Mol. Sci. 2017, 18, 2050. [Google Scholar] [CrossRef] [PubMed]
- Duthie, S.J.; Ma, A.; Ross, M.A.; Collins, A.R. Antioxidant Supplementation Decreases Oxidative DNA Damage in Human Lymphocytes. Cancer Res. 1996, 56, 1291–1295. [Google Scholar]
- Dean, R.T.; Fu, S.; Stocker, R.; Davies, M.J. Biochemistry and Pathology of Radical-Mediated Protein Oxidation. Biochem. J. 1997, 324 Pt 1, 1–18. [Google Scholar] [CrossRef]
- Pool-Zobel, B.L.; Bub, A.; Müller, H.; Wollowski, I.; Rechkemmer, G. Consumption of Vegetables Reduces Genetic Damage in Humans: First Results of a Human Intervention Trial with Carotenoid-Rich Foods. Carcinogenesis 1997, 18, 1847–1850. [Google Scholar] [CrossRef]
- Kazimírová, A.; Barancoková, M.; Krajcovicová-Kudlácková, M.; Volkovová, K.; Staruchová, M.; Valachovicová, M.; Pauková, V.; Blazícek, P.; Wsólová, L.; Dusinská, M. The Relationship between Micronuclei in Human Lymphocytes and Selected Micronutrients in Vegetarians and Non-Vegetarians. Mutat. Res. 2006, 611, 64–70. [Google Scholar] [CrossRef]
- Wasson, G.R.; McKelvey-Martin, V.J.; Downes, C.S. The Use of the Comet Assay in the Study of Human Nutrition and Cancer. Mutagenesis 2008, 23, 153–162. [Google Scholar] [CrossRef]
- Gerić, M.; Matković, K.; Gajski, G.; Rumbak, I.; Štancl, P.; Karlić, R.; Bituh, M. Adherence to Mediterranean Diet in Croatia: Lessons Learned Today for a Brighter Tomorrow. Nutrients 2022, 14, 3725. [Google Scholar] [CrossRef]
- Smolková, B.; Dusinská, M.; Raslová, K.; Barancoková, M.; Kazimírová, A.; Horská, A.; Spustová, V.; Collins, A. Folate Levels Determine Effect of Antioxidant Supplementation on Micronuclei in Subjects with Cardiovascular Risk. Mutagenesis 2004, 19, 469–476. [Google Scholar] [CrossRef]
- Herrmann, W.; Schorr, H.; Purschwitz, K.; Rassoul, F.; Richter, V. Total Homocysteine, Vitamin B(12), and Total Antioxidant Status in Vegetarians. Clin. Chem. 2001, 47, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Obersby, D.; Chappell, D.C.; Dunnett, A.; Tsiami, A.A. Plasma Total Homocysteine Status of Vegetarians Compared with Omnivores: A Systematic Review and Meta-Analysis. Br. J. Nutr. 2013, 109, 785–794. [Google Scholar] [CrossRef]
- Nair-Shalliker, V.; Armstrong, B.K.; Fenech, M. Does Vitamin D Protect against DNA Damage? Mutat. Res. 2012, 733, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N. DNA Damage from Micronutrient Deficiencies Is Likely to Be a Major Cause of Cancer. Mutat. Res. 2001, 475, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N. Micronutrient Deficiencies: A Major Cause of DNA Damage. Ann. N. Y. Acad. Sci. 1999, 889, 87–106. [Google Scholar] [CrossRef]
- Kapiszewska, M. A Vegetable to Meat Consumption Ratio as a Relevant Factor Determining Cancer Preventive Diet. The Mediterranean versus Other European Countries. Forum Nutr. 2006, 59, 130–153. [Google Scholar] [CrossRef]
- Viegas, S.; Ladeira, C.; Costa-Veiga, A.; Perelman, J.; Gajski, G. Forgotten Public Health Impacts of Cancer—An Overview. Arh. Hig. Rada Toksikol. 2017, 68, 287–297. [Google Scholar] [CrossRef]
- Anonymous. Annotations: Irish Distressed Ladies.-Vegetarianism.-“Sweating”. Hospital 1888, 3, 446. [Google Scholar]
- Anonymous. Vegetarianism and Cancer. Hospital 1888, 5, 124. [Google Scholar]

| Vegetarians (n = 16) | Non-Vegetarians (n = 16) | ||||
|---|---|---|---|---|---|
| Omnivores (n = 8) | Pescatarians (n = 8) | ||||
| Age (years) | |||||
| Mean ± SD | 31.4 ± 3.1 | 31.0 ± 3.5 | 31.0 ± 3.9 | 31.0 ± 3.27 | |
| Range | 25–35 | 26–35 | 26–35 | 26–35 | |
| Vegetarianism (years) | |||||
| Mean ± SD | 10.7 ± 3.8 | - | - | - | |
| Range | 4–18 | - | - | - | |
| Tail intensity (% tail DNA) | |||||
| Mean ± SD | 3.6 ± 1.1 *,# | 2.8 ± 1.0 | 3.2 ± 0.8 | 2.4 ± 1.1 | |
| Range | 1.9–6.0 | 0.9–4.6 | 2.1–4.6 | 0.9–3.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gajski, G.; Matković, K.; Delić, L.; Gerić, M. Evaluation of Primary DNA Damage in Young Healthy Females Based on Their Dietary Preferences. Nutrients 2023, 15, 2218. https://doi.org/10.3390/nu15092218
Gajski G, Matković K, Delić L, Gerić M. Evaluation of Primary DNA Damage in Young Healthy Females Based on Their Dietary Preferences. Nutrients. 2023; 15(9):2218. https://doi.org/10.3390/nu15092218
Chicago/Turabian StyleGajski, Goran, Katarina Matković, Luka Delić, and Marko Gerić. 2023. "Evaluation of Primary DNA Damage in Young Healthy Females Based on Their Dietary Preferences" Nutrients 15, no. 9: 2218. https://doi.org/10.3390/nu15092218
APA StyleGajski, G., Matković, K., Delić, L., & Gerić, M. (2023). Evaluation of Primary DNA Damage in Young Healthy Females Based on Their Dietary Preferences. Nutrients, 15(9), 2218. https://doi.org/10.3390/nu15092218







