The Impact of Linoleic Acid on Infant Health in the Absence or Presence of DHA in Infant Formulas
Abstract
1. Introduction
2. Levels of Main PUFAs in Breast Milk across the World
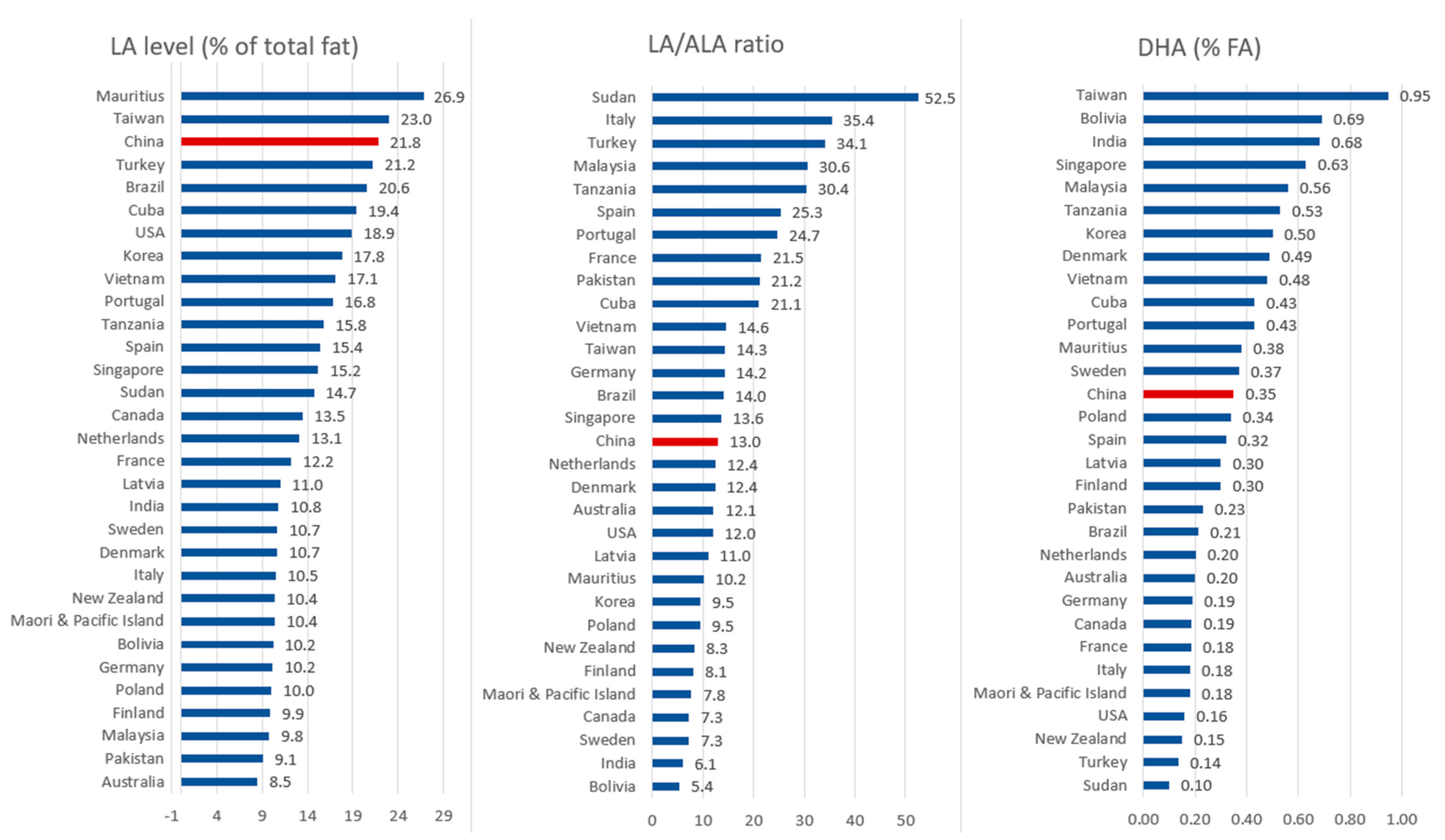
2.1. Levels of Main PUFAs in Breast Milk across Different Countries
2.2. Levels of Main PUFAs in Breast Milk across Different Regions in China
3. Levels of Special LA-Containing Triglycerides in BM
4. Changing LA Levels in BM over Time and the Influence of Diet
4.1. The Level of LA in BM Has Increased over the Past Decades
4.2. The Influence of Diet on PUFA Levels in BM
5. Role of LA and ALA in Infancy
5.1. Metabolism of LA and ALA
5.2. Preclinical Studies and Studies in Adults with LA and ALA
5.3. Intervention Studies with LA and ALA in Infants
5.3.1. Determination of the Minimum and Maximum Levels for LA Intake
| Author [Reference] | Tested Products | Study Population | Main Findings |
|---|---|---|---|
| Hansen et al. [57], Wiese et al. [58], Hansen et al. [59] | Five different IFs varying in content up to 7.3% EN LA | Term infants at birth (n = 428) | LA deficiency developed in infants who received a diet low in LA (<0.1% EN). Manifestations of the deficiency state disappeared when LA was given in IF at ≥1% EN. Deficiency symptoms: dryness of the skin with desquamation, thickening and later intertrigo. Growth was less in infants with low LA intakes, whereas it was normal in infants who received 1.3 to 7.3% EN LA |
| Naismith et al. [61] | IF with 0.55% EN LA vs. breastfed (BF) reference | Term infants (n = 40; n = 20 per group) | Growth in length and weight during the first 3 months of life were similar to BF infants. Voluntary food intakes followed the normal pattern. Clinical signs of deficiency were not observed, suggesting that the requirement for LA is less than was formerly believed (i.e., <0.55% EN) |
| Widdowson et al. [62] | Dutch IF with 58% LA, LA/ALA = 36:1 and British IF with 2% LA, LA/ALA = 2:1 vs. BM control with 8% LA, LA/ALA = 2.5:1 | Healthy term infants; 41 British infants; 37 Dutch infants; 2 BF infants | British babies fed IF never had more than 2% of LA in their body fat, and BF babies, 3–4%. In contrast, by 6 weeks the subcutaneous fat of Dutch infants had 25% FA as LA, and by 12 weeks, 46%. No obvious adverse effects were observed in infants raised on IF with close to 60% LA of total fat. |
| Putnam et al. [63] | Test IF with 45% LA compared to 14% LA in standard IF vs. BM control with 8.8% LA | Heathy term infants; n = 16 (Test IF); n = 15 (Standard IF); n = 9 (BM) | Concentrations of PUFAs in erythrocyte membranes of standard and test IF-fed infants were similar despite very significant differences in the amount of dietary LA. No obvious adverse effects were observed in infants raised on IF with close to 45% FA as LA. |
5.3.2. LA/ALA Interventions to Study the Effect on Growth and Tolerance
| Author [Reference] | Tested Products | Study Population | Main Findings |
|---|---|---|---|
| Makrides et al. [17] | IFs with an LA/ALA ratio of either 10:1 (16.9%:1.7%) or 5:1 (16.3%:3.3%) vs. BF reference | Term infants (n = 36–37/group), from near birth to 34 wks of age | Lowering the LA/ALA in IF from 10:1 to 5:1 resulted in a modest increase in plasma DHA but had no effect on visual evoked potential acuity or growth rate. Tolerance was the same for both formulas. |
| Rzehak et al. [67] | Partially [pHF-W] or extensively hydrolyzed-whey [eHF-W] vs. regular cow’s milk IF [CMF]. LA/ALA ratios: pHF-W (13:1), eHF-W (5:1) and CMF (5:1). | Infants from first wk after birth to ≥120 days of age (pHF-W: n = 35; eHF-W: n = 32; CMF: n = 49) | All tested IFs with an LA/ALA ratio ranging between 5:1 and 13:1 supported normal infant growth as assessed by weight and length gain. |
| Jensen et al. [70] | 4 IFs with LA (16% FA) and varying ALA concentrations (0.4%, 1.0%, 1.7%, or 3.2% FA) and LA/ALA ratios of 44:1, 18.2:1, 9.7:1 and 4.8:1 | Term infants from birth until 240 days of age | The lowest LA/ALA ratio (or highest ALA level) resulted in higher plasma phospholipid DHA but was not associated with improved visual function. Mean body weight of infants who received the lowest LA/ALA ratio was less at 120 days, suggesting that LA/ALA ratios < 4.8 should not be adopted. |
| Ponder et al. [69] | Soybean (SOY) and corn (CORN) oil IF with an LA/ALA ratio of 7:1 (31.5%:4.8%) or 39:1 (34.2%:0.8%), respectively | Infants from first wk after birth to 8 wks of age (in total n = 43; SOY: n = 11; CORN: n = 14; HM: n = 18) | Growth did not differ between groups. Plasma phospholipid and RBC phosphatidylethanolamine DHA was similar in the CORN and SOY formula groups at all ages. The formula content of LA or the LA/ALA ratio had no effect on RBC or plasma DHA levels of the infants. |
5.3.3. LA/ALA Interventions that Attempt to Match the DHA Status in BF Infants
| Author [Reference] | Tested Products | Study Population | Main Findings |
|---|---|---|---|
| Jensen et al. [72] | 4 IFs with LA (16% FA) and varying ALA concentrations (0.4%, 0.95%, 1.7%, or 3.2% FA) and LA/ALA ratios of 44:1, 18.2:1, 9.7:1 and 4.8:1) | Healthy term infants from shortly after birth until 120 days of age | Even though total n-3 FAs in plasma increased with higher levels of ALA, dietary LA/ALA ratios between 5 and 44 did not result in plasma levels of DHA similar to those at birth or in BF infants. |
| Chirouzo et al. [71] | First study: Two IFs with different FA compositions but no LCPUFAs. Second study: IF with or without DHA | Low-birth-weight infants after birth until the first 3 months of age | Both groups of IF-fed infants had significantly lower levels of DHA in RBCs compared with BF infants. In the second study, DHA remained stable in RBCs of infants supplemented with DHA, whereas it decreased in unsupplemented infants. Thus, adding DHA to IF is more effective in maintaining DHA levels in blood than increasing ALA content of IF. |
| Clark et al. [73] | Formula A: LA = 14%; ALA = 0.7%, Formula B: LA = 13%; ALA = 3.3% Formula C: LA= 3.5%; ALA = 1.1% | Healthy term infants from after birth until 10 weeks of age | Although DHA levels were higher in infants fed formula B and C with a low LA/ALA ratio compared to infants fed formula A with a high ratio, they did not reach the values observed in BF infants. |
| Libuda et al. [74] | Complementary foods with ALA-rich rapeseed oil, with DHA-rich salmon or with corn oil (control) | Healthy term infants from the age of 4 to 6 months until age of 10 months | Regular salmon consumption during complementary feeding enhanced infant EPA and DHA status, whereas use of rapeseed oil enhanced endogenous EPA synthesis, but didn’t affect DHA status compared to control. |
| Schwartz et al. [75] | Complementary meals with rapeseed oil (1.6 g/meal) rich in ALA (21% from study food) | Healthy term infants from 4 to 10 months. The control group (n = 53); the test group (n = 49). | After intervention, the plasma total n-3 FAs and n-3 LCPUFAs, but not ALA, were higher and the ratios of n-6/n-3 Fas were lower in the test group. Intervention with ALA favored n-3 LCPUFA synthesis in the complementary feeding period when LCPUFA intake from BM and formula was decreasing. |
| Sauerwald et al. [76] | Ifs with LA (16%), ALA (0.4%) and ARA (0.1%) but different DHA contents (from 0.04% to 0.52%) vs. BF with 11% LA, 0.1% ALA, 0.38% DHA | Preterms (n = 42, birth weight 1000–2200 g) From birth until day 28. | DHA supply increased plasma DHA in dose-dependent manner. IF DHA levels of 0.33% matched plasma DHA status of infants fed BM. LCPUFA synthesis was lower in BF infants than infants fed IF with different DHA and low ALA contents. With the LCPUFA supplementation used, DHA in formulas did not inhibit ARA or DHA synthesis. |
5.3.4. Cohort and LA/ALA Intervention Studies and Infant Health
| Author [Reference] | Tested Products | Study Population | Main Findings |
|---|---|---|---|
| Kim et al. [77] | LA/ALA were 9.7% FA ± 6.3 and 11.1% FA ± 6.9 | Pregnant women with a pre-pregnancy average BMI of 21.3 kg/m2 | Both the maternal dietary n-6/n-3 PUFAs and LA/ALA intake were strongly correlated with the mental (MDI) and psychomotor development (PDI) of infants at 6 months of age. Thus, maintaining low n-6/n-3 PUFAs and LA/ALA is encouraged for women during pregnancy. |
| Bernard et al. [78] | Never BF versus 5.4–9.7% and 9.7–15.9% of LA in colostrum | BF children | LA levels were negatively associated with motor and cognitive scores, independent of BF duration. Children BF with the highest levels of LA tended to score closer to the never BF children than children BF with the lowest levels of LA. |
| Auestad et al. [80] | Formulas with LA (20% FA) and ALA (2% FA) with or without ARA + DHA (ARA 0.46% and DHA 0.14% FA) | Infants after birth until 1 year of age | The findings did not support adding ARA + DHA to IF to enhance growth, visual acuity, information processing, general development, language, or temperament in healthy term infants during the first 14 months after birth. |
6. Regulatory Context
7. Gaps in Knowledge
8. Discussion
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Ballard, O.; Morrow, A.L. Human Milk Composition. Nutrients and Bioactive Factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.R.; Ling, P.-R.; Blackburn, G.L. Review of Infant Feeding: Key Features of Breast Milk and Infant Formula. Nutrients 2016, 8, 279. [Google Scholar] [CrossRef]
- Turck, D.; Bresson, J.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Scientific Opinion on the Safety and Suitability for Use by Infants of Follow-on Formulae with a Protein Content of at Least 1.6 g/100 Kcal. EFSA J. 2017, 15, e04781. [Google Scholar] [CrossRef] [PubMed]
- Patro-Golab, B.; Zalewski, B.M.; Kouwenhoven, S.M.; Karas, J.; Koletzko, B.; Bernard van Goudoever, J.; Szajewska, H. Protein Concentration in Milk Formula, Growth, and Later Risk of Obesity: A Systematic Review. J. Nutr. 2016, 146, 551–564. [Google Scholar] [CrossRef]
- Bode, L. Human Milk Oligosaccharides: Every Baby Needs a Sugar Mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef]
- Koletzko, B.; Agostoni, C.; Bergmann, R.; Ritzenthaler, K.; Shamir, R. Physiological Aspects of Human Milk Lipids and Implications for Infant Feeding: A Workshop Report. Acta Paediatr. 2011, 100, 1405–1415. [Google Scholar] [CrossRef]
- O’Brien, J.S.; Sampson, E.L. Lipid Composition of the Normal Human Brain: Gray Matter, White Matter, and Myelin. J. Lipid Res. 1965, 6, 537–544. [Google Scholar] [CrossRef]
- Benatti, P.; Nicolai, R.; Calvani, M.; Peluso, G. Polyunsaturated Fatty Acids: Biochemical, Nutritional and Epigenetic Properties. J. Am. Coll. Nutr. 2004, 23, 281–302. [Google Scholar] [CrossRef]
- Martínez, M.; Mougan, I. Fatty Acid Composition of Human Brain Phospholipids during Normal Development. J. Neurochem. 1998, 71, 2528–2533. [Google Scholar] [CrossRef]
- Floris, L.M.; Stahl, B.; Abrahamse-Berkeveld, M.; Teller, I.C. Human Milk Fatty Acid Profile across Lactational Stages after Term and Preterm Delivery: A Pooled Data Analysis. Prostaglandins Leukot. Essent. Fatty Acids 2020, 156, 102023. [Google Scholar] [CrossRef]
- Carlson, S.E.; Schipper, L.; Brenna, J.T.; Agostoni, C.; Calder, P.C.; Forsyth, S.; Legrand, P.; Abrahamse-Berkeveld, M.; van de Heijning, B.J.M.; van der Beek, E.M.; et al. Perspective: Moving Toward Desirable Linoleic Acid Content in Infant Formula. Adv. Nutr. 2021, 12, 2085–2098. [Google Scholar] [CrossRef]
- Ailhaud, G.; Massiera, F.; Weill, P.; Legrand, P.; Alessandri, J.M.; Guesnet, P. Temporal Changes in Dietary Fats: Role of n-6 Polyunsaturated Fatty Acids in Excessive Adipose Tissue Development and Relationship to Obesity. Prog. Lipid Res. 2006, 45, 203–236. [Google Scholar] [CrossRef] [PubMed]
- OECD-FAO Agricultural Outlook (Edition 2021)|OECD Agriculture Statistics|OECD ILibrary. Available online: https://www.oecd-ilibrary.org/agriculture-and-food/data/oecd-agriculture-statistics/oecd-fao-agricultural-outlook-edition-2021_4bde2d83-en?parentId=http%3A%2F%2Finstance.metastore.ingenta.com%2Fcontent%2Fcollection%2Fagr-data-en (accessed on 14 July 2022).
- Oléagineuse, L.F.; Asie, E.N.; Mittaine, J.-F. Oilseeds and Vegetable Oils in Asia: A World of Diversity. OCL 2016, 23, D602. [Google Scholar] [CrossRef]
- Delplanque, B.; Gibson, R.; Koletzko, B.; Lapillonne, A.; Strandvik, B. Lipid Quality in Infant Nutrition: Current Knowledge and Future Opportunities. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Delplanque, B.; Du, Q.; Martin, J.C.; Guesnet, P. Lipids for Infant Formulas. OCL—Oilseeds Fats Crops Lipids 2018, 25, 305. [Google Scholar] [CrossRef]
- Makrides, M.; Neumann, M.A.; Jeffrey, B.; Lien, E.L.; Gibson, R.A. A Randomized Trial of Different Ratios of Linoleic to α-Linolenic Acid in the Diet of Term Infants: Effects on Visual Function and Growth. Am. J. Clin. Nutr. 2000, 71, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Codex Alimentarius CODEX STAN 72-1981; Standard for Infant Formula and Formulas for Special Medicalpurposes Intended for Infants Sections A&B Revision 2007. Amended 2011. Codex Alimentarius Commission: Rome, Italy, 2011; pp. 1–21.
- CODEX STAN 156-1987; World Health Organisation (WHO) Standard for Follow-Up Formula. World Health Organisation (WHO): Geneva, Switzerland, 2017.
- Sun, H.; Ren, Q.; Zhao, X.; Tian, Y.; Pan, J.; Wei, Q.; Li, Y.; Chen, Y.; Zhang, H.; Zhang, W.; et al. Regional Similarities and Differences in Mature Human Milk Fatty Acids in Chinese Population: A Systematic Review. Prostaglandins Leukot. Essent. Fat. Acids 2020, 162, 102184. [Google Scholar] [CrossRef]
- Ren, Q.; Zhou, Y.; Zhang, W.; Tian, T.; Sun, H.; Zhao, X.; Xu, Y.; Jiang, S. Longitudinal changes in the bioactive proteins in human milk of the Chinese population: A systematic review. Food Sci. Nutr. 2021, 9, 25. [Google Scholar] [CrossRef]
- Peng, Y.; Zhou, T.; Wang, Q.; Liu, P.; Zhang, T.; Zetterström, R.; Strandvik, B. Fatty Acid Composition of Diet, Cord Blood and Breast Milk in Chinese Mothers with Different Dietary Habits. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Stark, A.H.; Crawford, M.A.; Reifen, R. Update on Alpha-Linolenic Acid. Nutr. Rev. 2008, 66, 326–332. [Google Scholar] [CrossRef]
- Miles, E.A.; Calder, P.C. The Influence of the Position of Palmitate in Infant Formula Triacylglycerols on Health Outcomes. Nutr. Res. 2017, 44, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tu, A.; Ma, Q.; Bai, H.; Du, Z. A Comparative Study of Triacylglycerol Composition in Chinese Human Milk within Different Lactation Stages and Imported Infant Formula by SFC Coupled with Q-TOF-MS. Food Chem. 2017, 221, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Wei, W.; Su, H.; Zou, X.; Wang, X. Evaluation of Sn-2 Fatty Acid Composition in Commercial Infant Formulas on the Chinese Market: A Comparative Study Based on Fat Source and Stage. Food Chem. 2018, 242, 29–36. [Google Scholar] [CrossRef]
- Kallio, H.; Nylund, M.; Boström, P.; Yang, B. Triacylglycerol Regioisomers in Human Milk Resolved with an Algorithmic Novel Electrospray Ionization Tandem Mass Spectrometry Method. Food Chem. 2017, 233, 351–360. [Google Scholar] [CrossRef]
- Yuan, T.; Qi, C.; Dai, X.; Xia, Y.; Sun, C.; Sun, J.; Yu, R.; Zhou, Q.; Jin, Q.; Wei, W.; et al. Triacylglycerol Composition of Breast Milk during Different Lactation Stages. J. Agric. Food Chem. 2019, 67, 2272–2278. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Balter, A.; Vodsky, V.; Odetallh, Y.; Ben-Dror, G.; Zhang, Y.; Zhao, A. Chinese Breast Milk Fat Composition and Its Associated Dietary Factors: A Pilot Study on Lactating Mothers in Beijing. Front. Nutr. 2021, 8, 606950. [Google Scholar] [CrossRef]
- Giuffrida, F.; Cruz-Hernandez, C.; Bertschy, E.; Fontannaz, P.; Elmelegy, I.M.; Tavazzi, I.; Marmet, C.; Sanchez-Bridge, B.; Thakkar, S.K.; de Castro, C.A.; et al. Temporal Changes of Human Breast Milk Lipids of Chinese Mothers. Nutrients 2016, 8, 715. [Google Scholar] [CrossRef]
- Martin, M.A.; Lassek, W.D.; Gaulin, S.J.C.; Evans, R.W.; Woo, J.G.; Geraghty, S.R.; Davidson, B.S.; Morrow, A.L.; Kaplan, H.S.; Gurven, M.D. Fatty Acid Composition in the Mature Milk of Bolivian Forager-Horticulturalists: Controlled Comparisons with a US Sample. Matern. Child. Nutr. 2012, 8, 404–418. [Google Scholar] [CrossRef]
- Szabó, É.; Boehm, G.; Beermann, C.; Weyermann, M.; Brenner, H.; Rothenbacher, D.; Decsi, T. Fatty Acid Profile Comparisons in Human Milk Sampled from the Same Mothers at the Sixth Week and the Sixth Month of Lactation. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 316–320. [Google Scholar] [CrossRef]
- Havlicekova, Z.; Jesenak, M.; Banovcin, P.; Kuchta, M. Beta-Palmitate—A Natural Component of Human Milk in Supplemental Milk Formulas. Nutr. J. 2016, 15, 28. [Google Scholar] [CrossRef]
- Zou, L.; Pande, G.; Akoh, C.C. Infant Formula Fat Analogs and Human Milk Fat: New Focus on Infant Developmental Needs. Annu. Rev. Food Sci. Technol. 2016, 7, 139–165. [Google Scholar] [CrossRef] [PubMed]
- Bar-Yoseph, F.; Lifshitz, Y.; Cohen, T. Review of Sn-2 Palmitate Oil Implications for Infant Health. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zeng, J.P.; Wu, Y.P.; Wei, M.; Zhang, H.; Zheng, L.; Deng, Z.Y.; Li, J. Human Milk Sn-2 Palmitate Triglyceride Rich in Linoleic Acid Had Lower Digestibility but Higher Absorptivity Compared with the Sn-2 Palmitate Triglyceride Rich in Oleic Acid In Vitro. J. Agric. Food Chem. 2021, 69, 9137–9146. [Google Scholar] [CrossRef] [PubMed]
- Sanders, T.A.B.; Reddy, S. The Influence of a Vegetarian Diet on the Fatty Acid Composition of Human Milk and the Essential Fatty Acid Status of the Infant. J. Pediatr. 1992, 120, S0022–S3476. [Google Scholar] [CrossRef] [PubMed]
- Chulei, R.; Xiaofang, L.; Hongsheng, M.; Xiulan, M.; Guizheng, L.; Gianhong, D.; DeFrancesco, C.A.; Connor, W.E. Milk Composition in Women from Five Different Regions of China: The Great Diversity of Milk Fatty Acids. J. Nutr. 1995, 125, 2993–2998. [Google Scholar] [CrossRef] [PubMed]
- Kneebone, G.M.; Kneebone, R.; Gibson, R.A. Fatty Acid Composition of Breast Milk from Three Racial Groups from Penang, Malaysia. Am. J. Clin. Nutr. 1985, 41, 765–769. [Google Scholar] [CrossRef]
- Fang, C.; Beghin, J.C. Urban Demand for Edible Oils and Fats in China: Evidence from Household Survey Data. J. Comp. Econ. 2002, 30, 732–753. [Google Scholar] [CrossRef]
- Miliku, K.; Duan, Q.L.; Moraes, T.J.; Becker, A.B.; Mandhane, P.J.; Turvey, S.E.; Lefebvre, D.L.; Sears, M.R.; Subbarao, P.; Field, C.J.; et al. Human Milk Fatty Acid Composition Is Associated with Dietary, Genetic, Sociodemographic, and Environmental Factors in the CHILD Cohort Study. Am. J. Clin. Nutr. 2019, 110, 1370–1383. [Google Scholar] [CrossRef]
- Liu, G.; Ding, Z.; Li, X.; Chen, X.; Wu, Y.; Xie, L. Relationship between Polyunsaturated Fatty Acid Levels in Maternal Diets and Human Milk in the First Month Post-Partum. J. Hum. Nutr. Diet. 2016, 29, 405–410. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Wang, L.; Perovic, M. The Investigation of Fatty Acid Composition of Breast Milk and Its Relationship with Dietary Fatty Acid Intake in 5 Regions of China. Medicine 2019, 98, e15855. [Google Scholar] [CrossRef]
- Tian, H.M.; Wu, Y.X.; Lin, Y.Q.; Chen, X.Y.; Yu, M.; Lu, T.; Xie, L. Dietary Patterns Affect Maternal Macronutrient Intake Levels and the Fatty Acid Profile of Breast Milk in Lactating Chinese Mothers. Nutrition 2019, 58, 83–88. [Google Scholar] [CrossRef]
- Chen, H.; Wang, P.; Han, Y.; Ma, J.; Troy, F.A.; Wang, B.; Wang, B. Evaluation of Dietary Intake of Lactating Women in China and Its Potential Impact on the Health of Mothers and Infants. BMC Womens Health 2012, 12, 18. [Google Scholar] [CrossRef]
- dos Santos, Q.; Sichieri, R.; Marchioni, D.M.; Verly Junior, E. Brazilian Pregnant and Lactating Women Do Not Change Their Food Intake to Meet Nutritional Goals. BMC Pregnancy Childbirth 2014, 14, 186. [Google Scholar] [CrossRef]
- Sotres-Alvarez, D.; Herring, A.H.; Siega-Riz, A.M. Latent Transition Models to Study Women’s Changing of Dietary Patterns from Pregnancy to 1 Year Postpartum. Am. J. Epidemiol. 2013, 177, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Cucó, G.; Fernández-Ballart, J.; Sala, J.; Viladrich, C.; Iranzo, R.; Vila, J.; Arija, V. Dietary Patterns and Associated Lifestyles in Preconception, Pregnancy and Postpartum. Eur. J. Clin. Nutr. 2006, 60, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Crozier, S.R.; Robinson, S.M.; Godfrey, K.M.; Cooper, C.; Inskip, H.M. Women’s Dietary Patterns Change Little from before to during Pregnancy. J. Nutr. 2009, 139, 1956–1963. [Google Scholar] [CrossRef]
- Bobiński, R.; Bobińska, J. Fatty Acids of Human Milk—A Review. Int. J. Vitam. Nutr. Res. 2020, 92, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Carnielli, V.P.; Wattimena, D.J.L.; Luijendijk, I.H.T.; Boerlage, A.; Degenhart, H.J.; Sauer, P.J.J. The Very Low Birth Weight Premature Infant Is Capable of Synthesizing Arachidonic and Docosahexaenoic Acids from Linoleic and Linolenic Acids. Pediatr. Res. 1996, 40, 169–174. [Google Scholar] [CrossRef]
- Sauerwald, T.U.; Hachey, D.L.; Jensen, C.L.; Chen, H.; Anderson, R.E.; Heird, W.C. Intermediates in Endogenous Synthesis of C22:6ω3 and C20:4ω6 by Term and Preterm Infants. Pediatr. Res. 1997, 41, 183–187. [Google Scholar] [CrossRef]
- Klevebro, S.; Juul, S.E.; Wood, T.R. A More Comprehensive Approach to the Neuroprotective Potential of Long-Chain Polyunsaturated Fatty Acids in Preterm Infants Is Needed—Should We Consider Maternal Diet and the n-6:N-3 Fatty Acid Ratio? Front. Pediatr. 2020, 7, 533. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Liu, R.; Chang, M.; Huang, J.; Wang, X. Dietary Linoleic Acid Intake and Blood Inflammatory Markers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Food Funct. 2017, 8, 3091–3103. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.H.; Fritsche, K. Effect of Dietary Linoleic Acid on Markers of Inflammation in Healthy Persons: A Systematic Review of Randomized Controlled Trials. J. Acad. Nutr. Diet. 2012, 112, 1041.e1–1041.e15. [Google Scholar] [CrossRef]
- Cole, R.M.; Puchala, S.; Ke, J.Y.; Abdel-Rasoul, M.; Harlow, K.; O’Donnell, B.; Bradley, D.; Andridge, R.; Borkowski, K.; Newman, J.W.; et al. Linoleic Acid–Rich Oil Supplementation Increases Total and High-Molecular-Weight Adiponectin and Alters Plasma Oxylipins in Postmenopausal Women with Metabolic Syndrome. Curr. Dev. Nutr. 2020, 4, nzaa136. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.E.; Haggard, M.E.; Boelsche, A.N.; Adam, D.J.; Wiese, H.F. Essential Fatty Acids in Infant Nutrition. III. Clinical Manifestations of Linoleic Acid Deficiency. J. Nutr. 1958, 66, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Wiese, H.F.; Hansen, A.E.; Adam, D.J. Essential Fatty Acids in Infant Nutrition. I. Linoleic Acid Requirement in Terms of Serum Di-, Tri- and Tetraenoic Acid Levels. J. Nutr. 1958, 66, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Hansen, E.; Wiese, F.; Adam, J.D. Role of Linoleic Acid in Infant Nutrition. Pediatrics 1963, 31, 171–192. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on Dietary Reference Values for Fats, Including Saturated Fatty Acids, Polyunsaturated Fatty Acids, Monounsaturated Fatty Acids, Trans Fatty Acids, and Cholesterol. EFSA J. 2016, 8, 1461. [Google Scholar] [CrossRef]
- Naismith, D.J.; Deeprose, S.P.; Supramaniam, G.; Williams, M.J.H. Reappraisal of Linoleic Acid Requirement of the Young Infant, with Particular Regard to Use of Modified Cows’ Milk Formulae. Arch. Dis. Child. 1978, 53, 845–849. [Google Scholar] [CrossRef]
- Widdowson, E.M.; Dauncey, M.J.; Gairdner, D.M.T.; Jonxis, J.H.P.; Pelikan-Filípková, M. Body Fat of British and Dutch Infants. Br. Med. J. 1975, 1, 653–655. [Google Scholar] [CrossRef]
- Putnam, J.C.; Carlson, S.E.; D.DeVoe, P.W.; Barness, L.A. The Effect of Variations in Dietary Fatty Acids on the Fatty Acid Composition of Erythrocyte Phosphatidylcholine and Phosphatidylethanolamine in Human Infants. Am. J. Clin. Nutr. 1982, 36, 106–114. [Google Scholar] [CrossRef]
- Choque, B.; Catheline, D.; Rioux, V.; Legrand, P. Linoleic Acid: Between Doubts and Certainties. Biochimie 2014, 96, 14–21. [Google Scholar] [CrossRef]
- Choque, B.; Catheline, D.; Delplanque, B.; Guesnet, P.; Legrand, P. Dietary Linoleic Acid Requirements in the Presence of α-Linolenic Acid Are Lower than the Historical 2% of Energy Intake Value, Study in Rats. Br. J. Nutr. 2015, 113, 1056–1068. [Google Scholar] [CrossRef] [PubMed]
- Widdowson, E.M. Upper Limits of Intakes of Total Fat and Polyunsaturated Fatty Acids in Infant Formulas1. J. Nutr. 1989, 119, 1814–1817. [Google Scholar] [CrossRef]
- Rzehak, P.; Koletzko, S.; Koletzko, B.; Sausenthaler, S.; Reinhardt, D.; Grübl, A.; Bauer, C.P.; Krämer, U.; Bollrath, C.; von Berg, A.; et al. Growth of Infants Fed Formula Rich in Canola Oil (Low Erucic Acid Rapeseed Oil). Clin. Nutr. 2011, 30, 339–345. [Google Scholar] [CrossRef]
- van Egmond, A.W.A.; Kosorok, M.R.; Koscik, R.; Laxova, A.; Farrell, P.M. Effect of Linoleic Acid Intake on Growth of Infants with Cystic Fibrosis. Am. J. Clin. Nutr. 1996, 63, 746–752. [Google Scholar] [CrossRef]
- Ponder, D.L.; Innis, S.M.; Benson, J.D.; Siegman, J.S. Docosahexaenoic Acid Status of Term Infants Fed Breast Milk or Infant Formula Containing Soy Oil or Corn Oil. Pediatr. Res. 1992, 32, 683–688. [Google Scholar] [CrossRef]
- Jensen, C.L.; Prager, T.C.; Fraley, J.K.; Chen, H.; Anderson, R.E.; Heird, W.C. Effect of Dietary Linoleic/Alpha-Linolenic Acid Ratio on Growth and Visual Function of Term Infants. J. Pediatr. 1997, 131, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Chirouze, V.; Lapillonne, A.; Putet, G.; Salle, B. Red Blood Cell Fatty Acid Composition in Low-birth-weight Infants Fed Either Human Milk or Formula during the First Months of Life. Acta Paediatr. 1994, 83, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.L.; Chen, H.; Fraley, J.K.; Anderson, R.E.; Heird, W.C. Biochemical Effects of Dietary Linoleic/α-Linolenic Acid Ratio in Term Infants. Lipids 1996, 31, 107–113. [Google Scholar] [CrossRef]
- Clark, K.J.; Makrides, M.; Neumann, M.A.; Gibson, R.A. Determination of the Optimal Ratio of Linoleic Acid to α-Linolenic Acid in Infant Formulas. J. Pediatr. 1992, 120, S151–S158. [Google Scholar] [CrossRef]
- Libuda, L.; Mesch, C.M.; Stimming, M.; Demmelmair, H.; Koletzko, B.; Warschburger, P.; Blanke, K.; Reischl, E.; Kalhoff, H.; Kersting, M. Fatty Acid Supply with Complementary Foods and LC-PUFA Status in Healthy Infants: Results of a Randomised Controlled Trial. Eur. J. Nutr. 2016, 55, 1633–1644. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.; Dube, K.; Sichert-Hellert, W.; Kannenberg, F.; Kunz, C.; Kalhoff, H.; Kersting, M. Modification of Dietary Polyunsaturated Fatty Acids via Complementary Food Enhances N-3 Long-Chain Polyunsaturated Fatty Acid Synthesis in Healthy Infants: A Double Blinded Randomised Controlled Trial. Arch. Dis. Child. 2009, 94, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Sauerwald, U.; Fink, M.M.; Demmelmair, H.; Schoenaich, P.V.; Rauh-Pfeiffer, A.A.M.; Koletzko, B. Effect of Different Levels of Docosahexaenoic Acid Supply on Fatty Acid Status and Linoleic and α-Linolenic Acid Conversion in Preterm Infants. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, H.; Lee, E.; Kim, Y.; Ha, E.H.; Chang, N. Association between Maternal Intake of N-6 to n-3 Fatty Acid Ratio during Pregnancy and Infant Neurodevelopment at 6 Months of Age: Results of the MOCEH Cohort Study. Nutr. J. 2017, 16, 23. [Google Scholar] [CrossRef] [PubMed]
- Bernard, J.Y.; Armand, M.; Garcia, C.; Forhan, A.; de Agostini, M.; Charles, M.-A.; Heude, B. The Association between Linoleic Acid Levels in Colostrum and Child Cognition at 2 and 3 y in the EDEN Cohort. Pediatr. Res. 2015, 77, 829–835. [Google Scholar] [CrossRef]
- Zou, R.; el Marroun, H.; Voortman, T.; Hillegers, M.; White, T.; Tiemeier, H. Maternal Polyunsaturated Fatty Acids during Pregnancy and Offspring Brain Development in Childhood. Am. J. Clin. Nutr. 2021, 114, 124–133. [Google Scholar] [CrossRef]
- Auestad, N.; Halter, R.; Hall, R.T.; Blatter, M.; Bogle, M.L.; Burks, W.; Erickson, J.R.; Fitzgerald, K.M.; Dobson, V.; Innis, S.M.; et al. Growth and Development in Term Infants Fed Long-Chain Polyunsaturated Fatty Acids: A Double-Masked, Randomized, Parallel, Prospective, Multivariate Study. Pediatrics 2001, 108, 372–381. [Google Scholar] [CrossRef]
- Brenna, J.T.; Carlson, S.E. Docosahexaenoic Acid and Human Brain Development: Evidence That Adietary Supply Is Needed for Optimal Development. J. Hum. Evol. 2014, 77, 99–106. [Google Scholar] [CrossRef]
- Monnard, C.; Fleith, M. Total Fat and Fatty Acid Intake among 1–7-Year-Old Children from 33 Countries: Comparison with International Recommendations. Nutrients 2021, 13, 3547. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products Nutrition and Allergies (NDA). Scientific Opinion on the Essential Composition of Infant and Follow-on Formulae. EFSA J. 2014, 12, 3760. [Google Scholar] [CrossRef]
- Koletzko, B.; Baker, S.; Cleghorn, G.; Fagundes Neto, U.; Gopalan, S.; Hernell, O.; Seng Hock, Q.; Jirapinyo, P.; Lonnerdal, B.; Pencharz, P.; et al. Global Standard for the Composition of Infant Formula: Recommendations of an ESPGHAN Coordinated International Expert Group Background of the Espghan Coordinated International Expert Group Consultation. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 584–599. [Google Scholar] [CrossRef]
- Xiang, M.; Alfvén, G.; Blennow, M.; Trygg, M.; Zetterström, R. Long-Chain Polyunsaturated Fatty Acids in Human Milk and Brain Growth during Early Infancy. Acta Paediatr. Int. J. Paediatr. 2000, 89, 142–147. [Google Scholar] [CrossRef]
- Mitoulas, L.R.; Gurrin, L.C.; Doherty, D.A.; Sherriff, J.L.; Hartmann, P.E. Infant Intake of Fatty Acids from Human Milk over the First Year of Lactation. Br. J. Nutr. 2003, 90, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Berenhauser, A.C.; Pinheiro Do Prado, A.C.; da Silva, R.C.; Gioielli, L.A.; Block, J.M. Fatty Acid Composition in Preterm and Term Breast Milk. Int. J. Food Sci. Nutr. 2012, 63, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.Y.; de Castro, G.S.F.; Jordão, A.A.; Sartorelli, D.S. Breast Milk Fatty Acid Composition of Women Living Far from the Coastal Area in Brazil. J. Pediatr. (Rio J.) 2013, 89, 263–268. [Google Scholar] [CrossRef]
- Patin, R.V.; Vítolo, M.R.; Valverde, M.A.; Carvalho, P.O.; Pastore, G.M.; Ancona Lopez, F. The Influence of Sardine Consumption on the Omega-3 Fatty Acid Content of Mature Human Milk. J. Pediatr. 2006, 82, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Innis, S.M.; King, D.J. Trans Fatty Acids in Human Milk Are Inversely Associated with Concentrations of Essential All-Cis n-6 and n-3 Fatty Acids and Determine Trans, but Not n-6 and n-3, Fatty Acids in Plasma Lipids of Breast-Fed Infants. Am. J. Clin. Nutr. 1999, 70, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Tijerina-Sáenz, S.; Innis, S.M.; Kitts, D.D. Antioxidant Capacity of Human Milk and Its Association with Vitamins A and E and Fatty Acid Composition. Acta Paediatr. 2009, 98, 1793–1798. [Google Scholar] [CrossRef]
- Krasevec, J.M.; Jones, P.J.; Cabrera-Hernandez, A.; Luisa Mayer, D.; Connor, W.E. Maternal and Infant Essential Fatty Acid Status in Havana, Cuba. Am. J. Clin. Nutr. 2002, 76, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Hørby Jørgensen, M.; Hernell, O.; Lund, P.; Hølmer, G.; Fleischer Michaelsen, K. Visual Acuity and Erythrocyte Docosahexaenoic Acid Status in Breast-Fed and Formula-Fed Term Infants during the First Four Months of Life. Lipids 1996, 31, 99–105. [Google Scholar] [CrossRef]
- Zou, X.; Huang, J.; Jin, Q.; Guo, Z.; Liu, Y.; Cheong, L.; Xu, X.; Wang, X. Lipid Composition Analysis of Milk Fats from Different Mammalian Species: Potential for Use as Human Milk Fat Substitutes. J. Agric. Food Chem. 2013, 61, 7070–7080. [Google Scholar] [CrossRef]
- Luukkainen, P.; Salo, M.K.; Nikkari, T. Changes in the Fatty Acid Composition of Preterm and Term Human Milk from 1 Week to 6 Months of Lactation. J. Pediatr. Gastroenterol. Nutr. 1994, 18, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Maurage, C.; Guesnet, P.; Pinault, M.; Rochettede Lempdes, J.-B.; Durand, G.; Antoine, J.-M.; Couet, C. Effect of Two Types of Fish Oil Supplementation on Plasma and Erythrocyte Phospholipids in Formula-Fed Term Infants. Biol. Neonate 1998, 74, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Pugo-Gunsam, P.; Guesnet, P.; Subratty, A.H.; Rajcoomar, D.A.; Maurage, C.; Couet, C. Fatty Acid Composition of White Adipose Tissue and Breast Milk of Mauritian and French Mothers and Erythrocyte Phospholipids of Their Full-Term Breast-Fed Infants. Br. J. Nutr. 1999, 82, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.C.; Bougnoux, P.; Fignon, A.; Theret, V.; Antoine, J.-M.; Lamisse, F.; Couet, C. Dependence of Human Milk Essential Fatty Acids on Adipose Stores during Lactation. Am. J. Clin. Nutr. 1993, 58, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Szabó, E.; Boehm, G.; Beermann, C.; Weyermann, M.; Brenner, H.; Rothenbacher, D.; Decsi, T. Trans Octadecenoic Acid and Trans Octadecadienoic Acid Are Inversely Related to Long-Chain Polyunsaturates in Human Milk: Results of a Large Birth Cohort Study. Am. J. Clin. Nutr. 2007, 85, 1320–1326. [Google Scholar] [CrossRef] [PubMed]
- Genzel-Boroviczény, O.; Wahle, J.; Koletzko, B. Fatty Acid Composition of Human Milk during the 1st Month after Term and Preterm Delivery. Eur. J. Pediatr. 1997, 156, 143–147. [Google Scholar] [CrossRef]
- Roy, S.; Dhar, P.; Ghosh, S. Comparative Evaluation of Essential Fatty Acid Composition of Mothers’ Milk of Some Urban and Suburban Regions of West Bengal, India. Int. J. Food Sci. Nutr. 2012, 63, 895–901. [Google Scholar] [CrossRef]
- Haddad, I.; Mozzon, M.; Frega, N.G. Trends in Fatty Acids Positional Distribution in Human Colostrum, Transitional, and Mature Milk. Eur. Food Res. Technol. 2012, 2, 325–332. [Google Scholar] [CrossRef]
- Scopesi, F.; Ciangherotti, S.; Lantieri, P.B.; Risso, D.; Bertini, I.; Campone, F.; Pedrotti, A.; Bonacci, W.; Serra, G. Maternal Dietary PUFAs Intake and Human Milk Content Relationships during the First Month of Lactation. Clin. Nutr. 2001, 20, 393–397. [Google Scholar] [CrossRef]
- Nguyen, M.T.T.; Kim, J.; Seo, N.; Lee, A.H.; Kim, Y.K.; Jung, J.A.; Li, D.; To, X.H.M.; Huynh, K.T.N.; van Le, T.; et al. Comprehensive Analysis of Fatty Acids in Human Milk of Four Asian Countries. J. Dairy Sci. 2021, 104, 6496–6507. [Google Scholar] [CrossRef]
- Aumeistere, L.; Ciproviča, I.; Zavadska, D.; Andersons, J.; Volkovs, V.; Ceļmalniece, K. Impact of Maternal Diet on Human Milk Composition among Lactating Women in Latvia. Medicina 2019, 55, 173. [Google Scholar] [CrossRef]
- Khor, G.L.; Tan, S.S.; Stoutjesdijk, E.; Ng, K.W.T.; Khouw, I.; Bragt, M.; Schaafsma, A.; Dijck-Brouwer, D.A.J.; Muskiet, F.A.J. Temporal Changes in Breast Milk Fatty Acids Contents: A Case Study of Malay Breastfeeding Women. Nutrients 2021, 13, 101. [Google Scholar] [CrossRef] [PubMed]
- Butts, C.A.; Hedderley, D.I.; Herath, T.D.; Paturi, G.; Glyn-Jones, S.; Wiens, F.; Stahl, B.; Gopal, P. Human Milk Composition and Dietary Intakes of Breastfeeding Women of Different Ethnicity from the Manawatu-Wanganui Region of New Zealand. Nutrients 2018, 10, 1231. [Google Scholar] [CrossRef] [PubMed]
- van de Heijning, B.J.M.; Stahl, B.; Schaart, M.W.; van der Beek, E.M.; Rings, E.H.H.M.; Mearin, M.L. Fatty Acid and Amino Acid Content and Composition of Human Milk in the Course of Lactation. Adv. Pediatr. Res. 2017, 4, 2–15. [Google Scholar] [CrossRef]
- Van Beusekom, C.M.; Nijeboer, H.J.; van der Veere, C.N.; Luteyn, A.J.; Offringa, P.J.; Muskiet, F.A.; Boersma, E.R. Indicators of Long Chain Polyunsaturated Fatty Acid Status of Exclusively Breastfed Infants at Delivery and after 20–22 Days. Early Hum. Dev. 1993, 32, 207–218. [Google Scholar] [CrossRef]
- Huisman, M.; van Beusekom, C.M.; Lanting, C.I.; Nijeboer, H.J.; Muskiet, F.A.; Boersma, E.R. Triglycerides, Fatty Acids, Sterols, Mono- and Disaccharides and Sugar Alcohols in Human Milk and Current Types of Infant Formula Milk. Eur. J. Clin. Nutr. 1996, 50, 255–260. [Google Scholar]
- Bobiński, R.; Mikulska, M.; Mojska, H.; Simon, M. Comparison of the Fatty Acid Composition of Transitional and Mature Milk of Mothers Who Delivered Healthy Full-Term Babies, Preterm Babies and Full-Term Small for Gestational Age Infants. Eur. J. Clin. Nutr. 2013, 67, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Szlagatys-Sidorkiewicz, A.; Martysiak-Zurowska, D.; Krzykowski, G.; Zagierski, M.; Kamińska, B. Maternal Smoking Modulates Fatty Acid Profile of Breast Milk. Acta Paediatr. Int. J. Paediatr. 2013, 102. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Balcao, V.; Guimaraes, H.; Rocha, G.; Moutinho, C.; Matos, C.; Almeida, C.; Casal, S.; Guerra, A. Fatty Acid Profile of Human Milk of Portuguese Lactating Women: Prospective Study from the 1st to the 16th Week of Lactation. Ann. Nutr. Metab. 2008, 53, 50–56. [Google Scholar] [CrossRef]
- Cruz-Hernandez, C.; Goeuriot, S.; Giuffrida, F.; Thakkar, S.K.; Destaillats, F. Direct Quantification of Fatty Acids in Human Milk by Gas Chromatography. J. Chromatogr. A 2013, 1284, 174–179. [Google Scholar] [CrossRef]
- Sánchez-Hernández, S.; Esteban-Muñoz, A.; Giménez-Martínez, R.; Aguilar-Cordero, M.J.; Miralles-Buraglia, B.; Olalla-Herrera, M. A Comparison of Changes in the Fatty Acid Profile of Human Milk of Spanish Lactating Women during the First Month of Lactation Using Gas Chromatography-Mass Spectrometry. A Comparison with Infant Formulas. Nutrients 2019, 11, 3055. [Google Scholar] [CrossRef] [PubMed]
- Moltó-Puigmartí, C.; Castellote, A.I.; Carbonell-Estrany, X.; López-Sabater, M.C. Differences in Fat Content and Fatty Acid Proportions among Colostrum, Transitional, and Mature Milk from Women Delivering Very Preterm, Preterm, and Term Infants. Clin. Nutr. 2011, 30, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Sala-Vila, A.; Castellote-Bargalló, A.I.; Rodriguez-Palmero, M.; Campoy; López-Sabater, M.C. Lipid Composition in Human Breast Milk from Granada (Spain): Changes during Lactation. Nutrition 2005, 21, 467–473. [Google Scholar] [CrossRef]
- Sala-Vila, A.; Campoy, C.; Castellote, A.I.; Garrido, F.; Rivero, M.; Rodríguez-Palmero, M.; López-Sabater, M.C. Influence of Dietary Source of Docosahexaenoic and Arachidonic Acids on Their Incorporation into Membrane Phospholipids of Red Blood Cells in Term Infants. Prostaglandins Leukot Essent Fat. Acids 2006, 74, 143–148. [Google Scholar] [CrossRef]
- López-López, A.; López-Sabater, M.C.; Campoy-Folgoso, C.; Rivero-Urgell, M.; Castellote-Bargalló, A.I. Fatty Acid and Sn-2 Fatty Acid Composition in Human Milk from Granada (Spain) and in Infant Formulas. Eur. J. Clin. Nutr. 2002, 56, 1242–1254. [Google Scholar] [CrossRef]
- Sala-Vila, A.; Castellote, A.I.; Campoy, C.; Rivero, M.; Rodriguez-Palmero, M.; López-Sabater, M.C. The Source of Long-Chain PUFA in Formula Supplements Does Not Affect the Fatty Acid Composition of Plasma Lipids in Full-Term Infants. J. Nutr. 2004, 134, 868–873. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, R.; Díaz-Bao, M.; Cepeda, A.; Regal, P.; Fente, C.A. Fatty Acid Composition of Breast Milk in Galicia (NW Spain): A Cross-Country Comparison. Prostaglandins Leukot Essent Fat. Acids 2018, 135, 102–114. [Google Scholar] [CrossRef]
- Rueda, R.; Ramírez, M.; García-Salmerón, J.L.; Maldonado, J.; Gil, G. Gestational Age and Origin of Human Milk Influence Total Lipid and Fatty Acid Contents. Ann. Nutr. Metab. 1998, 42, 12–22. [Google Scholar] [CrossRef]
- Nyuar, K.B.; Min, Y.; Dawood, M.; Abukashawa, S.; Daak, A.; Ghebremeskel, K. Regular Consumption of Nile River Fish Could Ameliorate the Low Milk DHA of Southern Sudanese Women Living in Khartoum City Area. Prostaglandins Leukot Essent Fat. Acids 2013, 89, 65–69. [Google Scholar] [CrossRef]
- Storck Lindholm, E.; Strandvik, B.; Altman, D.; Möller, A.; Palme Kilander, C. Different Fatty Acid Pattern in Breast Milk of Obese Compared to Normal-Weight Mothers. Prostaglandins Leukot Essent Fat. Acids 2013, 88, 211–217. [Google Scholar] [CrossRef]
- Huang, H.-L.; Chuang, L.-T.; Li, H.-H.; Lin, C.-P.; Glew, R.H. Docosahexaenoic Acid in Maternal and Neonatal Plasma Phospholipids and Milk Lipids of Taiwanese Women in Kinmen: Fatty Acid Composition of Maternal Blood, Neonatal Blood and Breast Milk. Lipids Health Dis. 2013, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.-C.; Lau, B.-H.; Chen, P.-H.; Wu, L.-T.; Tang, R.-B. Fatty Acid Composition of Taiwanese Human Milk. J. Chin. Med. Assoc. 2010, 73, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, R.S.; Luxwolda, M.F.; Dijck-Brouwer, D.A.J.; Muskiet, F.A.J. Fatty Acid Compositions of Preterm and Term Colostrum, Transitional and Mature Milks in a Sub-Saharan Population with High Fish Intakes. Prostaglandins Leukot Essent Fat. Acids 2012, 86, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Samur, G.; Topcu, A.; Turan, S. Trans Fatty Acids and Fatty Acid Composition of Mature Breast Milk in Turkish Women and Their Association with Maternal Diet’s. Lipids 2009, 44, 405–413. [Google Scholar] [CrossRef]
| China | EU | Codex | |||||
|---|---|---|---|---|---|---|---|
| Nutrient/100 kcal | Stage 1 0–6 mo | Stage 2 6–12 mo | Stage 3 12–36 mo | Stage 1 0–6 mo | Stage 2 6–12 mo | Stage 1 0–6 mo | Stage 2 6–12 mo |
| Fat, g | 4.4–6.0 | 3.5–6.0 | 3.5–6.0 | 4.4–6.0 | 4.4–6.0 | 4.4–6.0 | 3.0–6.0 |
| LA, g | 0.3–1.4 | 0.3–1.4 | 0.3–1.4 | 0.5–1.2 | 0.5–1.2 | 0.3–1.4 1 | 0.3–N.S. |
| ALA, mg | 50–N.S. | 50–N.S. | 50–N.S. | 50–100 | 50–100 | 50-N.S. | N.S. |
| LA/ALA | 5:1–15:1 | 5:1–15:1 | 5:1–15:1 | N.S. | N.S. | 5:1–15:1 | N.S. |
| DHA, mg | 15–40 | 15–40 | N.S.–40 | 20–50 | 20–50 | N.S.–22 2 | N.S. |
| ARA, mg | N.S.–80 | N.S.–80 | N.S.–80 | N.S. | N.S. | N.S.2 | N.S. |
| Company A 1 | Company B 2 | Company C 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Nutrient | Stage 1 0–6 mo | Stage 2 6–12 mo | Stage 3 12–36 mo | Stage 1 0–6 mo | Stage 2 6–12 mo | Stage 3 12–36 mo | Stage 1 0–6 mo | Stage 2 6–12 mo | Stage 3 12–36 mo |
| Fat, g/100 kcal | 5.1 | 4.6 | 4.2 | 5.4 | 4.5 | 3.7 | 5.4 | 4.6 | 4.2 |
| LA, mg/100 kcal | 543 | 418 | 376 | 794 | 627 | 711 | 794 | 543 | 418 |
| ALA, mg/100 kcal | 59 | NL | NL | 64 | NL | NL | 79 | NL | NL |
| LA/ALA | 9.2 | 12.4 | 10.1 | ||||||
| DHA % FA | 0.22 | 0.18 | 0.05 | 0.36 | 0.4 | 0.34 | 0.17 | 0.12 | 0.15 |
| ARA % FA | 0.28 | 0.24 | 0.11 | 0.36 | 0.4 | 0.09 | 0.35 | 0.15 | 0.30 |
| Company Y 4 | Company Z 5 | |||||
|---|---|---|---|---|---|---|
| Nutrient | Stage 1 0–6 mo | Stage 2 6–12 mo | Stage 3 12–36 mo | Stage 1 0–6 mo | Stage 2 6–12 mo | Stage 3 12–36 mo |
| Fat, g/100 kcal | 5.4 | 4.6 | 4.6 | 5.2 | 4.8 | 3.7 |
| LA, mg/100 kcal | 920 | 586 | 586 | 1046 | 920 | 711 |
| ALA, mg/100 kcal | 90 | NL | NL | 110 | NL | NL |
| LA/ALA | 10 | - | - | 10 | - | - |
| DHA % FA | 0.32 | 0.30 | 0.32 | 0.21 | 0.18 | 0.21 |
| ARA % FA | 0.64 | 0.60 | 0.64 | 0.38 | 0.33 | 0.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Einerhand, A.W.C.; Mi, W.; Haandrikman, A.; Sheng, X.-Y.; Calder, P.C. The Impact of Linoleic Acid on Infant Health in the Absence or Presence of DHA in Infant Formulas. Nutrients 2023, 15, 2187. https://doi.org/10.3390/nu15092187
Einerhand AWC, Mi W, Haandrikman A, Sheng X-Y, Calder PC. The Impact of Linoleic Acid on Infant Health in the Absence or Presence of DHA in Infant Formulas. Nutrients. 2023; 15(9):2187. https://doi.org/10.3390/nu15092187
Chicago/Turabian StyleEinerhand, Alexandra W. C., Wiola Mi, Alfred Haandrikman, Xiao-Yang Sheng, and Philip C. Calder. 2023. "The Impact of Linoleic Acid on Infant Health in the Absence or Presence of DHA in Infant Formulas" Nutrients 15, no. 9: 2187. https://doi.org/10.3390/nu15092187
APA StyleEinerhand, A. W. C., Mi, W., Haandrikman, A., Sheng, X.-Y., & Calder, P. C. (2023). The Impact of Linoleic Acid on Infant Health in the Absence or Presence of DHA in Infant Formulas. Nutrients, 15(9), 2187. https://doi.org/10.3390/nu15092187






