Associations between Maternal Nutrition and the Concentrations of Human Milk Oligosaccharides in a Cohort of Healthy Australian Lactating Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Maternal Investigations
2.2. Milk Sampling Protocol
2.3. Human Milk Compositional Analyses
2.4. Statistical Analyses
3. Results
3.1. Human Milk Composition
3.2. Associations between Human Milk Composition and Maternal Variables
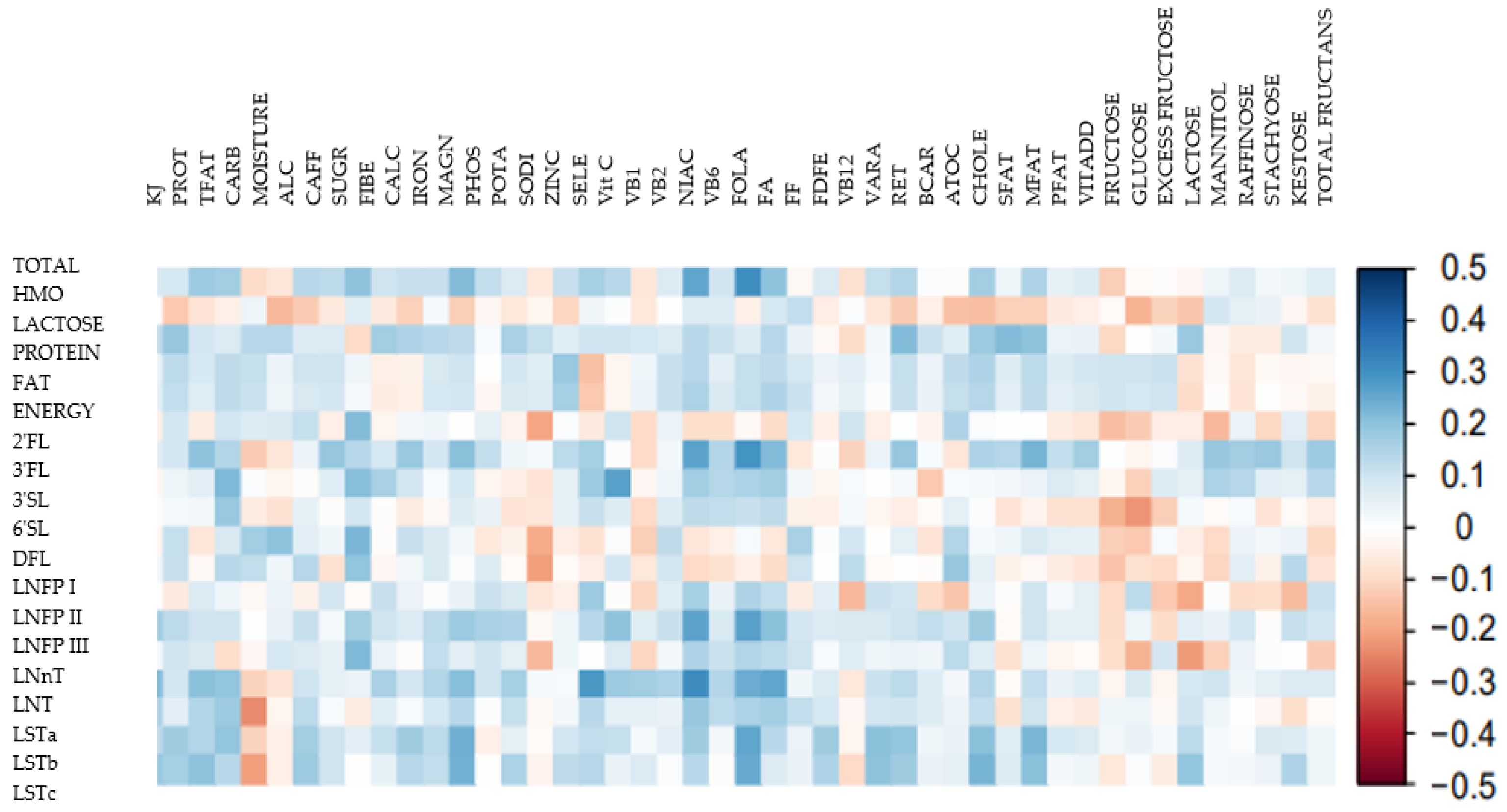
3.3. Associations between HMO Profiles and Maternal Dietary Intake
3.4. Associations between HMO Profiles and Maternal Anthropometry
3.5. Comparison of Maternal Body Composition Variables and Human Milk Values
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef]
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef] [PubMed]
- Kunz, C.; Rudloff, S. Compositional analysis and metabolism of human milk oligosaccharides in infants. Nestlé Nutr. Inst. Workshop Ser. 2017, 88, 137–147. [Google Scholar] [PubMed]
- Jantscher-Krenn, E.; Bode, L. Human milk oligosaccharides and their potential benefits for the breast-fed neonate. Minerva Pediatr. 2012, 64, 83–99. [Google Scholar] [PubMed]
- Thurl, S.; Munzert, M.; Henker, J.; Boehm, G.; Müller-Werner, B.; Jelinek, J.; Stahl, B. Variation of human milk oligosaccharides in relation to milk groups and lactational periods. Br. J. Nutr. 2010, 104, 1261–1271. [Google Scholar] [CrossRef]
- Coppa, G.V.; Gabrielli, O.; Pierani, P.; Catassi, C.; Carlucci, A.; Giorgi, P.L. Changes in carbohydrate composition in human milk over 4 months of lactation. Pediatrics 1993, 91, 637–641. [Google Scholar] [CrossRef]
- McGuire, M.K.; Meehan, C.L.; McGuire, M.A.; Williams, J.E.; Foster, J.; Sellen, D.W.; Kamau-Mbuthia, E.W.; Kamundia, E.W.; Mbugua, S.; Moore, S.E.; et al. What’s normal? Oligosaccharide concentrations and profiles in milk produced by healthy women vary geographically. Am. J. Clin. Nutr. 2017, 105, 1086–1100. [Google Scholar] [CrossRef]
- Azad, M.B.; Robertson, B.; Atakora, F.; Becker, A.B.; Subbarao, P.; Moraes, T.J.; Mandhane, P.J.; Turvey, S.E.; Lefebvre, D.L.; Sears, M.R.; et al. Human Milk Oligosaccharide Concentrations Are Associated with Multiple Fixed and Modifiable Maternal Characteristics, Environmental Factors, and Feeding Practices. J. Nutr. 2018, 148, 1733–1742. [Google Scholar] [CrossRef]
- Ferreira, A.L.; Alves, R.; Figueiredo, A.; Alves-Santos, N.; Freitas-Costa, N.; Batalha, M.; Yonemitsu, C.; Manivong, N.; Furst, A.; Bode, L.; et al. Human Milk Oligosaccharide Profile Variation Throughout Postpartum in Healthy Women in a Brazilian Cohort. Nutrients 2020, 12, 790. [Google Scholar] [CrossRef]
- Samuel, T.M.; Binia, A.; de Castro, C.A.; Thakkar, S.K.; Billeaud, C.; Agosti, M.; Al-Jashi, I.; Costeire, M.J.; Marchinr, G.; Martinez-Costa, C.; et al. Impact of maternal characteristics on human milk oligosaccharide composition over the first 4 months of lactation in a cohort of healthy European mothers. Sci. Rep. 2019, 9, 11767. [Google Scholar] [CrossRef]
- Davis, J.C.; Lewis, Z.T.; Krishnan, S.; Bernstein, R.M.; Moore, S.E.; Prentice, A.M.; Mills, D.A.; Lebrilla, C.B.; Zivkovic, A.M. Growth and Morbidity of Gambian Infants are Influenced by Maternal Milk Oligosaccharides and Infant Gut Microbiota. Sci. Rep. 2017, 7, 40466. [Google Scholar] [CrossRef]
- Biddulph, C.; Holmes, M.; Kuballa, A.; Davies, P.S.W.; Koorts, P.; Carter, R.J.; Maher, J. Human Milk Oligosaccharide Profiles and Associations with Maternal Nutritional Factors: A Scoping Review. Nutrients 2021, 13, 965. [Google Scholar] [CrossRef] [PubMed]
- Selma-Royo, M.; González, S.; Gueimonde, M.; Chang, M.; Fürst, A.; Martínez-Costa, C.; Bode, L.; Collado, M.C. Maternal Diet Is Associated with Human Milk Oligosaccharide Profile. Mol. Nutr. Food Res. 2022, 66, e2200058. [Google Scholar] [CrossRef] [PubMed]
- Seferovic, M.D.; Mohammad, M.; Pace, R.M.; Engevik, M.; Versalovic, J.; Bode, L.; Haymond, M.; Aagaard, K.M. Maternal diet alters human milk oligosaccharide composition with implications for the milk metagenome. Sci. Rep. 2020, 10, 22092. [Google Scholar] [CrossRef]
- Quin, C.; Vicaretti, S.D.; Mohtarudin, N.A.; Garner, A.M.; Vollman, D.M.; Gibson, D.L.; Zandberg, W.F. Influence of sulfonated and diet-derived human milk oligosaccharides on the infant microbiome and immune markers. J. Biol. Chem. 2020, 295, 4035–4048. [Google Scholar] [CrossRef]
- Li, X.; Mao, Y.; Liu, S.; Wang, J.; Li, X.; Zhao, Y.; Hill, D.R.; Wang, S. Vitamins, Vegetables and Metal Elements Are Positively Associated with Breast Milk Oligosaccharide Composition among Mothers in Tianjin, China. Nutrients 2022, 14, 4131. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Feng, J.; Yang, J.; Gu, G. The relationship between dietary vitamin A intake and the levels of sialic acid in the breast milk of lactating women. J. Nutr. Sci. Vitaminol. 2013, 59, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Isganaitis, E.; Venditti, S.; Matthews, T.J.; Lerin, C.; Demerath, E.W.; Fields, D.A. Maternal obesity and the human milk metabolome: Associations with infant body composition and postnatal weight gain. Am. J. Clin. Nutr. 2019, 110, 111–120. [Google Scholar] [CrossRef]
- Larsson, M.W.; Lind, M.V.; Laursen, R.P.; Yonemitsu, C.; Larnkjær, A.; Mølgaard, C.; Michaelsen, K.F.; Bode, L. Human Milk Oligosaccharide Composition Is Associated with Excessive Weight Gain during Exclusive Breastfeeding—An Explorative Study. Front. Pediatr. 2019, 7, 521. [Google Scholar] [CrossRef]
- Tonon, K.M.; de Morais, M.B.; Abrão, A.C.F.V.; Miranda, A.; Morais, T.B. Maternal and Infant Factors Associated with Human Milk Oligosaccharides Concentrations According to Secretor and Lewis Phenotypes. Nutrients 2019, 11, 1358. [Google Scholar] [CrossRef]
- Garrow, J.S.; Webster, J. Quetelet’s index (W/H2) as a measure of fatness. Int. J. Obes. 1985, 9, 147–153. [Google Scholar]
- Schlaff, R.A.; Baruth, M.; Deere, S.J.; Boggs, A.; Odabasic, A. Associations between prenatal diet quality and gestational weight gain. Nutr. Health 2020, 26, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Widaman, A.M.; Keim, N.L.; Burnett, D.J.; Miller, B.; Witbracht, M.G.; Widaman, K.F.; Laugero, K.D. A Potential Tool for Clinicians; Evaluating a Computer-Led Dietary Assessment Method in Overweight and Obese Women during Weight Loss. Nutrients 2017, 9, 218. [Google Scholar] [CrossRef]
- Rhee, J.J.; Sampson, L.; Cho, E.; Hughes, M.D.; Hu, F.B.; Willett, W.C. Comparison of methods to account for implausible reporting of energy intake in epidemiologic studies. Am. J. Epidemiol. 2015, 181, 225–233. [Google Scholar] [CrossRef]
- Biesiekierski, J.R.; Rosella, O.; Rose, R.; Liels, K.; Barrett, J.S.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. Quantification of fructans, galacto-oligosacharides and other short-chain carbohydrates in processed grains and cereals. J. Hum. Nutr. Diet. 2011, 24, 154–176. [Google Scholar] [CrossRef]
- Muir, J.G.; Rose, R.; Rosella, O.; Liels, K.; Barrett, J.S.; Shepherd, S.J.; Gibson, P.R. Measurement of short-chain carbohydrates in common Australian vegetables and fruits by high-performance liquid chromatography (HPLC). J. Agric. Food Chem. 2009, 57, 554–565. [Google Scholar] [CrossRef]
- Muir, J.G.; Shepherd, S.J.; Rosella, O.; Rose, R.; Barrett, J.S.; Gibson, P.R. Fructan and free fructose content of common Australian vegetables and fruit. J. Agric. Food Chem. 2007, 55, 6619–6627. [Google Scholar] [CrossRef] [PubMed]
- Heyward, V.H.; Wagner, D.R. Applied Body Composition Assessment; Human Kinetics: Champaign, IL, USA, 2004. [Google Scholar]
- Shepherd, J.A.; Ng, B.K.; Sommer, M.J.; Heymsfield, S.B. Body composition by DXA. Bone 2017, 104, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Gridneva, Z.; Rea, A.; Tie, W.J.; Lai, C.T.; Kugananthan, S.; Ward, L.C.; Murray, K.; Hartmann, P.E.; Geddes, D.T. Carbohydrates in Human Milk and Body Composition of Term Infants during the First 12 Months of Lactation. Nutrients 2019, 11, 1472. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.E.; Price, W.J.; Shafii, B.; Yahvah, K.M.; Bode, L.; McGuire, M.A.; McGuire, M.K. Relationships among Microbial Communities, Maternal Cells, Oligosaccharides, and Macronutrients in Human Milk. J. Hum. Lact. 2017, 33, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Johnson, T.; Sahin, L.; Tassinari, M.S.; Anderson, P.O.; Baker, T.E.; Bucci-Rechtweg, C.; Burckart, G.J.; Chambers, C.D.; Hale, T.W.; et al. Evaluation of the Safety of Drugs and Biological Products Used during Lactation: Workshop Summary. Clin. Pharmacol. Ther. 2017, 101, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Mitoulas, L.R.; Kent, J.C.; Cox, D.B.; Owens, R.A.; Sherriff, J.L.; Hartmann, P.E. Variation in fat, lactose and protein in human milk over 24 h and throughout the first year of lactation. Br. J. Nutr. 2002, 88, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Keller, R.P.; Neville, M.C. Determination of total protein in human milk: Comparison of methods. Clin. Chem. 1986, 32, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A.; Gibbs, J.A.; Lyster, R.L.; Baum, J.D. Creamatocrit: Simple clinical technique for estimating fat concentration and energy value of human milk. Br. Med. J. 1978, 1, 1018–1020. [Google Scholar] [CrossRef]
- Perrin, M.T.; Belfort, M.B.; Hagadorn, J.I.; McGrath, J.M.; Taylor, S.N.; Tosi, L.M.; Brownell, E.A. The Nutritional Composition and Energy Content of Donor Human Milk: A Systematic Review. Adv. Nutr. 2020, 11, 960–970. [Google Scholar] [CrossRef] [PubMed]
- Fenton, T.R.; Elmrayed, S. The Importance of Reporting Energy Values of Human Milk as Metabolizable Energy. Front. Nutr. 2021, 8, 655026. [Google Scholar] [CrossRef] [PubMed]
- Tonon, K.M.; Miranda, A.; Abrão, A.; de Morais, M.B.; Morais, T.B. Validation and application of a method for the simultaneous absolute quantification of 16 neutral and acidic human milk oligosaccharides by graphitized carbon liquid chromatography-electrospray ionization-mass spectrometry. Food Chem. 2019, 274, 691–697. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, T.; Chen, X.; Pang, X.; Zhang, S.; Obaroakpo, J.U.; Shilong, J.; Lu, J.; Lv, J. Absolute quantification of twelve oligosaccharides in human milk using a targeted mass spectrometry-based approach. Carbohydr. Polym. 2019, 219, 328–333. [Google Scholar] [CrossRef]
- Rasmussen, K.M.; Yaktine, A.L. Weight Gain during Pregnancy: Reexamining the Guidelines; The National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- Kim, S.Y.; Yi, D.Y. Components of human breast milk: From macronutrient to microbiome and microRNA. Clin. Exp. Pediatr. 2020, 63, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Picciano, M.F. Nutrient composition of human milk. Pediatr. Clin. N. Am. 2001, 48, 53–67. [Google Scholar] [CrossRef]
- Hale, T.W.; Hartmann, P.E. Hale & Hartmann’s Textbook of Human Lactation; Hale Pub: Amarillo, TX, USA, 2007. [Google Scholar]
- National Health and Medical Research Council. Nutrient Reference Values for Australia and New Zealand. In Commonwealth Department of Health and Ageing: Australia; National Health and Medical Research Council: Canberra, Australia, 2006. [Google Scholar]
- Thum, C.; Wall, C.R.; Weiss, G.A.; Wang, W.; Szeto, I.M.-Y.; Day, L. Changes in HMO Concentrations throughout Lactation: Influencing Factors, Health Effects and Opportunities. Nutrients 2021, 13, 2272. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.H. B vitamins in breast milk: Relative importance of maternal status and intake, and effects on infant status and function. Adv. Nutr. 2012, 3, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Food Standards Australia New Zealand. AUSNUT 2011–13–Australian Food Composition Database; Food Standards Australia New Zealand: Territory, Australia, 2014. [Google Scholar]
- Lee, Y.Q.; Loh, J.; Ang, R.S.E.; Chong, M.F.-F. Tracking of Maternal Diet from Pregnancy to Postpregnancy: A Systematic Review of Observational Studies. Curr. Dev. Nutr. 2020, 4, nzaa118. [Google Scholar] [CrossRef]
- Nuttall, F.Q. Body Mass Index: Obesity, BMI, and Health: A Critical Review. Nutr. Today 2015, 50, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Bravi, F.; Wiens, F.; Decarli, A.; Dal Pont, A.; Agostoni, C.; Ferraroni, M. Impact of maternal nutrition on breast-milk composition: A systematic review. Am. J. Clin. Nutr. 2016, 104, 646–662. [Google Scholar] [CrossRef] [PubMed]


| Variable and Category | Results |
|---|---|
| Age: | |
| 20–30 years | 35% |
| 31–40 years | 64% |
| 40+ years | 1% |
| Ethnicity: | |
| Caucasian | 93% |
| Indigenous | 1% |
| Other | 6% |
| Parity: | |
| 1 | 42% |
| 2 | 44% |
| 3+ | 14% |
| Time postpartum (weeks) | 14.4 ± 4.4 |
| Gestation period (weeks) | 39.4 ± 1.4 |
| Sex of baby: | |
| Male | 67% |
| Female | 33% |
| Birthweight of baby (kg) | 3.5 ± 0.4 |
| Usual body weight (pre-pregnancy) (kg) | 66.3 ± 10.3 |
| Body weight at time of delivery (kg) | 80.7 ± 11.0 |
| Pre-pregnancy BMI: | |
| Underweight | 4% |
| Normal | 68% |
| Overweight | 24% |
| Obese | 4% |
| Gestational weight gain (total, kg): | |
| Meets IOM guidelines | 43% |
| Exceeded IOM guidelines | 40% |
| Below IOM guidelines | 17% |
| Current BMI at time of sampling: | |
| Underweight | 2% |
| Normal | 45% |
| Overweight | 45% |
| Obese | 8% |
| Milk Component | Mean ± SD | Reference Range [41,42,43] |
|---|---|---|
| Lactose (g/L) | 75.4 ± 4.1 | 67–78 |
| Protein (g/L) | 10.4 ± 1.5 | 8–10 |
| Fat (g/L) | 39.3 ± 12.6 | 35–40 |
| Energy (kcal/L) | 697.3 ± 111.8 | 650–700 |
| HMO | Mean Concentration (mg/L) All Mothers (n = 101) | Mean Concentration (mg/L) Secretor Mothers (n = 80) | Mean Concentration (mg/L) Non-Secretor Mothers (n = 21) | p-Value 1 |
|---|---|---|---|---|
| 2′FL | 1133 ± 982 | 1431 ± 889 | 1 ± 5 | <0.001 * |
| 3FL | 937 ± 937 | 665 ± 573 | 1970 ± 1297 | <0.001 * |
| 3′SL | 126 ± 87 | 127 ± 88 | 125 ± 87 | >0.9 |
| 6′SL | 127 ± 93 | 132 ± 97 | 105 ± 77 | 0.2 |
| DFL | 49 ± 57 | 59 ± 56 | 11 ± 45 | <0.001 * |
| LNFP I | 245 ± 267 | 308 ± 266 | 4 ± 11 | <0.001 * |
| LNFP II | 747 ± 823 | 530 ± 429 | 1571 ± 1327 | 0.002 * |
| LNFP III | 284 ± 204 | 281± 189 | 296 ± 259 | 0.8 |
| LNnT | 79 ± 108 | 88 ± 110 | 46 ± 94 | 0.088 |
| LNT | 438 ± 303 | 392 ± 272 | 614 ± 355 | 0.013 * |
| LSTa | 68 ± 60 | 61 ± 49 | 94 ± 88 | 0.12 |
| LSTb | 73 ± 70 | 68 ± 61 | 91 ± 97 | 0.3 |
| LSTc | 61 ± 59 | 56 ± 55 | 80 ± 70 | 0.14 |
| TOTAL HMO | 4365 ± 2142 | 4196 ± 1978 | 5009 ± 2632 | 0.2 |
| Total Fucosylated | 3394 ± 1723 | 3273 ± 1586 | 3854 ± 2152 | 0.3 |
| Total Sialylated | 454 ± 279 | 444 ± 256 | 495 ± 357 | 0.5 |
| Total Neutral Core | 517 ± 330 | 479 ± 314 | 660 ± 361 | 0.045 * |
| Nutrient | Median | HMO | HMO Concentrations (mg/L) at Low Intake (Below Median) | HMO Concentrations (mg/L) at High Intake (Above Median) | p-Value |
|---|---|---|---|---|---|
| Protein | 97 g/day | LNFP III | 244 ± 137 | 325 ± 250 | 0.048 |
| LSTa | 55 ± 33 | 82 ± 77 | 0.024 | ||
| LSTc | 43 ± 28 | 78 ± 75 | 0.003 | ||
| Carbohydrate | 236 g/day | DFL | 61 ± 70 | 36 ± 36 | 0.024 |
| Alcohol | 0 g/day | LSTa | 75 ± 64 | 46 ± 38 | 0.008 |
| LSTb | 78 ± 77 | 54 ± 38 | 0.043 | ||
| LSTc | 67 ± 63 | 41 ± 37 | 0.015 | ||
| Calcium | 929 mg/day | 3′SL | 105 ± 70 | 148 ± 97 | 0.013 |
| LNnT | 53 ± 87 | 105 ± 121 | 0.014 | ||
| Iron | 13 mg/day | 3′SL | 102 ± 67 | 151 ± 98 | 0.004 |
| LNFP III | 241 ± 148 | 327 ± 243 | 0.035 | ||
| Potassium | 3328 mg/day | 3FL | 749 ± 768 | 1128 ± 1057 | 0.042 |
| LNFP III | 235 ± 131 | 333 ± 250 | 0.016 | ||
| LNT | 364 ± 244 | 513 ± 340 | 0.013 | ||
| LSTb | 58 ± 42 | 87 ± 88 | 0.036 | ||
| LSTc | 47 ± 41 | 74 ± 70 | 0.021 | ||
| Sodium | 2517 mg/day | LNFP III | 242 ± 142 | 327 ± 247 | 0.038 |
| 2′FL | 931 ± 908 | 1339 ± 1020 | 0.036 | ||
| Zinc | 12 mg/day | LNFP III | 236 ± 143 | 333 ± 244 | 0.017 |
| Selenium | 92 mg/day | 2′FL | 1342 ± 1001 | 920 ± 924 | 0.03 |
| Vit B2 | 2 mg/day | 3′SL | 108 ± 69 | 145 ± 99 | 0.033 |
| Vit B6 | 1.5 mg/day | LNFP III | 242 ± 148 | 327 ± 243 | 0.038 |
| LNT | 378 ± 295 | 499 ± 302 | 0.044 | ||
| Folate (total) 1 | 506 µg/day | Total HMO | 3802 ± 1951 | 4940 ± 2193 | 0.007 |
| 3FL | 722 ± 710 | 1155 ± 1087 | 0.02 | ||
| LNFP II | 565 ± 500 | 932 ± 1028 | 0.026 | ||
| LNFP III | 232 ± 170 | 337 ± 224 | 0.009 | ||
| LNT | 337 ± 269 | 541 ± 304 | <0.001 | ||
| Folate, DFE | 600 µg/day | Total HMO | 3918 ± 2017 | 4821 ± 2188 | 0.033 |
| Folate, natural | 341 µg/day | Total HMO | 3627 ± 1621 | 5118 ± 2352 | <0.001 |
| 3FL | 676 ± 672 | 1202 ± 1090 | 0.005 | ||
| LNFP II | 574 ± 527 | 923 ± 1018 | 0.034 | ||
| LNFP III | 225 ± 126 | 343 ± 248 | 0.004 | ||
| LNT | 342 ± 218 | 536 ± 346 | 0.001 | ||
| LSTa | 53 ± 34 | 83 ± 76 | 0.012 | ||
| LSTb | 57 ± 45 | 89 ± 86 | 0.023 | ||
| LSTc | 47 ± 41 | 74 ± 70 | 0.021 | ||
| RAE 2 | 945 µg/day | LSTa | 55 ± 30 | 81 ± 78 | 0.031 |
| LSTc | 48 ± 39 | 73 ± 72 | 0.035 | ||
| Beta-Carotene | 2932 µg/day | LSTa | 54 ± 30 | 83 ± 78 | 0.018 |
| LSTb | 59 ± 43 | 87 ± 88 | 0.047 | ||
| A-tocopherol 3 | 13 mg/day | LSTb | 58 ± 45 | 87 ± 87 | 0.038 |
| LSTc | 47 ± 39 | 74 ± 71 | 0.022 | ||
| MUFA | 40 mg/day | LNFP III | 44 ± 136 | 325 ± 251 | 0.047 |
| LSTb | 58 ± 42 | 88 ± 88 | 0.035 | ||
| LSTc | 46 ± 34 | 75 ± 73 | 0.013 | ||
| Stachyose | 0.3 g/day | LNFP I | 190 ± 234 | 300 ± 289 | 0.038 |
| FM (kg) | FM (%) | FMI (kg/m2) | FFM (g) | FFMI (kg/m2) | FM:FFM Proportion | AFM:GFM Proportion | |
|---|---|---|---|---|---|---|---|
| Mean ± SD | 25.4 ± 8.2 | 36 ± 7 | 9.6 ± 3.1 | 42.7 ± 4.6 | 17.4 ± 1.3 | 0.6 ± 0.2 | 0.4 ± 0.1 |
| Range | 10.9–44.5 | 20–50 | 4–17 | 34.7–54.0 | 5.2–18.7 | 0.2–0.9 | 0.2–0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biddulph, C.; Holmes, M.; Tran, T.D.; Kuballa, A.; Davies, P.S.W.; Koorts, P.; Maher, J. Associations between Maternal Nutrition and the Concentrations of Human Milk Oligosaccharides in a Cohort of Healthy Australian Lactating Women. Nutrients 2023, 15, 2093. https://doi.org/10.3390/nu15092093
Biddulph C, Holmes M, Tran TD, Kuballa A, Davies PSW, Koorts P, Maher J. Associations between Maternal Nutrition and the Concentrations of Human Milk Oligosaccharides in a Cohort of Healthy Australian Lactating Women. Nutrients. 2023; 15(9):2093. https://doi.org/10.3390/nu15092093
Chicago/Turabian StyleBiddulph, Caren, Mark Holmes, Trong D. Tran, Anna Kuballa, Peter S. W. Davies, Pieter Koorts, and Judith Maher. 2023. "Associations between Maternal Nutrition and the Concentrations of Human Milk Oligosaccharides in a Cohort of Healthy Australian Lactating Women" Nutrients 15, no. 9: 2093. https://doi.org/10.3390/nu15092093
APA StyleBiddulph, C., Holmes, M., Tran, T. D., Kuballa, A., Davies, P. S. W., Koorts, P., & Maher, J. (2023). Associations between Maternal Nutrition and the Concentrations of Human Milk Oligosaccharides in a Cohort of Healthy Australian Lactating Women. Nutrients, 15(9), 2093. https://doi.org/10.3390/nu15092093







