FGF21 Depletion Attenuates Colitis through Intestinal Epithelial IL-22-STAT3 Activation in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. DSS-Induced Colitis
2.3. Human Subject
2.4. Mouse Blood Biochemical Assays
2.5. Myeloperoxidase (MPO) Activity
2.6. RNA Extraction and Quantitative Real-Time RT-PCR
2.7. Colon Organ Culture and Assessments of Proinflammatory Mediator
2.8. Antibodies
2.9. Isolation of Lamina Propria Lymphocytes (LPLs)
2.10. Flow Cytometry
2.11. Histology
2.12. Western Blot Analysis
2.13. TUNEL Assay
2.14. Statistical Analysis
3. Results
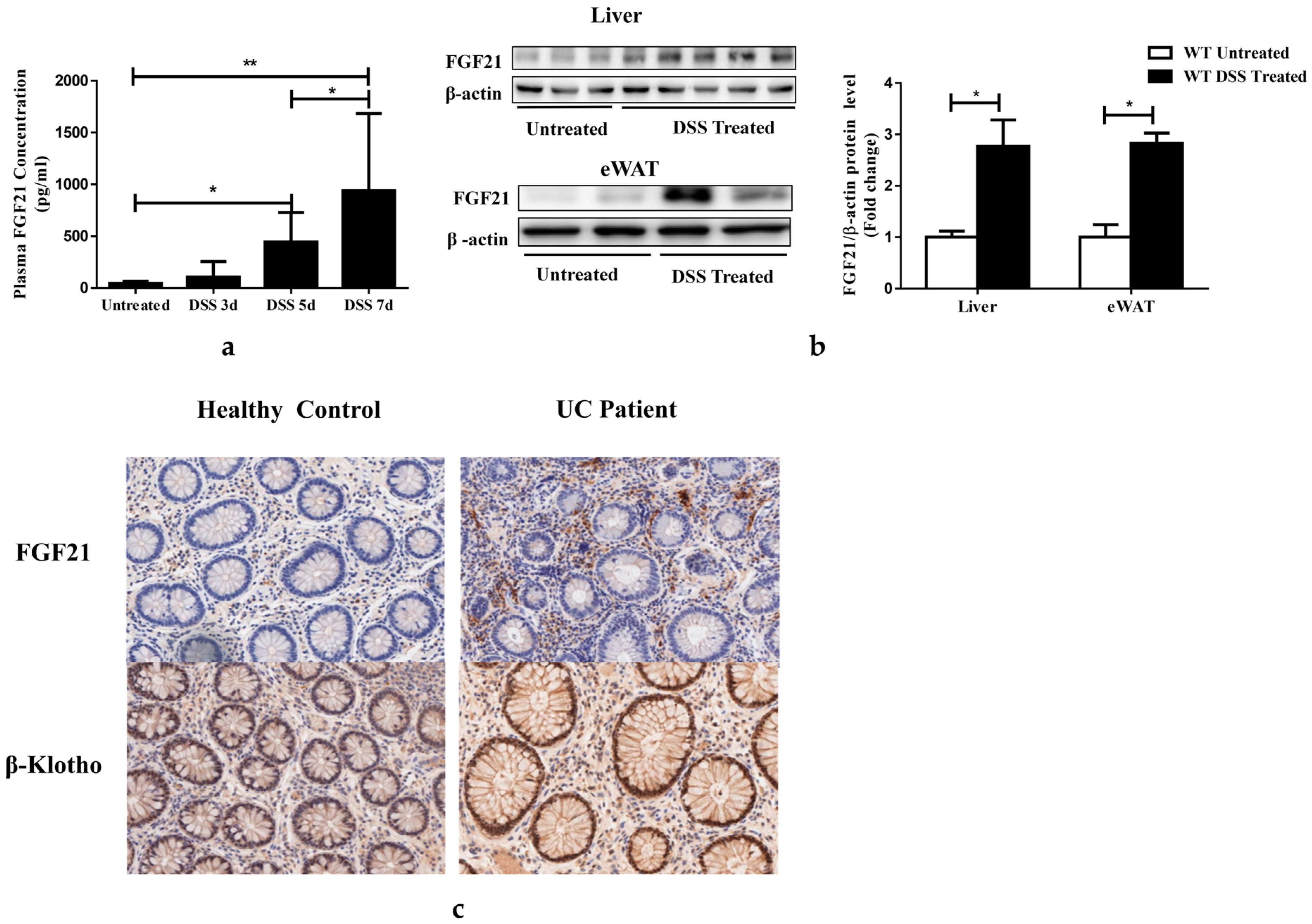
3.1. Serum FGF21 Levels Are Increased in Colitis
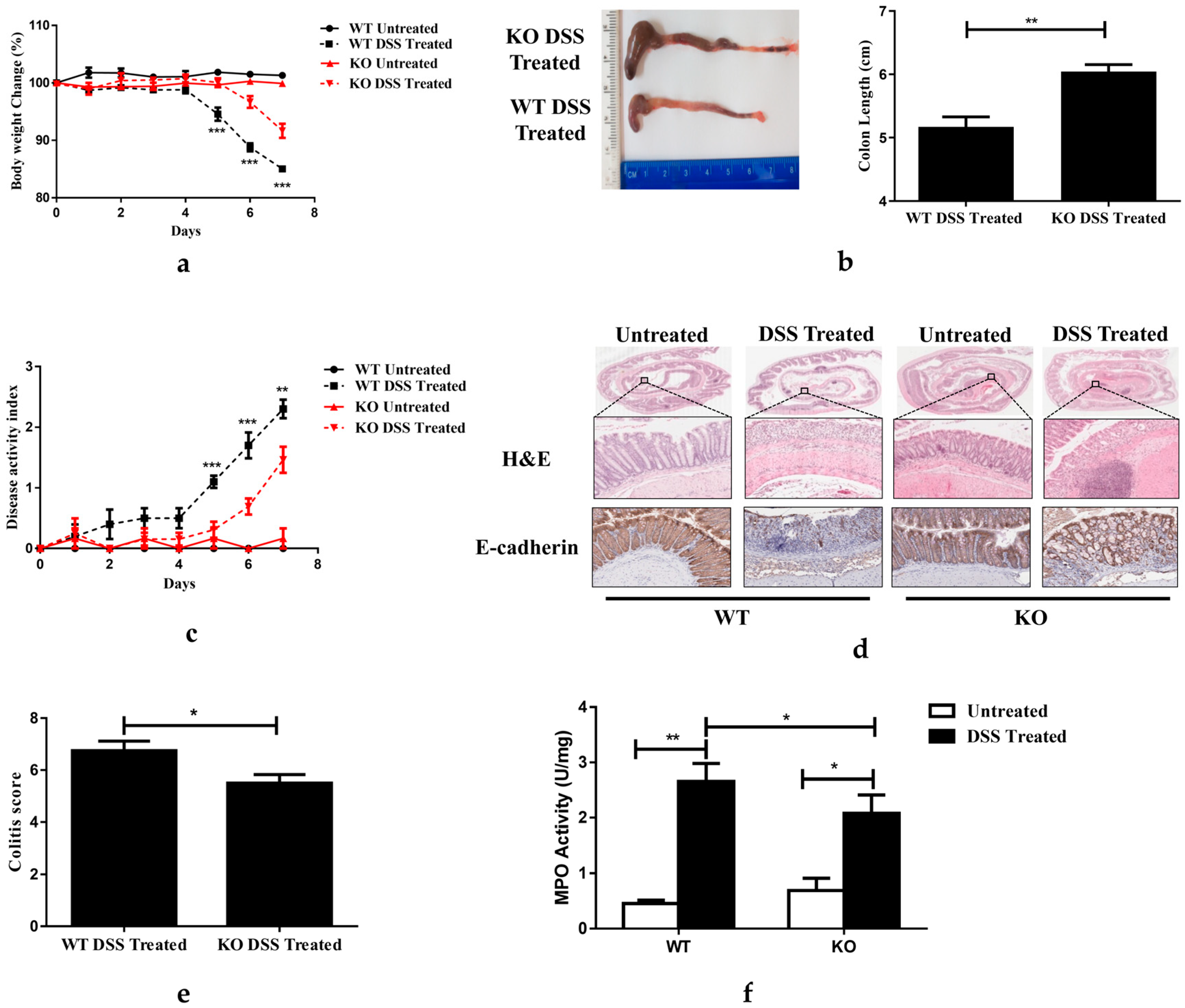
3.2. FGF21 Deficiency Protects Mice from DSS-Induced Acute Colitis
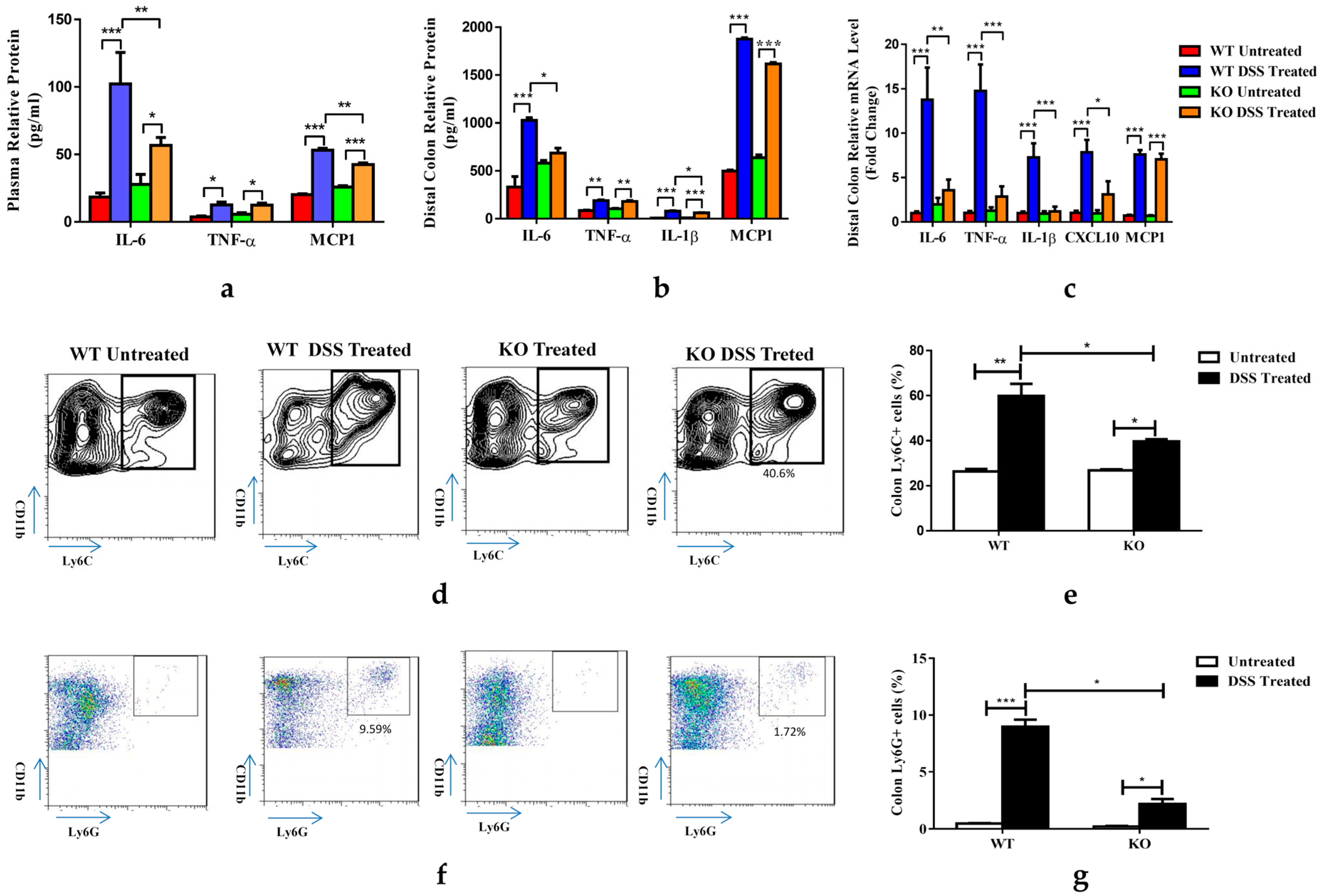
3.3. FGF21 Deficiency Decreases DSS-Induced Colon Inflammation
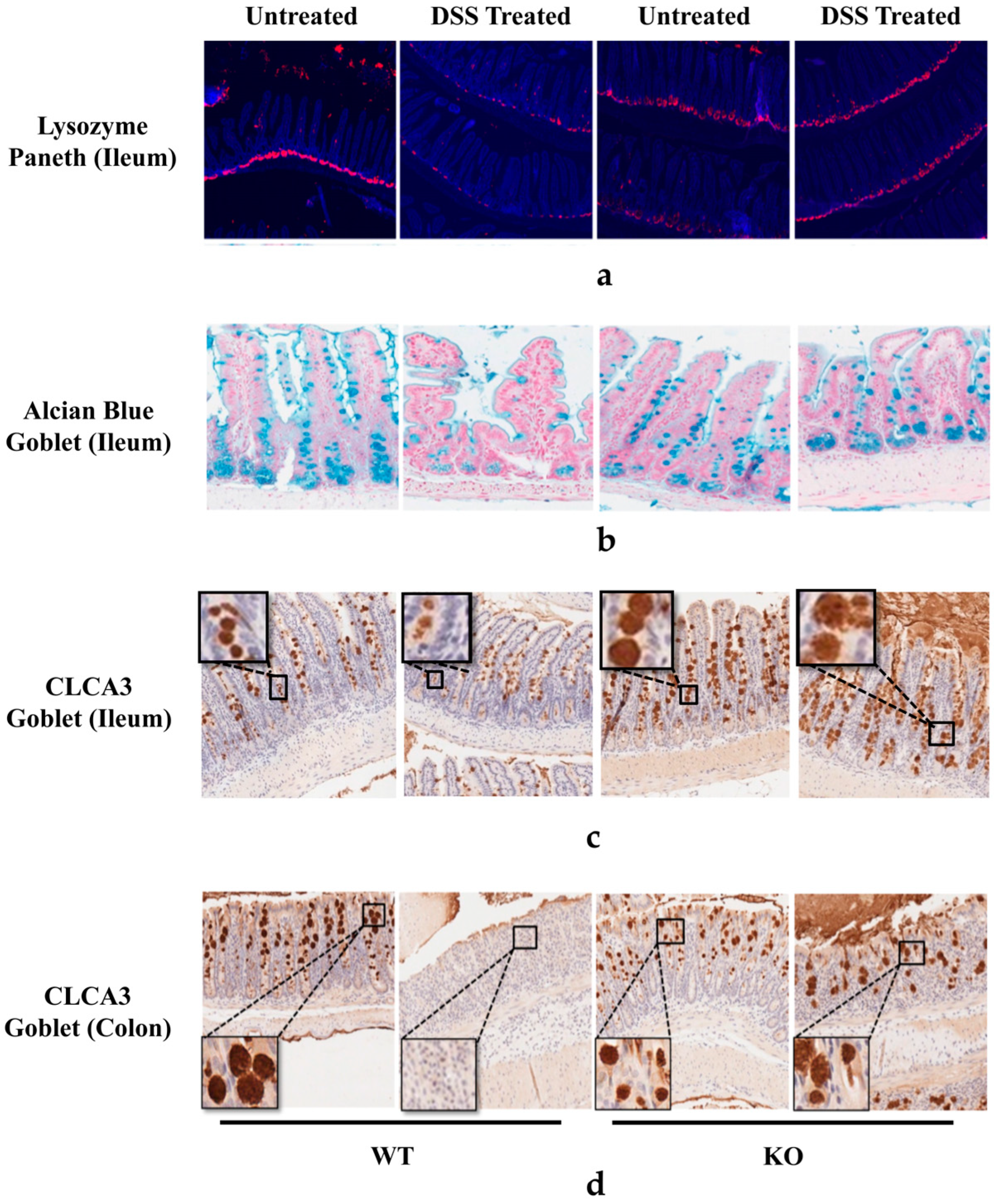
3.4. FGF21 Deficiency Prevents DSS-Induced Reduction of Paneth and Goblet Cells
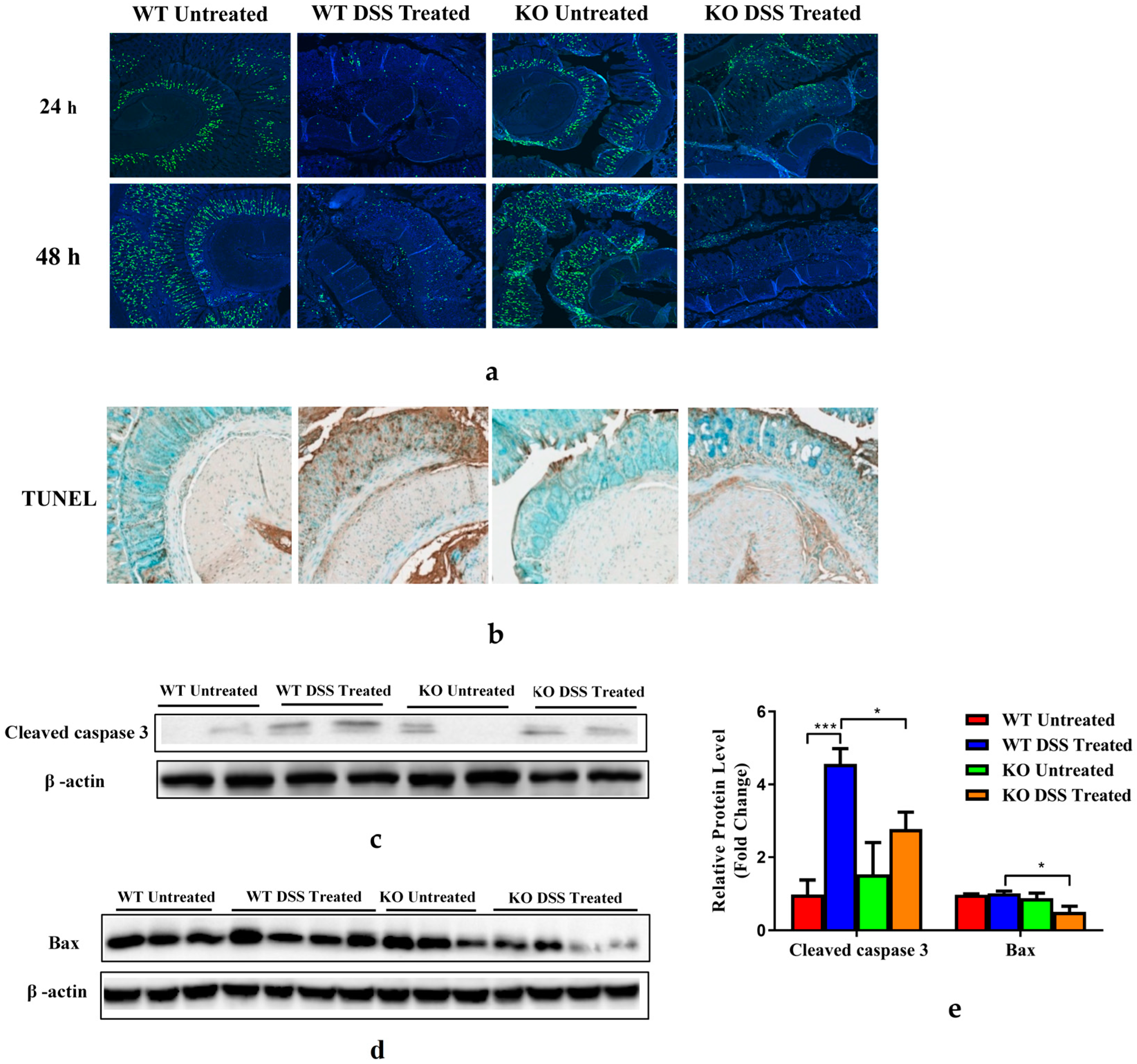
3.5. FGF21-Deficient Mice Were Protected against DSS-Induced Epithelial Cell Apoptosis and Impaired Proliferation
3.6. FGF21 Deficiency Enhances Distal Colon STAT3 Signaling
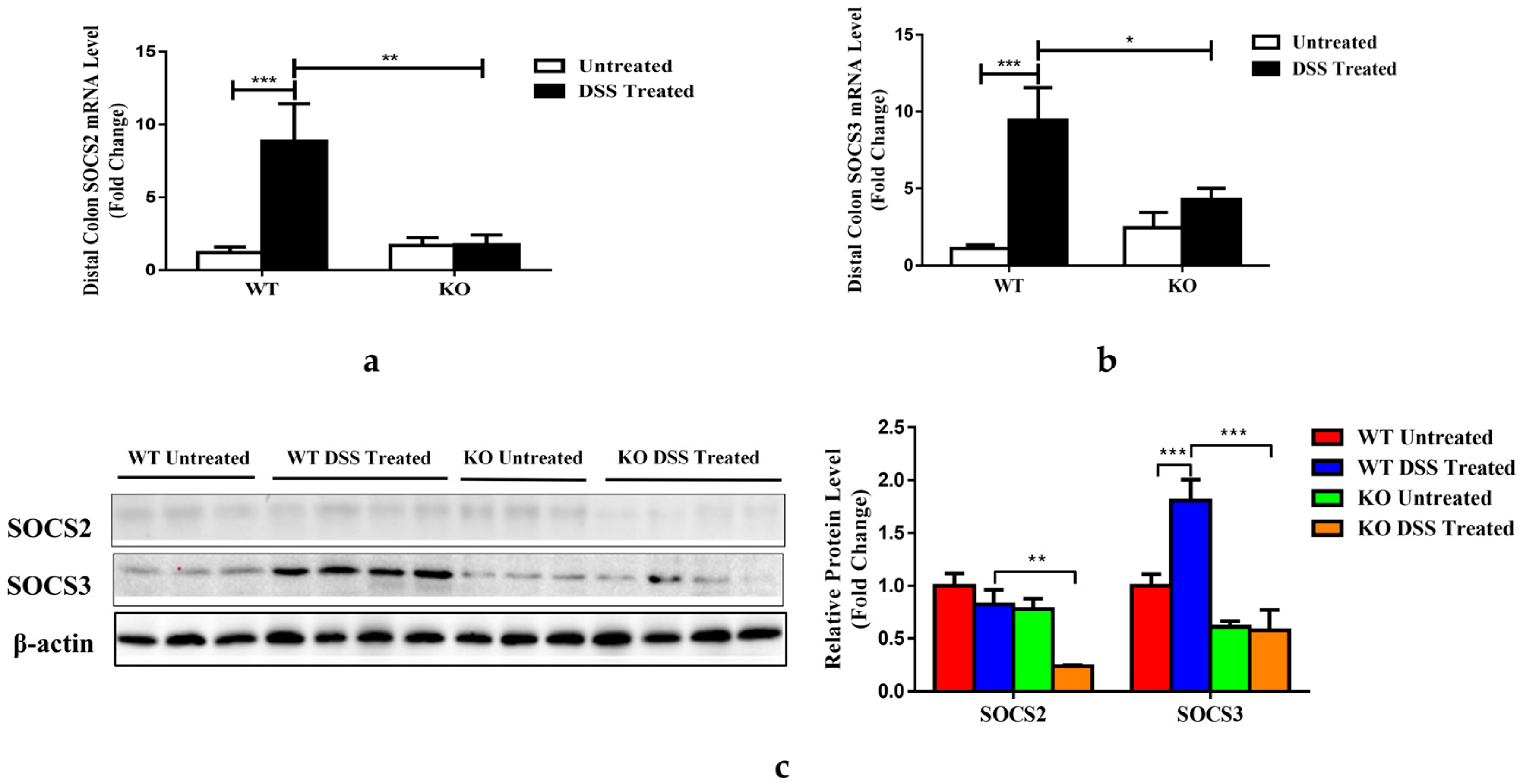
3.7. FGF21 Deficiency Decreases Colonic SOCS2/3 Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Strober, W.; Fuss, I.; Mannon, P. The fundamental basis of inflammatory bowel disease. J. Clin. Investig. 2007, 117, 514–521. [Google Scholar] [CrossRef]
- Szilagyi, A. Relationship(s) between obesity and inflammatory bowel diseases: Possible intertwined pathogenic mechanisms. Clin. J. Gastroenterol. 2020, 13, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Michalak, A.; Mosińska, P.; Fichna, J. Common links between metabolic syndrome and inflammatory bowel disease: Current overview and future perspectives. Pharmacol. Rep. 2016, 68, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Sartini, A.; Gitto, S.; Bianchini, M.; Verga, M.C.; Di Girolamo, M.; Bertani, A.; Del Buono, M.; Schepis, F.; Lei, B.; De Maria, N.; et al. Non-alcoholic fatty liver disease phenotypes in patients with inflammatory bowel disease. Cell Death Dis. 2018, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, R.; Abenavoli, L.; Corea, A.; Larussa, T.; Mancina, R.M.; Cosco, C.; Luzza, F.; Doldo, P. Multifaceted pathogenesis of liver steatosis in inflammatory bowel disease: A systematic review. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 5818–5825. [Google Scholar]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front. Endocrinol. 2016, 7, 30. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, C.; Yang, Y.; Kong, X.; Shao, T.; Ren, L.; Zhuang, X.; Yin, B.; Dryden, G.; McClain, C.; et al. Fibroblast Growth Factor 21 Deficiency Attenuates Experimental Colitis-Induced Adipose Tissue Lipolysis. Gastroenterol. Res. Pract. 2017, 2017, 3089378. [Google Scholar] [CrossRef]
- Fon Tacer, K.; Bookout, A.L.; Ding, X.; Kurosu, H.; John, G.B.; Wang, L.; Goetz, R.; Mohammadi, M.; Kuro-o, M.; Mangelsdorf, D.J.; et al. Research resource: Comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol. Endocrinol. 2010, 24, 2050–2064. [Google Scholar] [CrossRef]
- Ogawa, Y.; Kurosu, H.; Yamamoto, M.; Nandi, A.; Rosenblatt, K.P.; Goetz, R.; Eliseenkova, A.V.; Mohammadi, M.; Kuro-o, M. BetaKlotho is required for metabolic activity of fibroblast growth factor 21. Proc. Natl. Acad. Sci. USA 2007, 104, 7432–7437. [Google Scholar] [CrossRef]
- Owen, B.M.; Mangelsdorf, D.J.; Kliewer, S.A. Tissue-specific actions of the metabolic hormones FGF15/19 and FGF21. Trends Endocrinol. Metab. 2015, 26, 22–29. [Google Scholar] [CrossRef]
- Ge, X.; Chen, C.; Hui, X.; Wang, Y.; Lam, K.S.; Xu, A. Fibroblast growth factor 21 induces glucose transporter-1 expression through activation of the serum response factor/Ets-like protein-1 in adipocytes. J. Biol. Chem. 2011, 286, 34533–34541. [Google Scholar] [CrossRef] [PubMed]
- Kharitonenkov, A.; Shiyanova, T.L.; Koester, A.; Ford, A.M.; Micanovic, R.; Galbreath, E.J.; Sandusky, G.E.; Hammond, L.J.; Moyers, J.S.; Owens, R.A.; et al. FGF-21 as a novel metabolic regulator. J. Clin. Investig. 2005, 115, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Liu, Y.; Xiao, J.; Liu, L.; Chen, S.; Mohammadi, M.; McClain, C.J.; Li, X.; Feng, W. FGF21 mediates alcohol-induced adipose tissue lipolysis by activation of systemic release of catecholamine in mice. J. Lipid Res. 2015, 56, 1481–1491. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yuan, J.; Yu, Z.; Lin, L.; Jiang, Y.; Cao, Z.; Zhuang, P.; Whalen, M.J.; Song, B.; Wang, X.J.; et al. FGF21 Attenuates High-Fat Diet-Induced Cognitive Impairment via Metabolic Regulation and Anti-inflammation of Obese Mice. Mol. Neurobiol. 2018, 55, 4702–4717. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Solt, L.A.; Burris, T.P. Regulation of FGF21 expression and secretion by retinoic acid receptor-related orphan receptor alpha. J. Biol. Chem. 2010, 285, 15668–15673. [Google Scholar] [CrossRef]
- Feingold, K.R.; Grunfeld, C.; Heuer, J.G.; Gupta, A.; Cramer, M.; Zhang, T.; Shigenaga, J.K.; Patzek, S.M.; Chan, Z.W.; Moser, A.; et al. FGF21 is increased by inflammatory stimuli and protects leptin-deficient ob/ob mice from the toxicity of sepsis. Endocrinology 2012, 153, 2689–2700. [Google Scholar] [CrossRef]
- Singhal, G.; Fisher, F.M.; Chee, M.J.; Tan, T.G.; El Ouaamari, A.; Adams, A.C.; Najarian, R.; Kulkarni, R.N.; Benoist, C.; Flier, J.S.; et al. Fibroblast Growth Factor 21 (FGF21) Protects against High Fat Diet Induced Inflammation and Islet Hyperplasia in Pancreas. PLoS ONE 2016, 11, e0148252. [Google Scholar] [CrossRef]
- Fisher, F.M.; Chui, P.C.; Antonellis, P.J.; Bina, H.A.; Kharitonenkov, A.; Flier, J.S.; Maratos-Flier, E. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes 2010, 59, 2781–2789. [Google Scholar] [CrossRef]
- Hale, C.; Chen, M.M.; Stanislaus, S.; Chinookoswong, N.; Hager, T.; Wang, M.; Veniant, M.M.; Xu, J. Lack of overt FGF21 resistance in two mouse models of obesity and insulin resistance. Endocrinology 2012, 153, 69–80. [Google Scholar] [CrossRef]
- Al-Aqil, F.A.; Monte, M.J.; Peleteiro-Vigil, A.; Briz, O.; Rosales, R.; Gonzalez, R.; Aranda, C.J.; Ocon, B.; Uriarte, I.; de Medina, F.S.; et al. Interaction of glucocorticoids with FXR/FGF19/FGF21-mediated ileum-liver crosstalk. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2927–2937. [Google Scholar] [CrossRef]
- Potthoff, M.J.; Inagaki, T.; Satapati, S.; Ding, X.; He, T.; Goetz, R.; Mohammadi, M.; Finck, B.N.; Mangelsdorf, D.J.; Kliewer, S.A.; et al. FGF21 induces PGC-1α and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc. Natl. Acad. Sci. USA 2009, 106, 10853–10858. [Google Scholar] [CrossRef]
- Taylor, B.C.; Zaph, C.; Troy, A.E.; Du, Y.; Guild, K.J.; Comeau, M.R.; Artis, D. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J. Exp. Med. 2009, 206, 655–667. [Google Scholar] [CrossRef]
- Viennois, E.; Chen, F.; Laroui, H.; Baker, M.T.; Merlin, D. Dextran sodium sulfate inhibits the activities of both polymerase and reverse transcriptase: Lithium chloride purification, a rapid and efficient technique to purify RNA. BMC Res. Notes 2013, 6, 360. [Google Scholar] [CrossRef]
- Greten, F.R.; Eckmann, L.; Greten, T.F.; Park, J.M.; Li, Z.W.; Egan, L.J.; Kagnoff, M.F.; Karin, M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 2004, 118, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.S.; Severson, K.M.; Vaishnava, S.; Behrendt, C.L.; Yu, X.; Benjamin, J.L.; Ruhn, K.A.; Hou, B.; DeFranco, A.L.; Yarovinsky, F.; et al. Gammadelta intraepithelial lymphocytes are essential mediators of host-microbial homeostasis at the intestinal mucosal surface. Proc. Natl. Acad. Sci. USA 2011, 108, 8743–8748. [Google Scholar] [CrossRef] [PubMed]
- Vaishnava, S.; Yamamoto, M.; Severson, K.M.; Ruhn, K.A.; Yu, X.; Koren, O.; Ley, R.; Wakeland, E.K.; Hooper, L.V. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science 2011, 334, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhuang, X.; Deng, Z.B.; Jiang, H.; Mu, J.; Wang, Q.; Xiang, X.; Guo, H.; Zhang, L.; Dryden, G.; et al. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol. Ther. J. Am. Soc. Gene Ther. 2014, 22, 522–534. [Google Scholar] [CrossRef]
- Sidhu, M.; Cotoner, C.A.; Guleng, B.; Arihiro, S.; Chang, S.; Duncan, K.W.; Ajami, A.M.; Chau, M.; Reinecker, H.C. Small molecule tyrosine kinase inhibitors for the treatment of intestinal inflammation. Inflamm. Bowel Dis. 2011, 17, 2416–2426. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Liu, L.; Liu, Q.; Li, F.; Zhang, L.; Zhu, F.; Shao, T.; Barve, S.; Chen, Y.; Li, X.; et al. Fibroblast growth factor 21 is required for the therapeutic effects of Lactobacillus rhamnosus GG against fructose-induced fatty liver in mice. Mol. Metab. 2019, 29, 145–157. [Google Scholar] [CrossRef]
- Ryden, M. Fibroblast growth factor 21: An overview from a clinical perspective. Cell. Mol. Life Sci. CMLS 2009, 66, 2067–2073. [Google Scholar] [CrossRef]
- Muise, E.S.; Azzolina, B.; Kuo, D.W.; El-Sherbeini, M.; Tan, Y.; Yuan, X.; Mu, J.; Thompson, J.R.; Berger, J.P.; Wong, K.K. Adipose fibroblast growth factor 21 is up-regulated by peroxisome proliferator-activated receptor gamma and altered metabolic states. Mol. Pharmacol. 2008, 74, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Mathis, J.M.; Jennings, M.H.; Jordan, P.; Wang, Y.; Ando, T.; Joh, T.; Alexander, J.S. Reversal of experimental colitis disease activity in mice following administration of an adenoviral IL-10 vector. J. Inflamm. 2005, 2, 13. [Google Scholar] [CrossRef]
- Geng, H.; Bu, H.-F.; Liu, F.; Wu, L.; Pfeifer, K.; Chou, P.M.; Wang, X.; Sun, J.; Lu, L.; Pandey, A.; et al. In Inflamed Intestinal Tissues and Epithelial Cells, Interleukin 22 Signaling Increases Expression of H19 Long Noncoding RNA, Which Promotes Mucosal Regeneration. Gastroenterology 2018, 155, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K. Role of STAT3 in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 5110–5114. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, A.; Yano, A.; Himuro, H.; Ezaki, Y.; Sadanaga, T.; Mizoguchi, E. Clinical importance of IL-22 cascade in IBD. J. Gastroenterol. 2018, 53, 465–474. [Google Scholar] [CrossRef]
- Tomita, Y.; Ozawa, N.; Miwa, Y.; Ishida, A.; Ohta, M.; Tsubota, K.; Kurihara, T. Pemafibrate Prevents Retinal Pathological Neovascularization by Increasing FGF21 Level in a Murine Oxygen-Induced Retinopathy Model. Int. J. Mol. Sci. 2019, 20, 5878. [Google Scholar] [CrossRef]
- Pickert, G.; Neufert, C.; Leppkes, M.; Zheng, Y.; Wittkopf, N.; Warntjen, M.; Lehr, H.A.; Hirth, S.; Weigmann, B.; Wirtz, S.; et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J. Exp. Med. 2009, 206, 1465–1472. [Google Scholar] [CrossRef]
- Inagaki, T.; Lin, V.Y.; Goetz, R.; Mohammadi, M.; Mangelsdorf, D.J.; Kliewer, S.A. Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metab. 2008, 8, 77–83. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, C.; Xiao, J.; Liu, L.; Zhang, M.; Wang, C.; Wu, G.; Zheng, M.H.; Xu, L.M.; Chen, Y.P.; et al. Fibroblast growth factor 21 deficiency exacerbates chronic alcohol-induced hepatic steatosis and injury. Sci. Rep. 2016, 6, 31026. [Google Scholar] [CrossRef]
- Zeng, K.; Tian, L.; Patel, R.; Shao, W.; Song, Z.; Liu, L.; Manuel, J.; Ma, X.; McGilvray, I.; Cummins, C.L.; et al. Diet Polyphenol Curcumin Stimulates Hepatic Fgf21 Production and Restores Its Sensitivity in High-Fat-Diet-Fed Male Mice. Endocrinology 2017, 158, 277–292. [Google Scholar] [CrossRef]
- Yang, W.; Liu, L.; Wei, Y.; Fang, C.; Zhou, F.; Chen, J.; Han, Q.; Huang, M.; Tan, X.; Liu, Q.; et al. Exercise ameliorates the FGF21-adiponectin axis impairment in diet-induced obese mice. Endocr. Connect. 2019, 8, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.; Thiem, S.; Nguyen, P.M.; Eissmann, M.; Putoczki, T.L. Epithelial gp130/Stat3 functions: An intestinal signaling node in health and disease. Semin. Immunol. 2014, 26, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.M., Jr. The AKT genes and their roles in various disorders. Am. J. Med. Genet. A 2013, 161, 2931–2937. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yin, G.; Lu, Z.; Xie, P.; Zhou, H.; Liu, J.; Yu, L. Casticin prevents DSS induced ulcerative colitis in mice through inhibitions of NF-kappaB pathway and ROS signaling. Phytother. Res. 2018, 32, 1770–1783. [Google Scholar] [CrossRef]
- Ye, Z.; Li, Y.; She, Y.; Wu, M.; Hu, Y.; Qin, K.; Li, L.; Yu, H.; Zhao, Q.; Jin, Z.; et al. Renshen Baidu powder protects ulcerative colitis via inhibiting the PI3K/Akt/NF-kappaB signaling pathway. Front. Pharmacol. 2022, 13, 880589. [Google Scholar] [CrossRef]
- Farr, L.; Ghosh, S.; Jiang, N.; Watanabe, K.; Parlak, M.; Bucala, R.; Moonah, S. CD74 Signaling Links Inflammation to Intestinal Epithelial Cell Regeneration and Promotes Mucosal Healing. Cell Mol. Gastroenterol. Hepatol. 2020, 10, 101–112. [Google Scholar] [CrossRef]
- Iizuka, M.; Konno, S. Wound healing of intestinal epithelial cells. World J. Gastroenterol. 2011, 17, 2161–2171. [Google Scholar] [CrossRef]
- Yamamoto, T.; Nakahigashi, M.; Umegae, S.; Kitagawa, T.; Matsumoto, K. Impact of elemental diet on mucosal inflammation in patients with active Crohn’s disease: Cytokine production and endoscopic and histological findings. Inflamm. Bowel Dis. 2005, 11, 580–588. [Google Scholar] [CrossRef]
- West, N.R.; Hegazy, A.N.; Owens, B.M.J.; Bullers, S.J.; Linggi, B.; Buonocore, S.; Coccia, M.; Gortz, D.; This, S.; Stockenhuber, K.; et al. Oncostatin M drives intestinal inflammation and predicts response to tumor necrosis factor-neutralizing therapy in patients with inflammatory bowel disease. Nat. Med. 2017, 23, 579–589. [Google Scholar] [CrossRef]
- Nagalakshmi, M.L.; Rascle, A.; Zurawski, S.; Menon, S.; de Waal Malefyt, R. Interleukin-22 activates STAT3 and induces IL-10 by colon epithelial cells. Int. Immunopharmacol. 2004, 4, 679–691. [Google Scholar] [CrossRef]
- Zenewicz, L.A.; Yancopoulos, G.D.; Valenzuela, D.M.; Murphy, A.J.; Stevens, S.; Flavell, R.A. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity 2008, 29, 947–957. [Google Scholar] [CrossRef]
- Sugimoto, K.; Ogawa, A.; Mizoguchi, E.; Shimomura, Y.; Andoh, A.; Bhan, A.K.; Blumberg, R.S.; Xavier, R.J.; Mizoguchi, A. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J. Clin. Investig. 2008, 118, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Zenewicz, L.A.; Yin, X.; Wang, G.; Elinav, E.; Hao, L.; Zhao, L.; Flavell, R.A. IL-22 deficiency alters colonic microbiota to be transmissible and colitogenic. J. Immunol. 2013, 190, 5306–5312. [Google Scholar] [CrossRef] [PubMed]
- Bevins, C.L.; Salzman, N.H. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat. Rev. Microbiol. 2011, 9, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Ho, S.B. Intestinal goblet cells and mucins in health and disease: Recent insights and progress. Curr. Gastroenterol. Rep. 2010, 12, 319–330. [Google Scholar] [CrossRef]
- Zheng, X.; Tsuchiya, K.; Okamoto, R.; Iwasaki, M.; Kano, Y.; Sakamoto, N.; Nakamura, T.; Watanabe, M. Suppression of hath1 gene expression directly regulated by hes1 via notch signaling is associated with goblet cell depletion in ulcerative colitis. Inflamm. Bowel Dis. 2011, 17, 2251–2260. [Google Scholar] [CrossRef]
- Treveil, A.; Sudhakar, P.; Matthews, Z.J.; Wrzesinski, T.; Jones, E.J.; Brooks, J.; Olbei, M.; Hautefort, I.; Hall, L.J.; Carding, S.R.; et al. Regulatory network analysis of Paneth cell and goblet cell enriched gut organoids using transcriptomics approaches. Mol. Omics 2019, 16, 39–58. [Google Scholar] [CrossRef]
- Liu, T.C.; Gurram, B.; Baldridge, M.T.; Head, R.; Lam, V.; Luo, C.; Cao, Y.; Simpson, P.; Hayward, M.; Holtz, M.L.; et al. Paneth cell defects in Crohn’s disease patients promote dysbiosis. JCI Insight 2016, 1, e86907. [Google Scholar] [CrossRef]
- Sato, T.; van Es, J.H.; Snippert, H.J.; Stange, D.E.; Vries, R.G.; van den Born, M.; Barker, N.; Shroyer, N.F.; van de Wetering, M.; Clevers, H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 2011, 469, 415–418. [Google Scholar] [CrossRef]
- Clevers, H.C.; Bevins, C.L. Paneth cells: Maestros of the small intestinal crypts. Annu. Rev. Physiol. 2013, 75, 289–311. [Google Scholar] [CrossRef]
- Glal, D.; Sudhakar, J.N.; Lu, H.H.; Liu, M.C.; Chiang, H.Y.; Liu, Y.C.; Cheng, C.F.; Shui, J.W. ATF3 Sustains IL-22-Induced STAT3 Phosphorylation to Maintain Mucosal Immunity Through Inhibiting Phosphatases. Front. Immunol. 2018, 9, 2522. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.T.; Li, Y.Y.; Li, J.J.; Wang, K.; Han, Y.; Dong, W.Y.; Zhu, Z.F.; Xia, N.; Nie, S.F.; Zhang, M.; et al. Liver-heart crosstalk controls IL-22 activity in cardiac protection after myocardial infarction. Theranostics 2018, 8, 4552–4562. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Feng, D.; Gao, B. Interleukin-22 acts as a mitochondrial protector. Theranostics 2020, 10, 7836–7840. [Google Scholar] [CrossRef] [PubMed]
- Munyaka, P.M.; Rabbi, M.F.; Khafipour, E.; Ghia, J.E. Acute dextran sulfate sodium (DSS)-induced colitis promotes gut microbial dysbiosis in mice. J. Basic Microbiol. 2016, 56, 986–998. [Google Scholar] [CrossRef]
- Yee, S.M.; Choi, H.; Seon, J.E.; Ban, Y.J.; Kim, M.J.; Seo, J.E.; Seo, J.H.; Kim, S.; Moon, S.H.; Yun, C.H.; et al. Axl alleviates DSS-induced colitis by preventing dysbiosis of gut microbiota. Sci. Rep. 2023, 13, 5371. [Google Scholar] [CrossRef]

| Name | Sequences (Forward/Reverse 5′–3′) | |
|---|---|---|
| CXCL10 | GGTCTGAGTGGGACTCAAGG | GTGGCAATGATCTCAACACG |
| FGF21 | CCTCTAGGTTTCTTTGCCAACAG | AAGCTGCAGGCCTCAGGAT |
| IL-1β | TTCATCTTTGAAGAAGAGCCCAT | TCGGAGCCTGTAGTGCAGTT |
| IL-6 | TGGAAATGAGAAAAGAGTTGTGC | CCAGTTTGGTAGCATCCATCA |
| TNF-α | CACCACCATCAAGGACTCAA | AGGCAACCTGACCACTCTCC |
| MCP-1 | GGCTCAGCCAGATGCAGT | GAGCTTGGTGACAAAAACTACAG |
| Socs2 | TCCAGATGTGCAAGGATAAACG | AGGTACAGGTGAACAGTCCCATT |
| Socs3 | ATTTCGCTTCGGGACTAGCTC | AGCTGTCGCGGATAAGAAAGG |
| 18s | CTAACCCGTTGAACCCCATT | CCATCCAATCGGTAGTAGCG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Li, F.; Shao, T.; Zhang, L.; Lee, J.; Dryden, G.; McClain, C.J.; Zhao, C.; Feng, W. FGF21 Depletion Attenuates Colitis through Intestinal Epithelial IL-22-STAT3 Activation in Mice. Nutrients 2023, 15, 2086. https://doi.org/10.3390/nu15092086
Liu L, Li F, Shao T, Zhang L, Lee J, Dryden G, McClain CJ, Zhao C, Feng W. FGF21 Depletion Attenuates Colitis through Intestinal Epithelial IL-22-STAT3 Activation in Mice. Nutrients. 2023; 15(9):2086. https://doi.org/10.3390/nu15092086
Chicago/Turabian StyleLiu, Liming, Fengyuan Li, Tuo Shao, Lihua Zhang, Jiyeon Lee, Gerald Dryden, Craig J. McClain, Cuiqing Zhao, and Wenke Feng. 2023. "FGF21 Depletion Attenuates Colitis through Intestinal Epithelial IL-22-STAT3 Activation in Mice" Nutrients 15, no. 9: 2086. https://doi.org/10.3390/nu15092086
APA StyleLiu, L., Li, F., Shao, T., Zhang, L., Lee, J., Dryden, G., McClain, C. J., Zhao, C., & Feng, W. (2023). FGF21 Depletion Attenuates Colitis through Intestinal Epithelial IL-22-STAT3 Activation in Mice. Nutrients, 15(9), 2086. https://doi.org/10.3390/nu15092086






