Changes in the Gut Microbiota after the Use of Herbal Medicines in Overweight and Obese Individuals: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. The Materials
Registration and Protocol
2.2. Review Question
2.3. Search Methods for Identification of Studies
2.4. Study Selection
2.5. Data Extraction and Risk of Bias Assessment
2.6. Data Synthesis
3. Results
3.1. Results of the Search
3.2. Study Characteristics
3.3. Gut microbiota
3.4. Anthropometric and Biomarkers Data
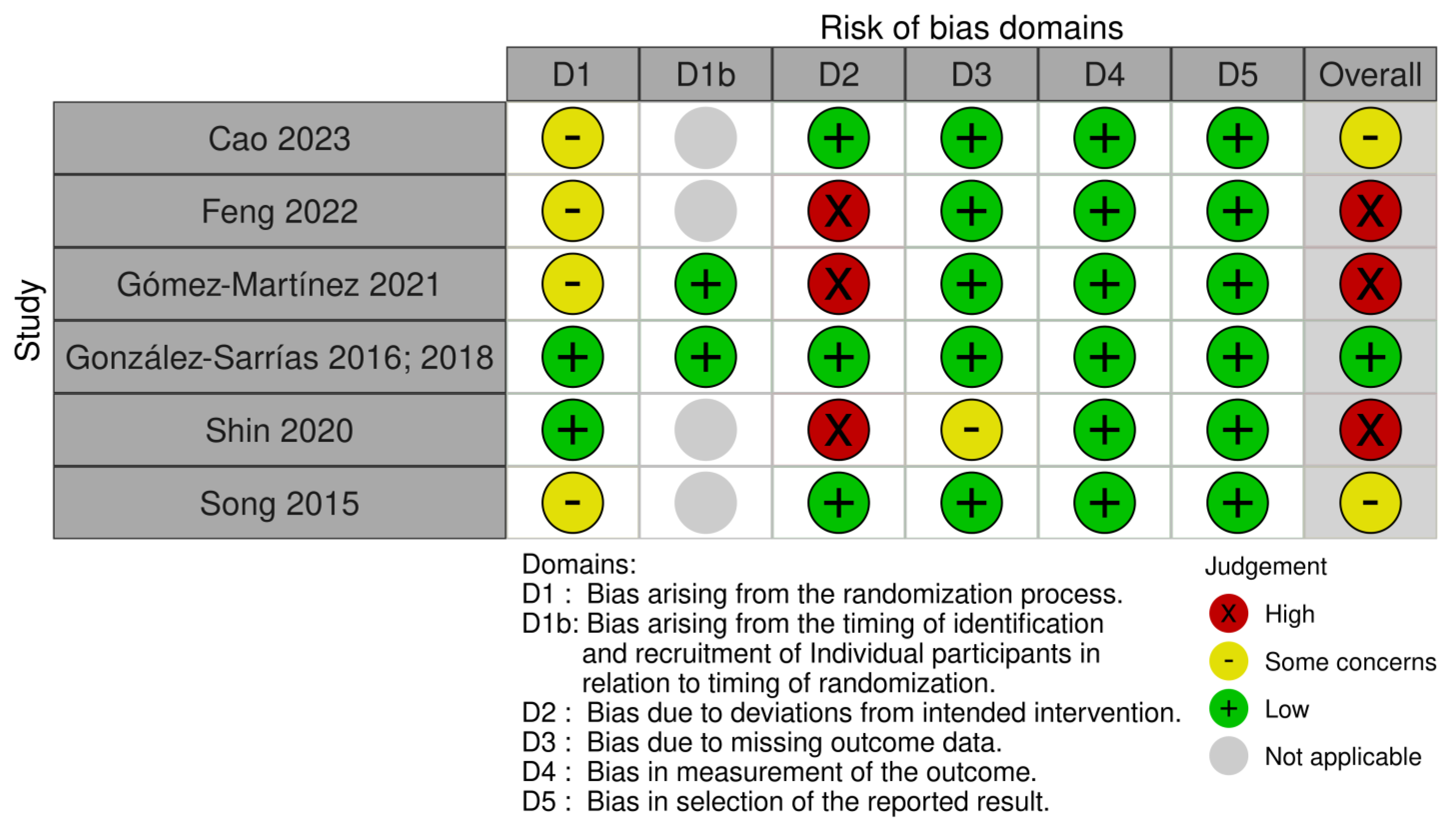
3.5. Risk of Bias in Included Studies
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1923–1994. [Google Scholar]
- Obesity and Overweight. Who.int. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 20 December 2022).
- Astell, K.J.; Mathai, M.L.; Su, X.Q. Plant extracts with appetite suppressing properties for body weight control: A systematic review of double blind randomized controlled clinical trials. Complement. Ther. Med. 2013, 21, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Apovian, C.; Aronne, L.J.; Bessesen, D.H.; McDonnell, M.E.; Murad, M.H.; Pagotto, U.; Ryan, D.H.; Still, C.D. Pharmacological management of obesity: An endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2015, 100, 342–362. [Google Scholar] [CrossRef] [PubMed]
- Mikail, H.; Mohammed, M.; Umar, H.D.; Suleiman, M.M. Secondary Metabolites: The Natural Remedies; IntechOpen: London, UK, 2022. [Google Scholar]
- Kumar, M.; Kaushik, D.; Kaur, J.; Proestos, C.; Oz, F.; Oz, E.; Gupta, P.; Kundu, P.; Kaur, A.; Anisha, A.; et al. A critical review on obesity: Herbal approach, bioactive compounds, and their mechanism. Appl. Sci. 2022, 12, 8342. [Google Scholar] [CrossRef]
- Ballini, A.; Scacco, S.; Boccellino, M.; Santacroce, L.; Arrigoni, R. Microbiota and obesity: Where are we now? Biology 2020, 9, 415. [Google Scholar] [CrossRef]
- Asadi, A.; Mehr, N.S.; Mohamadi, M.H.; Shokri, F.; Heidary, M.; Sadeghifard, N.; Khoshnood, S. Obesity and gut-microbiota-brain axis: A narrative review. J. Clin. Lab. Anal. 2022, 36, e24420. [Google Scholar] [CrossRef]
- Yang, Q.; Liang, Q.; Balakrishnan, B.; Belobrajdic, D.P.; Feng, Q.J.; Zhang, W. Role of dietary nutrients in the modulation of gut microbiota: A narrative review. Nutrients 2020, 12, 381. [Google Scholar] [CrossRef]
- Feng, W.; Ao, H.; Peng, C.; Yan, D. Gut microbiota, a new frontier to understand traditional Chinese medicines. Pharmacol. Res. 2019, 142, 176–191. [Google Scholar] [CrossRef]
- An, X.; Bao, Q.; Di, S.; Zhao, Y.; Zhao, S.; Zhang, H.; Zhang, H.; Lian, F.; Tong, X. The interaction between the gut microbiota and herbal medicines. Biomed. Pharmacother. 2019, 118, 109252. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Rebello, C.J.; Burton, J.; Heiman, M.; Greenway, F.L. Gastrointestinal microbiome modulator improves glucose tolerance in overweight and obese subjects: A randomized controlled pilot trial. J. Diabetes Complicat. 2015, 29, 1272–1276. [Google Scholar] [CrossRef]
- Cao, Z.; Wei, H.; Wen, C.; Song, Y.; Srivastava, K.; Yang, N.; Shi, Y.-M.; Miao, M.; Chung, D.; Li, X.-M. Clinical efficacy of weight loss herbal intervention therapy and lifestyle modifications on obesity and its association with distinct gut microbiome: A randomized double-blind phase 2 study. Front. Endocrinol. 2023, 14, 1054674. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Wang, Q.; Cao, H.; He, F.; Guan, Y.; Li, D.; Yan, J.; Yang, J.; Xia, Y.; Dong, M. White common bean extract remodels the gut microbiota and ameliorates type 2 diabetes and its complications: A randomized double-blinded placebo-controlled trial. Front. Endocrinol. 2022, 13, 999715. [Google Scholar] [CrossRef]
- Gómez-Martínez, S.; Díaz-Prieto, L.E.; Vicente Castro, I.; Jurado, C.; Iturmendi, N.; Martín-Ridaura, M.C.; Calle, N.; Dueñas, M.; Picón, M.J.; Marcos, A.; et al. Moringa oleifera leaf supplementation as a glycemic control strategy in subjects with prediabetes. Nutrients 2021, 14, 57. [Google Scholar] [CrossRef]
- Song, M.Y.; Wang, J.H.; Eom, T.; Kim, H. Schisandra chinensis fruit modulates the gut microbiota composition in association with metabolic markers in obese women: A randomized, double-blind placebo-controlled study. Nutr. Res. 2015, 35, 655–663. [Google Scholar] [CrossRef]
- González-Sarrías, A.; García-Villalba, R.; Romo-Vaquero, M.; Alasalvar, C.; Örem, A.; Zafrilla, P.; Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C. Clustering according to urolithin metabotype explains the interindividual variability in the improvement of cardiovascular risk biomarkers in overweight-obese individuals consuming pomegranate: A randomized clinical trial. Mol. Nutr. Food Res. 2017, 61, 1600830. [Google Scholar] [CrossRef]
- González-Sarrías, A.; Romo-Vaquero, M.; García-Villalba, R.; Cortés-Martín, A.; Selma, M.V.; Espín, J.C. The endotoxemia marker lipopolysaccharide-binding protein is reduced in overweight-obese subjects consuming pomegranate extract by modulating the gut microbiota: A randomized clinical trial. Mol. Nutr. Food Res. 2018, 62, e1800160. [Google Scholar] [CrossRef]
- Shin, N.R.; Gu, N.; Choi, H.S.; Kim, H. Combined effects of Scutellaria baicalensis with metformin on glucose tolerance of patients with type 2 diabetes via gut microbiota modulation. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E52–E61. [Google Scholar] [CrossRef]
- Deng, F.; Li, Y.; Zhao, J. The gut microbiome of healthy long-living people. Aging 2019, 11, 289–290. [Google Scholar] [CrossRef]
- Ahmad, A.; Yang, W.; Chen, G.; Shafiq, M.; Javed, S.; Ali Zaidi, S.S.; Shahid, R.; Liu, C.; Bokhari, H. Analysis of gut microbiota of obese individuals with type 2 diabetes and healthy individuals. PLoS ONE 2019, 14, e0226372. [Google Scholar] [CrossRef]
- Vetrani, C.; Maukonen, J.; Bozzetto, L.; Della Pepa, G.; Vitale, M.; Costabile, G.; Riccardi, G.; Rivellese, A.A.; Saarela, M.; Annuzzi, G. Diets naturally rich in polyphenols and/or long-chain n-3 polyunsaturated fatty acids differently affect microbiota composition in high-cardiometabolic-risk individuals. Acta Diabetol. 2020, 57, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Wang, F.; Yuan, J.; Li, J.; Jiang, D.; Zhang, J.; Li, H.; Wang, R.; Tang, J.; Huang, T.; et al. Effects of dietary fat on gut microbiota and faecal metabolites, and their relationship with cardiometabolic risk factors: A 6-month randomised controlled-feeding trial. Gut 2019, 68, 1417–1429. [Google Scholar] [CrossRef] [PubMed]
- Castaner, O.; Goday, A.; Park, Y.M.; Lee, S.H.; Magkos, F.; Shiow, S.A.T.E.; Schröder, H. The gut microbiome profile in obesity: A systematic review. Int. J. Endocrinol. 2018, 2018, 4095789. [Google Scholar] [CrossRef] [PubMed]
- Pinart, M.; Dötsch, A.; Schlicht, K.; Laudes, M.; Bouwman, J.; Forslund, S.K.; Pischon, T.; Nimptsch, K. Gut microbiome composition in obese and non-obese persons: A systematic review and meta-analysis. Nutrients 2021, 14, 12. [Google Scholar] [CrossRef]
- Fassarella, M.; Blaak, E.E.; Penders, J.; Nauta, A.; Smidt, H.; Zoetendal, E.G. Gut microbiome stability and resilience: Elucidating the response to perturbations in order to modulate gut health. Gut 2021, 70, 595–605. [Google Scholar] [CrossRef]
- Corb Aron, R.A.; Abid, A.; Vesa, C.M.; Nechifor, A.C.; Behl, T.; Ghitea, T.C.; Munteanu, M.A.; Fratila, O.; Andronie-Cioara, F.L.; Toma, M.M.; et al. Recognizing the benefits of pre-/probiotics in metabolic syndrome and type 2 diabetes mellitus considering the influence of Akkermansia muciniphila as a key gut bacterium. Microorganisms 2021, 9, 618. [Google Scholar] [CrossRef]
- Adithya, K.K.; Rajeev, R.; Selvin, J.; Seghal Kiran, G. Dietary influence on the dynamics of the human gut microbiome: Prospective implications in interventional therapies. ACS Food Sci. Technol. 2021, 1, 717–736. [Google Scholar] [CrossRef]
- Breton, J.; Galmiche, M.; Déchelotte, P. Dysbiotic gut bacteria in obesity: An overview of the metabolic mechanisms and therapeutic perspectives of next-generation probiotics. Microorganisms 2022, 10, 452. [Google Scholar] [CrossRef]
- Maunder, A.; Bessell, E.; Lauche, R.; Adams, J.; Sainsbury, A.; Fuller, N.R. Effectiveness of herbal medicines for weight loss: A systematic review and meta-analysis of randomized controlled trials. Diabetes Obes. Metab. 2020, 22, 891–903. [Google Scholar] [CrossRef]
- Whang, A.; Nagpal, R.; Yadav, H. Bi-directional drug-microbiome interactions of anti-diabetics. EBioMedicine 2019, 39, 591–602. [Google Scholar] [CrossRef]
- Lee, C.B.; Chae, S.U.; Jo, S.J.; Jerng, U.M.; Bae, S.K. The relationship between the gut microbiome and metformin as a key for treating type 2 diabetes mellitus. Int. J. Mol. Sci. 2021, 22, 3566. [Google Scholar] [CrossRef]
- Choo, J.M.; Leong, L.E.X.; Rogers, G.B. Sample storage conditions significantly influence faecal microbiome profiles. Sci. Rep. 2015, 5, 16350. [Google Scholar] [CrossRef]
- Plauzolles, A.; Toumi, E.; Bonnet, M.; Pénaranda, G.; Bidaut, G.; Chiche, L.; Allardet-Servent, J.; Retornaz, F.; Goutorbe, B.; Halfon, P. Human stool preservation impacts taxonomic profiles in 16S metagenomics studies. Front. Cell. Infect. Microbiol. 2022, 12, 722886. [Google Scholar] [CrossRef]
- Scherz, V.; Greub, G.; Bertelli, C. Building up a clinical microbiota profiling: A quality framework proposal. Crit. Rev. Microbiol. 2022, 48, 356–375. [Google Scholar] [CrossRef]
- Leeming, E.R.; Johnson, A.J.; Spector, T.D.; Le Roy, C.I. Effect of diet on the gut microbiota: Rethinking intervention duration. Nutrients 2019, 11, 2862. [Google Scholar] [CrossRef]
- Singh, N.; Stewart, R.A.H.; Benatar, J.R. Intensity and duration of lifestyle interventions for long-term weight loss and association with mortality: A meta-analysis of randomised trials. BMJ Open 2019, 9, e029966. [Google Scholar] [CrossRef]
- Klimenko, N.S.; Tyakht, A.V.; Popenko, A.S.; Vasiliev, A.S.; Altukhov, I.A.; Ischenko, D.S.; Shashkova, T.I.; Efimova, D.A.; Nikogosov, D.A.; Osipenko, D.A.; et al. Microbiome responses to an uncontrolled short-term diet intervention in the frame of the citizen science project. Nutrients 2018, 10, 576. [Google Scholar] [CrossRef]
- Ehrlich, S.D.; The MetaHIT Consortium. MetaHIT: The European Union Project on Metagenomics of the Human Intestinal Tract. In Metagenomics of the Human Body; Nelson, K.E., Ed.; Springer: New York, NY, USA, 2011. [Google Scholar]
- Biagini, F.; Calvigioni, M.; Lapomarda, A.; Vecchione, A.; Magliaro, C.; De Maria, C.; Montemurro, F.; Celandroni, F.; Mazzantini, D.; Mattioli-Belmonte, M.; et al. A novel 3D in vitro model of the human gut microbiota. Sci. Rep. 2020, 10, 21499. [Google Scholar] [CrossRef]

| Author, Country | Age (Years) | BMI (kg/m2) | Gender | Comorbities | nB/N Endpoint (% Dropout) | Intervention/Control | Dosage | Duration |
|---|---|---|---|---|---|---|---|---|
| Cao [14] China | 18 to 60 | 32.25 ± 1.4 (intervention) 34.04 ± 2.5 (control) | M: 24; F: 13 | - | 37/40 (7.5%) | W-LHIT | 9 to 15 capsules/day | 2 months |
| Feng [15] China | 35 to 75 | 27.9 ± 0.4 (intervention) 25.1 ± 0.5 (control) | M: 33; F: 50 | Diabetes T2D | 83/96 (13.5%) | WCBE | 1.5 g before each meal/day | 2 months |
| Gómez-Martínez [16] Spain | 45 to 70 | 28.6 ± 3.8 (intervention) 29.4 ± 4.0 (control) | M: 29; F: 36 | Prediabetes | 73/65 (11.0%) | Moringa oleifera | 2.4 g/day | 12 weeks |
| González-Sarrías, [18,19] Spain | >40 | 28.5 ± 1.1 overweight 33.2 ± 3.3 obese | M: 32; F: 17 | - | 50/49 (2.0%) | Punica granatum | 0.45 g/day (3 weeks) 1.8 g/day (3 weeks) | 24 weeks (3 weeks of wash out between dosage) |
| Shin [20] South Korea | 20 to 75 | 25.62 ± 0.64 (intervention) 25.69 ± 0.62 (control) | F and M | Diabetes T2D | 17/12 (29.4%) | Scutellaria baicalensis | 3.52 g/day | 8 weeks (4 weeks of wash out) |
| Song [17] South Korea | 25 to 45 | 29.99 ± 4.27 (intervention) 28.78 ± 3.47 (control) | F | - | 40/28 (30.0%) | Schisandra chinensis | 6.7 g/day | 12 weeks |
| Article | Intervention | Microbiota Analysis Method | GM Changes |
|---|---|---|---|
| Cao [14] China | 9 to 15 capsules W-LHIT/day | 16S rRNA | Increase in phylum Verrucomicrobia, and decrease in phylum Proteobacteria. Increase in genera Akkermansia and Enterococcus. Decrease in species Eubacterium rectale, Haemophilus parainfluenzae, and Faecalibacterium prausnitzii. |
| Feng [15] China | 1.5 g WBCE before each meal/day | 16S rRNA | Increase in genera Anaerostipes, Bifidobacterium, Faecalibacterium, Faecalitalea, Lactobacillus, and Romboutsia, and decrease in genera Adlercreutzia, Citrobacter, Cronobacter, Enterobacteriaceae, Fusobacterium, Klebsiella, and Weissella. |
| Gómez-Martínez [16] | 2.4 g dry extract MO/day 12 weeks | 16S rRNA | No significant change in Clostridium cluster IV and in genera Bifidobacterium and Lactobacillus. No significant change in species Blautia coccoides, Eubacterium rectale, Faecalibacterium prausnitzii, and Akkermansia muciniphila. |
| González-Sarrías [18] | 0.45 g dry extract PG/day 3 weeks 1.8 g dry extract PG/day 3 weeks | real-time qPCR | Increase in genera Gordonibacter and Bacteroides. Increase in species Escherichia coli. |
| González-Sarrías [19] | 0.45 g dry extract PG/day 3 weeks 1.8 g dry extract PG/day 3 weeks | 16S rRNA | Increase in phylum Bacteroidetes, and decrease in phylum Firmicutes. Increase in families Bacteroidaceae and Porphyromonadaceae, and decrease in families Peptostreptococcaceae, Clostridiaceae, and Coriobacteriaceae. Increase in genera Bacteroides and Faecalibacterium, and decrease in genera Romboutsia, Anaerostipes, Dorea, and Clostridium sensu stricto. No significant changes in bacterial diversity. |
| Shin [20] | 3.52 g dry extract SB/day 8 weeks | 16SrRNA | Increase in genera Lactobacillus, Weissella, and Akkermansia. No significant changes in bacterial diversity. |
| Song [17] | 6.7 g dry extract SC/day 12 weeks | qPCR | Increase in phylum Bacteroidetes, and decrease in phylum Firmicutes. Increase in genera Akkermansia, Roseburia, Bacteroides, Prevotella, and Bifidobacterium; decrease in genus Ruminococcus. |
| Study (N Intervention/N Control) | Intervention | Control | |||
|---|---|---|---|---|---|
| Cao 2023 (18/19) | Change | SD | Change | SD | Mean difference [CI 95%] |
| Glucose | −0.17 | 0.74 | 0.05 | 0.78 | −0.12 [−0.61, 0.37] |
| C-peptide | −0.5 | 1.23 | 0.1 | 1.33 | −0.40 [−1.22, 0.42] |
| Insulin | −3.87 | 9.78 | −2.1 | 13.33 | −1.77 [−9.28, 5.74] |
| BMI | −1.31 | 1.1 | −0.88 | 0.88 | −0.43 [−1.05, 0.19] |
| Gomez-Martinez 2021 (31/34) | Change | SD | Change | SD | Mean difference [CI 95%] |
| Glucose | −2.80 | 7.8 | 2.0 | 13.2 | −4.80 [−10.02, 0.42] |
| Insulin | 1.26 | 4.02 | 1.82 | 4.24 | −0.56 [−2.57, 1.45] |
| HbA1c | −0.09 | 0.30 | 0.04 | 0.34 | −0.13 [−0.29, 0.03] |
| HOMA | 0.24 | 1.06 | 0.57 | 1.4 | −0.33 [−0.93, 0.27] |
| GLP | −0.80 | 4.93 | −1.4 | 4.75 | 0.60 [−1.76, 2.96] |
| Ghrelin | −47.0 | 66.58 | −42.6 | 65.48 | −4.40 [−36.55, 27.75] |
| PYY | −6.0 | 17.08 | −7.33 | 19.61 | 1.33 [−7.59, 10.25] |
| Shin 2019 (6/6) | Change | SD | Change | SD | Mean difference (CI 95%) |
| Glucose | 3.2 | 4.66 | 5.3 | 4.51 | −2.10 [−7.29, 3.09] |
| Insulin | 0.58 | 0.52 | 0.72 | 0.87 | −0.14 [−0.95, 0.67] |
| HbA1c | 0.05 | 0.13 | 0.03 | 0.14 | 0.02 [−0.13, 0.17] |
| HOMA | 0.21 | 0.13 | 0.29 | 0.26 | −0.08 [−0.31, 0.15] |
| Weight | −0.05 | 1.72 | 0.46 | 1.65 | −0.51 [−2.42, 1.40] |
| BMI | 0.01 | 0.49 | 0.19 | 0.48 | −0.18 [−0.73, 0.37] |
| Waist | −0.22 | 1.28 | 0.54 | 1.28 | −0.76 [−2.21, 0.69] |
| Song 2015 (13/15) | Change | SD | Change | SD | Mean difference (CI 95%) |
| Glucose | −1.31 | 5.86 | 1.0 | 6.1 | −2.31 [−6.75, 2.13] |
| Insulin | −0.41 | 4.26 | −0.64 | 5.98 | 0.23 [−3.58, 4.04] |
| Cholesterol | −1.69 | 24.30 | −5.6 | 20.74 | 3.91 [−12.96, 20.78] |
| HDL | −1.15 | 8.97 | −8.4 | 20.42 | 7.25 [−4.18, 18.68] |
| TG | −27.46 | 109.8 | 11.6 | 45.84 | −39.06 [−103.10, 24.98] |
| Weight | −0.54 | 12.25 | −0.8 | 8.72 | 0.26 [−7.73, 8.25] |
| BMI | −0.2 | 3.42 | −0.33 | 2.82 | 0.13 [−2.21, 2.47] |
| Waist | −1.88 | 6.81 | −1.36 | 7.6 | −0.52 [−5.86, 4.82] |
| %fat | −2.39 | 4.19 | −1.35 | 3.11 | −1.04 [−3.81, 1.73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, M.; Cople-Rodrigues, C.d.S.; Waitzberg, D.L.; Rocha, I.M.G.d.; Curioni, C.C. Changes in the Gut Microbiota after the Use of Herbal Medicines in Overweight and Obese Individuals: A Systematic Review. Nutrients 2023, 15, 2203. https://doi.org/10.3390/nu15092203
Huang M, Cople-Rodrigues CdS, Waitzberg DL, Rocha IMGd, Curioni CC. Changes in the Gut Microbiota after the Use of Herbal Medicines in Overweight and Obese Individuals: A Systematic Review. Nutrients. 2023; 15(9):2203. https://doi.org/10.3390/nu15092203
Chicago/Turabian StyleHuang, Miguel, Cláudia dos Santos Cople-Rodrigues, Dan L. Waitzberg, Ilanna Marques Gomes da Rocha, and Cintia Chaves Curioni. 2023. "Changes in the Gut Microbiota after the Use of Herbal Medicines in Overweight and Obese Individuals: A Systematic Review" Nutrients 15, no. 9: 2203. https://doi.org/10.3390/nu15092203
APA StyleHuang, M., Cople-Rodrigues, C. d. S., Waitzberg, D. L., Rocha, I. M. G. d., & Curioni, C. C. (2023). Changes in the Gut Microbiota after the Use of Herbal Medicines in Overweight and Obese Individuals: A Systematic Review. Nutrients, 15(9), 2203. https://doi.org/10.3390/nu15092203






