Lysophosphatidylcholine-DHA Specifically Induces Cytotoxic Effects of the MDA-MB-231 Human Breast Cancer Cell Line In Vitro—Comparative Effects with Other Lipids Containing DHA
Abstract
1. Introduction
2. Materials and Methods
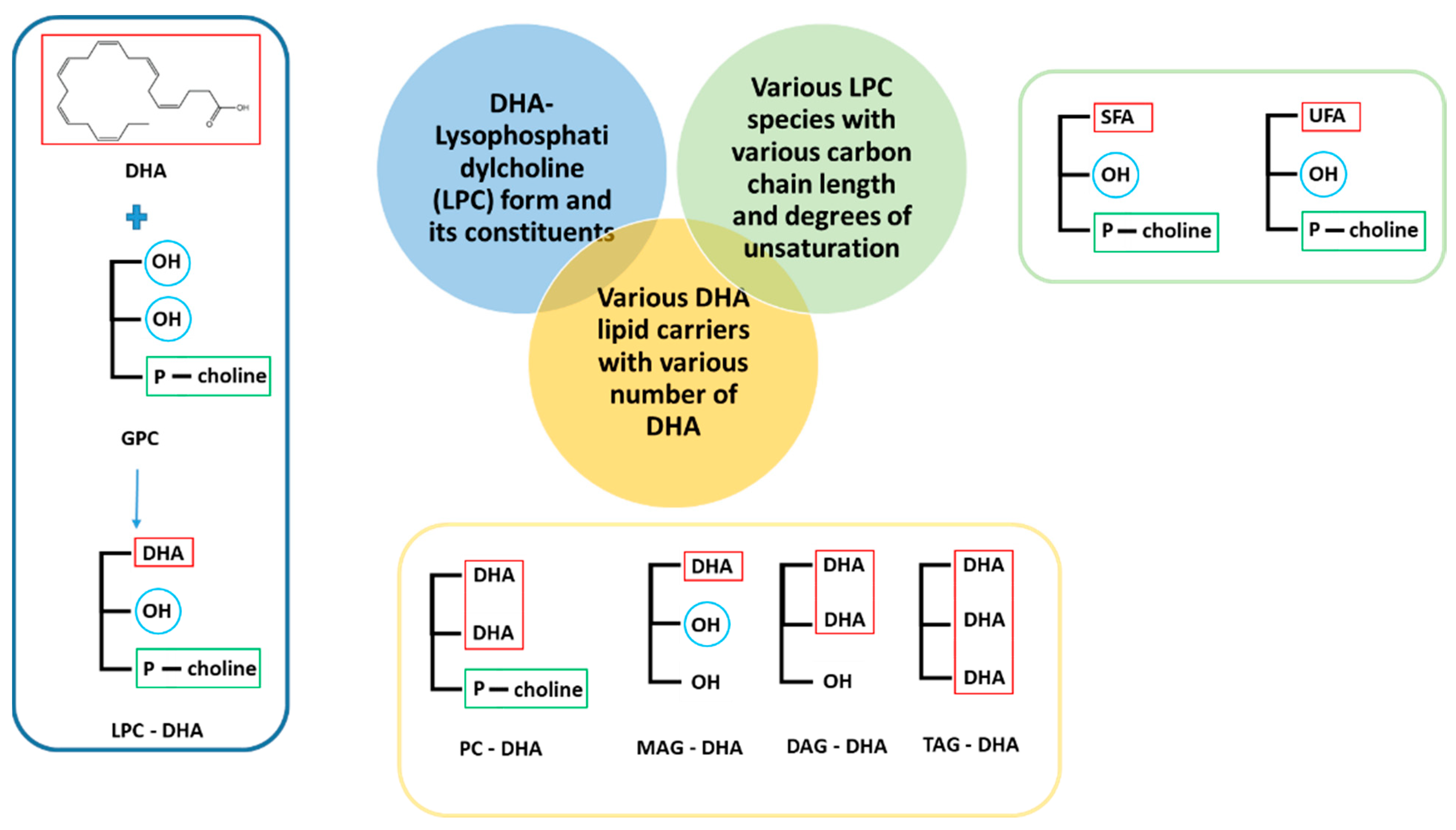
2.1. DHA and Other Tested Molecules
2.2. MDA-MB-231 Cell Culture
2.3. Treatment of MDA-MB-231 Cells with DHA and Other Molecules
2.4. Evaluation of Cell Viability by Neutral Red Uptake Test
2.5. Evaluation of Membrane Damage by the LDH Assay
2.6. Study of Cytotoxicity by High Content Analysis
2.7. Statistical Analysis
3. Results
3.1. Effect of LPC-DHA on MDA-MB-231 Human Breast Cancer Cells
3.1.1. Specificity of the Effect of LPC-DHA Compared to Free DHA and Other LPC
3.1.2. Specificity of the Effect of LPC-DHA Compared to Other DHA Lipid Carriers
3.2. Investigation of Cell Death Mechanism Induced by LPC-DHA in MDA-MB-231 Cells
3.2.1. LPC-DHA Did Not Induce Apoptotic or Autophagic Signaling and Did Not Provoke DNA Damage Response in MDA-MB-231 Cells
3.2.2. LPC-DHA Induces Oxidative Stress and Membrane Damage in MDA-MB-231 Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 Fatty Acids EPA and DHA: Health Benefits Throughout Life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Ackman, R.G.; Jangaard, P.M.; Hoyle, R.J.; Brockerhoff, H. Origin of Marine Fatty Acids. I. Analyses of the Fatty Acids Produced by the Diatom Skeletonema costatum. J. Fish. Res. Board Can. 1964, 21, 747–756. [Google Scholar] [CrossRef]
- Terme, N.; Chénais, B.; Fournière, M.; Bourgougnon, N.; Bedoux, G. Algal Derived Functional Lipids and Their Role in Promoting Health. In Recent Advances in Micro and Macroalgal Processing; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2021; pp. 370–417. ISBN 978-1-119-54265-0. [Google Scholar]
- Alasalvar, C.; Shahidi, F.; Quantick, P. Food and Health Applications of Marine Nutraceuticals: A Review. In Seafoods—Quality, Technology and Nutraceutical Applications; Alasalvar, C., Taylor, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2002; pp. 175–204. ISBN 978-3-662-09836-3. [Google Scholar]
- Berquin, I.M.; Edwards, I.J.; Chen, Y.Q. Multi-Targeted Therapy of Cancer by Omega-3 Fatty Acids. Cancer Lett. 2008, 269, 363–377. [Google Scholar] [CrossRef]
- Gerber, M. Omega-3 Fatty Acids and Cancers: A Systematic Update Review of Epidemiological Studies. Br. J. Nutr. 2012, 107 (Suppl. S2), S228–S239. [Google Scholar] [CrossRef] [PubMed]
- Sakai, M.; Kakutani, S.; Horikawa, C.; Tokuda, H.; Kawashima, H.; Shibata, H.; Okubo, H.; Sasaki, S. Arachidonic Acid and Cancer Risk: A Systematic Review of Observational Studies. BMC Cancer 2012, 12, 606. [Google Scholar] [CrossRef]
- Berquin, I.M.; Min, Y.; Wu, R.; Wu, J.; Perry, D.; Cline, J.M.; Thomas, M.J.; Thornburg, T.; Kulik, G.; Smith, A.; et al. Modulation of Prostate Cancer Genetic Risk by Omega-3 and Omega-6 Fatty Acids. J. Clin. Investig. 2007, 117, 1866–1875. [Google Scholar] [CrossRef]
- Newell, M.; Baker, K.; Postovit, L.M.; Field, C.J. A Critical Review on the Effect of Docosahexaenoic Acid (DHA) on Cancer Cell Cycle Progression. Int. J. Mol. Sci. 2017, 18, 1784. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Veigas, J.M.; Williams, P.J.; Fernandes, G.; Veigas, M. DHA Is a More Potent Inhibitor of Breast Cancer Metastasis to Bone and Related Osteolysis than EPA. Breast Cancer Res. Treat. 2013, 141, 341–352. [Google Scholar] [CrossRef]
- Bilyk, O.; Hamedi, B.; Dutta, I.; Newell, M.; Bukhari, A.B.; Gamper, A.M.; McVea, R.C.; Liu, J.; Schueler, J.; Siegers, G.M.; et al. Docosahexaenoic Acid in the Inhibition of Tumor Cell Growth in Preclinical Models of Ovarian Cancer. Nutr. Cancer 2021, 74, 1431–1445. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Zhou, W.; Liu, B.; Wang, X.; Chen, B. DHA Induces Apoptosis of Human Malignant Breast Cancer Tissues by the TLR-4/PPAR-α Pathways. Oncol. Lett. 2018, 15, 2967–2977. [Google Scholar] [CrossRef] [PubMed]
- Tamarindo, G.H.; Góes, R.M. Docosahexaenoic Acid Differentially Modulates the Cell Cycle and Metabolism- Related Genes in Tumor and Pre-Malignant Prostate Cells. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158766. [Google Scholar] [CrossRef]
- Ewaschuk, J.B.; Newell, M.; Field, C.J. Docosahexanoic Acid Improves Chemotherapy Efficacy by Inducing CD95 Translocation to Lipid Rafts in ER− Breast Cancer Cells. Lipids 2012, 47, 1019–1030. [Google Scholar] [CrossRef]
- Newell, M.; Patel, D.; Goruk, S.; Field, C.J. Docosahexaenoic Acid Incorporation Is Not Affected by Doxorubicin Chemotherapy in Either Whole Cell or Lipid Raft Phospholipids of Breast Cancer Cells in Vitro and Tumor Phospholipids in Vivo. Lipids 2020, 55, 549–565. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Qin, J.; Tian, C.; Cao, J.; Fida, G.; Wang, Z.; Chen, H.; Qian, Z.; Chen, W.R.; Gu, Y. The Targeting Mechanism of DHA Ligand and Its Conjugate with Gemcitabine for the Enhanced Tumor Therapy. Oncotarget 2014, 5, 3622–3635. [Google Scholar] [CrossRef] [PubMed]
- Ghasemifard, S.; Turchini, G.M.; Sinclair, A.J. Omega-3 Long Chain Fatty Acid “Bioavailability”: A Review of Evidence and Methodological Considerations. Prog. Lipid Res. 2014, 56, 92–108. [Google Scholar] [CrossRef]
- Burri, L.; Hoem, N.; Banni, S.; Berge, K. Marine Omega-3 Phospholipids: Metabolism and Biological Activities. Int. J. Mol. Sci. 2012, 13, 15401–15419. [Google Scholar] [CrossRef] [PubMed]
- Destaillats, F.; Oliveira, M.; Schmid, V.B.; Masserey-Elmelegy, I.; Giuffrida, F.; Thakkar, S.K.; Dupuis, L.; Gosoniu, M.L.; Cruz-Hernandez, C. Comparison of the Incorporation of DHA in Circulatory and Neural Tissue When Provided as Triacylglycerol (TAG), Monoacylglycerol (MAG) or Phospholipids (PL) Provides New Insight into Fatty Acid Bioavailability. Nutrients 2018, 10, 620. [Google Scholar] [CrossRef]
- Li, J.; Pora, B.L.R.; Dong, K.; Hasjim, J. Health Benefits of Docosahexaenoic Acid and Its Bioavailability: A Review. Food Sci. Nutr. 2021, 9, 5229–5243. [Google Scholar] [CrossRef]
- Sugasini, D.; Yalagala, P.C.; Goggin, A.; Tai, L.M.; Subbaiah, P.V. Enrichment of Brain Docosahexaenoic Acid (DHA) Is Highly Dependent upon the Molecular Carrier of Dietary DHA: Lysophosphatidylcholine Is More Efficient than Either Phosphatidylcholine or Triacylglycerol. J. Nutr. Biochem. 2019, 74, 108231. [Google Scholar] [CrossRef]
- Schuchardt, J.P.; Schneider, I.; Meyer, H.; Neubronner, J.; Von Schacky, C.; Hahn, A. Incorporation of EPA and DHA into Plasma Phospholipids in Response to Different Omega-3 Fatty Acid Formulations—A Comparative Bioavailability Study of Fish Oil vs. Krill Oil. Lipids Health Dis. 2011, 10, 145. [Google Scholar] [CrossRef]
- Schuchardt, J.P.; Hahn, A. Bioavailability of Long-Chain Omega-3 Fatty Acids. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 1–8. [Google Scholar] [CrossRef]
- Aursand, M.; Jørgensen, L.; Grasdalen, H. Positional Distribution of w3 Fatty Acids in Marine Lipid Triacylglycerols by High-Resolution13C Nuclear Magnetic Resonance Spectroscopy. J. Am. Oil Chem. Soc. 1995, 72, 293–297. [Google Scholar] [CrossRef]
- Laidlaw, M.; Cockerline, C.A.; Rowe, W.J. A Randomized Clinical Trial to Determine the Efficacy of Manufacturers’ Recommended Doses of Omega-3 Fatty Acids from Different Sources in Facilitating Cardiovascular Disease Risk Reduction. Lipids Health Dis. 2014, 13, 99. [Google Scholar] [CrossRef]
- Banno, F.; Doisaki, S.; Shimizu, N.; Fujimoto, K. Lymphatic Absorption of Docosahexaenoic Acid Given as Monoglyceride, Diglyceride, Triglyceride, and Ethyl Ester in Rats. J. Nutr. Sci. Vitaminol. 2002, 48, 30–35. [Google Scholar] [CrossRef]
- Cruz-Hernandez, C.; Destaillats, F.; Thakkar, S.K.; Goulet, L.; Wynn, E.; Grathwohl, D.; Roessle, C.; de Giorgi, S.; Tappy, L.; Giuffrida, F.; et al. Monoacylglycerol-Enriched Oil Increases EPA/DHA Delivery to Circulatory System in Humans with Induced Lipid Malabsorption Conditions. J. Lipid Res. 2016, 57, 2208–2216. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.M.; Hallaråker, H.; Sæbø, P.C.; Innis, S.M.; Kelley, K.M.; Sanoshy, K.D.; Berger, A.; Maki, K.C. Bioavailability of Long Chain Omega-3 Polyunsaturated Fatty Acids from Phospholipid-Rich Herring Roe Oil in Men and Women with Mildly Elevated Triacylglycerols. Prostaglandins Leukot. Essent. Fat. Acids 2016, 111, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Sugasini, D.; Yalagala, P.C.R.; Subbaiah, P.V. Efficient Enrichment of Retinal DHA with Dietary Lysophosphatidylcholine-DHA: Potential Application for Retinopathies. Nutrients 2020, 12, 3114. [Google Scholar] [CrossRef] [PubMed]
- Hossain, Z.; Hosokawa, M.; Takahashi, K. Growth Inhibition and Induction of Apoptosis of Colon Cancer Cell Lines by Applying Marine Phospholipid. Nutr. Cancer 2008, 61, 123–130. [Google Scholar] [CrossRef]
- Robinson, G.F.; Sooda, K.K.; Phillips, R.M.; Allison, S.J.; Javid, F.A. Investigation of the Cytotoxicity Induced by Didocosahexaenoin, an Omega 3 Derivative, in Human Prostate Carcinoma Cell Lines. Curr. Res. Pharmacol. Drug Discov. 2022, 3, 100085. [Google Scholar] [CrossRef]
- Morin, C.; Rousseau, É; Fortin, S. Anti-Proliferative Effects of a New Docosapentaenoic Acid Monoacylglyceride in Colorectal Carcinoma Cells. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 203–213. [Google Scholar] [CrossRef] [PubMed]
- González-Fernández, M.J.; Fabrikov, D.; Ramos-Bueno, R.P.; Guil-Guerrero, J.L.; Ortea, I. SWATH Differential Abundance Proteomics and Cellular Assays Show In Vitro Anticancer Activity of Arachidonic Acid- and Docosahexaenoic Acid-Based Monoacylglycerols in HT-29 Colorectal Cancer Cells. Nutrients 2019, 11, 2984. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Yang, Y.; Wang, F.; Yang, W.-G.; Zou, Z. MAG-DHA Induces Apoptosis and Autophagy in Breast Cancer Cells via Lipid Peroxidation-Mediated Endoplasmic Reticulum Stress. J. Food Sci. 2020. [Google Scholar] [CrossRef]
- Tsushima, T.; Matsubara, K.; Ohkubo, T.; Inoue, Y.; Takahashi, K. Docosahexaenoic- and Eicosapentaenoic Acid-Bound Lysophospholipids Are More Effective in Suppressing Angiogenesis than Conjugated Docosahexaenoic Acid. J. Oleo Sci. 2012, 61, 427–432. [Google Scholar] [CrossRef]
- Donovan, M.G.; Selmin, O.I.; Stillwater, B.J.; Neumayer, L.A.; Romagnolo, D.F. Do Olive and Fish Oils of the Mediterranean Diet Have a Role in Triple Negative Breast Cancer Prevention and Therapy? An Exploration of Evidence in Cells and Animal Models. Front. Nutr. 2020, 7, 571455. [Google Scholar] [CrossRef]
- Choi, A.M.; Alam, J. Heme Oxygenase-1: Function, Regulation, and Implication of a Novel Stress-Inducible Protein in Oxidant-Induced Lung Injury. Am. J. Respir. Cell Mol. Biol. 1996, 15, 9–19. [Google Scholar] [CrossRef]
- Melov, S.; Coskun, P.; Patel, M.; Tuinstra, R.; Cottrell, B.; Jun, A.S.; Zastawny, T.H.; Dizdaroglu, M.; Goodman, S.I.; Huang, T.-T.; et al. Mitochondrial Disease in Superoxide Dismutase 2 Mutant Mice. Proc. Natl. Acad. Sci. USA 1999, 96, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Ahmmed, M.K.; Ahmmed, F.; Tian, H.; Carne, A.; Bekhit, A.E. Marine Omega-3 (n-3) Phospholipids: A Comprehensive Review of Their Properties, Sources, Bioavailability, and Relation to Brain Health. Compr. Rev. Food Sci. Food Saf. 2020, 19, 64–123. [Google Scholar] [CrossRef]
- Hung, N.D.; Kim, M.R.; Sok, D.-E. 2-Polyunsaturated Acyl Lysophosphatidylethanolamine Attenuates Inflammatory Response in Zymosan A-Induced Peritonitis in Mice. Lipids 2011, 46, 893–906. [Google Scholar] [CrossRef]
- Khaddaj-Mallat, R.; Morin, C.; Rousseau, É. Novel N-3 PUFA Monoacylglycerides of Pharmacological and Medicinal Interest: Anti-Inflammatory and Anti-Proliferative Effects. Eur. J. Pharmacol. 2016, 792, 70–77. [Google Scholar] [CrossRef]
- Hachem, M.; Nacir, H. Emerging Role of Phospholipids and Lysophospholipids for Improving Brain Docosahexaenoic Acid as Potential Preventive and Therapeutic Strategies for Neurological Diseases. Int. J. Mol. Sci. 2022, 23, 3969. [Google Scholar] [CrossRef]
- Javadian, M.; Shekari, N.; Zangbar, M.S.S.; Mohammadi, A.; Mansoori, B.; Maralbashi, S.; Shanehbandi, D.; Baradaran, B.; Darabi, M.; Kazemi, T. Docosahexaenoic Acid Suppresses Migration of Triple-Negative Breast Cancer Cell through Targeting Metastasis-Related Genes and MicroRNA under Normoxic and Hypoxic Conditions. J. Cell. Biochem. 2020, 121, 2416–2427. [Google Scholar] [CrossRef]
- Chénais, B.; Cornec, M.; Dumont, S.; Marchand, J.; Blanckaert, V. Transcriptomic Response of Breast Cancer Cells MDA-MB-231 to Docosahexaenoic Acid: Downregulation of Lipid and Cholesterol Metabolism Genes and Upregulation of Genes of the Pro-Apoptotic ER-Stress Pathway. Int. J. Environ. Res. Public Health 2020, 17, 3746. [Google Scholar] [CrossRef]
- Rizzo, A.M.; Colombo, I.; Montorfano, G.; Zava, S.; Corsetto, P.A. Exogenous Fatty Acids Modulate ER Lipid Composition and Metabolism in Breast Cancer Cells. Cells 2021, 10, 175. [Google Scholar] [CrossRef]
- Glatz, J.F.C.; Luiken, J.J.F.P.; Bonen, A. Membrane Fatty Acid Transporters as Regulators of Lipid Metabolism: Implications for Metabolic Disease. Physiol. Rev. 2010, 90, 367–417. [Google Scholar] [CrossRef]
- Pencreac’h, G.; Ergan, F.; Poisson, L. DHA–Lysophospholipid Production. Curr. Org. Chem. 2013, 17, 793–801. [Google Scholar] [CrossRef]
- Wang, X.; Qin, X.; Li, X.; Zhao, Z.; Yang, B.; Wang, Y. An Efficient Synthesis of Lysophosphatidylcholine Enriched with n-3 Polyunsaturated Fatty Acids by Immobilized MAS1 Lipase. J. Agric. Food Chem. 2019, 68, 242–249. [Google Scholar] [CrossRef]
- Kim, Y.-L.; Im, Y.-J.; Ha, N.-C.; Im, D.-S. Albumin Inhibits Cytotoxic Activity of Lysophosphatidylcholine by Direct Binding. Prostaglandins Other Lipid Mediat. 2007, 83, 130–138. [Google Scholar] [CrossRef]
- Ross, T.; Jakubzig, B.; Grundmann, M.; Massing, U.; Kostenis, E.; Schlesinger, M.; Bendas, G. The Molecular Mechanism by Which Saturated Lysophosphatidylcholine Attenuates the Metastatic Capacity of Melanoma Cells. FEBS Open Bio 2016, 6, 1297–1309. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, Y.; Cai, W.; Guo, Y.; Xue, C.; Wang, J. DHA/EPA-Enriched Phosphatidylcholine Suppresses Tumor Growth and Metastasis via Activating Peroxisome Proliferator-Activated Receptor γ in Lewis Lung Cancer Mice. J. Agric. Food Chem. 2021, 69, 676–685. [Google Scholar] [CrossRef]
- Weltzien, H.U. Cytolytic and Membrane-Perturbing Properties of Lysophosphatidylcholine. Biochim. Biophys. Acta Rev. Biomembr. 1979, 559, 259–287. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, J.R.; Andresen, T.L.; Feldborg, L.N.; Duelund, L.; Ipsen, J.H. Understanding Detergent Effects on Lipid Membranes: A Model Study of Lysolipids. Biophys. J. 2010, 98, 2199–2205. [Google Scholar] [CrossRef] [PubMed]
- Hishikawa, D.; Valentine, W.J.; Iizuka-Hishikawa, Y.; Shindou, H.; Shimizu, T. Metabolism and Functions of Docosahexaenoic Acid-Containing Membrane Glycerophospholipids. FEBS Lett. 2017, 591, 2730–2744. [Google Scholar] [CrossRef] [PubMed]
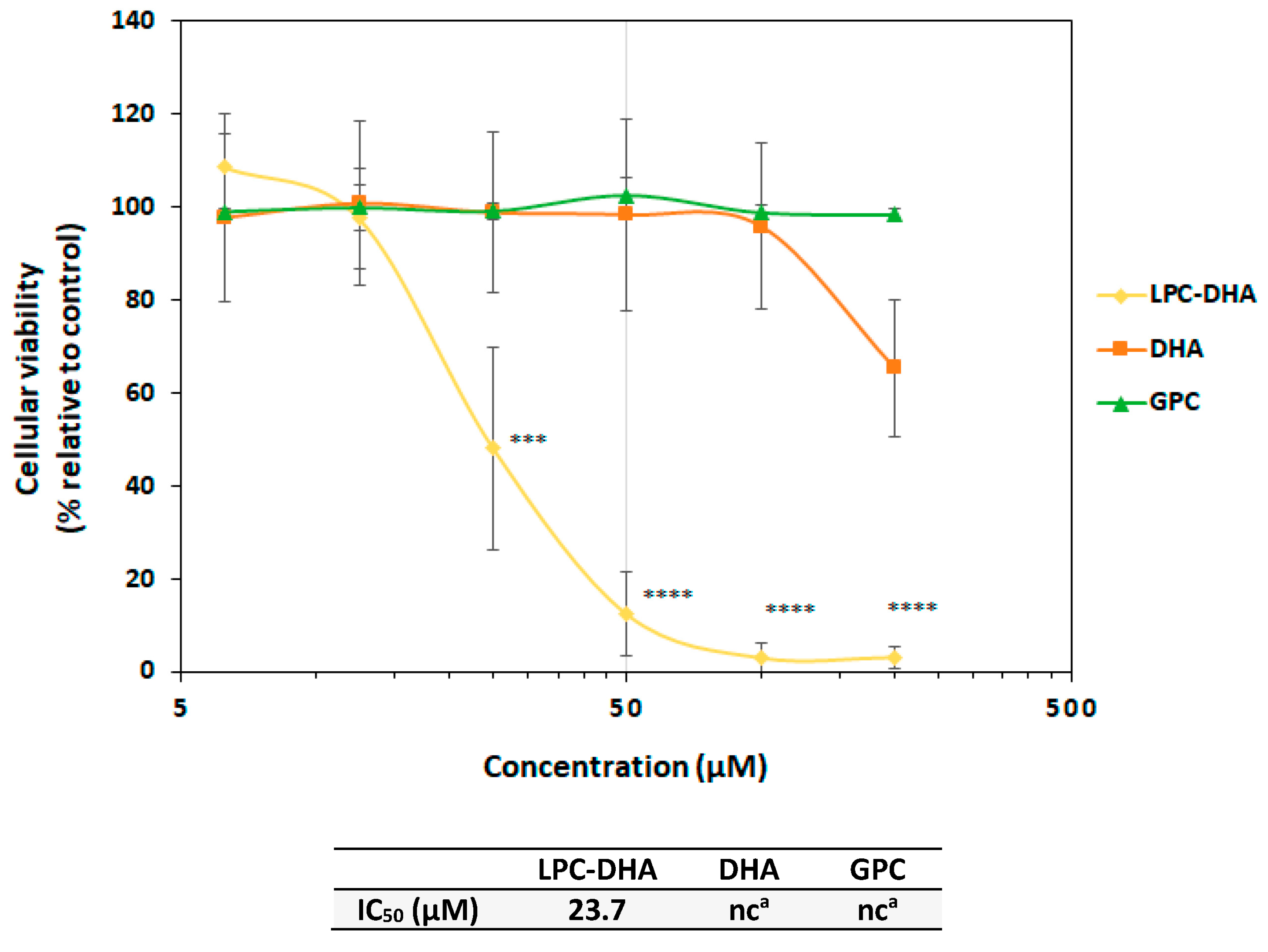
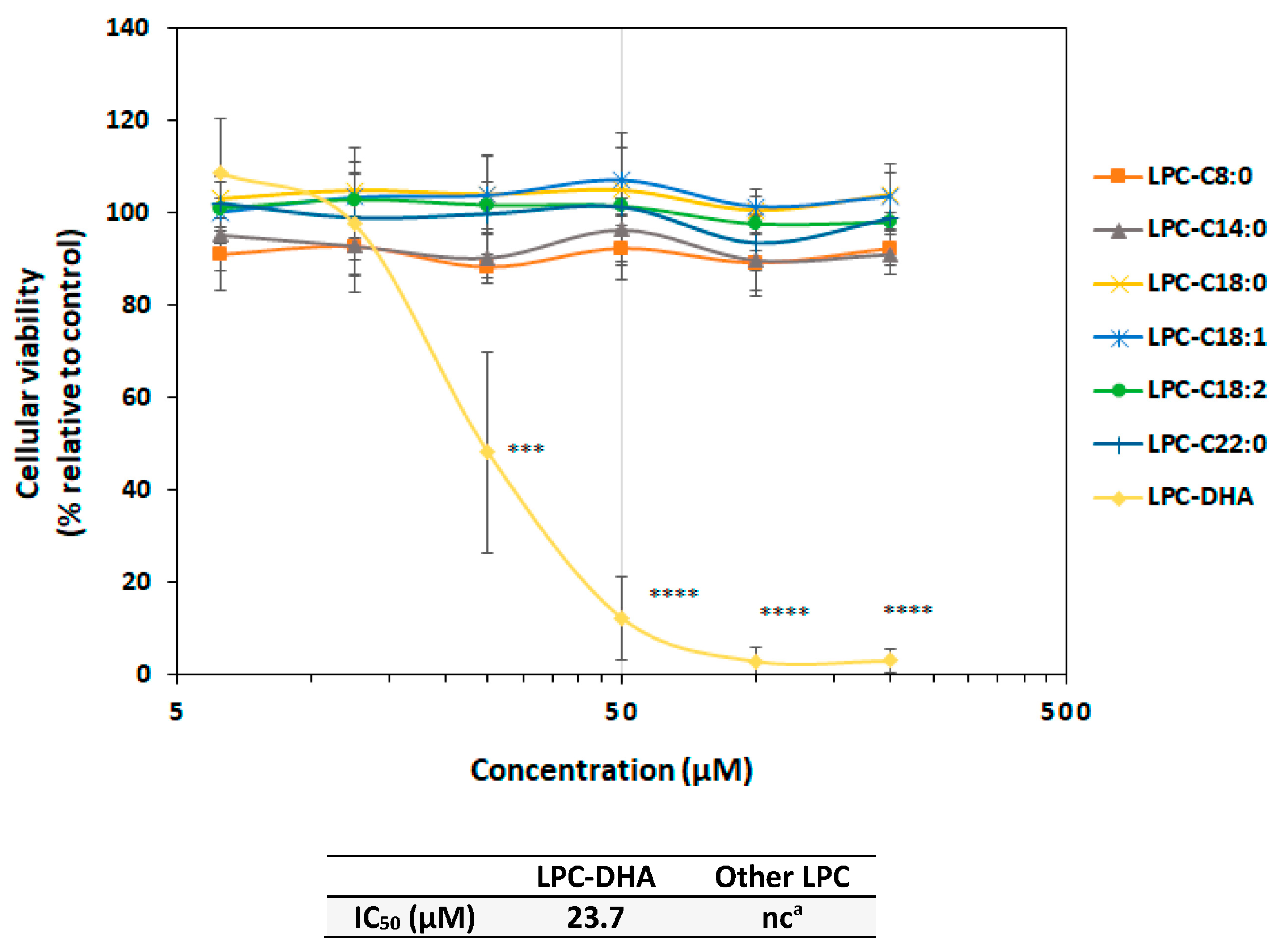


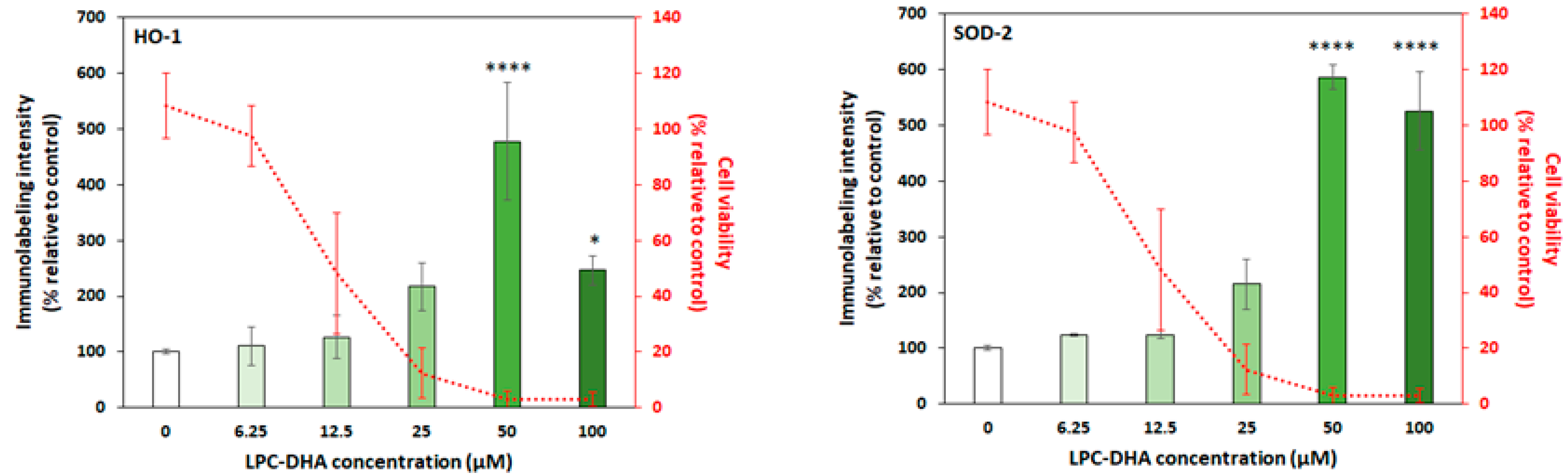

 ) are in relation to the IC50 values. The red cross on the line indicates no effect of the tested molecules.
) are in relation to the IC50 values. The red cross on the line indicates no effect of the tested molecules.
 ) are in relation to the IC50 values. The red cross on the line indicates no effect of the tested molecules.
) are in relation to the IC50 values. The red cross on the line indicates no effect of the tested molecules.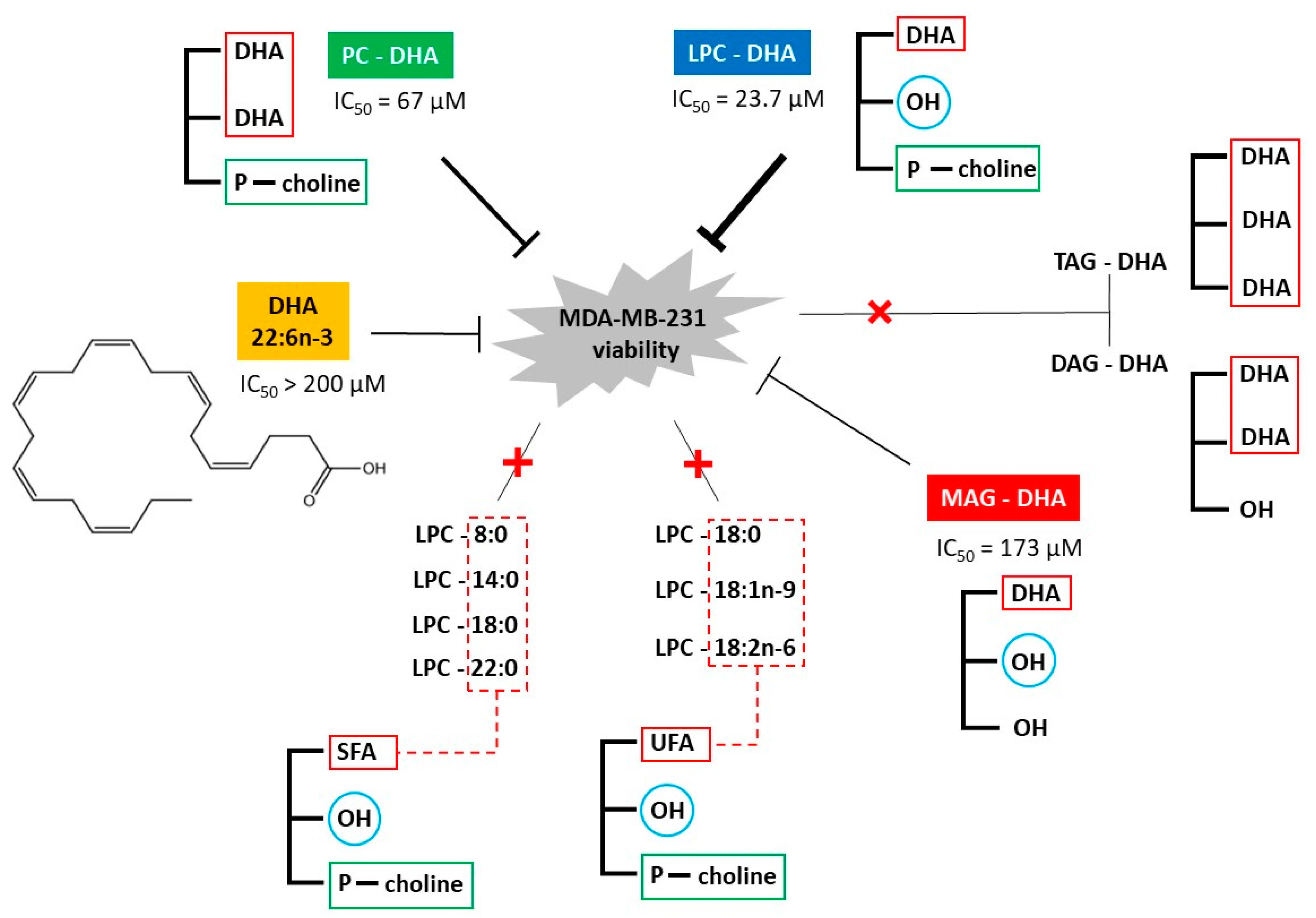
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamad Ali, D.; Hogeveen, K.; Orhant, R.-M.; Le Gal de Kerangal, T.; Ergan, F.; Ulmann, L.; Pencreac’h, G. Lysophosphatidylcholine-DHA Specifically Induces Cytotoxic Effects of the MDA-MB-231 Human Breast Cancer Cell Line In Vitro—Comparative Effects with Other Lipids Containing DHA. Nutrients 2023, 15, 2137. https://doi.org/10.3390/nu15092137
Mohamad Ali D, Hogeveen K, Orhant R-M, Le Gal de Kerangal T, Ergan F, Ulmann L, Pencreac’h G. Lysophosphatidylcholine-DHA Specifically Induces Cytotoxic Effects of the MDA-MB-231 Human Breast Cancer Cell Line In Vitro—Comparative Effects with Other Lipids Containing DHA. Nutrients. 2023; 15(9):2137. https://doi.org/10.3390/nu15092137
Chicago/Turabian StyleMohamad Ali, Dalal, Kevin Hogeveen, Rose-Marie Orhant, Tiphaine Le Gal de Kerangal, Françoise Ergan, Lionel Ulmann, and Gaëlle Pencreac’h. 2023. "Lysophosphatidylcholine-DHA Specifically Induces Cytotoxic Effects of the MDA-MB-231 Human Breast Cancer Cell Line In Vitro—Comparative Effects with Other Lipids Containing DHA" Nutrients 15, no. 9: 2137. https://doi.org/10.3390/nu15092137
APA StyleMohamad Ali, D., Hogeveen, K., Orhant, R.-M., Le Gal de Kerangal, T., Ergan, F., Ulmann, L., & Pencreac’h, G. (2023). Lysophosphatidylcholine-DHA Specifically Induces Cytotoxic Effects of the MDA-MB-231 Human Breast Cancer Cell Line In Vitro—Comparative Effects with Other Lipids Containing DHA. Nutrients, 15(9), 2137. https://doi.org/10.3390/nu15092137





