Abstract
Little is known about the association between sleep and diet in pregnancy, despite both behaviors impacting maternal and fetal health. We aimed to perform a systematic review of the available literature on associations between sleep characteristics and dietary intake and eating behaviors during pregnancy, reporting on both maternal and fetal outcomes. We followed the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and conducted our search on 27 May 2021 in the PubMed, EMBASE, and CINAHL databases. The search yielded 6785 unique articles, of which 25 met our eligibility criteria. The studies, mostly observational, published 1993–2021, include data from 168,665 participants. Studies included examinations of associations between various maternal sleep measures with a diverse set of diet-related measures, including energy or nutrient intake (N = 12), dietary patterns (N = 9), and eating behaviors (N = 11). Associations of maternal exposures with fetal/infant outcomes were also examined (N = 5). We observed considerable heterogeneity across studies precluding our ability to perform a meta-analysis or form strong conclusions; however, several studies did report significant findings. Results from this systematic review demonstrate the need for consistency in methods across studies to better understand relationships between diet and sleep characteristics during pregnancy.
1. Introduction
Sleep and diet are two important health behaviors that undergo changes during pregnancy and have implications for maternal and fetal health outcomes. Pregnant women report significant changes in their sleep over gestation including a reduction in sleep duration [1,2] and degradation of sleep quality [3], increase in wake after sleep onset, decrease in sleep efficiency, and a higher number of naps [4]. Sleep disturbances, including short sleep duration and poor sleep quality, have been linked to adverse perinatal outcomes such as a higher risk of gestational diabetes, preterm birth, greater gestational weight gain [5], longer labor, and a higher rate of Cesarean delivery [6].
Clinical sleep disorders have also been associated with adverse pregnancy and fetal outcomes. Specifically, restless leg syndrome has been linked to the development of hypertensive disorders of pregnancy [7]. Sleep-disordered breathing has been repeatedly associated with an increased risk for gestational diabetes [8,9], hypertensive disorders of pregnancy [8,10,11], preterm birth [8,11], severe maternal morbidity, and depressive symptoms [12]. Babies born to mothers with a diagnosis of obstructive sleep apnea are also more likely to be admitted to the intensive care unit, to require airway intubation and resuscitation, and to be diagnosed with a congenital anomaly [12,13]. Insomnia has been linked to severe maternal morbidity [14]. Disrupted circadian rhythm and blue light exposure are associated with gestational diabetes or abnormal glucose metabolism [15], and preterm birth [16].
During pregnancy, nutritional needs also demonstrate significant changes and increase to promote fetal growth and development, as well as maternal physiological adaptations of pregnancy (i.e., accumulation of fat reserves) [17,18]. Review of guidelines published by leading international organizations identifies marked consistency in the recommendation for extra calories and protein, especially during the second and third trimesters [18]. Limiting saturated fats, adequate amounts of long-chain fatty acids and possible supplementation with omega-3 fatty acids are also recommended [18]. Reduced consumption of simple sugars, particularly in the form of sugar-sweetened beverages, is recommended to limit excess calories [17].
Supplementation may be needed for adequate intake of iron, folate, Vitamin D, choline, calcium and iodine due to the importance of these in fetal development and the potential for insufficient intake via diet alone [17]. According to the WHO, over 38% of pregnancies are affected by anemia, indicating insufficient micronutrient consumption or absorption over gestation. Anemia, particularly in the first trimester, increases the risk for preterm birth [19] and low birth weight, as well as maternal and perinatal mortality [20]. Insufficient intake of iron and calcium in 91% and 55% of studies, respectively, and excess intake of dietary fat in 55% of studies was found in a meta-analysis of 18 studies of dietary intake pre-conceptually and perinatally [21]. A Cochrane review found that supplementation with iron and folic acid seems to have positive impacts on fetal growth and birth weight including lower small for gestational age (SGA) and preterm birth [22]. Limiting intake of caffeine during pregnancy to 300 mg/day is also recommended [17,18] according to a systematic review [23].
Beyond specific nutrients and supplements, dietary guidelines also recommend healthy diet in the form of diet quality. Poor diet quality is associated with an increased risk for obesity, gestational diabetes, hypertensive disorders of pregnancy, preterm delivery, congenital infections, abnormal fetal growth, and childhood obesity [19,24,25,26,27]. National and international guidelines provide recommendations about appropriate weight gain and healthy nutrition in pregnancy [28,29,30,31]. Consensus across dietary guidelines recommends high-quality dietary intake during pregnancy including consumption of nutrient-rich food groups, managing weight gain, and achieving adequate fluid intake. Specific dietary patterns, such as the Mediterranean Diet, the Dietary Approach to Stop Hypertension (DASH) and others have been studied during pregnancy [17,32,33,34]. The Mediterranean Diet, characterized by high intake of fruits, vegetables and olive oil, with moderate protein intake, has been associated with improvements in risk factors for metabolic syndrome [35] and cardiovascular disease and type 2 diabetes [36] among the general population, and with reduction in unhealthy pregnancy conditions such as gestational diabetes, childbirth complications, low birth weight and prematurity as well as better fetal growth [37].
There is evidence that sleep and diet may affect one another, yet the bulk of the existing literature originates in non-pregnant populations. For example, carbohydrates have been reported to decrease sleep onset latency, and consumption of dairy sources have been reported to increase sleep duration among athletes [38]. Conversely, caffeine increases sleep onset latency, and reduces sleep duration and sleep quality [39]. The timing of meals and meal quantity may affect circadian rhythm, where large portions of food later in the day have the potential to negatively impact sleep [40]. On the other hand, sleep is a pivotal modulator of metabolism and has been argued as a key therapeutic target in metabolic disorders such as obesity [41]. Indeed, in a systematic review, partial sleep deprivation was found to be associated with a positive energy balance (due to an increase in energy intake and no change in energy expenditure), a precursor of excessive weight gain and the development of obesity [42].
Despite the established bidirectional association between sleep and diet, and importance of both sleep and diet on maternal and fetal health, little is known about the associations between sleep and diet in pregnancy. A systematic review by Pauley et al. (2023) demonstrated the infancy of this line of research, identifying only three studies that examined an association between sleep and eating behavior [43]. The aim of this study was to perform a systematic review of the available literature on associations between sleep characteristics and dietary intake and eating behaviors during pregnancy. This study utilizes a comprehensive search strategy and reports on both maternal and fetal outcomes.
2. Methods
2.1. Protocol and Registration
The present review was conducted in accordance with the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [44]. We prospectively registered the study protocol in the PROSPERO database (CRD42021259982) prior to initiation of literature searches or data collection.
2.2. Search Strategy
With assistance from a research librarian, we created individually tailored search strategies for the PubMed, EMBASE, and CINAHL databases to capture studies examining associations between sleep- and diet-related variables during pregnancy. Each search strategy consisted of a search string for the concepts of pregnancy, sleep, and diet. We included search terms related to both nutrition and eating behaviors in the diet search string. The exclusion of animal studies was built into each search strategy as was an English language search limit. No date search limits were applied, and we ran the search on 27 May 2021. The full search strategy is available as Appendix A.
2.3. Screening and Eligibility Criteria
The search yielded 9139 hits, which were imported into Covidence, an online systematic review management system, for screening. After removing duplicates, we were left with 6785 unique articles to screen. We screened the titles and abstracts to identify potential studies for inclusion; articles deemed irrelevant were not reviewed at the full-text level. All authors contributed to screening at each level. At each level, articles were independently screened by two individuals. Conflicts were resolved by a third reviewer.
During the full-text screening, we assessed whether each article met eligibility criteria. Eligibility criteria in four areas were included: article type, population of interest, variables of interest, and relationship of interest. To meet eligibility criteria, articles needed to (1) present original research with quantitative outcomes; (2) be published in English in a peer-reviewed journal; and (3) include at least one sleep-related variable and one diet-related variable, at least one of which was a maternal measure during pregnancy. Subsequent variables could include infant measures as well as those during the postpartum period. Finally, (4) the analysis needed to include an examination of the association between at least one sleep- and one diet-related variable.
2.4. Data Extraction
All authors participated in data extraction, with two authors independently extracting data from each article in the final pool of studies. We extracted data related to study design, sample characteristics, measures for the variables of interest, and analyses and results related to the relationship of interest, and rated the Melnyk level of evidence for each article based on the analytical approach for the relationship of interest [45]. For example, if the analysis was conducted within the context of a prospective cohort study, but was cross-sectional in nature, the article’s level of evidence was rated as a 4 as opposed to a 3.
3. Results
We screened 6785 unique articles. Figure 1 (Prisma Flow Diagram) outlines the selection process which resulted in 25 studies included in the analysis, corresponding to 168,665 pregnant subjects. The characteristics of the 25 studies are presented in Table 1 (organized by year and then alphabetically). They were published between 1993 and 2021 and conducted on five different continents; 10 were conducted in the United States, 7 in Asia, 3 in Europe, 3 in South America and 1 in Australia). Two were randomized controlled trials [46,47] in which pregnant women with overweight or obesity were randomized to lifestyle interventions aimed to reduce gestational weight gain. The level of evidence varied among studies. Twenty-three were observational studies [48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70], which included fourteen that were level four (cross-sectional) [48,49,50,52,53,54,55,57,58,60,61,64,66,67,68,69], and eight that were level three (cohort studies) [51,52,56,59,62,63,65,70]. Only two were level two (experimental studies) [5,47]. Among the observational studies, 19 (83%) were based on relatively low-risk pregnancies [49,51,52,53,54,55,57,58,60,61,62,63,64,65,66,67,68,69,70], while six (25%) enrolled a special population: pregnant individuals with overweight or obese [5,47], overweight or obesity and low-income [48,56], low BMI and low-income [50], or history of major depression [59].
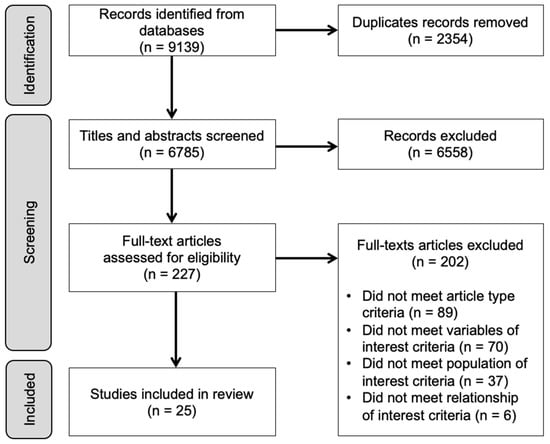
Figure 1.
PRISMA flow diagram.

Table 1.
Study and sample characteristics (n = 25).
A diverse set of sleep and diet-related measures were used across studies, and the research aims varied in whether the primary outcome(s) of interest were sleep- or diet-related. Thirteen studies framed diet as the exposure and sleep as the outcome [46,50,51,53,54,55,60,61,62,64,66,67,68]; six studies framed sleep as the exposure and diet as the outcome [46,49,50,64,66,70], and two studies examined the associations both ways [58,59], while four studies did not specify [48,53,57,60]. Study results are presented in Table 2, Table 3, Table 4 and Table 5 based on the type of association examined: maternal sleep with energy or nutrient intake (Table 2); maternal sleep with dietary patterns (Table 3); maternal sleep with eating behaviors (Table 4); and maternal exposures (diet or sleep) with fetal/infant outcomes (diet/feeding or sleep) (Table 5). Studies investigating more than one type of association are included in multiple tables. Descriptions of the study measures, which include a variety of objective and subjective measures, are also specified in Table 2, Table 3, Table 4 and Table 5.

Table 2.
Findings from studies with variables related to energy or nutrient intake (n = 12).

Table 3.
Findings from studies with variables related to dietary patterns (n = 9).

Table 4.
Findings from studies with variables related to eating behaviors (n = 10).

Table 5.
Findings from studies with fetal/infant outcomes (n = 5).
Measurement of maternal and infant sleep differed widely among the studies reviewed. Four studies assessed maternal sleep with actigraphy [46,47,56,59], while 19 utilized questionnaire items [48,49,50,53,54,55,57,58,60,61,62,63,64,65,66,67,68,69,70]. For fetal and infant sleep, two studies used direct observation with ultrasound or video recording during feeding [51,52]. For studies that administered questionnaires to mothers, the Pittsburgh Sleep Quality Index (PSQI) was commonly used (n = 8) [48,50,54,57,66,67,69,70], while six studies used single items [53,55,60,61,65,66].
Most studies also used questionnaires to assess diet, only two of which used single questionnaire items [52,61]. Six studies used 24 h dietary recall [53,54,58,60,66,67]. Two studies used standard food frequency questionnaires [49,57], and one a qualitative food frequency questionnaire [69], but protocols varied across studies.
3.1. Maternal Sleep with Energy or Nutrient Intake (N = 12)
Twelve studies investigated the association between maternal dietary intake or quality and sleep (Table 2) [5,47,49,50,52,53,54,56,60,66,67,68], seven of which assessed the relationship between total caloric/energy intake and various sleep outcomes [5,49,53,54,56,60,66].
3.2. Sleep Variables and Energy Intake
The GUSTO study measured diet with a single 24 h recall during the second trimester among 497 participants of the mother-offspring cohort study in Singapore [66] and the Peking University Birth Cohort measured diet with two non-consecutive 24 h recalls during the first trimester among 4352 participants [54]; neither found an association between energy intake and sleep quality. No association was found between energy intake, calculated based on metabolic parameters and weight (not dietary assessment), with awakenings or sleep duration among 31 overweight or obese participants [46]. Likewise, no association was found between energy intake assessed with two non-consecutive 24 h recalls and nighttime sleep duration among the 3692 participants of the same Peking University Birth Cohort mentioned above [54], and no association was found between energy intake, assessed using a single 24 h recall and nighttime sleep duration or bedtime among 1152 participants of the GUSTO study [60], also mentioned above.
Energy intake among 52 pregnant women with obesity, assessed in the first and third trimesters using energy expenditure (doubly labeled water) and fat deposition (changes in fat density), was not associated with total sleep time [56]. However, women who increased their time in bed were found to have lower energy intake across pregnancy compared to those who decreased their time in bed. Finally, a study of 437 participants in the Australian Longitudinal Study on Women’s Health (ALSWH) found that energy intake, assessed at any time during pregnancy using a food frequency questionnaire, was associated with sleeping behavior patterns that were identified via latent class analysis in crude models [49]. Women with average sleep duration and adverse sleep symptoms had higher energy intake compared to those with average sleep duration, but no adverse sleep-related symptoms [49]. These findings did not persist after adjustment for potential confounders [49].
An intervention study providing meal replacement for two meals per day aimed to restrict caloric intake to promote weight control and adherence to guideline recommendations of gestational weight gain [47]. No difference was found in actigraphy-measured sleep duration between the intervention and usual care comparison group [47].
3.3. Sleep Variables and Nutrient Intake
The relationship between nutrient intake and various aspects of sleep was also examined. Sleep and dietary fat were assessed by two studies [49,50]. In the first study, wherein 213 pregnant participants were recruited at Special Supplement Program for Women Infants and Children (WIC) sites in Michigan and assessed at a single time point, which included 36% in the first trimester, 32% in the second trimester and 32% in the third trimester. Dietary fat intake, measured by the Block screener was found to be directly associated with nighttime sleep disturbance in the overall cohort (p < 0.01) [50]. However, path analysis examining participants by trimester demonstrated that an association of dietary fat with depression (p < 0.05), and not nighttime sleep disturbance, was found during the first trimester [50]. That same study showed a path connecting sleep latency to dietary fat through the association with both fruit and vegetable intake (p < 0.05 for each leg of the path). Among second-trimester participants, an association of dietary fat with nighttime sleep disturbances (p < 0.01) and with fruit and vegetable intake (p < 0.05) was found, but none of these factors connected to depression [50]. Third-trimester participants were found to have an association between dietary fat and nighttime sleep disturbances (p < 0.05), which was also associated with depression (p < 0.01). An association of monounsaturated fat intake, but not overall fat intake, with sleep patterns was identified, as described above among the ALSWH sample [49]. Women with average sleep who also had adverse sleep symptoms consumed less monounsaturated fat than women with average sleep and no adverse sleep symptoms (p < 0.05) [49].
The relationship between carbohydrate intake and sleep was also assessed in two studies [49,67]. In cross-sectional analyses, the percentage of energy consumed as starch was found to be significantly higher among women with average sleep who also had adverse sleep symptoms, in comparison to women with average sleep and no adverse sleep symptoms (p < 0.05), though no differences in overall carbohydrate intake or sugar consumption were found between sleep pattern groups [49]. However, in multivariate models, overall carbohydrate intake was higher among women with average sleep who also had adverse sleep symptoms, in comparison to women with average sleep and no adverse sleep symptoms (p < 0.05), though no relationship was found for starch or sugar consumption with sleep patterns [49]. In the second study [67], intake of sugar-sweetened beverage, assessed by three 24 h recalls, was associated with short sleep duration and poor sleep quality, even after adjustment for potentially confounding variables. However, this cross-sectional study did not separate sugar-sweetened beverages that did or did not contain caffeine.
Additionally, the GUSTO mother-offspring cohort study in Singapore assessed discretionary calories as the sum of energy from caloric beverages (≥5 kcal; excluding plain water, diet soda, and unsweetened coffee, tea, and cow’s milk), local cakes, desserts, and snacks based on 24 h recall data. No association was found between discretionary calories with either sleep quality or duration assessed using the PSQI [66].
Caffeine consumption was assessed with maternal diet in two studies [52,68]. Maternal caffeine consumption during pregnancy was assessed with obstetrical outcomes among 750 participants recruited during the second trimester [52]. They reported that caffeine consumption during the second and third trimesters was associated with higher maternal depression and anxiety scores, and more cigarette use. Controlling for these variables, the investigators found that higher maternal caffeine consumption was significantly related to lower maternal sleep effectiveness as assessed by a Sleep Scale [52]. However, a study of 266 pregnant women in Poland late in pregnancy found no difference in insomnia between participants who did and did not abstain from coffee consumption during pregnancy [68].
3.4. Maternal Sleep with Dietary Patterns (N = 9 Studies)
Dietary intake was also assessed as food group consumption or dietary patterns (Table 3) [50,53,54,55,56,57,58,66,69]. Fruit and vegetable intake among Michigan WIC participants, assessed using a rapid food screener, was associated with sleep latency in the first and third trimester, but not in the second, and no association of fruit and vegetable intake with sleep quality or duration was found in any trimester [50]. In contrast, others assessing US respondents to the Behavioral Risk Factor Surveillance System (BRFSS) found that fruit and vegetable consumption as well as fruit consumption alone were associated with increased odds of meeting or exceeding sleep duration recommendations, but this association was only found when assessing sleep as an ordinal variable, while no association was found in linear models [55]. Several studies also examined associations between sleep and diet quality. Healthy Eating Index was calculated for two studies using very different dietary data collection. Diet was measured among pregnant women with obesity using food photography via the SmartIntake app collected in early (13–17 weeks’ gestation) and late (35–37 weeks’ gestation) pregnancy [56], whereas diet among women of any weight status was assessed using a single 24 h recall at 24–28 weeks’ gestation [66]. The first study using SmartIntake found no significant association between diet quality and time spent in bed or total sleep time [56]. The second that used 24 h recall also did not find an association between diet quality and sleep duration but did find a slightly higher HEI score (higher quality diet) among participants with good quality sleep (54.6) compared with those with poor quality sleep (52.0), though that association was no longer statistically significant when controlling for anxiety [66]. Chronotype (a person’s circadian preference in behavioral and biological rhythms relative to the external light–dark cycle) in the first trimester was associated with HEI scores calculated from three 24 h recalls such that higher whole grain and lower fruit HEI sub-scores were associated with higher HEI scores, though no difference in overall HEI score by chronotype was identified [58].
Four studies examined associations between various measures of sleep and several different types of dietary patterns [53,54,57,69]. A Mediterranean food pattern, assessed with a food frequency questionnaire among Spanish women at 11–13 weeks and 34 weeks’ gestation, was associated at both time periods with better sleep quality, assessed using the Spanish PSQI. The investigators also examined individual items of the Mediterranean diet, which showed that higher olive oil consumption at both time periods, and fruit early in pregnancy were associated with better sleep quality, while consumption of red meat and sub-products late in pregnancy was associated with lower sleep quality. A prospective study among pregnant Chinese women examined cross-sectional dietary intake and sleep early in pregnancy [53,54]. Dietary intake was measured with two days of 24 h recall from which total caloric intake was calculated, as described previously. However, the authors also categorized participants’ diets as balanced, more meat, veggie-rich, or vegan, though no methods for determining these categories were provided. Based on these non-specific methods, participants described as having a vegetarian or more-vegetables diet type were more likely to have poor sleep quality, as measured by the PSQI, than those with a balanced diet [54]. Additionally, those consuming a vegan diet were more likely to have either short or long sleep duration, which were self-reported. Dietary patterns of pregnant participants during their first trimester of gestation in the Chinese Pregnant Women Cohort Study [69] were assessed by principal components analysis of data derived from a 17-food group-based food frequency questionnaire. These analyses revealed five patterns: plant-based, vitamin-rich, animal protein-rich, bean products, and high fat, which were described by quartiles. Plant-based and vitamin-rich dietary patterns were associated with less sleep disturbance, and a high-fat dietary pattern was associated with greater sleep disturbance [69].
3.5. Maternal Sleep with Eating Behaviors (N = 11 Studies)
Eleven studies investigated the association between variables related to maternal eating behaviors with sleep (Table 4) [48,56,58,60,61,64,65,66,68,70], six of which included a measure related to nighttime eating or fasting [48,58,60,61,66,68]. Among African American women assessed between 14- and 24-weeks’ gestation, night eating was associated with lower sleep duration and quality, and higher sleep onset latency assessed using questions from the PSQI [48]. Among 673 s-trimester pregnant women in Singapore, night-eating was associated with a later bedtime, but did not find an association between the number of eating episodes with sleep duration, as reported in response to a single question [60]. The GUSTO mother-offspring cohort study in Singapore found no association between nighttime eating, nighttime fasting interval, and frequency of consumption episodes with either sleep quality or duration assessed using the PSQI [66]. Relatedly, an early pregnancy cross-sectional study in Brazil did not find an association between sleep chronotype and nightly fasting, eating duration, time of first or last meal, and number of meals [58].
Two studies specifically considered the association between nighttime eating and insomnia [61,68]. A study of third-trimester pregnant women in Poland found that women reporting insomnia, using the Athens Insomnia Scale, were more likely to eat at night [68]. Another study of pregnant women in Vietnam from 12 to 33 weeks’ gestation found that short meal-to-bed time, based on self-reported times, was a risk factor for reflux-related insomnia [61].
Four studies assessed eating behaviors including binge eating and food cravings [48,56,64,70]. One study of African American women with overweight or obesity between 14- and 24-weeks’ gestation found that sleep latency and quality (assessed with PSQI) were associated with overeating episodes, and poor sleep quality was associated with binge eating episodes. However, no associations were found between sleep duration and dietary restraint, overeating, or binge eating episodes [48]. The Norwegian Mother and Child Cohort Study (MoBa), which is a large, prospective population-based cohort study, assessed mothers with binge eating disorder (BED) symptoms and their associations with sleep, which was self-reported as sleep problem occurrence and during which weeks of gestation, sleep duration and satisfaction [65]. Participants reported BED symptoms before and during pregnancy. Participants reporting pre-pregnancy BED that remitted during pregnancy, or those reporting incident BED during pregnancy, were more likely to report sleep problems, and the likelihood of those problems increased with each trimester [65]. Incident BED during pregnancy was associated with both long and short sleep durations, whereas BED symptoms before or during pregnancy, or pre-pregnancy BED that remitted during pregnancy was not associated with long or short sleep. All three BED groups reported higher odds of sleep dissatisfaction than participants without any BED symptoms or history [65].
Food cravings were also examined with respect to sleep in two studies. One study of 52 pregnant women with obesity assessed sleep with wrist-worn actigraphy over six consecutive nights during early and late pregnancy. Pregnant women in this study who increased time in bed from early to late pregnancy also reported an increase in food cravings during that time compared to women who decreased time in bed or those who had no change in time in bed [56]. Another study of 245 pregnant women in Brazil assessed chronotype via mid-sleep time on free days with correction for calculated sleep debt across various times in pregnancy. Participants with sleep chronotype of evening types, which have ≥5 h difference between weekend and weekday average sleep duration, were more likely to report relief from negative states and feelings as a result of eating (as measured by the Food Craving Questionnaire-Trait) than those in non-evening chronotype (<5 h difference between weekend and week day average sleep duration). Compared to participants reporting morning type (<3.59 h difference between weekend and weekday average sleep duration), those with evening type reported both anticipation of relief from negative states and feelings as a result of eating, anticipation of positive reinforcement that may result from eating. Intense desire to eat was more likely to be found among evening type compared with morning type participants, whereas both intense desire to eat, and anticipation of positive reinforcement that may result from eating were found between evening type and non-evening type participants [64]. Additionally, The Pregnancy Eating Attributes Study (PEAS) followed 373 women from early pregnancy to one year postpartum [70]. Eating behaviors measured included cravings, which were assessed using questions developed by the authors wherein participants listed cravings and rated their strength; hedonic hunger, based on the Power of Food Scale; and addictive-like eating based on a modified Yale Food Addiction Scale. Sleep quality, assessed with the PSQI, was associated during pregnancy with cravings frequency and strength, but not with hedonic hunger or addictive-like eating. Additionally, change in sleep quality from pregnancy to post-partum was not associated with changes in hedonic hunger or changes in addictive-like eating [70].
3.6. Maternal Exposures with Fetal/Infant Outcomes (N = 5 Studies)
Three studies [51,52,62] examined the associations between maternal caffeine consumption during pregnancy on fetal and/or infant sleep (Table 4) [51,52,62]. The impact of high vs. low maternal caffeine consumption (reported use at study entry of >500mg (N = 10) vs. <200 mg (N = 10), respectively), on fetal behavioral states was assessed throughout the third trimester (30 to 40 weeks of gestational age) [51]. The fetuses of women who reported high caffeine consumption spent less time in active sleep and more time in arousal than fetuses of women with low caffeine consumption [52]. Caffeine consumption during the second and third trimesters was associated with higher maternal depression and anxiety scores, more cigarette use, and lower infant birth weight. Controlling these variables, higher maternal caffeine consumption was significantly related to higher amounts of infant active sleep, and more stress signs, and that caffeine consumption was correlated with lower maternal sleep effectiveness during the first 24 h after delivery [52]. In contrast, the last did not find any significant associations between maternal caffeine consumption during late pregnancy and infant sleep at 3 months of age [62].
One study [63] examined the association between maternal fermented food intake during pregnancy and infant sleep. The investigators found that intake of fermented food, especially miso, during the second and third trimesters, was associated with less infant sleep at one year of age [63].
Finally, another study examined the impact of a maternal sleep-related exposure on an infant diet-related outcome [59]. The investigators found that low maternal sleep efficiency in the third trimester was associated with lower likelihood to initiate breastfeeding, with a trend for a similar association between sleep efficiency and feeding status at 16 weeks postpartum.
4. Discussion
In this systematic review, we observed significant heterogeneity in studies examining associations between maternal sleep and diet. Differences in existing studies were observed in the selection of exposures and outcomes (sleep vs. diet) and their definitions. Participant samples range in numbers from less than 100 to over 17,000. Pregnant individuals were studied either during a specific trimester, or at any time during pregnancy. Samples also varied in terms of racial and ethnic representation with some studies including participants with a diverse range of demographics, while others included a more homogenous sample. Studies also varied in specific population of pregnant individuals assessed with some assessing participants with different weight statuses, or only including those with overweight or obesity pre-pregnancy. Further, the methodology used to examine sleep varied, but consisted of subjective questionnaires for most studies. Dietary variables of interest also varied with some studies examining diet quality and intake while others examined specific nutrient intake, food groups, or dietary patterns. This marked heterogeneity in the existing literature has precluded our ability to perform a meta-analysis of the data or even to form strong conclusions. Like findings from the Pauley et al. (2023) [43] review, our findings support the need for more longitudinal studies and randomized controlled trials. Notably, only two of the selected studies were designed as a randomized controlled trial. Additionally, a large proportion of data was obtained from low-risk samples, impacting the ability to extrapolate to higher-risk populations.
Energy intake was not found to be strongly associated with sleep among pregnant women. Most (5) of the seven studies that examined energy intake and sleep found no association of caloric intake with sleep quality [54,66] or duration [46,53,60]. The only randomized controlled trial of a partial meal-replacement to limit or control caloric intake also found no differences between intervention and comparison group in actigraphy-measured sleep outcomes [47]. Two studies found an association between energy intake with sleep variables, though methods and conclusions were very dissimilar. Women with obesity who increased their time in bed over the course of pregnancy had lower energy intake compared to those for whom time in bed decreased or stayed the same [56]. In another study, women with average sleep duration and adverse sleep symptoms had higher energy intake compared to those with average sleep duration, but no adverse sleep-related symptoms [49]; however, these findings did not persist in multivariate analysis.
Other studies of non-pregnant adults measuring energy intake and sleep duration have consistently shown that short sleep is associated with higher caloric intake, and that hormonal changes associated with short sleep may be responsible for the small changes in energy intake [71]. The set of papers in our review nicely highlights the wide variations in measurement that included first, second, third, or multiple trimesters. Energy intake was assessed using one or two 24 h recalls, a food frequency questionnaire, and several indirect measures using metabolic estimates of energy usage. Additionally, sleep was assessed in these studies as self-reported and actigraphy-measured sleep duration, self-reported sleep quality, and categories of sleep duration and/or sleep quality. So, though no associations were found between energy intake and sleep in these seven papers, the lack of consistency in methods challenges our ability to draw strong conclusions regarding this relationship.
The relationship between dietary fat intake and sleep among pregnant women is not clear from the two studies that examined this relationship. Neither assessment of sleep (pattern vs. sleep duration, quality, nighttime disturbances, latency) nor diet fat (screener vs. food frequency questionnaire) were assessed consistently across these studies. Higher overall dietary fat intake was associated with more sleep disturbance among Michigan WIC participants [50]. Additionally, women who reported average sleep with adverse sleep symptoms consumed less monounsaturated fat than women with average sleep and no adverse sleep symptoms [49]. Studies among non-pregnant adults of dietary fat with sleep outcomes are not common, but have collectively indicated that higher fat intake is associated with short sleep [71], and insomnia [72], and that saturated fat intake is overall negatively associated with sleep wellness [73]. Little to no literature is available to review the relationship between monounsaturated fat and sleep in other study populations.
Findings regarding the association between carbohydrate intake and sleep were scarce and did not support strong conclusions. The two studies that assessed carbohydrate intake showed higher carbohydrate consumption among women with average sleep, but adverse sleep symptoms, compared to those with average sleep without adverse sleep symptoms [49]. Additionally, higher sugar-sweetened beverage intake was associated with short sleep and poor sleep quality, though caffeine consumption was not taken into account [67]. Consistent association between carbohydrate consumption and sleep outcomes has also been inconclusive in studies of non-pregnant adults [73]. Differentiating between types of carbohydrate may shed light on this issue as intake of a high glycemic index diet or glycemic loads have been posited to be associated with higher risk of insomnia [73,74], which may be due to alterations in amino acid balance or a stimulated inflammatory response [74]. Beyond examining single nutrient diet composition, combinations of nutrients, such as high carbohydrate, low fat, have also been considered as potentially important in the relationship between diet and sleep [75].
Caffeine may be the single most studied substance in this literature. Caffeine consumption in our review was associated with lower maternal sleep effectiveness after controlling for depression and anxiety [52], though abstaining from coffee consumption was not associated with insomnia risk [68]. The impact of caffeine consumption on nighttime sleep is also not clear in non-pregnant adults [76,77], due to the cyclical impact of caffeine on performance enhancement and mitigating sleep deprivation and caffeine withdrawal [76].
A variety of dietary patterns were assessed with sleep in nine papers in this review. Higher fruit and vegetable consumption was inconsistently associated with sleep latency in one small study [50] and broadly associated with meeting or exceeding sleep duration recommendations in a larger, population-based study [55]. Higher fruit and vegetable consumption among non-pregnant adults has been consistently associated with achieving recommended sleep in observational studies, though experimental studies have been inconsistent [78].
Broadly assessing diet quality with sleep, HEI was not consistently found to be associated with sleep duration [56,66], but was associated with sleep quality in one study [66], and HEI sub-scores of higher whole grains and lower fruit consumption were associated with chronotype in another [58]. In non-pregnant adults, HEI has also been found to be associated with various sleep outcomes. HEI assessed using two 24 h recalls was significantly lower among short and long sleepers compared to those reporting the recommended 7–9 h of sleep among nationally representative NHANES study participants [79]. Additionally, among NHANES participants, higher HEI is associated with lower risk of sleep disturbance [80]. Broadly, a review of 29 studies indicates that higher dietary quality is associated with better sleep quality [81], though not all studies used the HEI index and methodological inconsistencies limit the strength of the evidence and preclude causal inference, as we have also found.
Nighttime eating is associated with misaligned circadian rhythms, or “eating jetlag”, which can be associated with positive energy balance and weight gain among shift workers and others who have milder shifts in eating behavior in the general population [84]. The relationship between nighttime eating and insomnia among pregnant women correlates these two nighttime occurrences, but it is unclear if pregnant women who experience insomnia awaken because of hunger, or eat because they were awake [68]. Insomnia has been found to be associated with intake of higher glycemic index (GI) diet in the Women’s Health Initiative Observational Study [74], which has been hypothesized to be mediated by inflammation [73,74]. GI, originally defined as the postprandial glucose response to food, [85] is calculated for and summed over all carbohydrate-containing foods [74].
Other dietary patterns, such as the Mediterranean diet, veggie-rich, meat-based, plant-based, vegetarian, vegan and other descriptors were mixed in association with various sleep outcomes, and inconclusive in our review. Research in non-pregnant populations has found that Mediterranean diet adherence is positively associated with sleep duration and indicators of better sleep quality [82]. Other studies have found changes in eating patterns associated with short sleep [83]. Among 15,199 adult participants of the NHANES from 2005 to 2010, short sleepers were found to eat earlier and later, consume more calories as snacks (than meals) and consume more sugar and caffeine than participants reporting longer sleep [83].
Sleep seems to also play an important role in eating disorders, including BED [86] and BED. For example, a systematic review by da Luz et al. (2023) [87] found that people who binge eat exhibit poorer overall sleep quality compared to people who do not binge eat, and may have more daytime sleepiness, insomnia, and difficulty falling asleep. BED has even been proposed as a possible circadian disorder [88]. More studies of these associations might explain these relationships as well as the findings among pregnant women reviewed here [48,65].
Cravings have been found to be associated with sleep in various non-pregnant populations. Lack of sleep has been found to be associated with cravings among women without obesity [89], and healthy young adults [90]. Food cravings were associated with poor sleep quality among shift-workers [91]. Higher sleep efficiency was found to be associated with lower sweet cravings among adolescents [92]. Chronotype was not associated with food cravings in several studies [93,94,95]. None of these findings are consistent with the associations of time in bed [56] and chronotype [64] with cravings found among pregnant women in this review.
Interpretation of these results must be considered with the limitations of the processes and studies themselves. As mentioned above, 60% of the studies had a low level of evidence (less than level 4) and only two of the studies were RCTs. Additionally, a wide range of methods and outcome measures were used, which limits the conclusions that can be drawn across studies. Further, most of the studies were conducted only with participants with low-risk pregnancies. However, the review of this research was conducted with systematic processes according to the PRISMA framework, so the literature presented is representative of the state of this research.
5. Conclusions
This literature review demonstrated the need for improved consistency or standardization in methods and a relative lack in longitudinal data, but also highlights great opportunities for future research. For example, higher-powered studies are needed to look both separately and across pregnancy trimesters to examine the relationship between maternal diet, sleep, and outcomes throughout the course of pregnancy. Additionally, more rigorous measures are likely needed to assess smaller components of diet such as monounsaturated fat, as day-to-day variations in intake require great precision of measurement; however, this will likely result in more burdensome assessment.
Author Contributions
T.v.A., L.S., M.H.B., G.B., A.S., S.P. and P.M.R. jointly conceptualized the review, completed screening, and abstracted the data. T.v.A. oversaw development of the search strategy, as well as screening and abstraction. M.H.B. and G.B. drafted the introduction, T.v.A. drafted the methods and tables, L.S. and P.M.R. drafted the results, and P.M.R. and A.S. drafted the discussion. All authors contributed to revising and finalizing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was not directly supported by a funding source.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors would like to acknowledge Chelsea Misquith, research librarian at the Brown University School of Public Health, for finalizing the search strategy and running the search. They would also like to thank Paola Solano and Melanie Morales for assisting in the screening process.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A. Search Strategy
PubMed:
| 1 | Birthing Centers/(819) |
| 2 | Maternal Health/(1765) |
| 3 | exp Maternal Health Services/(52223) |
| 4 | Maternal-Fetal Relations/(925) |
| 5 | Maternal-Child Nursing/(1939) |
| 6 | exp Pregnancy/(920493) |
| 7 | exp Pregnancy Complications/(438930) |
| 8 | Pregnant Women/or Surrogate Mothers/(11325) |
| 9 | exp delivery, obstetric/(83094) |
| 10 | (antenatal or gestat* or maternal* or maternity or perinatal or pregnan* or prenatal or surroga*).ti,ab,kf. (933301) |
| 11 | or/1–10 (1331767) |
| 12 | exp Sleep/(83550) |
| 13 | exp Sleep Wake Disorders/(94508) |
| 14 | Sleep Medicine Specialty/(387) |
| 15 | sleepiness/(455) |
| 16 | (sleep* or slept or somnolen*).ti,ab,kf. (201971) |
| 17 | (dream* or REM or rapid eye movement* or non-REM or NREM).ti,ab,kf. (31925) |
| 18 | (drowsy or drowsiness).ti,ab,kf. (7064) |
| 19 | or/12–18 (248547) |
| 20 | exp “diet, food, and nutrition”/(1778723) |
| 21 | exp Plants, Edible/(46593) |
| 22 | exp Dietary Services/(7054) |
| 23 | exp “Feeding and Eating Disorders”/(31946) |
| 24 | exp Nutrition Therapy/(104976) |
| 25 | nutritional sciences/or dietetics/or nutrigenomics/(18726) |
| 26 | exp Nutrition Policy/(11681) |
| 27 | exp Food Industry/(190037) |
| 28 | (diet* or supplement*).ti,ab,kf. (875322) |
| 29 | (eat or eaten or eats or eating or ate or cook*).ti,ab,kf. (143528) |
| 30 | (food* or fed or feed*).ti,ab,kf. (1035612) |
| 31 | (calorie* or caloric or high-calorie* or low-calorie* or high-caloric* or low-caloric*).ti,ab,kf. (46685) |
| 32 | (macro-nutrient* or macronutrient* or nutrition* or nutrient* or nutritive*).ti,ab,kf. (449174) |
| 33 | (consume? or consuming or consumption).ti,ab,kf. (475613) |
| 34 | energy.ti,ab,kf. (687974) |
| 35 | (intake or ingest*).ti,ab,kf. (373987) |
| 36 | (carbohydrate* or high-carb* or low-carb* or sugar*).ti,ab,kf. (266900) |
| 37 | (fat or fats or fat-rich or fat-heavy or fatty or high-fat* or low-fat* or omega-3* or omega-6* or oil or oils).ti,ab,kf. (657162) |
| 38 | (amino acid* or high-protein* or low-protein* or protein*).ti,ab,kf. (3425839) |
| 39 | (cholesterol or high-cholesterol or HDL or low-cholesterol or LDL or lipoprotein*).ti,ab,kf. (334576) |
| 40 | (dairy or fish or fruit* or vegetable* or meat or seafood or vegan* or vegetarian* or nonvegetarian* or non-vegetarian* or paleo* or pescatarian or keto* or mediterranean or meal or meals).ti,ab,kf. (648313) |
| 41 | (butter* or ghee* or milk*).ti,ab,kf. (149412) |
| 42 | (superfood* or probiotic*).ti,ab,kf. (28331) |
| 43 | (hunger or hungry or malnutrition).ti,ab,kf. (55056) |
| 44 | or/20–43 (7595460) |
| 45 | 11 and 19 and 44 (2520) |
EMBASE:
(‘maternity ward’/exp OR ‘maternal care’/exp OR ‘maternal health service’/exp OR ‘mother fetus relationship’/exp OR ‘maternal child health care’/exp OR ‘pregnancy’/exp OR ‘pregnancy disorder’/exp OR ‘pregnant woman’/exp OR ‘surrogate mother’/exp OR ‘obstetric delivery’/exp OR antenatal:ti,ab,kw OR gestat*:ti,ab,kw OR maternal*:ti,ab,kw OR maternity:ti,ab,kw OR perinatal:ti,ab,kw OR pregnan*:ti,ab,kw OR prenatal:ti,ab,kw OR surroga*:ti,ab,kw) AND (‘sleep’/exp OR ‘sleep disorder’/exp OR ‘sleep medicine’/exp OR ‘somnolence’/exp OR sleep*:ti,ab,kw OR slept:ti,ab,kw OR somnolen*:ti,ab,kw OR dream*:ti,ab,kw OR rem:ti,ab,kw OR ‘rapid eye movement*’:ti,ab,kw OR ‘non-rem’:ti,ab,kw OR nrem:ti,ab,kw OR drowsy:ti,ab,kw OR drowsiness:ti,ab,kw) AND (‘nutrition’/exp OR ‘edible plant’/exp OR ‘digestive function’/exp OR ‘dietary service’/exp OR ‘nutrition service’/exp OR ‘eating disorder’/exp OR ‘diet therapy’/exp OR ‘nutritional science’/exp OR ‘dietetics’/exp OR ‘nutrigenomics’/exp OR ‘nutrition policy’/exp OR ‘food handling’/exp OR ‘food insecurity’/exp OR diet*:ti,ab,kw OR supplement*:ti,ab,kw OR eat:ti,ab,kw OR eaten:ti,ab,kw OR eats:ti,ab,kw OR eating:ti,ab,kw OR ate:ti,ab,kw OR cook*:ti,ab,kw OR food*:ti,ab,kw OR fed:ti,ab,kw OR feed*:ti,ab,kw OR calorie*:ti,ab,kw OR caloric:ti,ab,kw OR ‘high-calorie*’:ti,ab,kw OR ‘low-calorie*’:ti,ab,kw OR ‘high-caloric*’:ti,ab,kw OR ‘low-caloric*’:ti,ab,kw OR ‘macro-nutrient*’:ti,ab,kw OR macronutrient*:ti,ab,kw OR nutrition*:ti,ab,kw OR nutrient*:ti,ab,kw OR nutritive*:ti,ab,kw OR consume$:ti,ab,kw OR consuming:ti,ab,kw OR consumption:ti,ab,kw OR energy:ti,ab,kw OR intake:ti,ab,kw OR ingest*:ti,ab,kw OR carbohydrate*:ti,ab,kw OR ‘high-carb*’:ti,ab,kw OR ‘low-carb*’:ti,ab,kw OR sugar*:ti,ab,kw OR fat:ti,ab,kw OR fats:ti,ab,kw OR ‘fat-rich’:ti,ab,kw OR ‘fat-heavy’:ti,ab,kw OR fatty:ti,ab,kw OR ‘high-fat*’:ti,ab,kw OR ‘low-fat*’:ti,ab,kw OR ‘omega-3*’:ti,ab,kw OR ‘omega-6*’:ti,ab,kw OR oil:ti,ab,kw OR oils:ti,ab,kw OR ‘amino acid*’:ti,ab,kw OR ‘high-protein*’:ti,ab,kw OR ‘low-protein*’:ti,ab,kw OR protein*:ti,ab,kw OR cholesterol:ti,ab,kw OR ‘high-cholesterol’:ti,ab,kw OR hdl:ti,ab,kw OR ‘low-cholesterol’:ti,ab,kw OR ldl:ti,ab,kw OR lipoprotein*:ti,ab,kw OR dairy:ti,ab,kw OR fish:ti,ab,kw OR fruit*:ti,ab,kw OR vegetable*:ti,ab,kw OR meat:ti,ab,kw OR seafood:ti,ab,kw OR vegan*:ti,ab,kw OR vegetarian*:ti,ab,kw OR nonvegetarian*:ti,ab,kw OR ‘non-vegetarian*’:ti,ab,kw OR paleo*:ti,ab,kw OR pescatarian:ti,ab,kw OR keto*:ti,ab,kw OR mediterranean:ti,ab,kw OR meal:ti,ab,kw OR meals:ti,ab,kw OR butter*:ti,ab,kw OR ghee*:ti,ab,kw OR milk*:ti,ab,kw OR superfood*:ti,ab,kw OR probiotic*:ti,ab,kw OR hunger:ti,ab,kw OR hungry:ti,ab,kw OR malnutrition:ti,ab,kw) NOT ( [animals]/lim NOT [humans]/lim) AND [english]/lim
CINAHL:
| S48 | S11 AND S18 AND S47 |
| S47 | S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 |
| S46 | TI (hunger or hungry or malnutrition) OR AB (hunger or hungry or malnutrition) |
| S45 | TI (superfood* or probiotic*) OR AB (superfood* or probiotic*) |
| S44 | TI (butter* or ghee* or milk*) OR AB (butter* or ghee* or milk*) |
| S43 | TI (dairy or fish or fruit* or vegetable* or meat or seafood or vegan* or vegetarian* or nonvegetarian* or non-vegetarian* or paleo* or pescatarian or keto* or mediterranean or meal or meals) OR AB (dairy or fish or fruit* or vegetable* or meat or seafood or vegan* or vegetarian* or nonvegetarian* or non-vegetarian* or paleo* or pescatarian or keto* or mediterranean or meal or meals) |
| S42 | TI (cholesterol or high-cholesterol or HDL or low-cholesterol or LDL or lipoprotein*) OR AB (cholesterol or high-cholesterol or HDL or low-cholesterol or LDL or lipoprotein*) |
| S41 | TI (amino acid* or high-protein* or low-protein* or protein*) OR AB (amino acid* or high-protein* or low-protein* or protein*) |
| S40 | TI (fat or fats or fat-rich or fat-heavy or fatty or high-fat* or low-fat* or omega-3* or omega-6* or oil or oils) OR AB (fat or fats or fat-rich or fat-heavy or fatty or high-fat* or low-fat* or omega-3* or omega-6* or oil or oils) |
| S39 | TI (carbohydrate* or high-carb* or low-carb* or sugar*) OR AB (carbohydrate* or high-carb* or low-carb* or sugar*) |
| S38 | TI (intake or ingest*) OR AB (intake or ingest*) |
| S37 | TI energy OR AB energy |
| S36 | TI (consume# or consuming or consumption) OR AB (consume# or consuming or consumption) |
| S35 | TI (macro-nutrient* or macronutrient* or nutrition* or nutrient* or nutritive*) OR AB (macro-nutrient* or macronutrient* or nutrition* or nutrient* or nutritive*) |
| S34 | TI (calorie* or caloric or high-calorie* or low-calorie* or high-caloric* or low-caloric*) OR AB (calorie* or caloric or high-calorie* or low-calorie* or high-caloric* or low-caloric*) |
| S33 | TI (food* or fed or feed*) OR AB (food* or fed or feed*) |
| S32 | TI (eat or eaten or eats or eating or ate or cook*) OR AB (eat or eaten or eats or eating or ate or cook*) |
| S31 | TI (diet* or supplement*) OR AB (diet* or supplement*) |
| S30 | (MH “Food Security”) |
| S29 | (MH “Food Industry+”) |
| S28 | (MH “Nutrition Policy+”) |
| S27 | (MH “Sports Nutritional Sciences”) OR (MH “Dietetics”) OR (MH “Research, Dietetics”) OR (MH “Nutrigenomics+”) |
| S26 | (MH “Diet Therapy+”) |
| S25 | (MH “Eating Disorders+”) |
| S24 | (MH “Nutrition Services+”) |
| S23 | (MH “Plants, Edible+”) |
| S22 | (MH “Eating Behavior+”) OR (MH “Drinking Behavior+”) |
| S21 | (MH “Nutritional Physiology”) OR (MH “Nutritive Value+”) OR (MH “Digestive System Physiology+”) |
| S20 | (MH “Food and Beverages+”) |
| S19 | (MH “Nutrition+”) |
| S18 | S12 OR S13 OR S14 OR S15 OR S16 OR S17 |
| S17 | TI ((drowsy or drowsiness)) OR AB ((drowsy or drowsiness)) |
| S16 | TI ((dream* or REM or “rapid eye movement*” or non-REM or NREM)) OR AB ((dream* or REM or “rapid eye movement*” or non-REM or NREM)) |
| S15 | I ((sleep* or slept or somnolen*)) OR AB ((sleep* or slept or somnolen*)) |
| S14 | (MH “Sleepiness”) |
| S13 | (MH “Sleep Disorders+”) |
| S12 | (MH “Sleep+”) |
| S11 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 |
| S10 | TI ((antenatal or gestat* or maternal* or maternity or perinatal or pregnan* or prenatal or surroga*)) OR AB ((antenatal or gestat* or maternal* or maternity or perinatal or pregnan* or prenatal or surroga*)) |
| S9 | (MH “Delivery, Obstetric+”) |
| S8 | (MH “Expectant Mothers”) OR (MH “Surrogate Mothers”) |
| S7 | (MH “Pregnancy Complications+”) |
| S6 | (MH “Pregnancy+”) |
| S5 | (MH “Maternal-Child Nursing”) OR (MH “Obstetric Nursing”) OR (MH “Perinatal Nursing”) |
| S4 | (MH “Prenatal Bonding”) |
| S3 | (MH “Maternal Health Services+”) |
| S2 | (MH “Obstetric Care+”) |
| S1 | (MH “Delivery Rooms+”) |
References
- Cai, S.; Tan, S.; Gluckman, P.D.; Godfrey, K.M.; Saw, S.M.; Teoh, O.H.; Chong, Y.-S.; Meaney, M.J.; Kramer, M.S.; Gooley, J.J.; et al. Sleep Quality and Nocturnal Sleep Duration in Pregnancy and Risk of Gestational Diabetes Mellitus. Sleep 2017, 40, zsw058. [Google Scholar] [CrossRef]
- Rawal, S.; Hinkle, S.N.; Zhu, Y.; Albert, P.S.; Zhang, C. A longitudinal study of sleep duration in pregnancy and subsequent risk of gestational diabetes: Findings from a prospective, multiracial cohort. Am. J. Obstet. Gynecol. 2017, 216, 399.e1–399.e8. [Google Scholar] [CrossRef]
- Sedov, I.D.; Cameron, E.E.; Madigan, S.; Tomfohr-Madsen, L.M. Sleep quality during pregnancy: A meta-analysis. Sleep Med. Rev. 2018, 38, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Pengo, M.F.; Won, C.H.; Bourjeily, G. Sleep in Women Across the Life Span. Chest 2018, 154, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Pauley, A.M.; Moore, G.A.; Mama, S.K.; Molenaar, P.; Symons Downs, D. Associations between prenatal sleep and psychological health: A systematic review. J. Clin. Sleep Med. 2020, 16, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Paine, S.J.; Signal, T.L.; Sweeney, B.; Priston, M.; Muller, D.; Smith, A.; Huthwaite, M.; Gander, P.; Lee, K. Maternal sleep disturbances in late pregnancy and the association with emergency caesarean section: A prospective cohort study. Sleep Health 2020, 6, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Zhang, X.; Wang, Y.; Li, J.; Xu, Y.; Song, X.; Su, S.; Zhu, X.; Vitiello, M.V.; Shi, J.; et al. Sleep disturbances during pregnancy and adverse maternal and fetal outcomes: A systematic review and meta-analysis. Sleep Med. Rev. 2021, 58, 101436. [Google Scholar] [CrossRef]
- Bourjeily, G.; Danilack, V.A.; Bublitz, M.H.; Lipkind, H.; Muri, J.; Caldwell, D.; Tong, I.; Rosene-Montella, K. Obstructive sleep apnea in pregnancy is associated with adverse maternal outcomes: A national cohort. Sleep Med. 2017, 38, 50–57. [Google Scholar] [CrossRef]
- Bourjeily, G.; Raker, C.A.; Chalhoub, M.; Miller, M.A. Pregnancy and fetal outcomes of symptoms of sleep-disordered breathing. Eur. Respir. J. 2010, 36, 849–855. [Google Scholar] [CrossRef]
- Facco, F.L.; Parker, C.B.; Reddy, U.M.; Silver, R.M.; Koch, M.A.; Louis, J.M.; Basner, R.C.; Chung, J.H.; Nhan-Chang, C.-L.; Pien, G.W.; et al. Association Between Sleep-Disordered Breathing and Hypertensive Disorders of Pregnancy and Gestational Diabetes Mellitus. Obstet. Gynecol. 2017, 129, 31–41. [Google Scholar] [CrossRef]
- Louis, J.M.; Mogos, M.F.; Salemi, J.L.; Redline, S.; Salihu, H.M. Obstructive sleep apnea and severe maternal-infant morbidity/mortality in the United States, 1998–2009. Sleep 2014, 37, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Bublitz, M.H.; Sharp, M.; Freeburg, T.; Sanapo, L.; Nugent, N.R.; Sharkey, K.; Bourjeily, G. Sleep Disordered Breathing Measures in Early Pregnancy Are Associated with Depressive Symptoms in Late Pregnancy. Diagnostics 2021, 11, 858. [Google Scholar] [CrossRef] [PubMed]
- Bourjeily, G.; Danilack, V.A.; Bublitz, M.H.; Muri, J.; Rosene-Montella, K.; Lipkind, H. Maternal obstructive sleep apnea and neonatal birth outcomes in a population based sample. Sleep Med. 2020, 66, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Kendle, A.M.; Salemi, J.L.; Jackson, C.L.; Buysse, D.J.; Louis, J.M. Insomnia during pregnancy and severe maternal morbidity in the united states: Nationally representative data from 2006 to 2017. Sleep 2022, 45, zsac175. [Google Scholar] [CrossRef] [PubMed]
- Izci Balserak, B.; Hermann, R.; Hernandez, T.L.; Buhimschi, C.; Park, C. Evening blue-light exposure, maternal glucose, and infant birthweight. Ann. N. Y. Acad. Sci. 2022, 1515, 276–284. [Google Scholar] [CrossRef]
- Facco, F.L.; Parker, C.B.; Hunter, S.; Reid, K.J.; Zee, P.P.; Silver, R.M.; Pien, G.; Chung, J.H.; Louis, J.M.; Haas, D.M.; et al. Later sleep timing is associated with an increased risk of preterm birth in nulliparous women. Am. J. Obstet. Gynecol. MFM 2019, 1, 100040. [Google Scholar] [CrossRef]
- Procter, S.B.; Campbell, C.G. Position of the Academy of Nutrition and Dietetics: Nutrition and lifestyle for a healthy pregnancy outcome. J. Acad. Nutr. Diet. 2014, 114, 1099–1103. [Google Scholar] [CrossRef]
- Tsakiridis, I.; Kasapidou, E.; Dagklis, T.; Leonida, I.; Leonida, C.; Bakaloudi, D.R.; Chourdakis, M. Nutrition in Pregnancy: A Comparative Review of Major Guidelines. Obstet. Gynecol. Surv. 2020, 75, 692–702. [Google Scholar] [CrossRef]
- Rahmati, S.; Azami, M.; Badfar, G.; Parizad, N.; Sayehmiri, K. The relationship between maternal anemia during pregnancy with preterm birth: A systematic review and meta-analysis. J. Matern. Fetal Neonatal Med. 2020, 33, 2679–2689. [Google Scholar] [CrossRef]
- Shi, H.; Chen, L.; Wang, Y.; Sun, M.; Guo, Y.; Ma, S.; Wang, X.; Jiang, H.; Wang, X.; Lu, J.; et al. Severity of Anemia During Pregnancy and Adverse Maternal and Fetal Outcomes. JAMA Netw. Open 2022, 5, e2147046. [Google Scholar] [CrossRef]
- Caut, C.; Leach, M.; Steel, A. Dietary guideline adherence during preconception and pregnancy: A systematic review. Matern. Child Nutr. 2020, 16, e12916. [Google Scholar] [CrossRef] [PubMed]
- Keats, E.C.; Haider, B.A.; Tam, E.; Bhutta, Z.A. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2019, 3, CD004905. [Google Scholar] [CrossRef] [PubMed]
- Wikoff, D.; Welsh, B.T.; Henderson, R.; Brorby, G.P.; Britt, J.; Myers, E.; Goldberger, J.; Lieberman, H.R.; O’Brien, C.; Peck, J.; et al. Systematic review of the potential adverse effects of caffeine consumption in healthy adults, pregnant women, adolescents, and children. Food Chem. Toxicol. 2017, 109 Pt 1, 585–648. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, R.; Dreibelbis, C.; Kingshipp, B.L.; Wong, Y.P.; Abrams, B.; Gernand, A.D.; Rasmussen, K.M.; Siega-Riz, A.M.; Stang, J.; O Casavale, K.; et al. Dietary patterns before and during pregnancy and maternal outcomes: A systematic review. Am. J. Clin. Nutr. 2019, 109 (Suppl. 7), 705s–728s. [Google Scholar] [CrossRef]
- Raghavan, R.; Dreibelbis, C.; Kingshipp, B.L.; Wong, Y.P.; Abrams, B.; Gernand, A.D.; Rasmussen, K.M.; Siega-Riz, A.M.; Stang, J.; O Casavale, K.; et al. Dietary patterns before and during pregnancy and birth outcomes: A systematic review. Am. J. Clin. Nutr. 2019, 109 (Suppl. 7), 729s–756s. [Google Scholar] [CrossRef]
- Shapiro, A.L.; Kaar, J.L.; Crume, T.L.; Starling, A.P.; Siega-Riz, A.M.; Ringham, B.M.; Glueck, D.H.; Norris, J.M.; A Barbour, L.; E Friedman, J.; et al. Maternal diet quality in pregnancy and neonatal adiposity: The Healthy Start Study. Int. J. Obes. (Lond.) 2016, 40, 1056–1062. [Google Scholar] [CrossRef]
- Khaled, K.; Hundley, V.; Tsofliou, F. Poor Dietary Quality and Patterns Are Associated with Higher Perceived Stress among Women of Reproductive Age in the UK. Nutrients 2021, 13, 2588. [Google Scholar] [CrossRef]
- Hanson, M.A.; Bardsley, A.; De-Regil, L.M.; Moore, S.E.; Oken, E.; Poston, L.; Ma, R.C.; McAuliffe, F.M.; Maleta, K.; Purandare, C.N.; et al. The International Federation of Gynecology and Obstetrics (FIGO) recommendations on adolescent, preconception, and maternal nutrition: “Think Nutrition First”. Int. J. Gynaecol. Obstet. 2015, 131 (Suppl. 4), S213–S253. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence, Royal College of Obstetricians & Gynaecologists. Antenatal Care (NG201) National Institute for Health and Care Excellence. 2021. Available online: www.nice.org.uk/guidance/ng201 (accessed on 29 April 2023).
- O’Connor, D.L.; Blake, J.; Bell, R.; Bowen, A.; Callum, J.; Fenton, S.; Gray-Donald, K.; Rossiter, M.; Adamo, K.; Brett, K.; et al. Canadian Consensus on Female Nutrition: Adolescence, Reproduction, Menopause, and Beyond. J. Obstet. Gynaecol. Can. 2016, 38, 508–554.e18. [Google Scholar] [CrossRef]
- World Health Organization. WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience Geneva; World Health Organization: Geneva, Switzerland, 2016; Available online: https://www.who.int/publications/i/item/9789241549912 (accessed on 29 April 2023).
- Abdollahi, S.; Soltani, S.; de Souza, R.J.; Forbes, S.C.; Toupchian, O.; Salehi-Abargouei, A. Associations between Maternal Dietary Patterns and Perinatal Outcomes: A Systematic Review and Meta-Analysis of Cohort Studies. Adv. Nutr. 2021, 12, 1332–1352. [Google Scholar] [CrossRef]
- Chia, A.R.; Chen, L.W.; Lai, J.S.; Wong, C.H.; Neelakantan, N.; van Dam, R.M.; Chong, M.F.-F. Maternal Dietary Patterns and Birth Outcomes: A Systematic Review and Meta-Analysis. Adv. Nutr. 2019, 10, 685–695. [Google Scholar] [CrossRef]
- Gonzalez-Nahm, S.; Marchesoni, J.; Maity, A.; Maguire, R.L.; House, J.S.; Tucker, R.; Atkinson, T.; Murphy, S.K.; Hoyo, C. Maternal Mediterranean Diet Adherence and Its Associations with Maternal Prenatal Stressors and Child Growth. Curr. Dev. Nutr. 2022, 6, nzac146. [Google Scholar] [CrossRef] [PubMed]
- Dayi, T.; Ozgoren, M. Effects of the Mediterranean diet on the components of metabolic syndrome. J. Prev. Med. Hyg. 2022, 63 (Suppl. 3), E56–E64. [Google Scholar]
- AlAufi, N.S.; Chan, Y.M.; Waly, M.I.; Chin, Y.S.; Mohd Yusof, B.N.; Ahmad, N. Application of Mediterranean Diet in Cardiovascular Diseases and Type 2 Diabetes Mellitus: Motivations and Challenges. Nutrients 2022, 14, 2777. [Google Scholar] [CrossRef] [PubMed]
- Zaragoza-Marti, A.; Ruiz-Rodenas, N.; Herranz-Chofre, I.; Sanchez-SanSegundo, M.; Serrano Delgado, V.C.; Hurtado-Sanchez, J.A. Adherence to the Mediterranean Diet in Pregnancy and Its Benefits on Maternal-Fetal Health: A Systematic Review of the Literature. Front. Nutr. 2022, 9, 813942. [Google Scholar] [CrossRef] [PubMed]
- Jeukendrup, A.E. Periodized Nutrition for Athletes. Sports Med. 2017, 47 (Suppl. 1), 51–63. [Google Scholar] [CrossRef] [PubMed]
- Tahara, Y.; Shibata, S. Chrono-biology, chrono-pharmacology, and chrono-nutrition. J. Pharmacol. Sci. 2014, 124, 320–335. [Google Scholar] [CrossRef] [PubMed]
- Peuhkuri, K.; Sihvola, N.; Korpela, R. Diet promotes sleep duration and quality. Nutr. Res. 2012, 32, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Pot, G.K. Sleep and dietary habits in the urban environment: The role of chrono-nutrition. Proc. Nutr. Soc. 2018, 77, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Al Khatib, H.K.; Harding, S.V.; Darzi, J.; Pot, G.K. The effects of partial sleep deprivation on energy balance: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2017, 71, 614–624. [Google Scholar] [CrossRef]
- Pauley, A.M.; Moore, G.A.; Mama, S.K.; Molenaar, P.; Downs, D.S. Systematic review of the associations between prenatal sleep behaviours and components of energy balance for regulating weight gain. J. Sleep Res. 2023, 32, e13619. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef]
- Melnyk, B.M.F.; Fineout-Overholt, E. Box 1.3: Rating system for the hierarchy of evidence for intervention/treatment questions. In Evidence-Based Practice in Nursing & Healthcare: A Guide to Best Practice, 3rd ed.; Wolters Kluwer Health: Philadelphia, PA, USA, 2015; p. 11. [Google Scholar]
- Pauley, A.M.; Hohman, E.E.; Leonard, K.S.; Guo, P.; McNitt, K.M.; Rivera, D.E.; Savage, J.S.; Downs, D.S. Short Nighttime Sleep Duration and High Number of Nighttime Awakenings Explain Increases in Gestational Weight Gain and Decreases in Physical Activity but Not Energy Intake among Pregnant Women with Overweight/Obesity. Clocks Sleep 2020, 2, 487–501. [Google Scholar] [CrossRef]
- Phelan, S.; Wing, R.R.; Brannen, A.; McHugh, A.; Hagobian, T.A.; Schaffner, A.; Jelalian, E.; Hart, C.N.; O Scholl, T.; Munoz-Christian, K.; et al. Randomized controlled clinical trial of behavioral lifestyle intervention with partial meal replacement to reduce excessive gestational weight gain. Am. J. Clin. Nutr. 2018, 107, 183–194. [Google Scholar] [CrossRef]
- Allison, K.C.; Wrotniak, B.H.; Paré, E.; Sarwer, D.B. Psychosocial Characteristics and Gestational Weight Change among Overweight, African American Pregnant Women. Obstet. Gynecol. Int. 2012, 2012, 878607. [Google Scholar] [CrossRef]
- Bennett, C.J.; Cain, S.W.; Blumfield, M.L. Monounsaturated fat intake is associated with improved sleep quality in pregnancy Short Nighttime Sleep Duration and High Number of Nighttime Awakenings Explain Increases in Gestational Weight Gain and Decreases in Physical Activity but Not Energy Intake among Pregnant Women with Overweight/Obesity. Midwifery 2019, 78, 64–70. [Google Scholar]
- Chang, M.W.; Brown, R.; Nitzke, S.; Smith, B.; Eghtedary, K. Stress, sleep, depression and dietary intakes among low-income overweight and obese pregnant women. Matern. Child Health J. 2015, 19, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Devoe, L.D.; Murray, C.; Arnaud, M. Maternal caffeine consumption and fetal behavior in normal third-trimester pregnancy. Am. J. Obstet. Gynecol. 1993, 168, 1105–1111; discussion 1111–1112. [Google Scholar] [CrossRef] [PubMed]
- Diego, M.F.T.; Hernandez-Reif, M.; Vera, Y.; Gil, K.; Gonzalez-Garcia, A. Caffeine Use Affects Pregnancy Outcome. J. Child Adolesc. Subst. Abus. 2008, 17, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Liu, J.; Han, N.; Zhao, Z.; Luo, S.; Wang, H. Association between sleep duration in early pregnancy and risk of gestational diabetes mellitus: A prospective cohort study. Diabetes Metab. 2021, 47, 101217. [Google Scholar] [CrossRef]
- Du, M.; Liu, J.; Han, N.; Zhao, Z.; Yang, J.; Xu, X.; Luo, S.; Wang, H. Maternal sleep quality during early pregnancy, risk factors and its impact on pregnancy outcomes: A prospective cohort study. Sleep Med. 2021, 79, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Duke, C.H.; Williamson, J.A.; Snook, K.R.; Finch, K.C.; Sullivan, K.L. Association Between Fruit and Vegetable Consumption and Sleep Quantity in Pregnant Women. Matern. Child Health J. 2017, 21, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, E.W.; Most, J.; Broskey, N.T.; Altazan, A.D.; Beyl, R.A.; Keadle, S.K.; Drews, K.L.; Singh, P.; Redman, L.M. Identification of changes in sleep across pregnancy and the impact on cardiometabolic health and energy intake in women with obesity. Sleep Med. 2021, 77, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Flor-Alemany, M.; Nestares, T.; Alemany-Arrebola, I.; Marin-Jimenez, N.; Borges-Cosic, M.; Aparicio, V.A. Influence of Dietary Habits and Mediterranean Diet Adherence on Sleep Quality during Pregnancy. The GESTAFIT Project. Nutrients 2020, 12, 3569. [Google Scholar] [CrossRef]
- Gontijo, C.A.; Cabral, B.B.M.; Balieiro, L.C.T.; Teixeira, G.P.; Fahmy, W.M.; Maia, Y.C.P.; Crispim, C.A. Time-related eating patterns and chronotype are associated with diet quality in pregnant women. Chronobiol. Int. 2019, 36, 75–84. [Google Scholar] [CrossRef]
- Gordon, L.K.; Mason, K.A.; Mepham, E.; Sharkey, K.M. A mixed methods study of perinatal sleep and breastfeeding outcomes in women at risk for postpartum depression. Sleep Health 2021, 7, 353–361. [Google Scholar] [CrossRef]
- Loy, S.L.; Cheung, Y.B.; Cai, S.; Colega, M.T.; Godfrey, K.M.; Chong, Y.S.; Shek, L.; Tan, K.H.; Chong, M.F.-F.; Yap, F.; et al. Maternal night-time eating and sleep duration in relation to length of gestation and preterm birth. Clin. Nutr. 2020, 39, 1935–1942. [Google Scholar] [CrossRef]
- Quach, D.T.; Le, Y.T.; Mai, L.H.; Hoang, A.T.; Nguyen, T.T. Short Meal-to-Bed Time Is a Predominant Risk Factor of Gastroesophageal Reflux Disease in Pregnancy Association Between Fruit and Vegetable Consumption and Sleep Quantity in Pregnant Women: A mixed methods study of perinatal sleep and breastfeeding outcomes in women at risk for postpartum depression. J. Clin. Gastroenterol. 2021, 55, 316–320. [Google Scholar]
- Santos, I.S.; Matijasevich, A.; Domingues, M.R. Maternal caffeine consumption and infant nighttime waking: Prospective cohort study. Pediatrics 2012, 129, 860–868. [Google Scholar] [CrossRef]
- Sugimori, N.; Hamazaki, K.; Matsumura, K.; Kasamatsu, H.; Tsuchida, A.; Inadera, H.; Japan Environment and Children’s Study Group. Association between maternal fermented food consumption and infant sleep duration: The Japan Environment and Children’s Study. PLoS ONE 2019, 14, e0222792. [Google Scholar] [CrossRef]
- Teixeira, G.P.; Balieiro, L.C.T.; Gontijo, C.A.; Fahmy, W.M.; Maia, Y.C.P.; Crispim, C.A. The association between chronotype, food craving and weight gain in pregnant women Time-related eating patterns and chronotype are associated with diet quality in pregnant women Sleep disturbances and binge eating disorder symptoms during and after pregnancy. J. Hum. Nutr. Diet. 2020, 33, 342–350. [Google Scholar] [PubMed]
- Ulman, T.F.; Von Holle, A.; Torgersen, L.; Stoltenberg, C.; Reichborn-Kjennerud, T.; Bulik, C.M. Sleep disturbances and binge eating disorder symptoms during and after pregnancy. Sleep 2012, 35, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Van Lee, L.; Chia, A.R.; Loy, S.L.; Colega, M.; Tham, E.K.H.; Cai, S.; Yap, F.; Godfrey, K.M.; Teoh, O.H.; Goh, D.; et al. Sleep and Dietary Patterns in Pregnancy: Findings from the GUSTO Cohort. Int. J. Environ. Res. Public Health 2017, 14, 1409. [Google Scholar] [CrossRef]
- Wang, M.L.; Libby, B.A.; Moore Simas, T.A.; Waring, M.E. Sugar-Sweetened Beverage Consumption and Sleep Duration and Quality Among Pregnant Women. J. Nutr. Educ. Behav. 2021, 53, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Wołyńczyk-Gmaj, D.; Różańska-Walędziak, A.; Ziemka, S.; Ufnal, M.; Brzezicka, A.; Gmaj, B.; Januszko, P.; Fudalej, S.; Czajkowski, K.; Wojnar, M. Insomnia in Pregnancy Is Associated With Depressive Symptoms and Eating at Night. J. Clin. Sleep Med. 2017, 13, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Ma, H.; Feng, Y.; Wang, Y.; Wu, S.; Cai, S.; Shi, Y.; Chen, Y.; Ma, L.; Jiang, Y. Dietary patterns in relation to gestational depression and sleep disturbance in Chinese pregnant women Identification of changes in sleep across pregnancy and the impact on cardiometabolic health and energy intake in women with obesity. J. Obstet. Gynaecol. Res. 2020, 77, 120–127. [Google Scholar]
- Betts, G.M.; Lipsky, L.M.; Temmen, C.D.; Siega-Riz, A.M.; Faith, M.S.; Nansel, T.R. Poorer mental health and sleep quality are associated with greater self-reported reward-related eating during pregnancy and postpartum: An observational cohort study. Int. J. Behav. Nutr. Phys. Act. 2021, 18, 58. [Google Scholar] [CrossRef]
- Dashti, H.S.; Scheer, F.A.; Jacques, P.F.; Lamon-Fava, S.; Ordovas, J.M. Short sleep duration and dietary intake: Epidemiologic evidence, mechanisms, and health implications. Adv. Nutr. 2015, 6, 648–659. [Google Scholar] [CrossRef]
- Sejbuk, M.; Mironczuk-Chodakowska, I.; Witkowska, A.M. Sleep Quality: A Narrative Review on Nutrition, Stimulants, and Physical Activity as Important Factors. Nutrients 2022, 14, 1912. [Google Scholar] [CrossRef]
- Zhao, M.; Tuo, H.; Wang, S.; Zhao, L. The Effects of Dietary Nutrition on Sleep and Sleep Disorders. Mediat. Inflamm. 2020, 2020, 3142874. [Google Scholar] [CrossRef]
- Gangwisch, J.E.; Hale, L.; St-Onge, M.P.; Choi, L.; LeBlanc, E.S.; Malaspina, D.; Opler, M.G.; Shadyab, A.H.; Shikany, J.M.; Snetselaar, L.; et al. High glycemic index and glycemic load diets as risk factors for insomnia: Analyses from the Women’s Health Initiative. Am. J. Clin. Nutr. 2020, 111, 429–439. [Google Scholar] [CrossRef]
- St-Onge, M.P.; Mikic, A.; Pietrolungo, C.E. Effects of Diet on Sleep Quality. Adv. Nutr. 2016, 7, 938–949. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, F.; Muurlink, O.; Reid, N. Effects of caffeine on sleep quality and daytime functioning. Risk Manag. Healthc. Policy 2018, 11, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Snel, J.; Lorist, M.M. Effects of caffeine on sleep and cognition. Prog. Brain Res. 2011, 190, 105–117. [Google Scholar] [PubMed]
- Noorwali, E.; Hardie, L.; Cade, J. Bridging the Reciprocal Gap between Sleep and Fruit and Vegetable Consumption: A Review of the Evidence, Potential Mechanisms, Implications, and Directions for Future Work. Nutrients 2019, 11, 1382. [Google Scholar] [CrossRef]
- Jansen, E.C.; Prather, A.; Leung, C.W. Associations between sleep duration and dietary quality: Results from a nationally-representative survey of US adults. Appetite 2020, 153, 104748. [Google Scholar] [CrossRef]
- Deng, M.G.; Nie, J.Q.; Li, Y.Y.; Yu, X.; Zhang, Z.J. Higher HEI-2015 Scores Are Associated with Lower Risk of Sleep Disorder: Results from a Nationally Representative Survey of United States Adults. Nutrients 2022, 14, 873. [Google Scholar] [CrossRef]
- Godos, J.; Grosso, G.; Castellano, S.; Galvano, F.; Caraci, F.; Ferri, R. Association between diet and sleep quality: A systematic review. Sleep Med. Rev. 2021, 57, 101430. [Google Scholar] [CrossRef]
- Scoditti, E.; Tumolo, M.R.; Garbarino, S. Mediterranean Diet on Sleep: A Health Alliance. Nutrients 2022, 14, 2998. [Google Scholar] [CrossRef]
- Kant, A.K.; Graubard, B.I. Association of self-reported sleep duration with eating behaviors of American adults: NHANES 2005-2010. Am. J. Clin. Nutr. 2014, 100, 938–947. [Google Scholar] [CrossRef]
- Boege, H.L.; Bhatti, M.Z.; St-Onge, M.P. Circadian rhythms and meal timing: Impact on energy balance and body weight. Curr. Opin. Biotechnol. 2021, 70, 1–6. [Google Scholar] [CrossRef]
- Neuhouser, M.L.; Tinker, L.F.; Thomson, C.; Caan, B.; Horn, L.V.; Snetselaar, L.; Parker, L.M.; Patterson, R.E.; Robinson-O’brien, R.; Beresford, S.A.A.; et al. Development of a glycemic index database for food frequency questionnaires used in epidemiologic studies. J. Nutr. 2006, 136, 1604–1609. [Google Scholar] [CrossRef] [PubMed]
- Manasse, S.M.; Lampe, E.W.; Gillikin, L.; Trainor, C.M.; Abber, S.R.; Fitzpatrick, B.; Sanchez, H.; Juarascio, A.S. An examination of daily sleep characteristics and subsequent eating disorder behavior among individuals with binge-spectrum eating disorders. Eat. Weight. Disord. 2022, 27, 3743–3749. [Google Scholar] [CrossRef]
- Da Luz, F.Q.; Sainsbury, A.; Salis, Z.; Hay, P.; Cordas, T.; Morin, C.M.; Paulos-Guarnieri, L.; Pascoareli, L.; El Rafihi-Ferreira, R. A systematic review with meta-analyses of the relationship between recurrent binge eating and sleep parameters. Int. J. Obes. (Lond.) 2023, 47, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Plano, S.A.; Soneira, S.; Tortello, C.; Golombek, D.A. Is the binge-eating disorder a circadian disorder? Front. Nutr. 2022, 9, 964491. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.L.; Schnepp, J.; Tucker, R.M. Increased Hunger, Food Cravings, Food Reward, and Portion Size Selection after Sleep Curtailment in Women Without Obesity. Nutrients 2019, 11, 663. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Finlayson, G.; Dando, R. Sleep, food cravings and taste. Appetite 2018, 125, 210–216. [Google Scholar] [CrossRef]
- Vidafar, P.; Cain, S.W.; Shechter, A. Relationship between Sleep and Hedonic Appetite in Shift Workers. Nutrients 2020, 12, 2835. [Google Scholar] [CrossRef]
- Kracht, C.L.; Chaput, J.P.; Martin, C.K.; Champagne, C.M.; Katzmarzyk, P.T.; Staiano, A.E. Associations of Sleep with Food Cravings, Diet, and Obesity in Adolescence. Nutrients 2019, 11, 2899. [Google Scholar] [CrossRef]
- Lai, P.P.; Say, Y.H. Associated Factors of Sleep Quality and Behavior among Students of Two Tertiary Institutions in Northern Malaysia. Med. J. Malays. 2013, 68, 195–203. [Google Scholar]
- Meule, A.; Roeser, K.; Randler, C.; Kubler, A. Skipping breakfast: Morningness-eveningness preference is differentially related to state and trait food cravings. Eat. Weight. Disord. 2012, 17, e304–e308. [Google Scholar] [PubMed]
- Yang, C.L.; Tucker, R.M. Snacking behavior differs between evening and morning chronotype individuals but no differences are observed in overall energy intake, diet quality, or food cravings Skipping breakfast: Morningness-eveningness preference is differentially related to state and trait food cravings Associated Factors of Sleep Quality and Behavior among Students of Two Tertiary Institutions in Northern Malaysia. Chronobiol. Int. 2022, 39, 616–625. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).