Phase Angle as a Prognostic Indicator of Survival in Institutionalized Psychogeriatric Patients
Highlights
- Phase angle (PhA) has been demonstrated to be a reliable clinical indicator of survival in psychogeriatric patients regardless of age, the presence of dementia, and BMI.
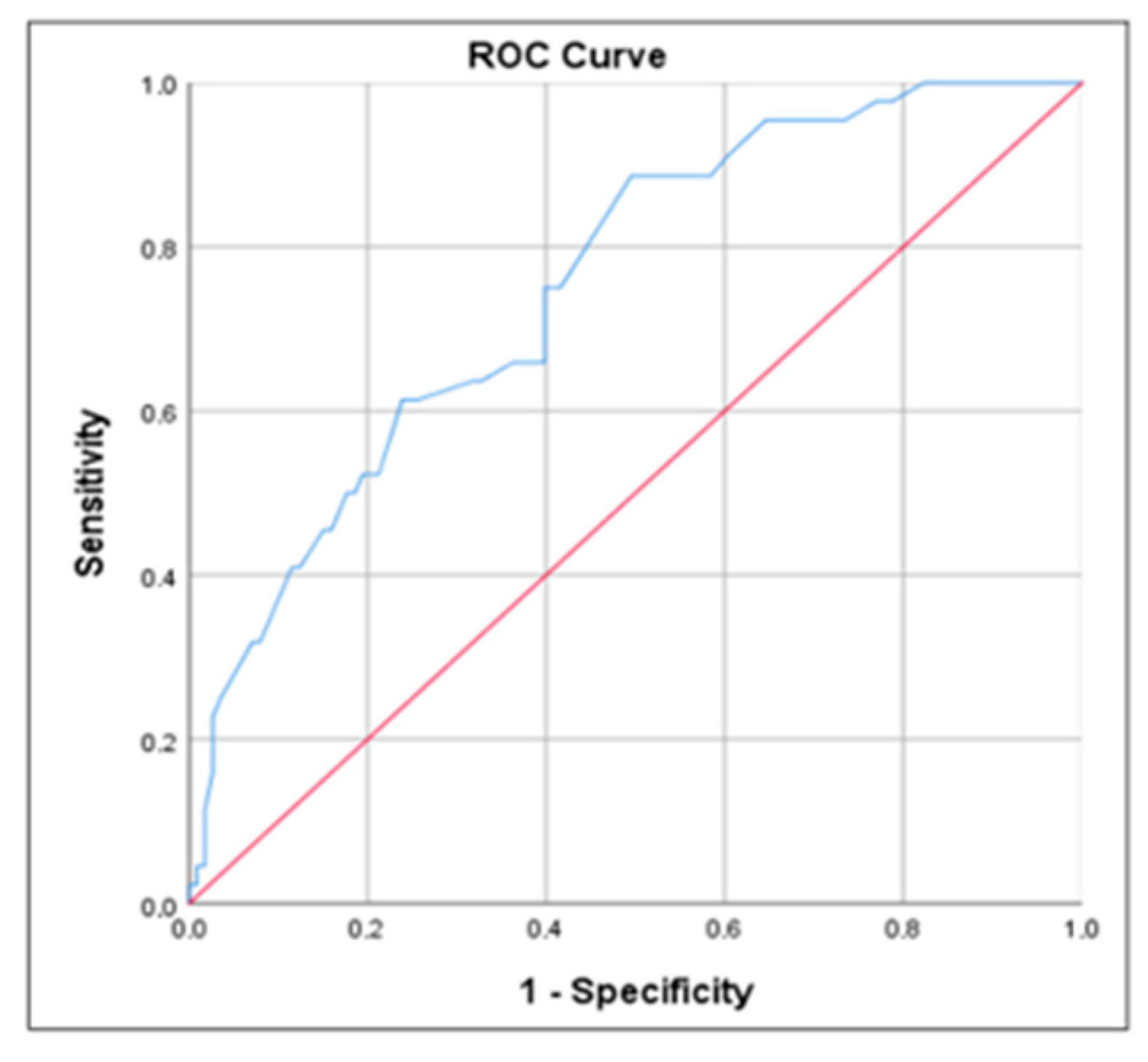
- The outcomes of this research indicated that the Z-PhA cutoff point as a survival indicator in institutionalized psychogeriatric patients was −0.81 (sensitivity: 0.75; specificity: 0.60).
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Participants’ Characteristics
3.2. Nutritional Status and Body Composition
3.3. Phase Angle, Functional and Nutritional Status, and Mortality Risk
4. Discussion
Limitations of This Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- García Peris, P. Prevalencia y factores asociados a malnutrición en ancianos hospitalizados. Ann. Med. Interna. 2004, 21, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Sieber, C.C. Malnutrition and sarcopenia. Aging Clin. Exp. Res. 2019, 31, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Cabello, A.; Vicente Rodríguez, G.; Vila-Maldonado, S.; Casajús, J.A.; Ara, I. Envejecimiento y composición corporal: La obesidad sarcopénica en España. Nutr. Hosp. 2012, 27, 22–30. [Google Scholar]
- Vega, M.M.; García Almeida, J.M.; Vegas, I.; Muñoz-Garach, A.; Gómez, A.M.; Cornejo, I.; Díaz, C.; Bellido, D. Revisión sobre los fundamentos teórico-práctico del ángulo de fase y su valor pronóstico en la práctica clínica. Nutr. Clin. Med. 2017, 11, 129–148. [Google Scholar]
- Ravasco, P.; Anderson, H.; Mardones, F. Métodos de valoración del estado nutricional. Nutr. Hosp. 2010, 25, 57–66. [Google Scholar]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.C.; Pirlich, M.; et al. The ESPEN Woring Group. Bioelectrical impedance analysis, part II: Utilization in clinical practice. Clin. Nutr. 2004, 23, 1430–1453. [Google Scholar] [CrossRef] [PubMed]
- Day, K.; Kwok, A.; Evans, A.; Mata, F.; Verdejo-García, A.; Hart, K.; Ward, L.C.; Truby, H. Comparison of a Bioelectrical Impedance Device against the reference method Dual Energy X Ray Absorptiometry and Anthropometry for evaluation of bod composition in adults. Nutrients 2018, 10, 1469. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ng, B.K.; Liu, Y.E.; Wang, W.; Kelly, T.L.; Wilson, K.E.; Schoeller, D.A.; Heymsfield, S.B.; Shepherd, J.A. Validation of rapid 4-component body composition assessment with the use of dual energy X Ray absorptiometry and bioelectrical impedance analysis. Am. J. Clin. Nutr. 2018, 104, 708–715. [Google Scholar] [CrossRef] [PubMed][Green Version]
- García Almeida, J.M.; García García, C.; Bellido Castañeda, V.; Bellido Guerrero, D. Nuevo enfoque de la Nutrición. Valoración del estado nutricional del paciente: Función y composición corporal. Nutr. Hosp. 2018, 35, 1–14. [Google Scholar] [CrossRef]
- Costa Moreira, O.; Alonso-Aubin, D.; Patrocinio de Oliveira, C.; Candia-Luján, R. Métodos de evaluación de la composición corporal: Una revisión actualizada de descripción, aplicación, ventajas y desventajas. Arch. Med. Deporte 2015, 32, 387–394. [Google Scholar]
- Reis de Lima, R.; Porto Sabino Pinho, C.; Galvao Rodrigues, I.; Moura Monteiro, J.G. Ángulo de fase como indicador del estado nutricional y pronóstico en pacientes críticos. Nutr. Hosp. 2015, 31, 1278–1285. [Google Scholar]
- Lukaski, H.C. Evolution of bioimpedance: A circuitous journey from estimation of physiological function to assessment of body composition and a return to clinical research. Eur. J. Clin. Nutr. 2013, 67, S2–S9. [Google Scholar] [CrossRef][Green Version]
- Norman, K.; Smoliner, C.; Valentini, L.; Lochs, H.; Pirlich, M. Is bioelectrical vector analysis of value in the elderly with malnutrition and impaired functionality? Nutrition 2007, 23, 564–569. [Google Scholar] [CrossRef]
- Buffa, R.; Floris, G.; Marini, E. Assessment of nutritional status in free-living elderly individuals by bioelectrical impedance vector analysis. Nutrition 2009, 25, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Norman, K.; Stobäus, N.; Pirlich, M.; Bosy-Westphal, A. Bioelectrical phase angle and impedance vector analysis. Clinical relevance and applicability of impedance parameters. Clin. Nutr. 2012, 31, 854–861. [Google Scholar] [CrossRef]
- Buffa, R.; Mereu, E.; Comandini, O.; Ibañez, M.E.; Marini, E. Bioelectrical impedance vector analysis (BIVA) for the assessment of two-compartment body composition. Eur. J. Clin. Nutr. 2014, 68, 1234–1240. [Google Scholar] [CrossRef][Green Version]
- Wilhelm-Leen, E.R.; Hall, Y.N.; Horwitz, R.I.; Chertow, G.M. Phase angle, frailty and mortality in older adults. J. Gen. Intern. Med. 2014, 29, 147–154. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bellido Castañeda, V. Relación del Ángulo de Fase Determinado por Bioimpedanciometría con Factor de Riesgo Cardiovascular, Adipocitoquinas, Antropometría e Ingesta Dietética de Pacientes Obesos. Ph.D. Thesis, Departamento de Ciencias de la Salud, Facultad de Enfermería y Podología, Universidade Da Coruña, Coruña, Spain, 2014. [Google Scholar]
- Abad, S.; Sotomayor, G.; Vega, A.; Pérez de José, A.; Verdalles Guzmán, V.; Jofré, R.; López Gómez, J.M. El ángulo de fase de la impedanciometría eléctrica es un predictor de supervivencia a largo plazo en pacientes en diálisis. Nefrología 2011, 31, 670–676. [Google Scholar] [PubMed]
- Marinho Esteves Pereira, M.; dos Santos Campello Queiroz, M.; Masiero Cavalcanti de Albuquerque, N.; Rodrigues, J.; Varea Maria Wiegert, E.; Calixto Lima, L.; Costa de Oliveira, L. Phase angle and nutritional status in individuals with advanced cancer in paliative. Nutr. Clin. Prac. 2018, 33, 813–824. [Google Scholar]
- Saragat, B.; Buffa, R.; Mereu, E.; Succa, V.; Cabras, S.; Mereu, R.M.; Succa, V.; Cabras, S.; Mereu, R.M.; Viale, D.; et al. Nutritional and psycho-functional status in elderly patients with Alzheimer disease. J. Nutr. Health Aging 2012, 16, 231–236. [Google Scholar] [CrossRef]
- de Sousa, O.V.; Mendes, J.; Amaral, T.F. Nutritional and functional indicators and their association with mortality among older adults with Alzheimer’s Disease. Am. J. Alzheimers Dis. Other Demen. 2020, 35, 1533317520907168. [Google Scholar] [CrossRef]
- American Psychiatric Association (APA). Manual Diagnóstico y Estadístico de los Trastornos Mentales, 5th ed.; (DSM-5); Panamericana: Madrid, Spain, 2014. [Google Scholar]
- World Health Organization. CIE 10: Clasificación Internacional de Enfermedades; 10th revision; Modificación Clínica; Ministerio de Sanidad, Servicios Sociales e Igualdad: Madrid, Spain, 2018.
- Reisberg, B. Functional assessment staging (FAST). Psychopharmacol. Bull. 1988, 24, 653–659. [Google Scholar]
- Gómez, B.X.; Martínez, M. Identificación de personas con enfermedades crónicas avanzadas y necesidad de cuidados paliativos en servicios sociosanitarios: Elaboración de NECPAL CCOMS-ICO herramienta. Med. Clin. 2013, 140, 241–245. [Google Scholar] [CrossRef]
- Morley, J.E.; Vellas, B.; Van Kan, G.A.; Anker, S.D.; Bauer, J.M.; Bernabé, R.; Cesari, M.; Chumlea, W.C.; Doehner, W.; Evans, J.; et al. Frailty consensus: A call to action. J. Am. Med. Dir. Asoc. 2013, 14, 392–397. [Google Scholar] [CrossRef][Green Version]
- Baztán, J.J.; Pérez del Molino, J.; Alarcón, T.; San Cristóbal, E.; Izquierdo, G.; Manzarbeitia, J. Índice de Barthel: Instrumento válido para la valoración funcional de pacientes con enfermedad cerebrovascular. Rev. Esp. Geriatr. Gerontol. 1993, 28, 32–40. [Google Scholar]
- Guigoz, Y.; Vellas, B.; Garry, P.J. Mini Nutritional Assessment: A practical assessment tool for grading the nutritional state of elderly patients. Facts Res. Gerontol. 1994, 12, 15–59. [Google Scholar]
- Canaslan, K.; Bulut, E.A.; Kocyigit, S.E.; Aydin, A.E.; Isik, A.T. Predictivity of the comorbidity indices for geriatric syndromes. BCM Geriatr. 2022, 22, 440. [Google Scholar] [CrossRef]
- NHANES; CDC. Anthropometry Procedures Manual; CDC: Atlanta, GA, USA, 2007.
- WHO. Report of a WHO Expert Committee. Physical Status: The Use and Interpretation of Anthropometry; WHO Technical Report Series; WHO: Geneva, Switzerland, 1995. [Google Scholar]
- Wanden-Berghe, C. Valoración antropométrica. In Valoración Nutricional en el Anciano. Recomendaciones Prácticas de los Expertos en Geriatría y Nutrición. (SENPE y SEGG); Planas, M., Ed.; Galénitas-Nigra Trea: Madrid, Spain, 2006; pp. 77–96. [Google Scholar]
- WHO. Clasificación Internacional Para Adultos de Bajo Peso, Sobrepeso y Obesidad en Relación al IMC; Criterios de la OMS; WHO: Geneva, Switzerland, 2006. [Google Scholar]
- WHO. Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ. Tech. Rep. Ser. 2000, 894, 1–253. [Google Scholar]
- Lukaski, H.C.; Bolonchuk, W.W.; Hall, C.B.; Siders, W.A. Validation of tetrapolar bioelectrical impedance method to assess human body composition. J. Appl. Physiol. 1986, 60, 1327–1332. [Google Scholar] [CrossRef][Green Version]
- Kyle, U.G.; Genton, L.; Slosman, D.O.; Pichard, C. Fat-free and fat mass percentiles in 5225 healthy subjects aged 15 to 98 years. Nutrition 2001, 17, 534–541. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Baumgartner, R.N.; Ross, R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J. Appl. Physiol. 2000, 89, 465–471. [Google Scholar] [CrossRef][Green Version]
- Camina MartÍn, M.A.; Barrera Ortega, S.; Domínguez Rodríguez, L.; Couceiro Muiño, C.; Mateo Silleras, B.; Redondo del Río, M.P. Presencia de malnutrición y riesgo de malnutrición en ancianos institucionalizados con demencia en función del tipo y estadio evolutivo. Nutr. Hosp. 2012, 27, 424–430. [Google Scholar]
- Dos Santos, T.B.N.; Fonseca, L.C.; Tedrus, G.M.A.S.; Delbue, J.L. Alzheimer’s disease: Nutritional status and cognitive aspects associated with disease severity. Nutr. Hosp. 2018, 35, 1298–1304. [Google Scholar] [CrossRef][Green Version]
- Buffa, R.; Mereu, E.; Putzu, P.; Mereu, R.M.; Marini, E. Lower lean mass and higher percent fat mass in patients with Alzheimer’s disease. Exp. Gerontol. 2014, 58, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Mereu, E.; Succa, V.; Buffa, R.; Sanna, C.; Mereu, R.M.; Catte, O.; Marini, E. Total body and arm bioimpedance in patients with Alzheimer’s disease. Exp. Gerontol. 2018, 102, 145–148. [Google Scholar] [CrossRef]
- Al-Sofiani, M.E.; Ganji, S.S.; Kalyani, R.R. Body composition changes in diabetes and aging. J. Diabetes Complicat. 2019, 33, 451–459. [Google Scholar] [CrossRef]
- Newberry, C.; Dakin, G. Nutrition and weight management in the elderly. Clin. Geriatr. Med. 2021, 37, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Mazereel, V.; Detraux, J.; Vancampfort, D.; van Winkel, R.; De Hert, M. Impact of psychotropic medication effects on obesity and the metabolic syndrome in people with serious mental illness. Front. Endocrinol. 2020, 11, 573479. [Google Scholar] [CrossRef] [PubMed]
- Camina Martín, M.A.; de Mateo Silleras, B.; Nescolarde Selva, L.; Barrera Ortega, S.; Domínguez Rodríguez, L.; Redondo Del Río, M.P. Bioimpedance vector analysis and conventional bioimpedance to assess body composition in older adults with dementia. Nutrition 2015, 31, 155–159. [Google Scholar] [CrossRef][Green Version]
- De Souto Barreto, P.; Andrade, L.P.; Cadroy, Y.; Kelaiditi, E.; Vellas, B.; Rolland, Y. The prognostic value of body-mass index on mortality in older adults with dementia living in nursing homes. Clin. Nutr. 2017, 36, 423–428. [Google Scholar] [CrossRef]
- Barbosa-Silva, M.C.G.; Barros, A.J.D.; Wang, J.; Heymsfield, S.B.; Pierson, R.N. Bioelectrical impedance analysis: Population reference values for phase angle by age and sex. Am. J. Clin. Nutr. 2005, 82, 49–52. [Google Scholar] [CrossRef]
- Llames, L.; Baldomero, V.; Iglesias, H.L.; Rodota, L.P. Valores del ángulo de fase por bioimpedanciometría eléctrica; estado nutricional y valor pronóstico. Nutr. Hosp. 2013, 28, 286–295. [Google Scholar]
- Mattiello, R.; Azambuja Amaral, M.; Mundstock, E.; Klarmann Ziegelmann, P. Reference values for the phase angle of the electrical bioimpedance: Systematic review and meta-analysis involving more than 250,000 subjects. Clin. Nutr. 2020, 39, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
- Bosy-Westphal, A.; Danielzik, S.; Dörhöfer, R.P.; Later, W.; Wiese, S.; Müller, M.J. Phase angle from bioelectrical impedance analysis: Population reference values by age, sex, and body mass index. JPEN 2006, 30, 309–316. [Google Scholar] [CrossRef]
- Dittmar, M. Reliability and variability of bioimpedance measures in normal adults: Effects of age, gender, and body mass. Am. J. Phis. Anthrop. 2003, 122, 361–370. [Google Scholar] [CrossRef]
- Buffa, R.; Floris, G.; Marini, E. Migration of the bioelectrical impedance vector in healthy elderly subjets. Nutrition 2003, 19, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Marino, L.V.; Ramos, L.F.; Chiarello, P.G. Nutritional status according to the stages of Alzheimer’s disease. Aging Clin. Exp. Res. 2015, 27, 507–513. [Google Scholar] [CrossRef]
- Genton, L.; Norman, K.; Spoerri, A.; Pichard, C.; Karsegard, V.L.; Herrmann, F.R.; Graf, C.E. Bioimpedance-derived phase angle and mortality among older people. Rejuvenation Res. 2017, 20, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Norman, K.; Herpich, C.; Müller-Werdan, U. Role of phase angle in older adults with focus on the geriatric syndromes sarcopenia and frailty. Rev. Endocr. Metab. Disord. 2022, 2, 1–9. [Google Scholar] [CrossRef]
- Garlini, L.M.; Alves, F.D.; Ceretta, L.B.; Perry, I.S.; Souza, G.C.; Clausell, N.O. Phase angle and mortality: A systematic review. Eur. J. Clin. Nutr. 2019, 73, 495–508. [Google Scholar] [CrossRef]
- Kwon, Y.E.; Lee, J.S.; Kim, J.Y.; Baeg, S.I.; Choi, H.M.; Kim, H.B.; Yang, J.Y.; Oh, D.J. Impact of sarcopenia and phase angle on mortality of the very elderly. J. Cachexia Sarcopenia Muscle 2023, 14, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Genton, L.; Herrmann, F.R.; Spörri, A.; Graf, C.E. Association of mortality and phase angle measured by different bioelectrical impedance analysis (BIA) devices. Clin. Nutr. 2018, 37, 1066–1069. [Google Scholar] [CrossRef] [PubMed]
- Zekry, D.; Herrmann, F.R.; Grandjean, R.; Meinet, M.P.; Michel, J.P.; Gold, G.; Krause, K.H. Demented versus non demented very old impatients: The same comorbilities but poorer functional and nutritional status. Age Ageing 2008, 37, 83–89. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Variables | All | Dementia | Schizophrenia | OPP | |
|---|---|---|---|---|---|
| n (%) | 157 (100) | 73 (46.5) | 69 (43.9) | 15 (9.6) | |
| Age (y.) (mean (SD)) | 76.9 (8.9) | 80.3 (9.5) # | 74.3 (7.0) | 72.5 (8.2) | |
| Length of stay (y.) (mean (SD)) | 21.5 (19.8) | 10.8 (12.9) | 34 (19.6) * | 16.2 (15.6) | |
| FRAIL test (n (%)) | Frailty | 70 (44.6) | 52 (71.2) # | 15 (21.7) | 3 (20.0) |
| Risk of frailty | 64 (40.8) | 19 (26.0) | 40 (58.0) * | 5 (33.3) | |
| Non frailty | 23 (14.6) | 2 (2.7) | 14 (20.3) | 7 (46.7) | |
| Barthel test (n (%)) | Mild dependency | 85 (54.1) | 15 (20.5) | 59 (85.5) * | 11 (73.3) |
| Moderate dependency | 50 (31.8) | 40 (54.8) # | 7 (10.1) | 3 (20.0) | |
| Severe dependency | 7 (4.5) | 4 (5.5) | 2 (2.9) | 1 (6.7) | |
| Total dependency | 15 (9.6) | 14 (19.2) # | 1 (1.4) | 0 (0) | |
| High comorbidity (CCI) (n (%)) | 96 (61.1) | 65 (89.0) # | 29 (42.0) | 2 (13.3) | |
| Polypharmacy (n (%)) | 142 (90.4) | 71 (97.3) & | 58 (84.1) | 13 (86.7) | |
| Indicator | Variable/Category | Psychiatric Pathology N (%) If No Other Is Indicated | ||
|---|---|---|---|---|
| Dementia | Schizophrenia | OPP | ||
| MNA | MNA (points) (mean (SD)) | 17.8 (3.3) * | 21.6 (3.7) | 20.0 (4.4) |
| MN | 22 (30.1) | 3 (4.3) | 1 (6.7) | |
| Risk of MN | 42 (57.5) | 11 (15.9) | 5 (33.3) | |
| Normal nutritional status | 9 (12.3) | 55 (79.9) | 9 (60.0) | |
| BMI | BMI (kg/m2) (mean (SD)) | 23.4 (4.2) * | 25.2 (3.8) | 26.5 (3.8) |
| MN (BMI < 18.5 kg/m2) | 9 (12.3) | 1 (1.4) | 0 (0) | |
| Risk of MN (BMI: 18.5–21.9 kg/m2) | 16 (21.9) | 15 (21.7) | 2 (13.3) | |
| Normal (BMI: 22–26.9 kg/m2) | 37 (50.7) | 32 (46.4) | 6 (40.0) | |
| Overweight (BMI: 27–29.9 kg/m2) | 1 (1.4) | 14 (20.3) | 3 (20.0) | |
| Obesity (BMI: 30 kg/m2) | 10 (13.7) | 7 (10.1) | 4 (26.7) | |
| Waist circumference | Risk of metabolic complications | 24 (33.8) | 21 (31.8) | 6 (40.0) |
| No risk | 47 (66.2) | 45 (68.2) | 9 (60.0) | |
| Body Composition Variables (Mean (SD)) | All Subjects | Groups | ||
|---|---|---|---|---|
| Dementia | Schizophrenia | OPP | ||
| Z-FFMI | −0.95 (1.2) | −1.11 (1.1) | −0.87 (1.2) | −0.56 (1.3) |
| Z-FMI | 0.39 (1.2) | 0.03 (1.1) * | 0.64 (1.1) | 0.99 (1.4) |
| Z-SMMI | −0.87 (0.97) | −0.90 (0.82) | −0.90 (1.1) | −0.58 (1.2) |
| Variables | Exitus | ||
|---|---|---|---|
| No (n = 44) | Yes (n = 113) | ||
| Age (years) (mean (SD)) | 71.5 (6.6) | 79.1 (8.8) * | |
| BMI (kg/m2) (mean (SD)) | 26.1 (3.6) | 23.9 (4.1) * | |
| R (Ohm) (mean (SD)) | 550.3 (86.5) | 587.0 (90.5) * | |
| Xc (Ohm) (mean (SD)) | 48.2 (8.9) | 41.2 (8.7) * | |
| Z-PhA (mean (SD)) | −0.24 (0.79) | −1.03 (0.84) * | |
| MNA (points) (mean (SD)) | 20.9 (4.0) | 17.8 (3.3) * | |
| Nutritional status (n (%)) | Undernutrition | 3 (11.5) | 23 (88.5) # |
| Malnutrition risk | 8 (13.8) | 50 (86.2) # | |
| Normal | 33 (45.2) | 40 (54.8) | |
| Frailty (n (%)) | Frailty | 11 (15.7) | 59 (84.3) # |
| Frailty risk | 20 (31.3) | 44 (68.8) # | |
| Non frailty | 13 (56.5) | 10 (43.5) | |
| Dependency (n (%)) | Total | 0 (0) | 15 (100) # |
| Severe | 0 (0) | 7 (100) # | |
| Moderade | 6 (12.0) | 44 (88) # | |
| Mild | 38 (44.7) | 47 (55.3) | |
| Variable | HR (Survival) | 95% CI | p | |
|---|---|---|---|---|
| Z-Phase angle | 0.47 | 0.36–0.60 | <0.001 | |
| Age | 1.07 | 1.04–1.09 | <0.001 | |
| Pathology | Dementia | 1.00 | <0.001 | |
| Schizophrenia | 0.32 | 0.21–0.47 | <0.001 | |
| Others | 0.30 | 0.15–0.64 | 0.002 | |
| FRAIL | Normal | 1.00 | <0.001 | |
| Fragility risk | 2.29 | 1.12–4.71 | 0.024 | |
| Fragility | 4.69 | 2.31–9.54 | <0.001 | |
| Barthel | Low dependence | 1.00 | <0.001 | |
| Moderate dependence | 2.92 | 1.92–4.44 | <0.001 | |
| High dependence | 14.67 | 6.19–34.75 | <0.001 | |
| Total dependence | 5.25 | 2.90–9.51 | <0.001 | |
| BMI | 0.90 | 0.85–0.95 | <0.001 | |
| MNA | 0.91 | 0.84–0.97 | 0.006 | |
| Variable | HR (Survival) | 95% CI | p | |
|---|---|---|---|---|
| Z-Phase angle (≤−0.81 vs. >−0.81) | 1.90 | 1.27–2.85 | 0.002 | |
| Age | 1.04 | 1.01–1.06 | 0.005 | |
| Pathology | Dementia | 1.00 | 0.001 | |
| Schizophrenia | 0.45 | 0.29–0.70 | <0.001 | |
| Others | 0.48 | 0.22–1.03 | 0.061 | |
| BMI | 0.94 | 0.89–0.99 | 0.011 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrera Ortega, S.; Redondo del Río, P.; Carreño Enciso, L.; de la Cruz Marcos, S.; Massia, M.N.; de Mateo Silleras, B. Phase Angle as a Prognostic Indicator of Survival in Institutionalized Psychogeriatric Patients. Nutrients 2023, 15, 2139. https://doi.org/10.3390/nu15092139
Barrera Ortega S, Redondo del Río P, Carreño Enciso L, de la Cruz Marcos S, Massia MN, de Mateo Silleras B. Phase Angle as a Prognostic Indicator of Survival in Institutionalized Psychogeriatric Patients. Nutrients. 2023; 15(9):2139. https://doi.org/10.3390/nu15092139
Chicago/Turabian StyleBarrera Ortega, Sara, Paz Redondo del Río, Laura Carreño Enciso, Sandra de la Cruz Marcos, María Noel Massia, and Beatriz de Mateo Silleras. 2023. "Phase Angle as a Prognostic Indicator of Survival in Institutionalized Psychogeriatric Patients" Nutrients 15, no. 9: 2139. https://doi.org/10.3390/nu15092139
APA StyleBarrera Ortega, S., Redondo del Río, P., Carreño Enciso, L., de la Cruz Marcos, S., Massia, M. N., & de Mateo Silleras, B. (2023). Phase Angle as a Prognostic Indicator of Survival in Institutionalized Psychogeriatric Patients. Nutrients, 15(9), 2139. https://doi.org/10.3390/nu15092139






