Association of Vitamin D Receptor Gene Polymorphisms with Cardiometabolic Phenotypes in Hispanics: A Life Course Approach
Abstract
1. Introduction
2. Materials and Methods
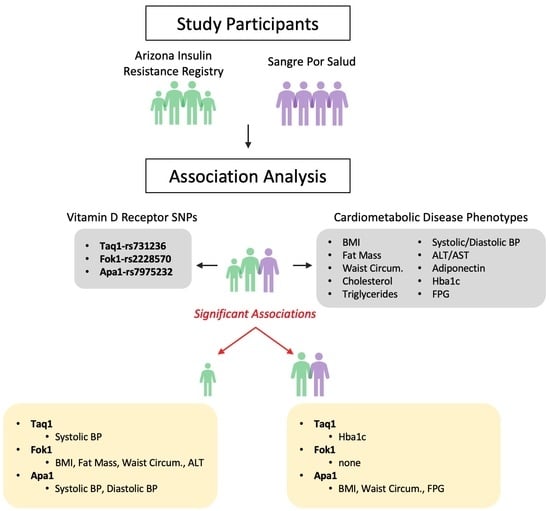
2.1. Participants and Cohorts
2.2. Single Nucleotide Polymorphism (SNP) Genotyping
2.3. AIR Registry Phenotypes Used for Genetic Analysis
2.4. SPS Biobank Phenotypes for Genetic Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Taq1-rs731236 | AA 290 (0.58) | AG 185 (0.37) | GG 23 (0.05) | p-value * | p-value ** |
| NS | NS | NS | |||
| Fok1-rs2228570 | GG 157 (0.31) | GA 225 (0.45) | AA 117 (0.23) | p-value * | p-value ** |
| NS | NS | NS | |||
| Apa1-rs7975232 | CC 154 (0.31) | CA 263 (0.53) | AA 76 (0.15) | p-value * | p-value ** |
| Fasting plasma glucose, mg/dL | 96.3 | 93.7 | 93.5 | 0.04 | 0.02 |
| Taq1-rs731236 | AA 586 (0.59) | AG 339 (0.34) | GG 63 (0.06) | p-value * | p-value ** |
| Hemoglobin A1c, % | 6.2 | 6.0 | 5.8 | 0.01 | 0.007 |
| Fok1-rs2228570 | GG 275 (0.28) | GA 507 (0.51) | AA 208 (0.21) | p-value * | p-value ** |
| NS | NS | NS | |||
| Apa1-rs7975232 | CC 344 (0.35) | CA 440 (0.45) | AA 200 (0.20) | p-value * | p-value ** |
| Body mass index, kg/m2 | 30.0 | 30.4 | 31.5 | 0.005 | 0.006 |
| Waist circumference, cm | 99.8 | 100.3 | 103.4 | 0.01 | 0.01 |
References
- Tuoresmäki, P.; Väisänen, S.; Neme, A.; Heikkinen, S.; Carlberg, C. Patterns of genome-wide VDR locations. PLoS ONE 2014, 9, e96105. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C. Molecular endocrinology of vitamin D on the epigenome level. Mol. Cell. Endocrinol. 2017, 453, 14–21. [Google Scholar] [CrossRef]
- Khashim Alswailmi, F.; Shah, S.I.A.; Nawaz, H.; Al-Mazaideh, G.M. Molecular Mechanisms of Vitamin D-Mediated Immunomodulation. Galen Med. J. 2021, 10, e2097. [Google Scholar] [CrossRef]
- Anderson, P.H.; May, B.K.; Morris, H.A. Vitamin D metabolism: New concepts and clinical implications. Clin. Biochem. Rev. 2003, 24, 13–26. [Google Scholar]
- Jones, G.; Strugnell, S.A.; DeLuca, H.F. Current understanding of the molecular actions of vitamin D. Physiol. Rev. 1998, 78, 1193–1231. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D: Production, Metabolism and Mechanisms of Action. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Hofland, J., Dungan, K., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Bikle, D. Nonclassic actions of vitamin D. J. Clin. Endocrinol. Metab. 2009, 94, 26–34. [Google Scholar] [CrossRef]
- Wang, H.; Chen, W.; Li, D.; Yin, X.; Zhang, X.; Olsen, N.; Zheng, S.G. Vitamin D and Chronic Diseases. Aging Dis. 2017, 8, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Lorentzon, M.; Lorentzon, R.; Nordström, P. Vitamin D receptor gene polymorphism is associated with birth height, growth to adolescence, and adult stature in healthy caucasian men: A cross-sectional and longitudinal study. J. Clin. Endocrinol. Metab. 2000, 85, 1666–1670. [Google Scholar] [CrossRef]
- Karras, S.N.; Dursun, E.; Alaylıoğlu, M.; Gezen-Ak, D.; Annweiler, C.; Skoutas, D.; Evangelidis, D.; Kiortsis, D. Diverse Effects of Combinations of Maternal-Neonatal VDR Polymorphisms and 25-Hydroxyvitamin D Concentrations on Neonatal Birth Anthropometry: Functional Phenocopy Variability Dependence, Highlights the Need for Targeted Maternal 25-Hydroxyvitamin D Cut-Offs during Pregnancy. Nutrients 2021, 13, 443. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A.; Borchers, M.; Gudat, F.; Dürmüller, U.; Stähelin, H.B.; Dick, W. Vitamin D receptor expression in human muscle tissue decreases with age. J. Bone Miner. Res. 2004, 19, 265–269. [Google Scholar] [CrossRef]
- Prentice, A.; Goldberg, G.R.; Schoenmakers, I. Vitamin D across the lifecycle: Physiology and biomarkers. Am. J. Clin. Nutr. 2008, 88, 500s–506s. [Google Scholar] [CrossRef] [PubMed]
- Akter, R.; Afrose, A.; Sharmin, S.; Rezwan, R.; Rahman, M.R.; Neelotpol, S. A comprehensive look into the association of vitamin D levels and vitamin D receptor gene polymorphism with obesity in children. Biomed. Pharmacother. 2022, 153, 113285. [Google Scholar] [CrossRef] [PubMed]
- Alathari, B.E.; Sabta, A.A.; Kalpana, C.A.; Vimaleswaran, K.S. Vitamin D pathway-related gene polymorphisms and their association with metabolic diseases: A literature review. J. Diabetes Metab. Disord. 2020, 19, 1701–1729. [Google Scholar] [CrossRef]
- Standage-Beier, C.S.; Bakhshi, B.; Parra, O.D.; Soltani, L.; Spegman, D.J.; Molina, P.; Pereira, E.; Landes, L.; Mandarino, L.J.; Kohler, L.N. Fruit, Vegetable, and Physical Activity Guideline Adherence and Metabolic Syndrome in El Banco por Salud. Nutrients 2022, 14, 1767. [Google Scholar] [CrossRef] [PubMed]
- Kirk, E.P.; Klein, S. Pathogenesis and pathophysiology of the cardiometabolic syndrome. J. Clin. Hypertens. 2009, 11, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef]
- Walsh, J.S.; Bowles, S.; Evans, A.L. Vitamin D in obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 389–394. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, X.; Huang, S.Y.; Gong, L.; Cui, J.H.; Shen, H.W.; Ye, X.H.; He, X.F. Evaluation of association studies and a systematic review and meta-analysis of VDR polymorphisms in type 2 diabetes mellitus risk. Medicine 2021, 100, e25934. [Google Scholar] [CrossRef]
- Faghfouri, A.H.; Faghfuri, E.; Maleki, V.; Payahoo, L.; Balmoral, A.; Khaje Bishak, Y. A comprehensive insight into the potential roles of VDR gene polymorphism in obesity: A systematic review. Arch. Physiol. Biochem. 2022, 128, 1645–1657. [Google Scholar] [CrossRef]
- Aravindhan, S.; Almasoody, M.F.M.; Selman, N.A.; Andreevna, A.N.; Ravali, S.; Mohammadi, P.; Eslami, M.M.; Razi, B.; Aslani, S.; Imani, D. Vitamin D Receptor gene polymorphisms and susceptibility to type 2 diabetes: Evidence from a meta-regression and meta-analysis based on 47 studies. J. Diabetes Metab. Disord. 2021, 20, 845–867. [Google Scholar] [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. National Diabetes Statistics Report 2020; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2020.
- Jin, T.; Lu, W.; Gong, X.; Zhou, J.; Wu, F. Association of vitamin D receptor polymorphisms with metabolic syndrome-related components: A cross-sectional study. J. Clin. Lab. Anal. 2021, 35, e23829. [Google Scholar] [CrossRef] [PubMed]
- Shaibi, G.Q.; Coletta, D.K.; Vital, V.; Mandarino, L.J. The design and conduct of a community-based registry and biorepository: A focus on cardiometabolic health in Latinos. Clin. Transl. Sci. 2013, 6, 429–434. [Google Scholar] [CrossRef]
- Shaibi, G.; Singh, D.; De Filippis, E.; Hernandez, V.; Rosenfeld, B.; Otu, E.; Montes de Oca, G.; Levey, S.; Radecki Breitkopf, C.; Sharp, R.; et al. The Sangre Por Salud Biobank: Facilitating Genetic Research in an Underrepresented Latino Community. Public Health Genom. 2016, 19, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Roe, J.D.; Garcia, L.A.; Klimentidis, Y.C.; Coletta, D.K. Association of PNPLA3 I148M with Liver Disease Biomarkers in Latinos. Hum. Hered. 2021, 86, 21–27. [Google Scholar] [CrossRef] [PubMed]
- DeMenna, J.; Puppala, S.; Chittoor, G.; Schneider, J.; Kim, J.Y.; Shaibi, G.Q.; Mandarino, L.J.; Duggirala, R.; Coletta, D.K. Association of common genetic variants with diabetes and metabolic syndrome related traits in the Arizona Insulin Resistance registry: A focus on Mexican American families in the Southwest. Hum. Hered. 2014, 78, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, G.K.; Remus, L.S.; Jurutka, P.W.; Zitzer, H.; Oza, A.K.; Dang, H.T.; Haussler, C.A.; Galligan, M.A.; Thatcher, M.L.; Encinas Dominguez, C.; et al. Functionally relevant polymorphisms in the human nuclear vitamin D receptor gene. Mol. Cell. Endocrinol. 2001, 177, 145–159. [Google Scholar] [CrossRef]
- McGrath, J.J.; Saha, S.; Burne, T.H.; Eyles, D.W. A systematic review of the association between common single nucleotide polymorphisms and 25-hydroxyvitamin D concentrations. J. Steroid Biochem. Mol. Biol. 2010, 121, 471–477. [Google Scholar] [CrossRef]
- Awasthi, R.; Manger, P.T.; Khare, R.K. Fok I and Bsm I gene polymorphism of vitamin D receptor and essential hypertension: A mechanistic link. Clin. Hypertens. 2023, 29, 5. [Google Scholar] [CrossRef]
- Correa-Rodríguez, M.; Carrillo-Ávila, J.A.; Schmidt-RioValle, J.; González-Jiménez, E.; Vargas, S.; Martín, J.; Rueda-Medina, B. Genetic association analysis of vitamin D receptor gene polymorphisms and obesity-related phenotypes. Gene 2018, 640, 51–56. [Google Scholar] [CrossRef]
- Al-Hazmi, A.S.; Al-Mehmadi, M.M.; Al-Bogami, S.M.; Shami, A.A.; Al-Askary, A.A.; Alomery, A.M.; Al-Shehri, S.S.; Dahlawi, H.; Abdulrazag, K.; Ali, T.; et al. Vitamin D receptor gene polymorphisms as a risk factor for obesity in Saudi men. Electron. Physician 2017, 9, 5427–5433. [Google Scholar] [CrossRef] [PubMed]
- Bienertová-Vašků, J.; Zlámal, F.; Pohořalá, A.; Mikeš, O.; Goldbergová-Pávková, M.; Novák, J.; Šplíchal, Z.; Pikhart, H. Allelic variants in vitamin D receptor gene are associated with adiposity measures in the central-European population. BMC Med. Genet. 2017, 18, 90. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.W.; Choi, J.; Jeon, H.J.; Oh, T.K.; Lee, D.H. The Associations Between Vitamin D Receptor BsmI and ApaI Polymorphisms and Obesity in Korean Patients with Type 2 Diabetes Mellitus. Diabetes Metab. Syndr. Obes. 2021, 14, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, W.N.A.; Mohd Yunus, N.; Yaacob, N.M.; Omar, J.; Wan Mohamed, W.M.I.; Sirajudeen, K.N.S.; Tuan Ismail, T.S. Association between Vitamin D Receptor Polymorphisms (BsmI and FokI) and Glycemic Control among Patients with Type 2 Diabetes. Int. J. Environ. Res. Public Health 2021, 18, 1595. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, F.; Ostadsharif, M. Association of VDR gene ApaI polymorphism with obesity in Iranian population. Biomedica 2021, 41, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.T.; Kaelber, D.C.; Baker-Smith, C.M.; Blowey, D.; Carroll, A.E.; Daniels, S.R.; de Ferranti, S.D.; Dionne, J.M.; Falkner, B.; Flinn, S.K.; et al. Subcommittee on Screening and Management of High Blood Pressure in Children. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics 2017, 140, e20171904, Erratum in Pediatrics 2018, 142, e20181739. [Google Scholar] [CrossRef]
- Gartlehner, G.; Vander Schaaf, E.B.; Orr, C.; Kennedy, S.M.; Clark, R.; Viswanathan, M.U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews. In Screening for Hypertension in Children and Adolescents: Systematic Review for the U.S. Preventive Services Task Force; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2020. [Google Scholar]
- Wang, D.; Su, K.; Ding, Z.; Zhang, Z.; Wang, C. Association of Vitamin D Receptor Gene Polymorphisms with Metabolic Syndrome in Chinese Children. Int. J. Gen. Med. 2021, 14, 57–66. [Google Scholar] [CrossRef]
- Deurenberg, P.; Deurenberg-Yap, M.; Guricci, S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes. Rev. 2002, 3, 141–146. [Google Scholar] [CrossRef]
- Qin, X.; Qiu, L.; Tang, G.; Tsoi, M.F.; Xu, T.; Zhang, L.; Qi, Z.; Zhu, G.; Cheung, B.M.Y. Prevalence of metabolic syndrome among ethnic groups in China. BMC Public Health 2020, 20, 297. [Google Scholar] [CrossRef]
- Rivera-Leon, E.A.; Palmeros-Sanchez, B.; Llamas-Covarrubias, I.M.; Fernandez, S.; Armendariz-Borunda, J.; Gonzalez-Hita, M.; Bastidas-Ramirez, B.E.; Zepeda-Moreno, A.; Sanchez-Enriquez, S. Vitamin-D receptor gene polymorphisms (TaqI and ApaI) and circulating osteocalcin in type 2 diabetic patients and healthy subjects. Endokrynol. Pol. 2015, 66, 329–333. [Google Scholar] [CrossRef]
- Piña-Aguero, M.I.; Maldonado-Hernández, J.; Sebastián-Medina, L.; Tejero-Barrera, M.E.; Robledo-Pérez, R.M.; Villalpando-Hernández, S.; Ventura-Bravo, Z.A.; Morales-Ramírez, L.K. Vitamin D Receptor Gene Polymorphisms, β-cell Function, and Vitamin D Status in Non-obese Mexican Adults. Arch. Med. Res. 2022, 53, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, E.T.; Alberts, D.S.; Foote, J.A.; Green, S.B.; Hollis, B.W.; Yu, Z.; Martínez, M.E. Vitamin D insufficiency in southern Arizona. Am. J. Clin. Nutr. 2008, 87, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin. Proc. 2006, 81, 353–373. [Google Scholar] [CrossRef]
- Cui, A.; Xiao, P.; Ma, Y.; Fan, Z.; Zhou, F.; Zheng, J.; Zhang, L. Prevalence, trend, and predictor analyses of vitamin D deficiency in the US population, 2001–2018. Front. Nutr. 2022, 9, 965376. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.W.; Shin, H.T.; Seo, J. Risk Allele Frequency Analysis of Single-Nucleotide Polymorphisms for Vitamin D Concentrations in Different Ethnic Group. Genes 2021, 12, 1530. [Google Scholar] [CrossRef]
- Sawicki, C.M.; Van Rompay, M.I.; Au, L.E.; Gordon, C.M.; Sacheck, J.M. Sun-Exposed Skin Color Is Associated with Changes in Serum 25-Hydroxyvitamin D in Racially/Ethnically Diverse Children. J. Nutr. 2016, 146, 751–757. [Google Scholar] [CrossRef]
- Usategui-Martín, R.; De Luis-Román, D.A.; Fernández-Gómez, J.M.; Ruiz-Mambrilla, M.; Pérez-Castrillón, J.L. Vitamin D Receptor (VDR) Gene Polymorphisms Modify the Response to Vitamin D Supplementation: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 360. [Google Scholar] [CrossRef]
- Coletta, D.K.; Hlusko, L.J.; Scott, G.R.; Garcia, L.A.; Vachon, C.M.; Norman, A.D.; Funk, J.L.; Shaibi, G.Q.; Hernandez, V.; De Filippis, E.; et al. Association of EDARV370A with breast density and metabolic syndrome in Latinos. PLoS ONE 2021, 16, e0258212. [Google Scholar] [CrossRef]
- Hlusko, L.J.; McNelis, M.G. Evolutionary adaptation highlights the interconnection of fatty acids, sunlight, inflammation and epithelial adhesion. Acta Paediatr. 2022, 111, 1313–1318. [Google Scholar] [CrossRef]
| Phenotypes | AIR–Children (<18 Age, Years) | AIR–Adults (≥18 Age, Years) | SPS–Adults (≥18 Age, Years) |
|---|---|---|---|
| n = 121 | n = 499 | n = 990 | |
| Gender (Female/Male) | 61/60 | 322/177 | 686/304 |
| Age, years | 14.1 ± 0.2 | 36.4 ± 0.5 | 45.8 ± 0.5 |
| Body Mass Index, kg/m2 | 24.2 ± 0.6 | 29.8 ± 0.3 | 30.5 ± 0.3 |
| Fat Mass, % | 15.5 ± 1.0 | 24.3 ± 0.5 | - |
| Waist Circumference, cm | 83.3 ± 1.6 | 98.6 ± 0.6 | 100.7 ± 0.7 |
| Hip Circumference, cm | 97.4 ± 1.4 | 108.6 ± 0.5 | - |
| Cholesterol, mg/dL | 143.8 ± 2.7 | 174.3 ± 1.6 | 186.3 ± 1.6 |
| Triglycerides, mg/dL | 92.1 ± 4.5 | 136.5 ± 3.5 | 144.8 ± 4.2 |
| High-density lipoprotein, mg/dL | 45.2 ± 0.9 | 44.0 ± 0.5 | 49.96 ± 0.7 |
| Low-density lipoprotein, mg/dL | 82.8 ± 2.2 | 106.9 ± 1.3 | 108.1 ± 1.4 |
| Very low-density lipoprotein, mg/dL | 15.5 ± 0.7 | 21.8 ± 0.5 | - |
| Systolic blood pressure, mm Hg | 110.6 ± 1.1 | 120.1 ± 0.7 | - |
| Diastolic blood pressure, mm Hg | 67.5 ± 0.8 | 76.4 ± 0.4 | - |
| Alanine aminotransferase, IU/L | 17.2 ± 0.8 | 26.4 ± 0.8 | - |
| Aspartate aminotransferase, IU/L | 22.2 ± 0.7 | 24.0 ± 0.5 | - |
| Adiponectin, ug/ml | 8.7 ± 0.3 | 6.4 ± 0.1 | - |
| Hemoglobin A1c, % | 5.5 ± 0.03 | 5.6 ± 0.02 | 6.1 ± 0.1 |
| Fasting plasma glucose, mg/dL | 91.3 ± 0.6 | 94.4 ± 0.5 | 95.2 ± 1.1 |
| 2hOGTT, mg/dL | 119.3 ± 2.8 | 136.96 ± 2.3 | 122.2 ± 2.9 |
| Taq1-rs731236 | AA 74 (0.62) | AG 44 (0.37) | GG 2 (0.02) | p-value * | p-value ** |
| Systolic blood pressure, mmHg | 113.1 | 106.8 | 95.3 | 0.0008 | 0.005 |
| Fok1-rs2228570 | AA 29 (0.24) | AG 64 (0.53) | GG 28 (0.23) | p-value * | p-value ** |
| Body mass index, kg/m2 | 26.6 | 23.9 | 22.2 | 0.01 | 0.02 |
| Fat mass, % | 20.7 | 14.4 | 12.4 | 0.005 | 0.007 |
| Waist circumference, cm | 92.8 | 81.1 | 78.4 | 0.002 | 0.004 |
| Hip circumference, cm | 102.9 | 96.4 | 93.8 | 0.01 | 0.03 |
| Alanine aminotransferase, IU/L | 21.7 | 15.5 | 16.5 | 0.03 | 0.03 |
| Apa1-rs7975232 | CC 37 (0.31) | CA 68 (0.56) | AA 16 (0.13) | p-value * | p-value ** |
| Systolic blood pressure, mmHg | 114.2 | 110.3 | 103.9 | 0.003 | 0.01 |
| Diastolic blood pressure, mmHg | 69.7 | 67.0 | 64.6 | 0.03 | 0.05 |
| Taq1-rs731236 | AA 876 (0.59) | AG 524 (0.35) | GG 86 (0.06) | p-value * | p-value ** |
| Hemoglobin A1c, % | 6.0 | 5.9 | 5.7 | 0.005 | 0.004 |
| Fok1-rs2228570 | AA 432 (0.29) | AG 732 (0.49) | GG 325 (0.22) | p-value * | p-value ** |
| NS | NS | NS | |||
| Apa1-rs7975232 | CC 498 (0.34) | CA 703 (0.48) | AA 276 (0.19) | p-value * | p-value ** |
| Body mass index, kg/m2 | 30.0 | 30.2 | 31.0 | 0.03 | 0.04 |
| Waist circumference, cm | 99.5 | 99.6 | 102.0 | 0.04 | 0.05 |
| Fasting plasma glucose, mg/dL | 96.7 | 94.0 | 94.2 | 0.04 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Standage-Beier, C.S.; Garcia, L.A.; De Filippis, E.; Shaibi, G.Q.; Mandarino, L.J.; Coletta, D.K. Association of Vitamin D Receptor Gene Polymorphisms with Cardiometabolic Phenotypes in Hispanics: A Life Course Approach. Nutrients 2023, 15, 2118. https://doi.org/10.3390/nu15092118
Standage-Beier CS, Garcia LA, De Filippis E, Shaibi GQ, Mandarino LJ, Coletta DK. Association of Vitamin D Receptor Gene Polymorphisms with Cardiometabolic Phenotypes in Hispanics: A Life Course Approach. Nutrients. 2023; 15(9):2118. https://doi.org/10.3390/nu15092118
Chicago/Turabian StyleStandage-Beier, Carrie S., Luis A. Garcia, Eleanna De Filippis, Gabriel Q. Shaibi, Lawrence J. Mandarino, and Dawn K. Coletta. 2023. "Association of Vitamin D Receptor Gene Polymorphisms with Cardiometabolic Phenotypes in Hispanics: A Life Course Approach" Nutrients 15, no. 9: 2118. https://doi.org/10.3390/nu15092118
APA StyleStandage-Beier, C. S., Garcia, L. A., De Filippis, E., Shaibi, G. Q., Mandarino, L. J., & Coletta, D. K. (2023). Association of Vitamin D Receptor Gene Polymorphisms with Cardiometabolic Phenotypes in Hispanics: A Life Course Approach. Nutrients, 15(9), 2118. https://doi.org/10.3390/nu15092118