Proposal to Screen for Zinc and Selenium in Patients with IgA Deficiency
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Participants
2.3. Dietary Intake
2.4. Anthropometric Evaluation
2.5. Laboratory Evaluation
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Inzaghi, E.; Pampanini, V.; Deodati, A.; Cianfarani, S. The Effects of Nutrition on Linear Growth. Nutrients 2022, 14, 1752. [Google Scholar] [CrossRef] [PubMed]
- The Global Burden of Disease Diet Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef] [PubMed]
- Visiolia, F.; Marangonic, F.; Polic, A.; Ghisellid, A.; Martini, D. Nutrition and health or nutrients and health? Int. J. Food Sci. Nutr. 2022, 73, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Chen, O.; Mah, E.; Dioum, E.; Marwaha, A.; Shanmugam, S.; Malleshi, N.; Sudha, V.; Gayathri, R.; Unnikrishnan, R.; Anjana, R.M.; et al. The Role of Oat Nutrients in the Immune System: A Narrative Review. Nutrients 2021, 13, 1048. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Nutrition and immunity: Lessons for COVID-19. Eur. J. Clin. Nutr. 2021, 75, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Verduci, E.; Köglmeier, J. Immunomodulation in Children: The Role of the Diet. J. Pediatr. Gastroenterol. Nutr. 2021, 73, 293–298. [Google Scholar] [CrossRef]
- Childs, C.E.; Calder, P.C.; Miles, E.A. Diet and Immune Function. Nutrients 2019, 11, 1933. [Google Scholar] [CrossRef]
- Katona, P.; Katona-Apte, J. The interaction between nutrition and infection. Clin. Infect. Dis. 2008, 46, 1582–1588. [Google Scholar] [CrossRef]
- Kouhkan, A.; Pourpak, Z.; Moin, M.; Dorosty, A.R.; Safaralizadeh, R.; Teimorian, S.; Farhoudi, A.; Aghamohammadi, A.; Mesdaghi, M.; Kazemnejad, A. A study of malnutrition in Iranian patients with primary antibody deficiency. Iran. J. Allergy Asthma Immunol. 2004, 3, 189–196. [Google Scholar]
- Eggersdorfer, M.; Akobundu, U.; Bailey, R.L.; Shlisky, J.; Beaudreault, A.R.; Bergeron, G.; Blancato, R.B.; Blumberg, J.B.; Bourassa, M.W.; Gomes, F.; et al. Hidden Hunger: Solutions for America’s Aging Populations. Nutrients 2018, 10, 1210. [Google Scholar] [CrossRef]
- Costa, M.I.; Sarmento-Ribeiro, A.B.; Gonçalves, A.C. Zinc: From Biological Functions to Therapeutic Potential. Int. J. Mol. Sci. 2023, 24, 4822. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Jiang, W.; Wang, X.; Shahid, S.; Saba, N.; Ahmad, M.; Dar, A.; Masood, S.U.; Imran, M.; Mustafa, A. Mechanistic Impact of Zinc Deficiency in Human Development. Rev. Front. Nutr. 2022, 9, 717064. [Google Scholar] [CrossRef] [PubMed]
- Salgueiro, M.J.; Zubillaga, M.B.; Lysionek, A.E.; Caro, R.A.; Weill, R.; Boccio, J.R. The Role of Zinc in the Growth and Development of Children. Nutrition 2002, 18, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, P.; Benedetti, G.; Albarède, F.; Miossec, P. Zinc and its role in immunity and inflammation. Autoimmun. Rev. 2015, 14, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Weyh, C.; Krüger, K.; Peeling, P.; Castell, L. The Role of Minerals in the Optimal Functioning of the Immune System. Nutrients 2022, 14, 644. [Google Scholar] [CrossRef]
- Hoffmann, P.R.; Berry, M.J. The influence of selenium on immune responses. Mol. Nutr. Food Res. 2008, 52, 1273–1280. [Google Scholar] [CrossRef]
- Xia, X.; Zhang, X.; Liu, M.; Duan, M.; Zhang, S.; Wei, X.; Liu, X. Toward improved human health: Efficacy of dietary selenium on immunity at the cellular level. Food Funct. 2021, 12, 976–989. [Google Scholar] [CrossRef]
- Barchielli, G.; Capperucci, A.; Tanini, D. The Role of Selenium in Pathologies: An Updated Review. Antioxidants 2022, 11, 251. [Google Scholar] [CrossRef]
- Razaghi, A.; Poorebrahim, M.; Sarhan, D.; Björnstedt, M. Selenium stimulates the antitumour immunity: Insights to future research. Eur. J. Cancer 2021, 155, 256–267. [Google Scholar] [CrossRef]
- Delves, P.J.; Roitt, I.M. The immune system. First of two parts. N. Engl. J. Med. 2000, 343, 37–49. [Google Scholar] [CrossRef]
- Bruzeau, C.; Cook-Moreau, J.; Pinaud, E.; Le Noir, S. Contribution of Immunoglobulin Enhancers to B Cell Nuclear Organization. Front. Immunol. 2022, 13, 877930. [Google Scholar] [CrossRef]
- Odineal, D.D.; Gershwin, M.E. The Epidemiology and Clinical Manifestations of Autoimmunity in Selective IgA Deficiency. Clin. Rev. Allergy Immunol. 2020, 58, 107–133. [Google Scholar] [CrossRef] [PubMed]
- Morawska, I.; Kurkowska, S.; Bębnowska, D.; Hrynkiewicz, R.; Becht, R.; Michalski, A.; Piwowarska-Bilska, H.; Birkenfeld, B.; Załuska-Ogryzek, K.; Grywalska, E.; et al. The Epidemiology and Clinical Presentations of Atopic Diseases in Selective IgA Deficiency. J. Clin. Med. 2021, 10, 3809. [Google Scholar] [CrossRef] [PubMed]
- Tangye, S.G.; Al-Herz, W.; Bousfiha, A.; Cunningham-Rundles, C.; Franco, J.L.; Holland, S.M.; Klein, C.; Morio, T.; Oksenhendler, E.; Picard, C.; et al. Human Inborn Errors of Immunity: 2022 Update on the Classification from the International Union of Immunological Societies Expert Committee. J. Clin. Immunol. 2022, 42, 1473–1507. [Google Scholar] [CrossRef] [PubMed]
- Reust, C.E. Evaluation of primary immunodeficiency disease in children. Am. Fam. Physician 2013, 87, 773–778. [Google Scholar] [PubMed]
- Elsink, K.; van Montfrans, J.M.; van Gijn, M.E.; Blom, M.; van Hagen, P.M.; Kuijpers, T.W.; Frederix, G.W.J. Cost and impact of early diagnosis in primary immunodeficiency disease: A literature review. Clin. Immunol. 2020, 213, 108359. [Google Scholar] [CrossRef] [PubMed]
- Yel, L. Selective IgA Deficiency. J. Clin. Immunol. 2010, 30, 10–16. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Child Growth Standards. 2007. Available online: http://www.who.int/childgrowth/en/index.html (accessed on 15 January 2023).
- WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age. Methods and Development; WHO: Geneva, Switzerland, 2006.
- Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation on Obesity; WHO Technical Report Series n. 894; WHO: Geneva, Switzerland, 2000.
- Lohman, G.L.; Roche, A.F.; Martorell, R. Antropometric Standardization Reference Manual; Human Kinetics Books: Champaign, IL, USA, 1988. [Google Scholar]
- Frisancho, A. Anthropometric Standards for the Assessment of Growth and Nutritional Status; University of Michigan: Ann Arbor, MI, USA, 1990. [Google Scholar]
- Lee, S.W. Methods for testing statistical differences between groups in medical research: Statistical standard and guideline of Life Cycle Committee. Life Cycle 2022, 2, e1. [Google Scholar] [CrossRef]
- Mafra, D.; Maria, S.; Cozzolino, F. The importance of zinc in human nutrition. Rev. Nutr. Campinas 2004, 17, 79–87. [Google Scholar] [CrossRef]
- Millán Adame, E.; Florea, D.; Sáez Pérez, L.; Molina López, J.; López-González, B.; Pérez de la Cruz, A.; Planells del Pozo, E. Deficient selenium status of a healthy adult Spanish population. Nutr. Hosp. 2012, 27, 524–528. [Google Scholar] [CrossRef]
- Ghosh, S.; Kurpad, A.V.; Sachdev, H.S.; Thomas, T. A risk-based approach to measuring population micronutrient status from blood biomarker concentrations. Front. Nutr. 2022, 9, 991707. [Google Scholar] [CrossRef] [PubMed]
- dos Santos-Valente, E.C.; da Silva, R.; de Moraes-Pinto, M.I.; Sarni, R.O.; Costa-Carvalho, B.T. Assessment of nutritional status: Vitamin A and zinc in patients with common variable immunodeficiency. J. Investig. Allergol. Clin. Immunol. 2012, 22, 427–431. [Google Scholar] [PubMed]
- Mariz, C.A.; de Albuquerque, M.F.P.M.; Ximenes, R.A.A.; de Melo, H.R.L.M.; Bandeira, F.; Braga e Oliveira, T.G.; de Carvalho, E.H.; da Silva, A.P.; Miranda Filho, D.B. Body mass index in individuals with HIV infection and factors associated with thinness and overweight/obesity. Cad. Saude Publica 2011, 27. [Google Scholar] [CrossRef] [PubMed]
- Pecora, F.; Persico, F.; Argentiero, A.; Neglia, C.; Esposito, S. The Role of Micronutrients in Support of the Immune Response against Viral Infections. Nutrients 2020, 12, 3198. [Google Scholar] [CrossRef]
- Sadeghsoltani, F.; Mohammadzadeh, I.; Safari, M.M.; Hassanpour, P.; Izadpanah, M.; Qujeq, D.; Moein, S.; Vaghari-Tabari, M. Zinc and Respiratory Viral Infections: Important Trace Element in Anti-viral Response and Immune Regulation. Biol. Trace Elem. Res. 2022, 200, 2556–2571. [Google Scholar] [CrossRef]
- Maywald, M.; Rink, L. Zinc in Human Health and Infectious Diseases. Biomolecules 2022, 12, 1748. [Google Scholar] [CrossRef]
- Vlieg-Boerstra, B.; de Jong, N.; Meyer, R.; Agostoni, C.; De Cosmi, V.; Grimshaw, K.; Milani, G.P.; Muraro, A.; Oude Elberink, H.; Pali-Schöl, I.; et al. Nutrient supplementation for prevention of viral respiratory tract infections in healthy subjects: A systematic review and meta-analysis. Allergy 2022, 77, 1373–1388. [Google Scholar] [CrossRef]
- Hariharan, S.; Dharmaraj, S. Selenium and selenoproteins: It’s role in regulation of inflammation. Inflammopharmacology 2020, 28, 667–695. [Google Scholar] [CrossRef]
- Zemrani, B.; Bines, J.E. Recent insights into trace element deficiencies: Causes, recognition and correction. Curr. Opin. Gastroenterol. 2020, 36, 110–117. [Google Scholar] [CrossRef]
- Al Fify, M.; Nichols, B.; Arailoudi Alexiadou, L.; Stefanowicz, F.; Armstrong, J.; Russell, R.K.; Raudaschl, A.; Pinto, N.; Duncan, A.; Catchpole, A.; et al. Development of age-dependent micronutrient centile charts and their utility in children with chronic gastrointestinal conditions at risk of deficiencies: A proof-of-concept study. Clin. Nutr. 2022, 41, 931–936. [Google Scholar] [CrossRef]
- de Bortoli, M.C.; Cozzolino, S.M.F. Zinc and selenium nutritional status in vegetarians. Biol. Trace Elem. Res. 2009, 127, 228–233. [Google Scholar] [CrossRef] [PubMed]
| Variables | n (%) |
|---|---|
| Sex | |
| Male | 9 (56) |
| Female | 7 (44) |
| Age Distribution | |
| Children | 4 (25) |
| Adolescent | 8 (50) |
| Adult | 4 (25) |
| Outpatient infections in the last 12 months | |
| Acute otitis media | 3 (19) |
| Sinusitis | 8 (50) |
| Diarrhea | 5 (31) |
| Pneumonia | 2 (12) |
| Other | 7 (44) |
| 4–10 Years Mean ± SD | 11–19 Years Mean ± SD | 20–25 Years Mean ± SD | |
|---|---|---|---|
| Energy (kcal/day) | 1533.3 ± 338.6 | 1852.5 ± 865.1 | 2304.1 ± 379.9 |
| DRI (kcal/day) | 1759.5 ± 171.5 | 2272.6 ± 588.5 | 3193.87 ± 634.3 |
| Protein (g/day) | 65.4 ± 20.5 | 74.8 ± 21.9 | 100.11 ± 22.6 |
| DRI (g/day) | 20.7 ± 4.6 | 37.9 ± 11.4 | 58.2 ± 8.3 |
| Protein (%) | 16.8 ± 1.9 | 16.6 ± 4.7 | 16.6 ± 1.9 |
| DRI (%) | 10–35 | 10–35 | 10–35 |
| Carbohydrates (%) | 57.2 ± 4.9 | 57.6 ± 5.9 | 61.3 ± 7.2 |
| DRI (%) | 45–65 | 45–65 | 45–65 |
| Lipids (%) | 26.9 ± 3.1 | 26.0 ± 1.2 | 22.5 ± 5.6 |
| DRI (%) | 25–35 | 25–35 | 20–35 |
| Zn (mg/day) | 8.2 ± 2.7 | 9.8 ± 3.2 | 11.3 ± 2.6 |
| DRI (mg/day) | 4–7 (EAR) | 7–8.5 (EAR) | 6.8–9.4 (EAR) |
| Se (µg/day) | 64.5 ± 26.1 | 69.7 ± 21.4 | 96.7 ± 14.4 |
| DRI (µg/day) | 23–35 (EAR) | 35–45 (EAR) | 45 (EAR) |
| Groups | |||
|---|---|---|---|
| Nutritional Status | Children * n (%) | Adolescents * n (%) | Adults ** n (%) |
| Low weight | 1 (25) | - | - |
| Eutrophy | 3 (75) | 5 (62) | - |
| Overweight | - | 2 (25) | 2 (50) |
| Obesity | - | 1 (12) | 2 (50) |
| Zn | Children | Adolescents | Adults | |||
|---|---|---|---|---|---|---|
| Plasma | Erythrocytes | Plasma | Erythrocytes | Plasma | Erythrocytes | |
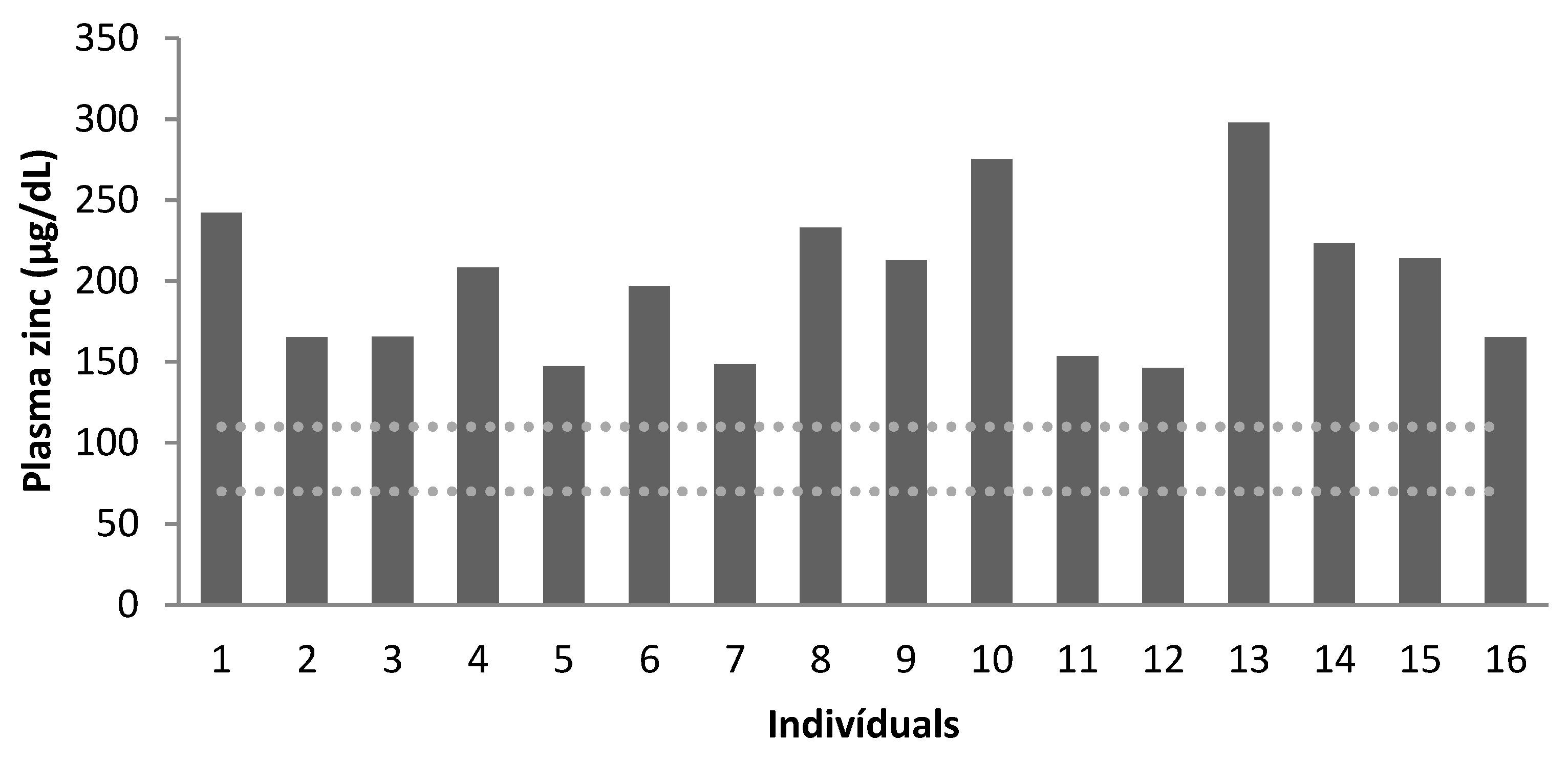
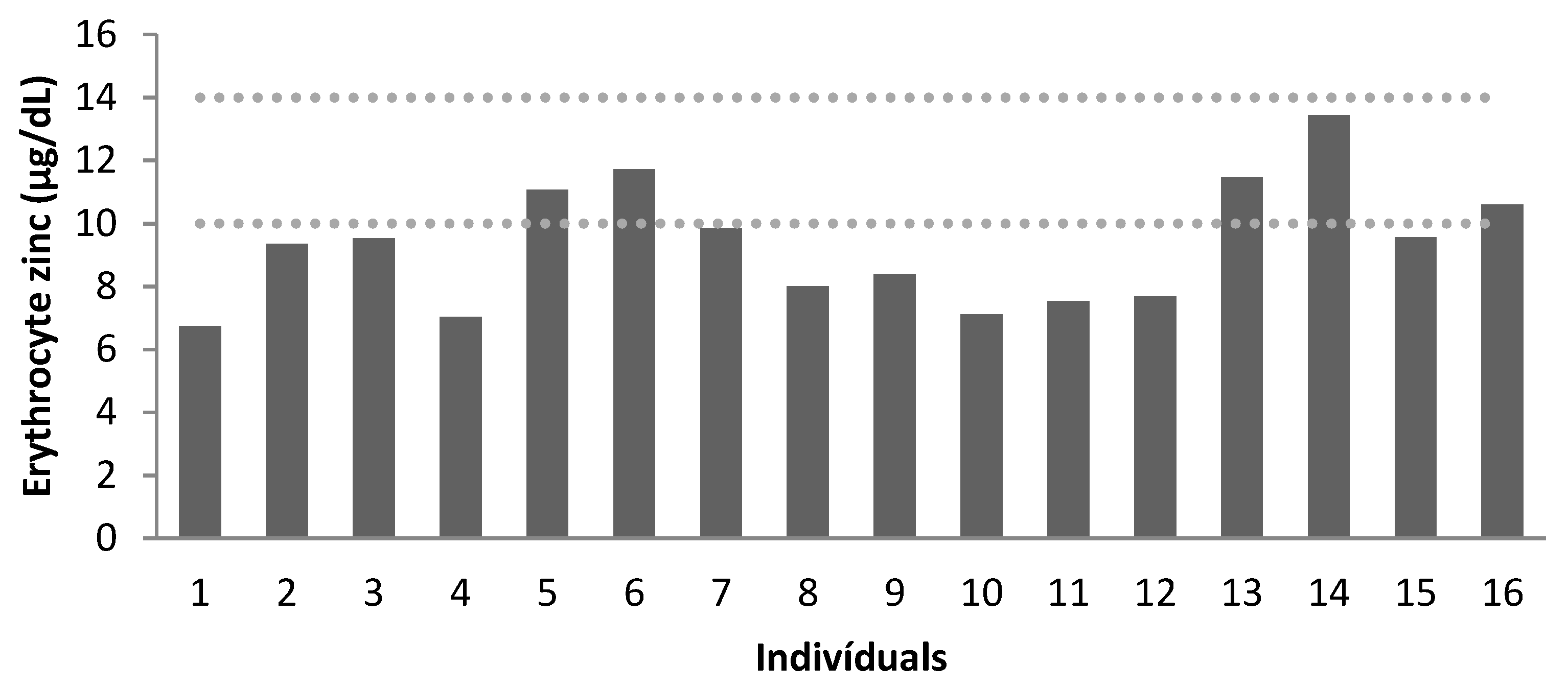
| Recommendation * | 70–110 | 10–14 | 70–110 | 10–14 | 70–110 | 10–14 |
| Mean | 195 | 8 | 189 | 9 | 225 | 11 |
| Below Normal | 0 | 100% | 0 | 75% | 0 | 25% |
| Normal | 100% | 0 | 100% | 25% | 100% | 75% |
| Se | Children | Adolescents | Adults | |||
|---|---|---|---|---|---|---|
| Plasma | Erythrocytes | Plasma | Erythrocytes | Plasma | Erythrocytes | |
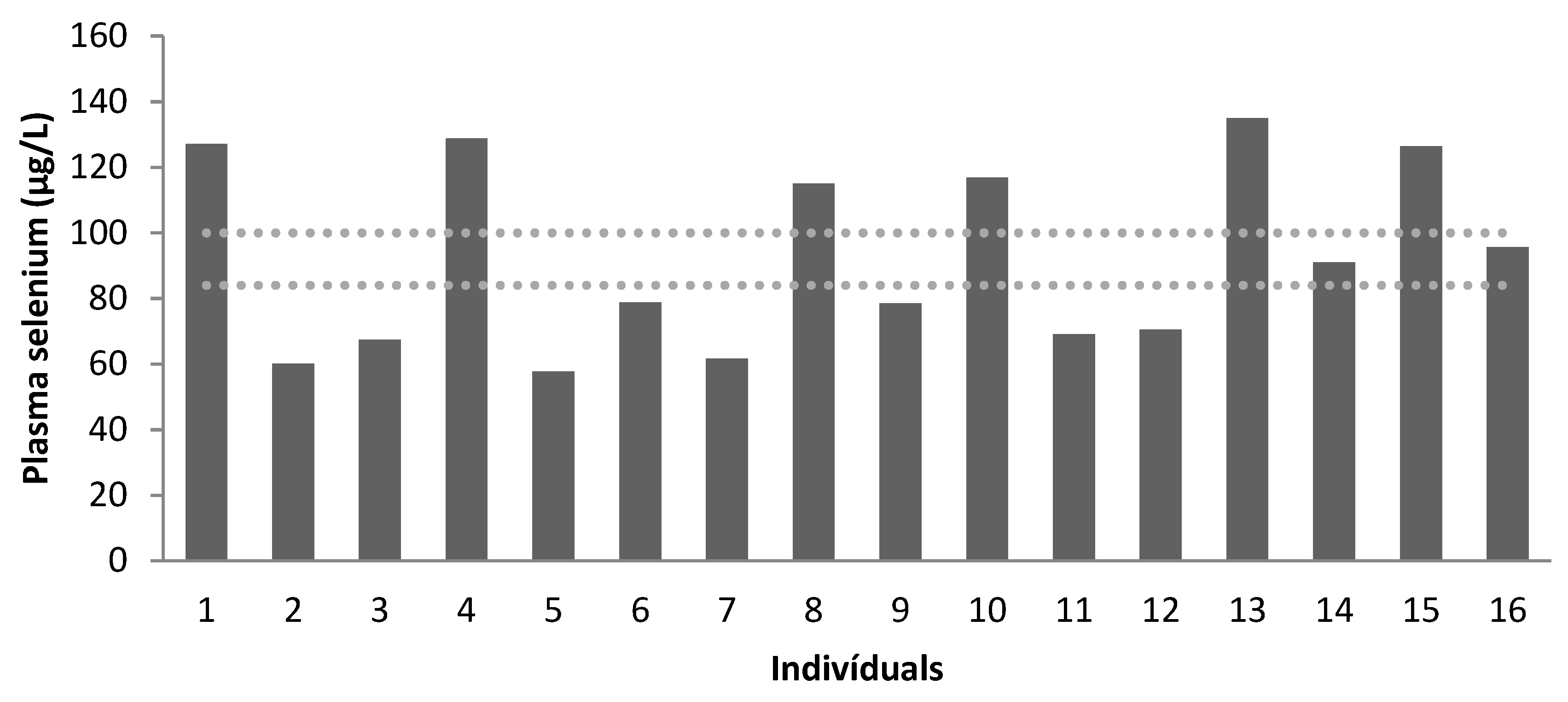
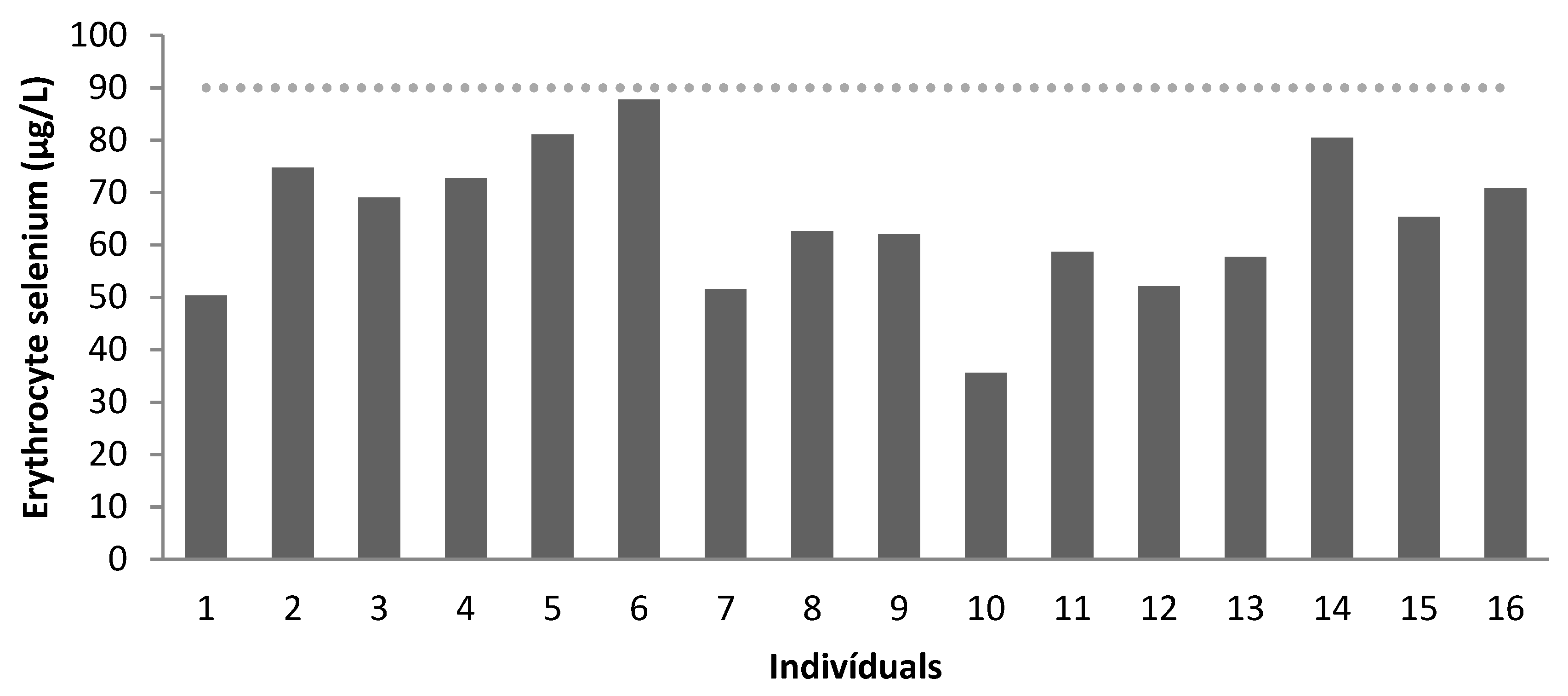
| Recommendation * | 84–100 | >90 | 84–100 | >90 | 84–100 | >90 |
| Mean | 95.86 | 66.70 | 81.03 | 61.43 | 111.96 | 68.57 |
| Below Normal | 50% | 100% | 75% | 100% | 0 | 100% |
| Normal | 50% | 0 | 25% | 0% | 100% | 0% |
| Children | Adolescents | Adults | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Hemoglobin (g/dL) | 13.28 | 0.83 | 12.94 | 0.52 | 16.23 | 2.09 |
| Hematocrit (%) | 40.50 | 2.65 | 38.88 | 1.36 | 48.75 | 6.50 |
| MCV (µ/mm3) | 79.75 | 10.53 | 87.75 | 6.09 | 90.75 | 5.68 |
| MCH (pg) | 26.28 | 3.59 | 29.15 | 1.93 | 30.40 | 1.79 |
| Leukocytes (cells × 103/mm3) | 8.42 | 4.36 | 7.72 | 2.62 | 7.82 | 0.95 |
| Total lymphocytes (cells × 103/mm3) | 2.35 | 0.97 | 2.54 | 0.78 | 2.14 | 0.8.5 |
| Total proteins (g/dL) | 7.35 | 0.29 | 7.55 | 0.41 | 7.16 | 0.73 |
| Albumin (g/dL) | 4.27 | 0.44 | 4.21 | 0.40 | 4.13 | 0.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu Jamra, S.R.; Komatsu, C.G.; Barbosa, F., Jr.; Roxo-Junior, P.; Navarro, A.M. Proposal to Screen for Zinc and Selenium in Patients with IgA Deficiency. Nutrients 2023, 15, 2145. https://doi.org/10.3390/nu15092145
Abu Jamra SR, Komatsu CG, Barbosa F Jr., Roxo-Junior P, Navarro AM. Proposal to Screen for Zinc and Selenium in Patients with IgA Deficiency. Nutrients. 2023; 15(9):2145. https://doi.org/10.3390/nu15092145
Chicago/Turabian StyleAbu Jamra, Soraya Regina, Camila Gomes Komatsu, Fernando Barbosa, Jr., Persio Roxo-Junior, and Anderson Marliere Navarro. 2023. "Proposal to Screen for Zinc and Selenium in Patients with IgA Deficiency" Nutrients 15, no. 9: 2145. https://doi.org/10.3390/nu15092145
APA StyleAbu Jamra, S. R., Komatsu, C. G., Barbosa, F., Jr., Roxo-Junior, P., & Navarro, A. M. (2023). Proposal to Screen for Zinc and Selenium in Patients with IgA Deficiency. Nutrients, 15(9), 2145. https://doi.org/10.3390/nu15092145







