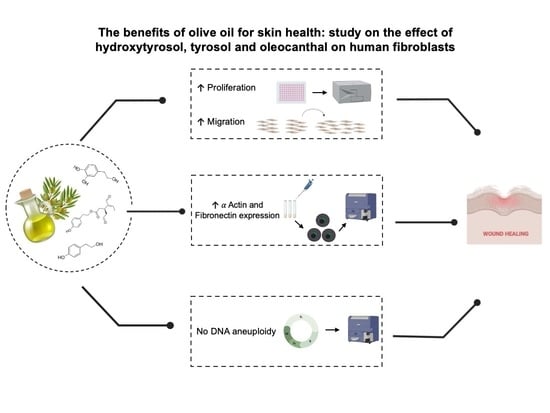
The Benefits of Olive Oil for Skin Health: Study on the Effect of Hydroxytyrosol, Tyrosol, and Oleocanthal on Human Fibroblasts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Products
2.2. Cell Culture
2.3. Cell Proliferation Assay
2.4. Effects of Phenolic Compounds on Cell Migration
2.5. Cell Cycle Assay
2.6. Antigenic Profile by Flow Cytometry
2.7. Immunofluorescence
2.8. Statistical Analysis
3. Results
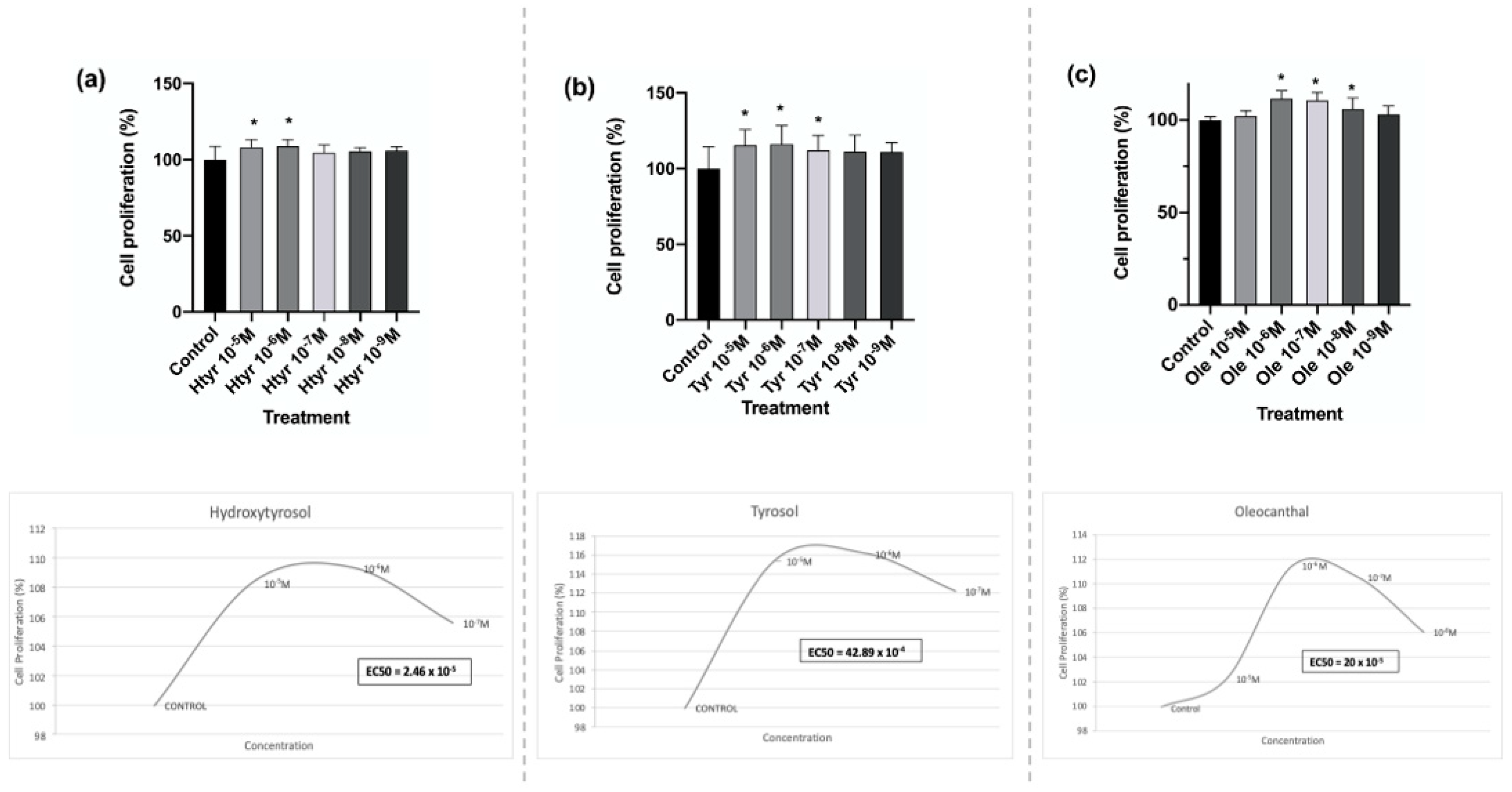
3.1. Effects of Htyr, Tyr, and Ole on the Proliferation of Human Fibroblasts in Culture
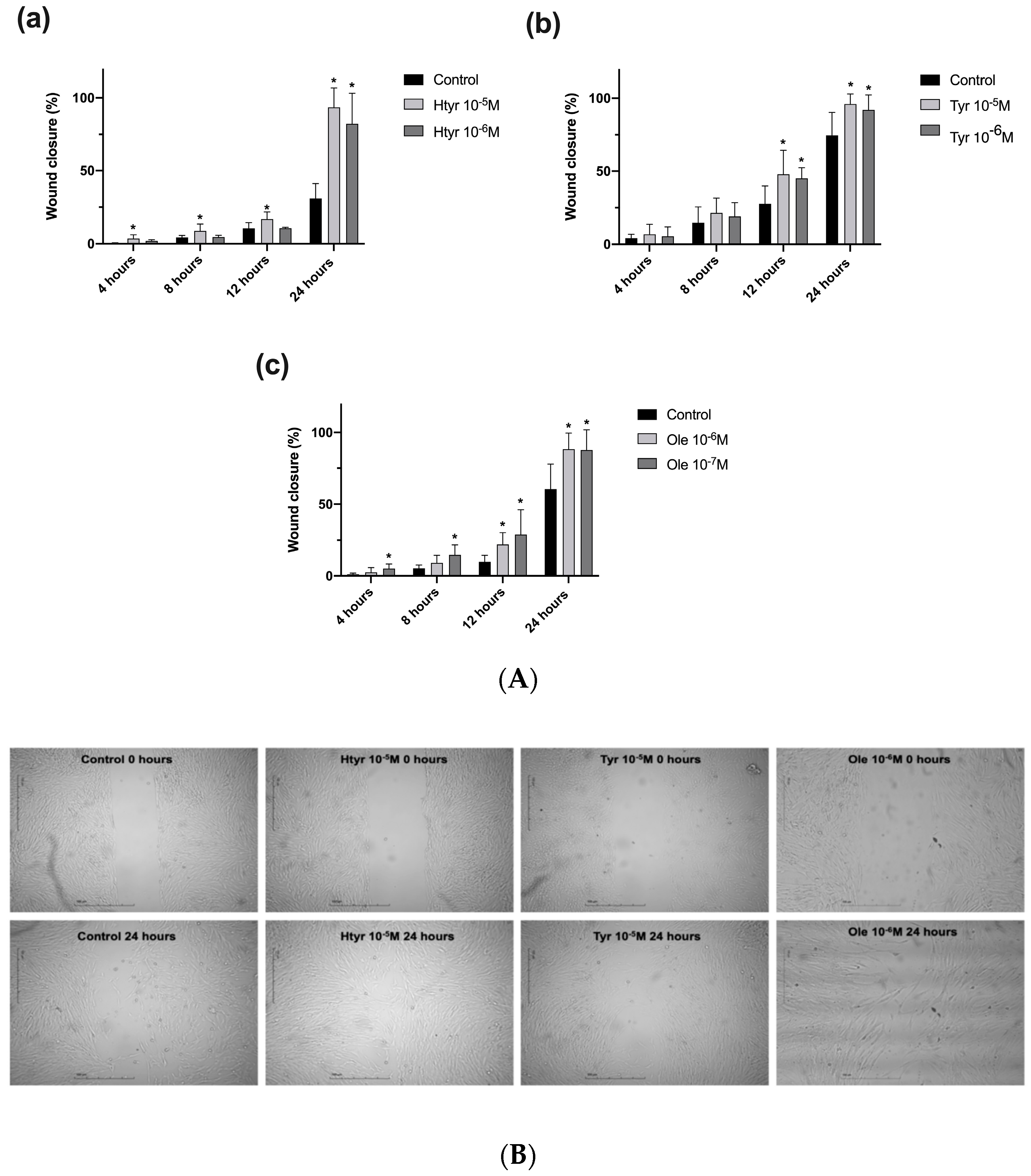
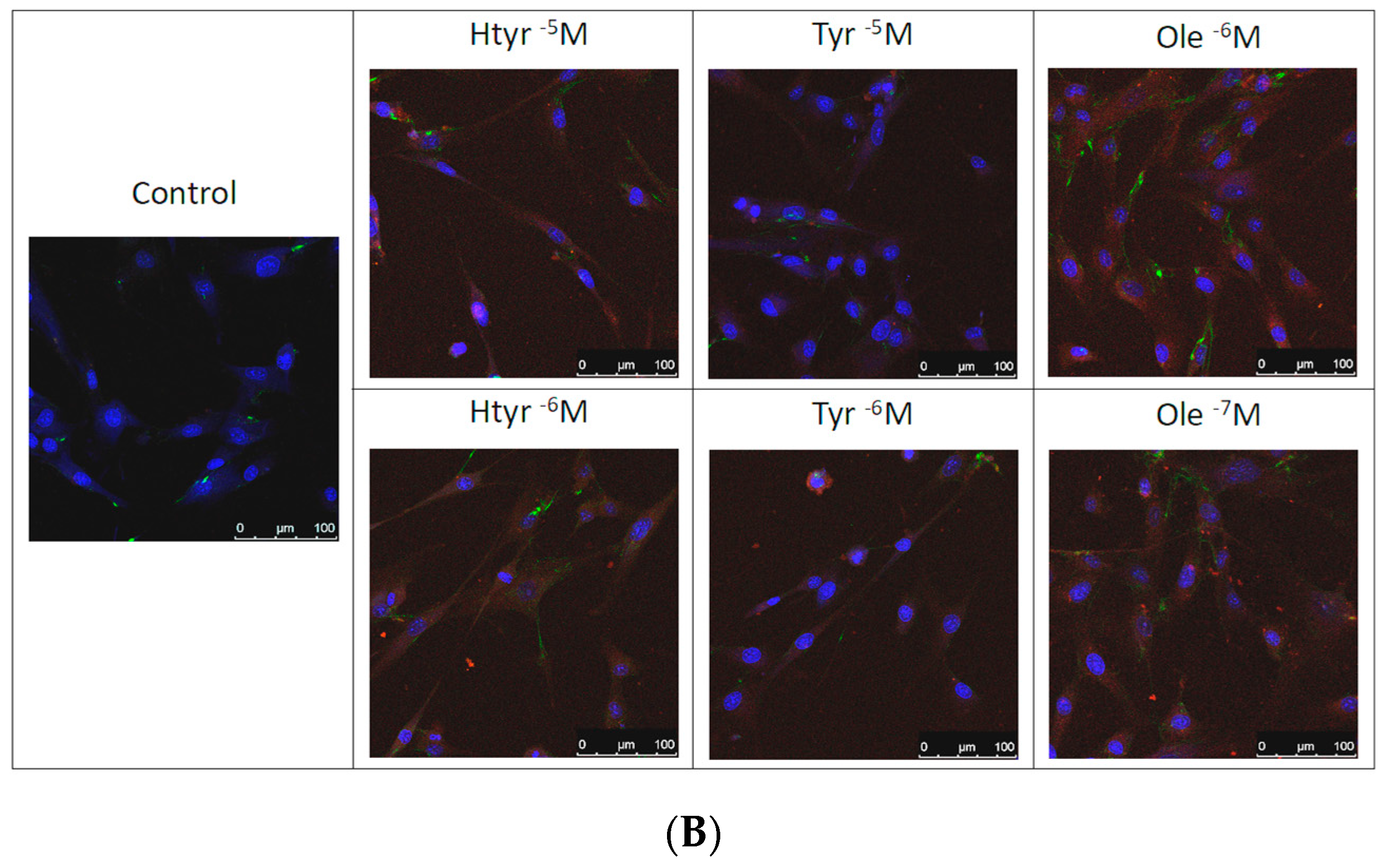
3.2. Effects of Htyr, Tyr, and Ole on the Migratory Capacity of Cultured Human Fibroblasts
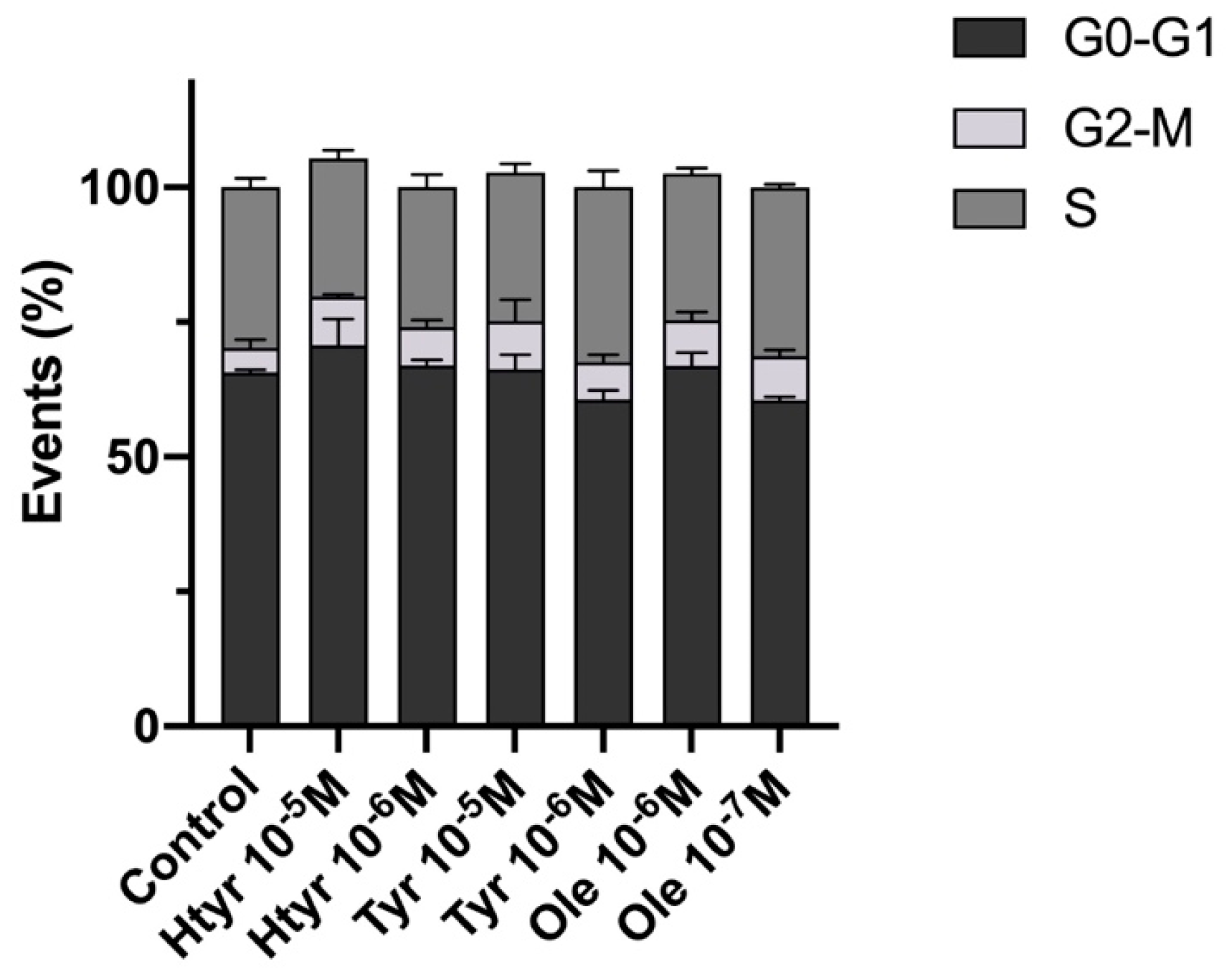
3.3. Effects of Htyr, Tyr, and Ole on the Cell Cycle of Cultured Human Fibroblasts
3.4. Effects of Htyr, Tyr, and Ole on the Antigenic Profile of Human Fibroblasts in Culture
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Imran, H.; Ahmad, M.; Yaqeen, Z.; Sohail, T.; Fatima, N.; Iqbal, W.; Yaqeen, S.S. Evaluation of Wound Healing Effects between Salvadora Persica Ointment and Solcoseryl Jelly in Animal Model. Pak. J. Pharm. Sci. 2015, 4, 1777–1780. [Google Scholar]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Larouche, J.; Sheoran, S.; Maruyama, K.; Martino, M.M. Immune Regulation of Skin Wound Healing: Mechanisms and Novel Therapeutic Targets. Adv. Wound Care 2018, 7, 209–231. [Google Scholar] [CrossRef]
- Martin, P. Wound Healing—Aiming for Perfect Skin Regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Wynn, T.A.; Martin, P. Inflammation and Metabolism in Tissue Repair and Regeneration. Science 2017, 356, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Reinke, J.M.; Sorg, H. Wound Repair and Regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef]
- Lindley, L.E.; Stojadinovic, O.; Pastar, I.; Tomic-Canic, M. Biology and Biomarkers for Wound Healing. Plast. Reconstr. Surg. 2016, 138, 18S. [Google Scholar] [CrossRef]
- Lynch, M.D.; Watt, F.M. Fibroblast Heterogeneity: Implications for Human Disease. J. Clin. Investig. 2018, 128, 26–35. [Google Scholar] [CrossRef]
- Bainbridge, P. Wound Healing and the Role of Fibroblasts. J. Wound Care 2013, 22, 407–408, 410–412. [Google Scholar] [CrossRef]
- Janson, D.; Rietveld, M.; Mahé, C.; Saintigny, G.; El Ghalbzouri, A. Differential Effect of Extracellular Matrix Derived from Papillary and Reticular Fibroblasts on Epidermal Development In Vitro. Eur. J. Dermatol. EJD 2017, 27, 237–246. [Google Scholar] [CrossRef]
- Pilcher, B.K.; Dumin, J.A.; Sudbeck, B.D.; Krane, S.M.; Welgus, H.G.; Parks, W.C. The Activity of Collagenase-1 Is Required for Keratinocyte Migration on a Type I Collagen Matrix. J. Cell Biol. 1997, 137, 1445–1457. [Google Scholar] [CrossRef] [PubMed]
- Sternlicht, M.D.; Werb, Z. How Matrix Metalloproteinases Regulate Cell Behavior. Annu. Rev. Cell Dev. Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef] [PubMed]
- Senger, D.R.; Ledbetter, S.R.; Claffey, K.P.; Papadopoulos-Sergiou, A.; Peruzzi, C.A.; Detmar, M. Stimulation of Endothelial Cell Migration by Vascular Permeability Factor/Vascular Endothelial Growth Factor through Cooperative Mechanisms Involving the Alphavbeta3 Integrin, Osteopontin, and Thrombin. Am. J. Pathol. 1996, 149, 293–305. [Google Scholar] [PubMed]
- Gabbiani, G. The Myofibroblast in Wound Healing and Fibrocontractive Diseases. J. Pathol. 2003, 200, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Phan, S.H.; Thannickal, V.J.; Prunotto, M.; Desmoulière, A.; Varga, J.; De Wever, O.; Mareel, M.; Gabbiani, G. Recent Developments in Myofibroblast Biology. Am. J. Pathol. 2012, 180, 1340–1355. [Google Scholar] [CrossRef]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and Mechano-Regulation of Connective Tissue Remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef]
- Phan, S.H. Biology of Fibroblasts and Myofibroblasts. Proc. Am. Thorac. Soc. 2008, 5, 334–337. [Google Scholar] [CrossRef]
- Servili, M.; Esposto, S.; Fabiani, R.; Urbani, S.; Taticchi, A.; Mariucci, F.; Selvaggini, R.; Montedoro, G.F. Phenolic Compounds in Olive Oil: Antioxidant, Health and Organoleptic Activities According to Their Chemical Structure. Inflammopharmacology 2009, 17, 76–84. [Google Scholar] [CrossRef]
- Boskou, D.; Blekas, G.; Tsimidou, M. Olive Oil Composition; Academic Press and AOCS Press: Cambridge, MA, USA, 2006. [Google Scholar]
- Tuck, K.L.; Hayball, P.J. Major Phenolic Compounds in Olive Oil: Metabolism and Health Effects. J. Nutr. Biochem. 2002, 13, 636–644. [Google Scholar] [CrossRef]
- Amiot, M.J.; Fleuriet, A.; Macheix, J.J. Importance and Evolution of Phenolic Compounds in Olive during Growth and Maturation. J. Agric. Food Chem. 1986, 34, 823–826. [Google Scholar] [CrossRef]
- Martínez Nieto, L.; Hodaifa, G.; Lozano Peña, J.L. Changes in Phenolic Compounds and Rancimat Stability of Olive Oils from Varieties of Olives at Different Stages of Ripeness. J. Sci. Food Agric. 2010, 90, 2393–2398. [Google Scholar] [CrossRef] [PubMed]
- Uceda, M.; Hermoso, M.; García-Ortiz, A.; Jiménez, A.; Beltrán, G. Intraspecific Variation of Oil Contents and the Characteristics of Oils in Olive Cultivars. Acta Hortic. 1999, 474, 659–662. [Google Scholar] [CrossRef]
- Inglese, P.; Famiani, F.; Galvano, F.; Servili, M.; Esposto, S.; Urbani, S. Factors Affecting Extra-Virgin Olive Oil Composition. In Horticultural Reviews; Wiley-Blackwell: Hoboken, NJ, USA, 2011; pp. 83–147. ISBN 978-0-470-87237-6. [Google Scholar]
- Bucciantini, M.; Leri, M.; Nardiello, P.; Casamenti, F.; Stefani, M. Olive Polyphenols: Antioxidant and Anti-Inflammatory Properties. Antioxidants 2021, 10, 1044. [Google Scholar] [CrossRef] [PubMed]
- Bender, C.; Candi, I.; Rogel, E. Efficacy of Hydroxytyrosol-Rich Food Supplements on Reducing Lipid Oxidation in Humans. Int. J. Mol. Sci. 2023, 24, 5521. [Google Scholar] [CrossRef] [PubMed]
- Bahrani, H.M.H.; Ghobeh, M.; Homayouni Tabrizi, M. The Anticancer, Anti-Oxidant, and Antibacterial Activities of Chitosan-lecithin-Coated Parthenolide/Tyrosol Hybrid Nanoparticles. J. Biomater. Sci. Polym. Ed. 2023, 20, 1–15. [Google Scholar] [CrossRef]
- Aparicio-Soto, M.; Sánchez-Hidalgo, M.; Rosillo, M.Á.; Castejón, M.L.; Alarcón-de-la-Lastra, C. Extra Virgin Olive Oil: A Key Functional Food for Prevention of Immune-Inflammatory Diseases. Food Funct. 2016, 7, 4492–4505. [Google Scholar] [CrossRef]
- Santangelo, C.; Vari, R.; Scazzocchio, B.; De Sanctis, P.; Giovannini, C.; D’Archivio, M.; Masella, R. Anti-Inflammatory Activity of Extra Virgin Olive Oil Polyphenols: Which Role in the Prevention and Treatment of Immune-Mediated Inflammatory Diseases? Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 36–50. [Google Scholar] [CrossRef]
- Lucas, L.; Russell, A.; Keast, R. Molecular Mechanisms of Inflammation. Anti-Inflammatory Benefits of Virgin Olive Oil and the Phenolic Compound Oleocanthal. Curr. Pharm. Des. 2011, 17, 754–768. [Google Scholar] [CrossRef]
- Han, J.; Talorete, T.P.N.; Yamada, P.; Isoda, H. Anti-Proliferative and Apoptotic Effects of Oleuropein and Hydroxytyrosol on Human Breast Cancer MCF-7 cells. Cytotechnology 2009, 59, 45–53. [Google Scholar] [CrossRef]
- Cao, K.; Xu, J.; Zou, X.; Li, Y.; Chen, C.; Zheng, A.; Li, H.; Li, H.; Szeto, I.M.-Y.; Shi, Y.; et al. Hydroxytyrosol prevents Diet-Induced Metabolic Syndrome and Attenuates Mitochondrial Abnormalities in Obese Mice. Free Radic. Biol. Med. 2014, 67, 396–407. [Google Scholar] [CrossRef]
- Ebaid, G.M.X.; Seiva, F.R.F.; Rocha, K.K.H.R.; Souza, G.A.; Novelli, E.L.B. Effects of Olive Oil and Its Minor Phenolic Constituents on Obesity-Induced Cardiac Metabolic Changes. Nutr. J. 2010, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Rosillo, M.Á.; Alcaraz, M.J.; Sánchez-Hidalgo, M.; Fernández-Bolaños, J.G.; Alarcón-de-la-Lastra, C.; Ferrándiz, M.L. Anti-inflammatory and Joint Protective Effects of Extra-Virgin Olive-Oil Polyphenol Extract in Experimental Arthritis. J. Nutr. Biochem. 2014, 25, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Rosillo, M.A.; Sánchez-Hidalgo, M.; González-Benjumea, A.; Fernández-Bolaños, J.G.; Lubberts, E.; Alarcón-de-la-Lastra, C. Preventive Effects of Dietary Hydroxytyrosol Acetate, an Extra Virgin Olive Oil Polyphenol in murine Collagen-Induced Arthritis. Mol. Nutr. Food Res. 2015, 59, 2537–2546. [Google Scholar] [CrossRef] [PubMed]
- Michalsen, A.; Eddin, O.; Salama, A. A Case Series of the Effects of a Novel Composition of a Traditional Natural Preparation for the Treatment of Psoriasis. J. Tradit. Complement. Med. 2016, 6, 395–398. [Google Scholar] [CrossRef]
- Hussain, Z.; Katas, H.; Mohd Amin, M.C.I.; Kumolosasi, E.; Buang, F.; Sahudin, S. Self-Assembled Polymeric Nanoparticles for Percutaneous Co-Delivery of Hydrocortisone/Hydroxytyrosol: An Ex Vivo and In Vivo Study Using an NC/Nga Mouse Model. Int. J. Pharm. 2013, 444, 109–119. [Google Scholar] [CrossRef]
- Segura Palacios, J.M.; Blázquez Sánchez, N.; Rivas Ruiz, F.; Aguilar Bernier, M.; Ramírez López, B.; Sánchez, M.E.F.; de Troya Martín, M. Topical Treatment with Oleocanthal Extract in Reducing Inflammatory Reactions after Photodynamic Therapy: A Prospective Quasi-Experimental Pilot Study. Complement. Ther. Med. 2019, 42, 298–301. [Google Scholar] [CrossRef]
- García-Martínez, O.; Luna-Bertos, E.D.; Ramos-Torrecillas, J.; Ruiz, C.; Milia, E.; Lorenzo, M.L.; Jimenez, B.; Sánchez-Ortiz, A.; Rivas, A. Phenolic Compounds in Extra Virgin Olive Oil Stimulate Human Osteoblastic Cell Proliferation. PLoS ONE 2016, 11, e0150045. [Google Scholar] [CrossRef]
- Illescas-Montes, R.; Melguizo-Rodríguez, L.; Manzano-Moreno, F.J.; García-Martínez, O.; Ruiz, C.; Ramos-Torrecillas, J. Cultured Human Fibroblast Biostimulation Using a 940 nm Diode Laser. Materials 2017, 10, 793. [Google Scholar] [CrossRef]
- Zhang, J.; Williams, T.D.; Abdallah, M.A.-E.; Harrad, S.; Chipman, J.K.; Viant, M.R. Transcriptomic and Metabolomic Approaches to Investigate the Molecular Responses of Human Cell Lines Exposed to the Flame Retardant Hexabromocyclododecane (HBCD). Toxicol. In Vitro Int. J. Publ. Assoc. BIBRA 2015, 29, 2116–2123. [Google Scholar] [CrossRef]
- Cappiello, F.; Casciaro, B.; Mangoni, M.L. A Novel In Vitro Wound Healing Assay to Evaluate Cell Migration. J. Vis. Exp. JoVE 2018, 133, 56825. [Google Scholar] [CrossRef]
- Manzano-Moreno, F.J.; Ramos-Torrecillas, J.; De Luna-Bertos, E.; Ruiz, C.; García-Martínez, O. High Doses of Bisphosphonates Reduce Osteoblast-Like Cell Proliferation by Arresting the Cell Cycle and Inducing Apoptosis. J. Cranio-Maxillofac. Surg. 2015, 43, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Torrecillas, J.; Luna-Bertos, E.; de Manzano-Moreno, F.J.; García-Martínez, O.; Ruiz, C. Human Fibroblast-Like Cultures in the Presence of Platelet-Rich Plasma as a Single Growth Factor Source: Clinical Implications. Adv. Skin Wound Care 2014, 27, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Batarfi, W.A.; Mohd Yunus, M.H.; Hamid, A.A. The Effect of Hydroxytyrosol in Type II Epithelial-Mesenchymal Transition in Human Skin Wound Healing. Molecules 2023, 28, 2652. [Google Scholar] [CrossRef] [PubMed]
- de S Ribeiro, B.C.; de C Faria, R.V.; de S Nogueira, J.; Valença, S.S.; Chen, L.; Romana-Souza, B. Olive Oil Promotes the Survival and Migration of Dermal Fibroblasts through Nrf2 Pathway Activation. Lipids 2023, 58, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Astrof, S.; Hynes, R.O. Fibronectins in Vascular Morphogenesis. Angiogenesis 2009, 12, 165–175. [Google Scholar] [CrossRef]
- Kumar, V.B.S.; Viji, R.I.; Kiran, M.S.; Sudhakaran, P.R. Angiogenic Response of Endothelial Cells to Fibronectin. In Biochemical Roles of Eukaryotic Cell Surface Macromolecules; Sudhakaran, P.R., Surolia, A., Eds.; Springer: New York, NY, USA, 2012; pp. 131–151. [Google Scholar]
- Hynes, R.O. Cell-Matrix Adhesion in Vascular Development. J. Thromb. Haemost. JTH 2007, 5 (Suppl. S1), 32–40. [Google Scholar] [CrossRef]
- Sarrazy, V.; Billet, F.; Micallef, L.; Coulomb, B.; Desmoulière, A. Mechanisms of pathological scarring: Role of myofibroblasts and current developments. Wound Repair Regen. 2011, 19, s10–s15. [Google Scholar] [CrossRef]
- Chitturi, R.T.; Balasubramaniam, A.M.; Parameswar, R.A.; Kesavan, G.; Haris, K.T.M.; Mohideen, K. The Role of Myofibroblasts in Wound Healing, Contraction and its Clinical Implications in Cleft Palate Repair. J. Int. Oral Health JIOH 2015, 7, 75–80. [Google Scholar]
- Darby, I.A.; Laverdet, B.; Bonté, F.; Desmoulière, A. Fibroblasts and Myofibroblasts in Wound Healing. Clin. Cosmet. Investig. Dermatol. 2014, 7, 301–311. [Google Scholar] [CrossRef]
- Kohnen, G.; Kertschanska, S.; Demir, R.; Kaufmann, P. Placental Villous Stroma as a Model System for Myofibroblast Differentiation. Histochem. Cell Biol. 1996, 105, 415–429. [Google Scholar] [CrossRef]
- Gabbiani, G.; Ryan, G.B.; Majne, G. Presence of Modified Fibroblasts in Granulation Tissue and Their Possible Role in wound Contraction. Experientia 1971, 27, 549–550. [Google Scholar] [CrossRef] [PubMed]
- Darby, I.; Skalli, O.; Gabbiani, G. Alpha-Smooth Muscle Actin Is Transiently Expressed by Myofibroblasts during Experimental Wound Healing. Lab. Investig. J. Tech. Methods Pathol. 1990, 63, 21–29. [Google Scholar]
- Grinnell, F. Fibronectin and Wound Healing. J. Cell. Biochem. 1984, 26, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, A.B. Fibronectin in Acute and Chronic Wounds. J. Nurs. Off. Publ. Int. Assoc. Enteros. Ther. 1992, 19, 166–170. [Google Scholar]
- Clark, R.A. Potential Roles of Fibronectin in Cutaneous Wound Repair. Arch. Dermatol. 1988, 124, 201–206. [Google Scholar] [CrossRef]
- Nasiri, M.; Fayazi, S.; Jahani, S.; Yazdanpanah, L.; Haghighizadeh, M.H. The Effect of Topical Olive Oil on the Healing of Foot Ulcer in Patients with Type 2 Diabetes: A Double-Blind Randomized Clinical Trial Study in Iran. J. Diabetes Metab. Disord. 2015, 14, 38. [Google Scholar] [CrossRef]
- Karimi, Z.; Behnammoghadam, M.; Rafiei, H.; Abdi, N.; Zoladl, M.; Talebianpoor, M.S.; Arya, A.; Khastavaneh, M. Impact of Olive Oil and Honey on Healing of Diabetic Foot: A Randomized Controlled Trial. Clin. Cosmet. Investig. Dermatol. 2019, 12, 347–354. [Google Scholar] [CrossRef]
- Cicerale, S.; Lucas, L.J.; Keast, R.S.J. Antimicrobial, Antioxidant and Anti-Inflammatory Phenolic Activities in Extra Virgin Olive Oil. Curr. Opin. Biotechnol. 2012, 23, 129–135. [Google Scholar] [CrossRef]
- Rafehi, H.; Ververis, K.; Karagiannis, T.C. Mechanisms of Action of Phenolic Compounds in Olive. J. Diet. Suppl. 2012, 9, 96–109. [Google Scholar] [CrossRef]
- Melguizo-Rodríguez, L.; Illescas-Montes, R.; Costela-Ruiz, V.J.; Ramos-Torrecillas, J.; de Luna-Bertos, E.; García-Martínez, O.; Ruiz, C. Antimicrobial Properties of Olive Oil Phenolic Compounds and Their Regenerative Capacity towards Fibroblast Cells. J. Tissue Viability 2021, 30, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth Factors and Cytokines in Wound Healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.C. The Role of Growth Factors in the Healing of Chronic Wounds. Wound Repair Regen. 1997, 5, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Yager, D.R.; Chen, S.M.; Ward, S.I.; Olutoye, O.O.; Diegelmann, R.F.; Kelman Cohen, I. Ability of Chronic Wound Fluids to Degrade Peptide Growth Factors Is Associated with Increased Levels of Elastase Activity and Diminished Levels of Proteinase Inhibitors. Wound Repair Regen. 1997, 5, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Rosillo, M.Á.; Alarcón-de-la-Lastra, C.; Castejón, M.L.; Montoya, T.; Cejudo-Guillén, M.; Sánchez-Hidalgo, M. Polyphenolic Extract from Extra Virgin Olive Oil Inhibits the Inflammatory Response in IL-1β-Activated Synovial Fibroblasts. Br. J. Nutr. 2019, 121, 55–62. [Google Scholar] [CrossRef]
- Menendez, J.A.; Joven, J.; Aragonès, G.; Barrajón-Catalán, E.; Beltrán-Debón, R.; Borrás-Linares, I.; Camps, J.; Corominas-Faja, B.; Cufí, S.; Fernández-Arroyo, S.; et al. Xenohormetic and Anti-Aging Activity of Secoiridoid Polyphenols Present in Extra Virgin Olive Oil: A New Family of Gerosuppressant Agents. Cell Cycle Georget. Tex 2013, 12, 555–578. [Google Scholar] [CrossRef]
- Song, Y.; Zeng, R.; Hu, L.; Maffucci, K.G.; Ren, X.; Qu, Y. In vivo Wound Healing and In Vitro Antioxidant Activities of Bletilla Striata Phenolic Extracts. Biomed. Pharmacother. 2017, 93, 451–461. [Google Scholar] [CrossRef]
- Kaptaner İğci, B.; Aytaç, Z. An Investigation on the In Vitro Wound Healing Activity and Phytochemical Composition of Hypericum pseudolaeve N. Robson Growing in Turkey. Turk. J. Pharm. Sci. 2020, 17, 610–619. [Google Scholar] [CrossRef]
- de Oliveira Rodrigues, R.; Yaochite, J.N.U.; Sasahara, G.L.; Albuquerque, A.A.; da Cruz Fonseca, S.G.; de Vasconcelos Araújo, T.D.; Santiago, G.M.P.; de Sousa, L.M.; de Carvalho, J.L.; Alves, A.P.N.N.; et al. Antioxidant, Anti-Inflammatory and Healing Potential of Ethyl Acetate Fraction of Bauhinia ungulata L. (Fabaceae) on In Vitro and In Vivo Wound Model. Mol. Biol. Rep. 2020, 47, 2845–2859. [Google Scholar] [CrossRef]
- Hecker, A.; Schellnegger, M.; Hofmann, E.; Luze, H.; Nischwitz, S.P.; Kamolz, L.-P.; Kotzbeck, P. The Impact of Resveratrol on Skin Wound Healing, Scarring, and Aging. Int. Wound J. 2022, 19, 9–28. [Google Scholar] [CrossRef]
- Akbik, D.; Ghadiri, M.; Chrzanowski, W.; Rohanizadeh, R. Curcumin as a Wound Healing Agent. Life Sci. 2014, 116, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kadyrbayeva, G.; Zagórska, J.; Grzegorczyk, A.; Gaweł-Bęben, K.; Strzępek-Gomółka, M.; Ludwiczuk, A.; Czech, K.; Kumar, M.; Koch, W.; Malm, A.; et al. The Phenolic Compounds Profile and Cosmeceutical Significance of Two Kazakh Species of Onions: Alliumgalanthum and A. turkestanicum. Mol. Basel Switz. 2021, 26, 5491. [Google Scholar] [CrossRef] [PubMed]
- Aichele, K.; Bubel, M.; Deubel, G.; Pohlemann, T.; Oberringer, M. Bromelain Down-Regulates Myofibroblast Differentiation in an In Vitro Wound Healing Assay. Naunyn. Schmiedebergs Arch. Pharmacol. 2013, 386, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Tsakiroglou, P.; VandenAkker, N.E.; Del Bo’, C.; Riso, P.; Klimis-Zacas, D. Role of Berry Anthocyanins and Phenolic Acids on Cell Migration and Angiogenesis: An Updated Overview. Nutrients 2019, 11, 1075. [Google Scholar] [CrossRef] [PubMed]
- Melguizo-Rodríguez, L.; García-Recio, E.; Ruiz, C.; De Luna-Bertos, E.; Illescas-Montes, R.; Costela-Ruiz, V.J. Biological Properties and Therapeutic Applications of Garlic and Its Components. Food Funct. 2022, 13, 2415–2426. [Google Scholar] [CrossRef]
- Addis, R.; Cruciani, S.; Santaniello, S.; Bellu, E.; Sarais, G.; Ventura, C.; Maioli, M.; Pintore, G. Fibroblast Proliferation and Migration in Wound Healing by Phytochemicals: Evidence for a Novel Synergic Outcome. Int. J. Med. Sci. 2020, 17, 1030–1042. [Google Scholar] [CrossRef]
- Giampieri, F.; Alvarez-Suarez, J.M.; Mazzoni, L.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Gonzàlez-Paramàs, A.M.; Santos-Buelga, C.; Quiles, J.L.; Bompadre, S.; Mezzetti, B.; et al. Polyphenol-Rich Strawberry Extract Protects Human Dermal Fibroblasts against Hydrogen Peroxide Oxidative Damage and Improves Mitochondrial Functionality. Molecules 2014, 19, 7798–7816. [Google Scholar] [CrossRef]
- Perker, M.C.; Yilmaz, B.O.; Yildizbayrak, N.; Aydin, Y.; Erkan, M. Protective Effects of Curcumin on Biochemical and Molecular Changes in Sodium Arsenite-Induced Oxidative Damage in Embryonic Fibroblast Cells. J. Biochem. Mol. Toxicol. 2019, 33, e22320. [Google Scholar] [CrossRef]
- Abate, M.; Citro, M.; Pisanti, S.; Caputo, M.; Martinelli, R. Keratinocytes Migration Promotion, Proliferation Induction, and Free Radical Injury Prevention by 3-Hydroxytirosol. Int. J. Mol. Sci. 2021, 22, 2438. [Google Scholar] [CrossRef]
- Li, W.; Sun, Y.N.; Yan, X.T.; Yang, S.Y.; Kim, S.; Chae, D.; Hyun, J.W.; Kang, H.K.; Koh, Y.-S.; Kim, Y.H. Anti-Inflammatory and Antioxidant Activities of Phenolic Compounds from Desmodium Caudatum Leaves and Stems. Arch. Pharm. Res. 2014, 37, 721–727. [Google Scholar] [CrossRef]
- Aparicio-Soto, M.; Redhu, D.; Sánchez-Hidalgo, M.; Fernández-Bolaños, J.G.; Alarcón-de-la-Lastra, C.; Worm, M.; Babina, M. Olive-Oil-Derived Polyphenols Effectively Attenuate Inflammatory Responses of Human Keratinocytes by Interfering with the NF-κB Pathway. Mol. Nutr. Food Res. 2019, 63, e1900019. [Google Scholar] [CrossRef] [PubMed]
- Soeur, J.; Eilstein, J.; Léreaux, G.; Jones, C.; Marrot, L. Skin Resistance to Oxidative Stress Induced by Resveratrol: From Nrf2 Activation to GSH Biosynthesis. Free Radic. Biol. Med. 2015, 78, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Fasano, E.; Serini, S.; Mondella, N.; Trombino, S.; Celleno, L.; Lanza, P.; Cittadini, A.; Calviello, G. Antioxidant and Anti-Inflammatory Effects of Selected Natural Compounds Contained in a Dietary Supplement on Two Human Immortalized Keratinocyte Lines. BioMed Res. Int. 2014, 2014, 327452. [Google Scholar] [CrossRef] [PubMed]

| mAbs | Fluorochromes | Supplier |
|---|---|---|
| Control PE | PE | Caltag (Burlingame, CA, USA) |
| Control FITC | FITC | Caltag |
| Anti-human fibronectin fluorescein | FITC | R&D Systems |
| Anti-human α-actin PE | PE | R&D Systems |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Acedo, A.; Ramos-Torrecillas, J.; Illescas-Montes, R.; Costela-Ruiz, V.J.; Ruiz, C.; Melguizo-Rodríguez, L.; García-Martínez, O. The Benefits of Olive Oil for Skin Health: Study on the Effect of Hydroxytyrosol, Tyrosol, and Oleocanthal on Human Fibroblasts. Nutrients 2023, 15, 2077. https://doi.org/10.3390/nu15092077
González-Acedo A, Ramos-Torrecillas J, Illescas-Montes R, Costela-Ruiz VJ, Ruiz C, Melguizo-Rodríguez L, García-Martínez O. The Benefits of Olive Oil for Skin Health: Study on the Effect of Hydroxytyrosol, Tyrosol, and Oleocanthal on Human Fibroblasts. Nutrients. 2023; 15(9):2077. https://doi.org/10.3390/nu15092077
Chicago/Turabian StyleGonzález-Acedo, Anabel, Javier Ramos-Torrecillas, Rebeca Illescas-Montes, Víctor J. Costela-Ruiz, Concepción Ruiz, Lucía Melguizo-Rodríguez, and Olga García-Martínez. 2023. "The Benefits of Olive Oil for Skin Health: Study on the Effect of Hydroxytyrosol, Tyrosol, and Oleocanthal on Human Fibroblasts" Nutrients 15, no. 9: 2077. https://doi.org/10.3390/nu15092077
APA StyleGonzález-Acedo, A., Ramos-Torrecillas, J., Illescas-Montes, R., Costela-Ruiz, V. J., Ruiz, C., Melguizo-Rodríguez, L., & García-Martínez, O. (2023). The Benefits of Olive Oil for Skin Health: Study on the Effect of Hydroxytyrosol, Tyrosol, and Oleocanthal on Human Fibroblasts. Nutrients, 15(9), 2077. https://doi.org/10.3390/nu15092077