Abstract
Coronavirus disease 2019 (COVID-19) restrictions have been correlated with vitamin D deficiency in children, but some uncertainties remain. We retrospectively studied vitamin 25-(OH) D blood levels in 2182 Italian children/adolescents hospitalized for various chronic diseases in the year before (n = 1052) and after (n = 1130) the nationwide lockdown. The type of underlying disease, gender, and mean age (91 ± 55 and 91 ± 61 months, respectively) of patients included in the two periods were comparable. Although mean levels were the same (p = 0.24), deficiency status affected a significantly higher number of subjects during the lockdown period than in the pre-COVID period (p = 0.03), particularly in summer (p = 0.02), and there was also a smoothing of seasonal variations in vitamin D levels. Particularly at risk were males (OR = 1.22; p = 0.03), the 1–5 year age group (OR = 1.57; p < 0.01) and the 6–12 year age group (OR = 1.30; p = 0.04). Infants appeared not to be affected (p = 1.00). In the post-COVID period, the risk of vitamin D deficiency was unchanged in disease-specific groups. However, the proportion of deficiency or severe deficiency differed significantly in the subgroup with endocrinopathy (higher; Chi-square p = 0.04), and with respiratory problems and obesity (lower; Chi-square p = 0.01 and p < 0.01, respectively). Conflicting/opposite literature results advocate for further studies to clearly indicate the need for supplementation during possible future periods of confinement.
1. Introduction
An optimal serum vitamin D status is fundamental for the promotion of health both at the pediatric age and in adulthood [1]. Beyond regulating the calcium-phosphate metabolism, vitamin D has been found to have a vital role at all ages concerning bone mass and metabolism, and also multiple central extra-skeletal effects on several target organs, such as adipose tissue, blood cells, the immune system, skin, muscles, endocrine pancreas, and blood vessels. Recent years’ relentless interest raising in vitamin D has been made possible and fueled by the discovery of the molecular mechanisms by which this molecule acts as a hormone. Its receptor (VDR) mediating the hormonal function in several physiological processes is expressed in almost all organs and acts via the genomic associated (nuclear VDR) and the non-genomic pathways (membrane VDR). The human body’s vitamin D is generated in large part through the skin synthesis of the provitamin dehydrocholesterol under the influence of ultraviolet radiation. Diet is a secondary minor source of cholecalciferol/vitamin D3 and ergocalciferol/vitamin D2 forms of vitamin D in animal and in plant products, respectively. Vitamin D so obtained is biologically inert and must undergo two hydroxylation reactions in the body to become active. The first occurs in the liver and converts vitamin D to 25-hydroxyvitamin D [25(OH)D], also known as “calcidiol”; the second happens mostly in the kidney and produces the physiologically active 1,25-dihydroxyvitamin D [1,25(OH)2D], also known as “calcitriol” [2,3].
Having said that, it appears clear that factors that may influence vitamin D status therefore include sun exposure, skin pigmentation/race, season, BMI, and nutritional factors (consumption of vitamin D-rich foods or vitamin supplements). In recent decades, adolescents have seemed to be particularly at risk for hypovitaminosis D, due to an increased tendency to conduct a sedentary indoor life with an increased access to computers, televisions and smartphones [4,5]. More recently, the coronavirus disease 2019 (COVID-19) pandemic determined the further confinement of adults and children for a very long period, with possible significant short-term and long-term effects on health. Confinement definitely reduced exposure time to sunlight, especially for people who do not live in rural areas, determining decreased cholecalciferol synthesis in the lower layers of the epidermis, which is the major source of vitamin D at all ages. Epidemiological studies have reported that the COVID-19 pandemic changed vitamin D levels in school and pre-school children [6,7,8]. Patients suffering from chronic diseases such as obesity, intestinal or hepatobiliary/pancreatic pathologies, pharmacologically treated epilepsy, and cancer have been described as being at an even higher risk of vitamin D deficiency [9,10], which could have been worsened during pandemics, but data from the COVID-19 period are lacking or conflicting. Our study aimed to fill these existing gaps and analyze the effects of the COVID-19 global-pandemic-related lockdown on the serum levels and status of 25-(OH)D in a cohort of children seen at a single pediatric third level hospital. We evaluated subgroups of children affected by several types of chronic diseases, also considering age, gender, season.
2. Materials and Methods
On 21 February 2020 the first case of COVID-19 was diagnosed in Italy, and a few weeks later, on 11 March 2020, the WHO officially declared it a pandemic. The Italian government officially announced a nationwide lockdown on 9 March 2020; this decree implied the total closure of schools, universities, public squares and all shops except supermarkets, grocery stores and pharmacies, as well as clearly defined travel restrictions [11]. We retrospectively enrolled children aged 1 month to 17 years of age that had performed a routine measurement of serum vitamin D levels during a hospitalization or a day in hospital in any ward of the third level Pediatric Hospital Santobono–Pausilipon in Naples between 11 March 2019 and 11 March 2021. Enrolled subjects were subsequently assigned to one of two groups, depending on when they had been hospitalized: the “pre-COVID group” for children hospitalized between March 2019 and March 2020, and the “post-COVID group” for children hospitalized between March 2020 and March 2021.
For the purpose of statistical analysis, each group was then split into further subgroups based on age range (0–12 months, 1–5 years, 6–12 years, >12 years; Table S1), gender (male, female; Table S2) or underlying pathology (endocrine, short stature, hepatic, nephrological, hematological, pneumological, obesity, neurological, oncological, gastroenterological, or other; Table S3). To evaluate the effects of the COVID-19-related lockdown on the serum concentration of vitamin D during different seasons, we performed separate analyses for each season, thus comparing pre-pandemic mean values to post-pandemic ones in the same season of the year. Serum samples were stored at 2–8 °C before assay.
Due to its quite long circulating half-life, serum concentrations of 25(OH)D rather than those of 1,25(OH)2D are the main acknowledged indicator of vitamin D status, reflecting vitamin D produced endogenously and vitamin D obtained from foods and supplements [2]. We measured the 25(OH) D concentration using automated electrochemiluminescence assay (ECLIA). For patients who had received more than one measurement of vitamin D, only the first collected value was considered for the analysis. Following the most recent recommendations released by international organizations [12] and Italian pediatric scientific societies [13], the serum vitamin D status of each study subject was evaluated in good agreement with those of the expert committee of the Food and Nutrition Board (FNB) at the National Academies of Sciences, Engineering, and Medicine (NASEM) [2]. In fact, 25(OH)D serum levels of 30 ng/mL (75 nmol/L) or greater were deemed sufficient, levels between 20 ng/mL (50 nmol/L) and 29 ng/mL were considered insufficient, subjects with serum 25(OH)D values below 20 ng/mL were considered as having vitamin deficiency, and levels below 10 ng/mL were classified as severe vitamin D deficiency.
Statistical analysis was performed using STATA software version 17 (StataCorp LP, College Station, TX, USA). Data are presented as absolute numbers, percentages, mean values and standard deviations. Taking into account that the obtained data did not display a normal distribution in our study population, and that there was a significant difference in the size of the groups, the non-parametric Kruskal–Wallis test was used to ensure the equality of the mean values amongst the groups. A Chi-square test was used to compare percentages amongst the different groups. The risk estimation between vitamin D status and the pre- and post-pandemic periods for the main potentially associated factors was analyzed. The response variable was dichotomized (vitamin D deficiency vs. non-deficiency) and the odds ratios with their corresponding p-value were calculated. The serum vitamin D level is asymmetrical and not normally distributed. In order to assess the effect on vitamin D levels of gender, age groups, seasons, and period (pre-COVID and post-COVID), generalized linear models (GLM) were applied with gamma distribution and inverse link function [14]. Furthermore, the presence of any interactions between the pandemic period and the other covariates was evaluated. A p-value level < 0.05 was considered statistically significant.
3. Results
Over the period under investigation, 2182 patients underwent a first serum vitamin D level measurement. Of them, 1052 patients had been hospitalized in the “pre-COVID” period (males = 549; females = 503; mean age 91 ± 55.08 months), while 1130 subjects had been hospitalized in the “post-COVID” period (males = 588; females = 542; mean age 91 ± 61.95 months). There were no statistically significant differences in age (p = 0.83) and gender (p = 0.94) distribution between the two groups.
Mean levels of vitamin D in the pre- and post-COVID groups (26.37 ng/mL vs. 25.67 ng/mL; p = 0.24) were comparable; however, there were more subjects with vitamin D severe deficiency or deficiency in the post-COVID group compared to the pre-COVID group (severe deficiency or deficiency: 40% vs. 36%; OR = 1.38, p = 0.01). (Figure 1) When studying different age groups, the 1–5 years and 6–12 years subgroups showed a significantly larger proportion of subjects with severely deficient or deficient vitamin D in the post-COVID period, with a higher risk of vitamin D deficiency (1–5 years: severe deficiency or deficiency: 39% vs. 29%; OR = 1.57, p < 0.01; 6–12 years: severe deficiency or deficiency: 48% vs. 41%; OR = 1.30, p = 0.04). (Figure 1) (Table S1) On the other hand, infants did not show abnormal values in either period. By analyzing the two genders separately, males had a significantly higher level of vitamin D severe deficiency or deficiency in the post-COVID group compared to the pre-COVID group (severe deficiency or deficiency: 41% vs. 34%; OR = 1.22, p = 0.03) (Figure 1) (Table S2).
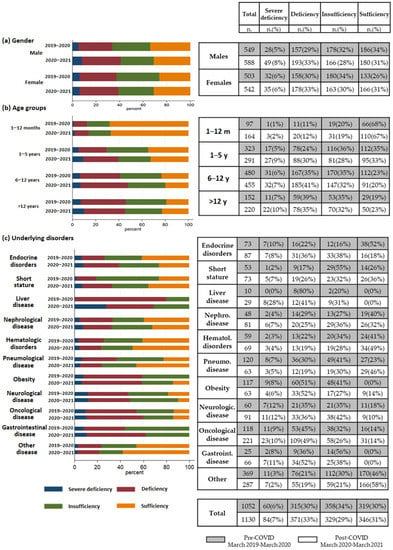
Figure 1.
Comparison of serum vitamin D status by gender (a), age groups (b) and underlying disorders (c) in the pre- and post-COVID period (absolute frequencies, percentages).
Regarding the underlying diseases of our study population in the two periods, they were characterized by 11 subgroups: endocrinopathies (n = 73 and 87); short stature (n = 53 and 73); hepatopathy (n = 10 and 29); nephropathy (n = 48 and 81); hematological disorders (n = 59 and 69); pulmonary disease (n = 120 and 63); obesity (n = 117 and 63); neurological disorders (n = 60 and 91); oncological disease (n = 118 and 221); gastroenterological disorders (n = 25 and 66); and other diseases (n = 369 and 287). The vitamin D status of each of these subgroups is shown in Figure 1 and detailed in Table S3. The statistical analysis of the subgroups based on the underlying disorders of the studied subjects showed a relationship between vitamin D status and period, with higher percentages of vitamin D deficiency or severe deficiency in individuals affected by endocrine disorders (severe deficiency: 8% vs. 6%; deficiency: 31% vs. 20%; Chi-squared p = 0.04). (Figure 1). Furthermore, a significant association was observed between vitamin D status and pandemic period, with a lower and similar proportion of deficiency and severe deficiency in the post-COVID period, respectively, for patients with respiratory problems and obesity (Chi-squared p = 0.01 and p < 0.01, respectively). However, by calculating the odds ratios, no disease-specific group showed a statistically significant change in the risk of vitamin D deficiency during the post-pandemic period.
Lastly, by comparing values obtained over the course of different seasons, only during the summer season were there significantly lower concentrations of serum vitamin D in the post-pandemic group compared to the previous period (p = 0.02). This was accompanied by a perceptible smoothing of the vitamin D levels peak of the summer seasonal variability versus the other periods in the same year (Figure 2).
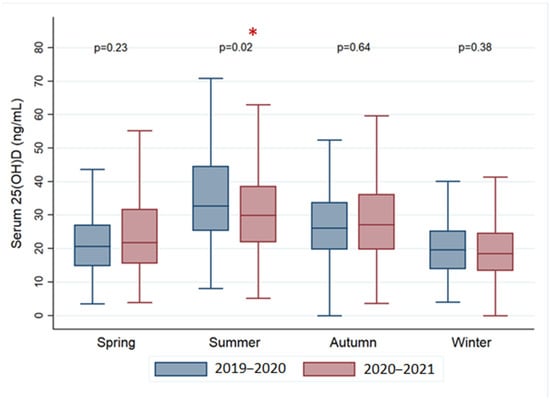
Figure 2.
Comparison of the mean concentrations of serum vitamin D during the different seasons in the pre- and post-COVID period. * statistically significant.
In a multivariate analysis, we assessed the effect of gender, age group, season, and period on vitamin D levels (Table 1).

Table 1.
Multiple generalized linear model.
After adjusting for the covariates, the impact of the pandemic period on vitamin D levels is evident for gender (p < 0.05) and age (p < 0.01), with the first year of life showing significantly higher levels (mean 39.28 mg/dL). A significant interaction between seasonality and the difference between the pre- and post-pandemic periods (p < 0.01) emerges, as well.
4. Discussion
The importance of vitamin D in maintaining overall health cannot be ignored. It plays, in fact, a central role, especially in calcium absorption, bone mineralization, and immune system function, so that its deficiency is deemed responsible for a range of health issues which include, among others, rickets, osteoporosis, and increased susceptibility to autoimmune diseases and infections. The COVID-19 pandemic-related lockdown therefore brought some attention to the impact of confinement on the health of children during this exceptional period. Our retrospective, monocentric investigation was aimed at examining the correlation between the Italian nationwide lockdown measures implemented during the COVID-19 pandemic and the risk of vitamin D deficiency in a pediatric population. The study draws on a large cohort, therefore providing robust evidence to support the findings that the incidence of hypovitaminosis D during the pandemic was, in general, higher than in the pre-COVID period, with a greater prevalence among males and children aged 1–5 and 6–12 years. The above differences were particularly evident during the summer season, suggesting that these age groups may have been most affected due to the confinement-related reduced opportunities for outdoor activities and exposure to sunlight.
Seasonal fluctuations in serum vitamin D usually demonstrate the lowest values in winter and autumn and the highest values in summer and spring. Although in most geographic regions seasonal changes by themselves do not cause significant decreases in serum vitamin D, they can, however, result in lower levels in individuals at risk of vitamin D deficiency. In all cases, it has also been shown that the seasonal difference can be corrected to some degree with the help of vitamin D supplementation [15]. In this regard, it is worth noting that the usual routine supplementation of vitamin D for Italian infants under 12 months suggested by the scientific pediatric societies [13,16] may have therefore played a likely protective role, explaining the lack of significant differences in this age group between the pre- and post-COVID periods.
Previous surveys in the pediatric population have already reported a correlation between the global pandemic and vitamin D deficiency. A recent meta-analysis of a cohort of approximately 2000 individuals per period reported in five studies (of which four were retrospective and two focused specifically on infants) showed a significant decrease in vitamin D levels during the pandemic in all pediatric age groups except infants, as compared to the pre-pandemic period [17]. However, when the meta-analysis considered different classes based on their vitamin D status, the proportions of cases with vitamin deficiency or insufficiency remained unchanged [17], whereas we could register a severe deficiency of vitamin D only in the 1–5 and 6–12 year subgroups. Some differences among studies (e.g., smoothing of the vitamin D levels’ seasonal variability only in some cohorts) [18] may be attributable to the geographical heterogeneity of the studies included in the meta-analysis (Greece, Poland, Hong Kong, Korea, China), national differences in lockdown severity/duration, and vitamin D prescription habits by pediatricians, as well. Other factors which may have influenced vitamin D levels during the lockdown-related closure of schools likely comprise sociocultural and dietary aspects, including the consumption of processed and/or highly caloric foods and an excessive intake of sodas [19]. House confinement during the COVID-19 pandemic led, in fact, to dramatic shifts in the usual household food dynamics, likely impacting the health of all the family members. In this regard, a recent systematic literature review of parents’ perspectives on the changes in meal preparation and eating routines and other nutrition-related behaviors has shown that, in general, families were eating more varied foods and balanced home-cooked meals. On the other hand, however, these virtuous changes were combined with overeating and increased snacking on high-calorie snacks, desserts, and sweets. Moreover, food insecurity increased among the lowest-income families [20]. Altogether, all these elements are in agreement with the increased incidence of overweight, obesity and type 2 diabetes mellitus detected during the lockdown confinement [21].
When our results were broken down by the category of patients’ underlying disease, a surprising lower prevalence of vitamin D deficiency in children with obesity or respiratory problems emerged. Traditionally, obesity has been shown to have a negative effect on vitamin D levels due to a reduced bioavailability as a result of its adiponectin-mediated deposition in the adipose tissue compartments at all ages. Our study, instead, found a lower percentage of vitamin D deficiency in this group of patients, contrary to what had been previously observed by others [22,23,24]. However, when interpreting these findings, one should consider the likely possibility of a self-prescribed or medically recommended vitamin D supplementation. In fact, because individuals with obesity and chronic respiratory disorders, particularly atopic ones [13], were recognized to be at higher risk for more severe COVID-19 infections, vitamin D supplementation has become a widely practiced measure among these two categories of patients [25,26,27,28].
Limitations of the Study
Despite the large sample size, representing a major strength of our study, there are some potential limitations that should be acknowledged. One possible drawback that we have already mentioned is the lack of comprehensive patient clinical information, including regarding their possible vitamin D supplementation. Additionally, the generalizability of our findings may be limited to populations with a similar sun exposure index and food/nutraceutical intake. Furthermore, the observational nature of the study, while demonstrates associations, does not allow the drawing of definitive conclusions about causality. Careful consideration of these limitations is therefore necessary when interpreting the results of our study, and further research is needed to confirm and extend our findings.
5. Conclusions
In conclusion, the global pandemic and its associated lockdowns have had a significant impact on the health of children, and one area of concern is the effect on serum vitamin D levels in this population. Our research confirms that the lockdown did indeed affect these levels, with a higher incidence of hypovitaminosis D in schoolers and pre-schoolers. Interestingly, infants appeared to be less affected, with normal values being maintained, likely due to the vitamin D supplementation recommended in this age group regardless of whether they are breastfed or formula-fed [13]. Similarly, the unexpected [29] higher percentage of vitamin D sufficiency observed in children with obesity and respiratory disorders may be due to the therapeutic or preventive administration of vitamins in these patients groups, as suggested by Italian primary care pediatric recommendations [13]. Much is probably also due to the wave of recent literature reports regarding these categories’ risks during the pandemic [26,27,28]. These issues should be considered in the contingency of future lockdowns.
The findings from our research add to the growing body of evidence regarding the vitamin D status of schoolers and pre-schoolers during periods of lockdown. This highlights the need for pediatricians to be vigilant about monitoring vitamin D levels in their patients, and to prescribe supplementation when necessary. Policymakers should consider the potential health consequences of future prolonged pediatric age individuals’ confinement, including their exposure to sunlight and opportunities for outdoor activities. Obesity prevention and treatment initiatives, as well as ongoing efforts to address nutritional management, parental feeding practices, and food insecurity, may account for these changes in the future [20]. Moreover, since optimal serum levels of vitamin D for bone and general health may not be the same and are also likely to fluctuate with life stages, race, ethnicity, and the specific measures used for assessing a wide variety of physiological processes, further studies appear necessary to determine which are the optimal indications for vitamin D supplementation [2,3].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15092089/s1, Table S1: Comparison of serum vitamin D status in different age groups in the pre- and post-pandemic period; Table S2: Comparison of the serum vitamin D levels between males and females in the pre-COVID and post-COVID periods; Table S3: Comparison of vitamin D serum levels/status based on underlying disorders in the pre- and post-COVID era.
Author Contributions
Conceptualization, C.M. (Claudia Mandato), C.M. (Caterina Mosca) and A.C.; Formal analysis, F.S. and C.C.; Investigation, C.M. (Caterina Mosca), G.R., M.D.B., A.P. and A.M. (Antonio Maglione); Writing—original draft preparation, A.M. (Annalisa Morelli) and C.M. (Claudia Mandato); Writing—review and editing, C.M. (Claudia Mandato) and P.V.; Visualization, A.C., P.V. and C.M. (Claudia Mandato); Project administration, F.S. and C.M. (Claudia Mandato). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Santobono-Pausilipon Children’s Hospital (protocol code 0023956–03/12/2021).
Informed Consent Statement
At our institution, data treatment includes all patients giving their informed consent to perform diagnostic tests which may be useful for their health status at the discretion of the ward doctors.
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Marino, R.; Misra, M. Extra-Skeletal Effects of Vitamin D. Nutrients 2019, 11, 1460. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary Reference Intakes for Calcium and Vitamin D; Ross, A.C., Taylor, C.L., Yaktine, A.L., Del Valle, H.B., Eds.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Office of Dietary Supplements. Vitamin D Fact Sheet for Health Professionals. Available online: https://Ods.Od.Nih.Gov/Factsheets/Vitamind-HealthProfessional (accessed on 21 April 2023).
- Cesareo, R.; Attanasio, R.; Caputo, M.; Castello, R.; Chiodini, I.; Falchetti, A.; Guglielmi, R.; Papini, E.; Santonati, A.; Scillitani, A. Italian Association of Clinical Endocrinologists (AME) and Italian Chapter of the American Association of Clinical Endocrinologists (AACE) Position Statement: Clinical Management of Vitamin D Deficiency in Adults. Nutrients 2018, 10, 546. [Google Scholar] [CrossRef] [PubMed]
- Vierucci, F.; Del Pistoia, M.; Fanos, M.; Gori, M.; Carlone, G.; Erba, P.; Massimetti, G.; Federico, G.; Saggese, G. Vitamin D Status and Predictors of Hypovitaminosis D in Italian Children and Adolescents: A Cross-Sectional Study. Eur. J. Pediatr. 2013, 172, 1607–1617. [Google Scholar] [CrossRef] [PubMed]
- Beyazgül, G.; Bağ, Ö.; Yurtseven, İ.; Coşkunol, F.; Başer, S.; Çiçek, D.; Kanberoğlu, G.İ.; Çelik, F.; Nalbantoğlu, Ö.; Özkan, B. How Vitamin D Levels of Children Changed during COVID-19 Pandemic: A Comparison of Pre-Pandemic and Pandemic Periods. J. Clin. Res. Pediatr. Endocrinol. 2022, 14, 188. [Google Scholar] [CrossRef] [PubMed]
- Bertoldo, F.; Cianferotti, L.; Di Monaco, M.; Falchetti, A.; Fassio, A.; Gatti, D.; Gennari, L.; Giannini, S.; Girasole, G.; Gonnelli, S. Definition, Assessment, and Management of Vitamin D Inadequacy: Suggestions, Recommendations, and Warnings from the Italian Society for Osteoporosis, Mineral Metabolism and Bone Diseases (SIOMMMS). Nutrients 2022, 14, 4148. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.S.; Tung, K.T.; So, H.-K.; Wong, W.H.; Wong, S.Y.; Tsang, H.W.; Tung, J.Y.; Chua, G.T.; Ho, M.H.; Wong, I.C. Impact of COVID-19 Pandemic on Serum Vitamin D Level among Infants and Toddlers: An Interrupted Time Series Analysis and before-and-after Comparison. Nutrients 2021, 13, 1270. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, R.; Locci, C.; Clemente, M.G.; Chicconi, E.; Antonucci, L. Vitamin D Deficiency in Childhood: Old Lessons and Current Challenges. J. Pediatr. Endocrinol. Metab. 2018, 31, 247–260. [Google Scholar] [CrossRef]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D Deficiency 2.0: An Update on the Current Status Worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef]
- Valitutti, F.; Zenzeri, L.; Mauro, A.; Pacifico, R.; Borrelli, M.; Muzzica, S.; Boccia, G.; Tipo, V.; Vajro, P. Effect of Population Lockdown on Pediatric Emergency Room Demands in the Era of COVID-19. Front. Pediatr. 2020, 8, 521. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Saggese, G.; Vierucci, F.; Prodam, F.; Cardinale, F.; Cetin, I.; Chiappini, E.; de’Angelis, G.L.; Massari, M.; Miraglia Del Giudice, E.; Miraglia Del Giudice, M. Vitamin D in Pediatric Age: Consensus of the Italian Pediatric Society and the Italian Society of Preventive and Social Pediatrics, Jointly with the Italian Federation of Pediatricians. Ital. J. Pediatr. 2018, 44, 1–40. [Google Scholar] [CrossRef] [PubMed]
- McCullagh, P. Generalized Linear Models; Routledge: New York, NY, USA, 2019; ISBN 0-203-75373-9. [Google Scholar]
- Won, J.W.; Jung, S.K.; Jung, I.A.; Lee, Y. Seasonal Changes in Vitamin D Levels of Healthy Children in Mid-Latitude, Asian Urban Area. Pediatr. Gastroenterol. Hepatol. Nutr. 2021, 24, 207. [Google Scholar] [CrossRef] [PubMed]
- Munns, C.F.; Shaw, N.; Kiely, M.; Specker, B.L.; Thacher, T.D.; Ozono, K.; Michigami, T.; Tiosano, D.; Mughal, M.Z.; Mäkitie, O. Global Consensus Recommendations on Prevention and Management of Nutritional Rickets. Horm. Res. Paediatr. 2016, 85, 83–106. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Zhai, Y.; Wang, S.; Ding, K.; Yang, Z.; Tian, Y.; Huo, T. Effect of the COVID-19 Pandemic on Serum Vitamin D Levels in People under Age 18 Years: A Systematic Review and Meta-Analysis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2022, 28, e935823-1. [Google Scholar] [CrossRef] [PubMed]
- Rustecka, A.; Maret, J.; Drab, A.; Leszczyńska, M.; Tomaszewska, A.; Lipińska-Opałka, A.; Będzichowska, A.; Kalicki, B.; Kubiak, J.Z. The Impact of COVID-19 Pandemic during 2020–2021 on the Vitamin D Serum Levels in the Paediatric Population in Warsaw, Poland. Nutrients 2021, 13, 1990. [Google Scholar] [CrossRef]
- Di Renzo, L.; Gualtieri, P.; Pivari, F.; Soldati, L.; Attinà, A.; Cinelli, G.; Leggeri, C.; Caparello, G.; Barrea, L.; Scerbo, F. Eating Habits and Lifestyle Changes during COVID-19 Lockdown: An Italian Survey. J. Transl. Med. 2020, 18, 229. [Google Scholar] [CrossRef]
- Titis, E. Parental Perspectives of the Impact of COVID-19 Lockdown on Food-Related Behaviors: Systematic Review. Foods 2022, 11, 2851. [Google Scholar] [CrossRef]
- Capra, M.E.; Stanyevic, B.; Giudice, A.; Monopoli, D.; Decarolis, N.M.; Esposito, S.; Biasucci, G. The Effects of COVID-19 Pandemic and Lockdown on Pediatric Nutritional and Metabolic Diseases: A Narrative Review. Nutrients 2023, 15, 88. [Google Scholar] [CrossRef]
- Rockell, J.E.; Green, T.J.; Skeaff, C.M.; Whiting, S.J.; Taylor, R.W.; Williams, S.M.; Parnell, W.R.; Scragg, R.; Wilson, N.; Schaaf, D. Season and Ethnicity Are Determinants of Serum 25-Hydroxyvitamin D Concentrations in New Zealand Children Aged 5–14 y. J. Nutr. 2005, 135, 2602–2608. [Google Scholar] [CrossRef]
- Katz, A.; Nambi, S.S.; Mather, K.; Baron, A.D.; Follmann, D.A.; Sullivan, G.; Quon, M.J. Quantitative Insulin Sensitivity Check Index: A Simple, Accurate Method for Assessing Insulin Sensitivity in Humans. J. Clin. Endocrinol. Metab. 2000, 85, 2402–2410. [Google Scholar] [CrossRef]
- Chen, H.; Sullivan, G.; Quon, M.J. Assessing the Predictive Accuracy of QUICKI as a Surrogate Index for Insulin Sensitivity Using a Calibration Model. Diabetes 2005, 54, 1914–1925. [Google Scholar] [CrossRef] [PubMed]
- Mauro, A.; Improda, N.; Zenzeri, L.; Valitutti, F.; Vecchione, E.; Esposito, S.; Tipo, V. Infection Control Strategy and Primary Care Assistance in Campania Region during the National Lockdown Due to COVID-19 Outbreak: The Experience of Two Tertiary Emergency Centers. Ital. J. Pediatr. 2021, 47, 19. [Google Scholar] [CrossRef] [PubMed]
- Radujkovic, A.; Hippchen, T.; Tiwari-Heckler, S.; Dreher, S.; Boxberger, M.; Merle, U. Vitamin D Deficiency and Outcome of COVID-19 Patients. Nutrients 2020, 12, 2757. [Google Scholar] [CrossRef]
- Wang, Q.; Ying, Q.; Zhu, W.; Chen, J. Vitamin D and Asthma Occurrence in Children: A Systematic Review and Meta-Analysis. J. Pediatr. Nurs. 2022, 62, e60–e68. [Google Scholar] [CrossRef] [PubMed]
- Martineau, A.R.; Cates, C.J.; Urashima, M.; Jensen, M.; Griffiths, A.P.; Nurmatov, U.; Sheikh, A.; Griffiths, C.J. Vitamin D for the Management of Asthma. Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef]
- Jaybhaye, A.P.; Sangle, A.L.; Ugra, D.; Chittal, R.Y. A Hospital-Based Study of Vitamin D Levels in Children with Recurrent Respiratory Infections. Cureus 2022, 14, e27864. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).