Integrated Omic Analysis of Human Plasma Metabolites and Microbiota in a Hypertension Cohort
Abstract
1. Introduction
2. Results
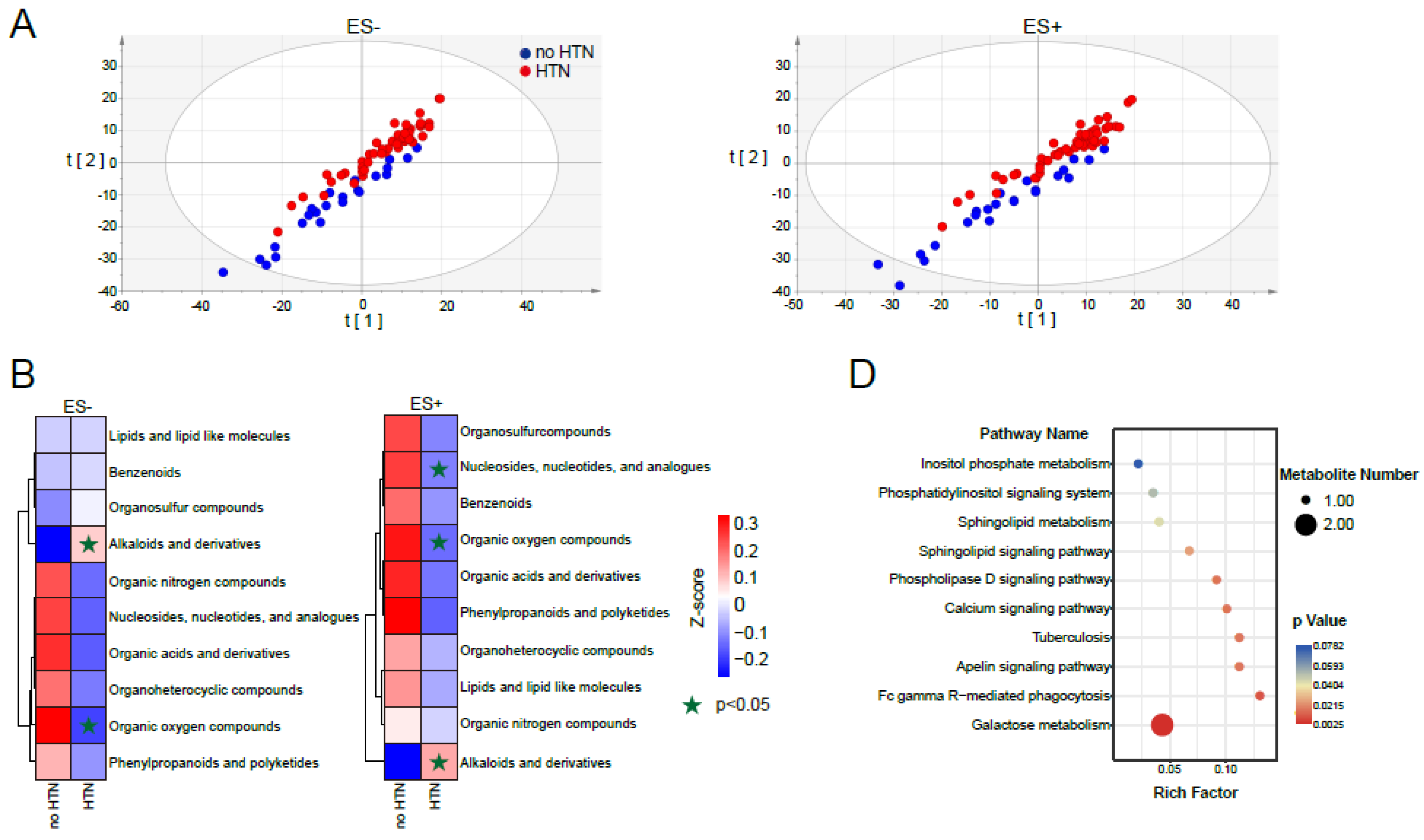
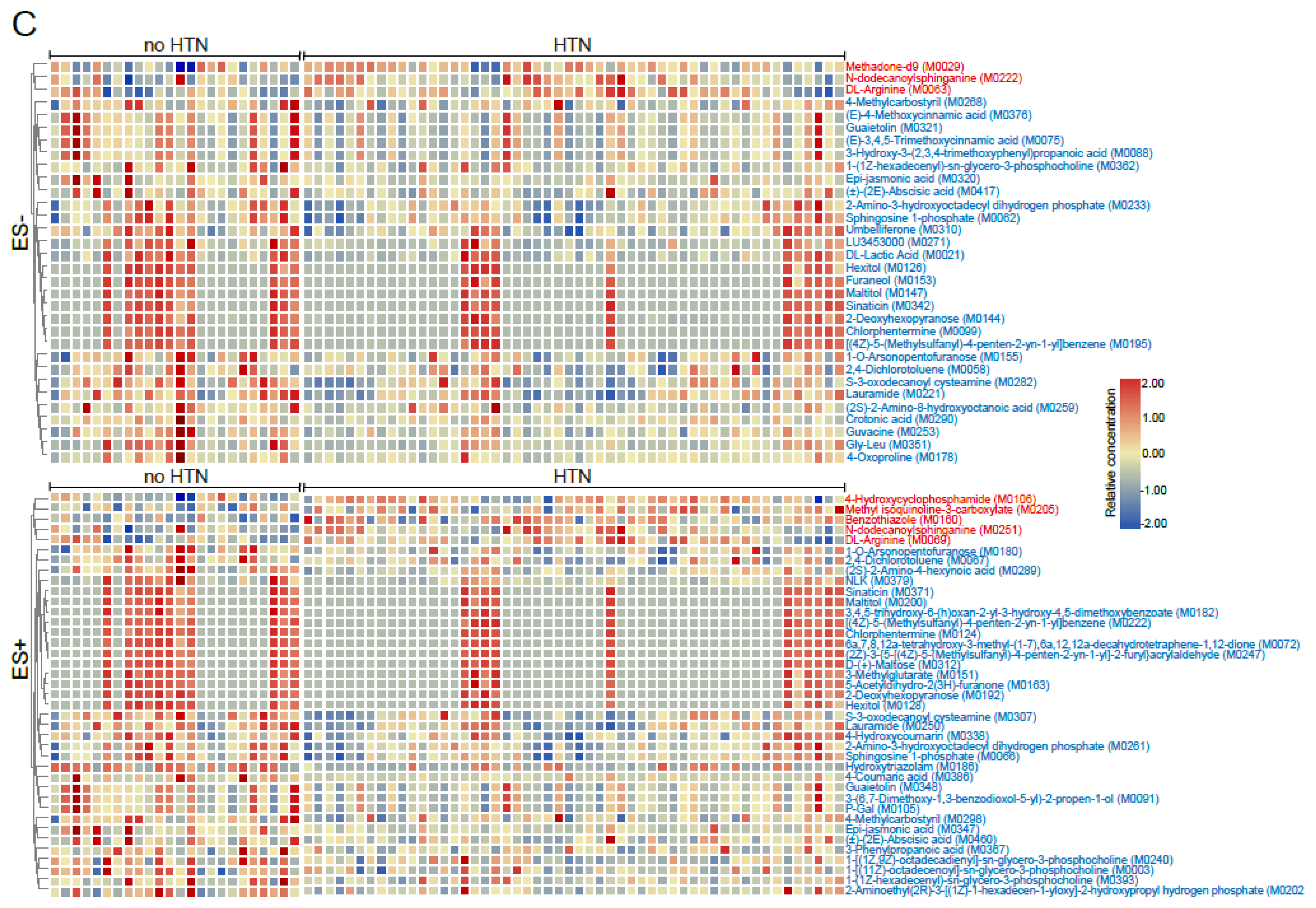
2.1. Metabolomic Profile of Fasting Plasma from No HTN and HTN Participants
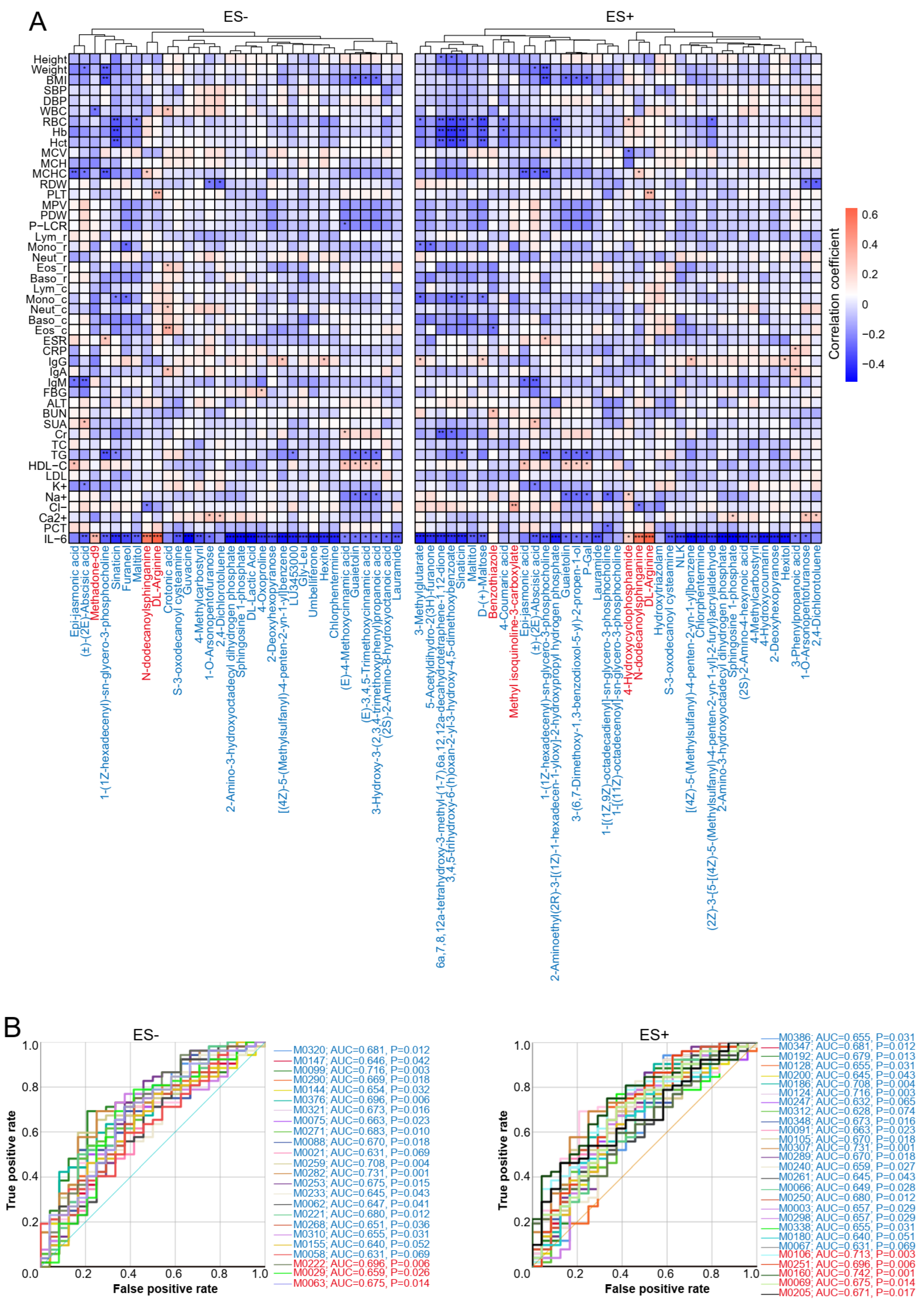
2.2. Correlations between Plasma Metabolites and Clinical Parameters
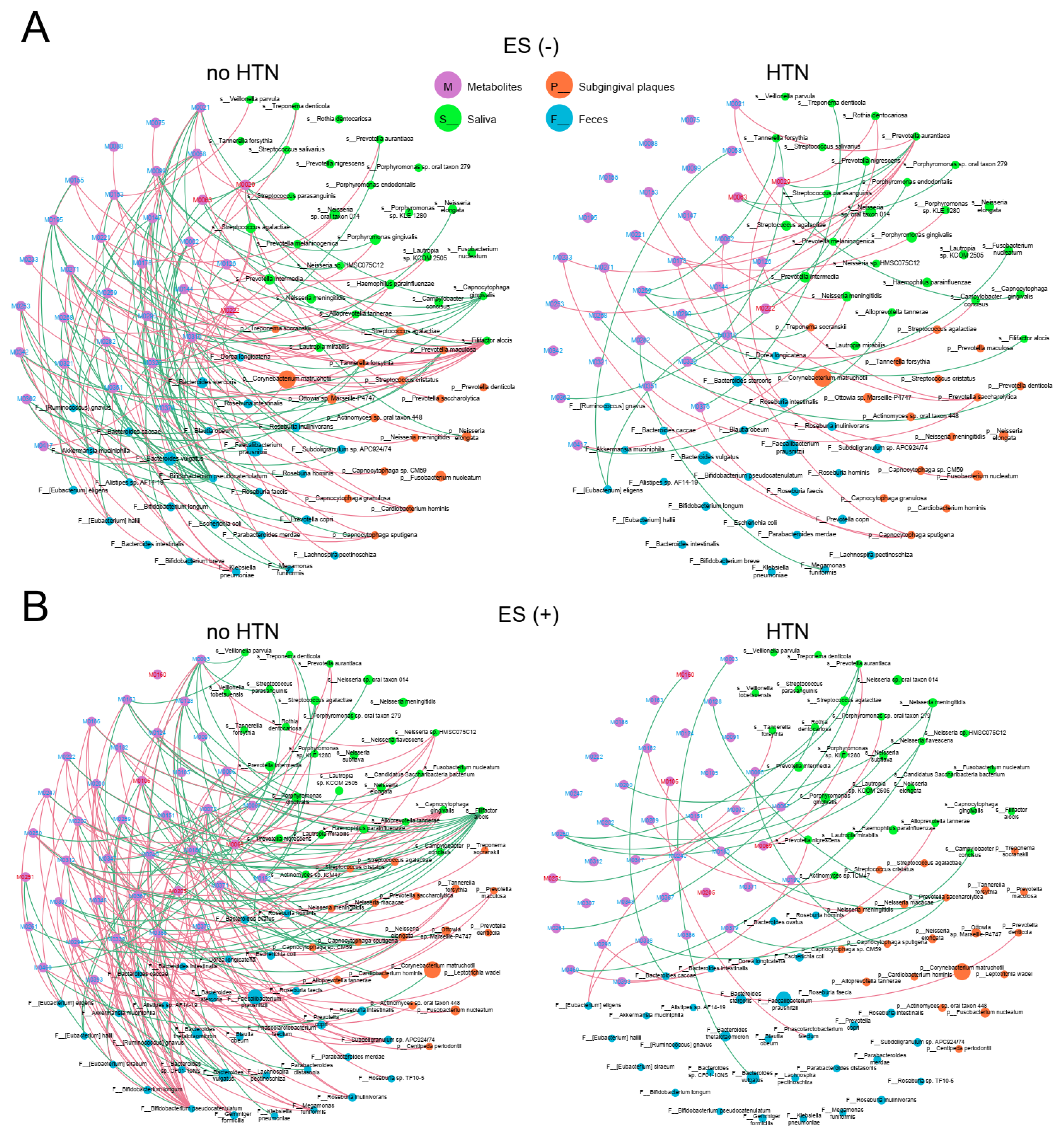
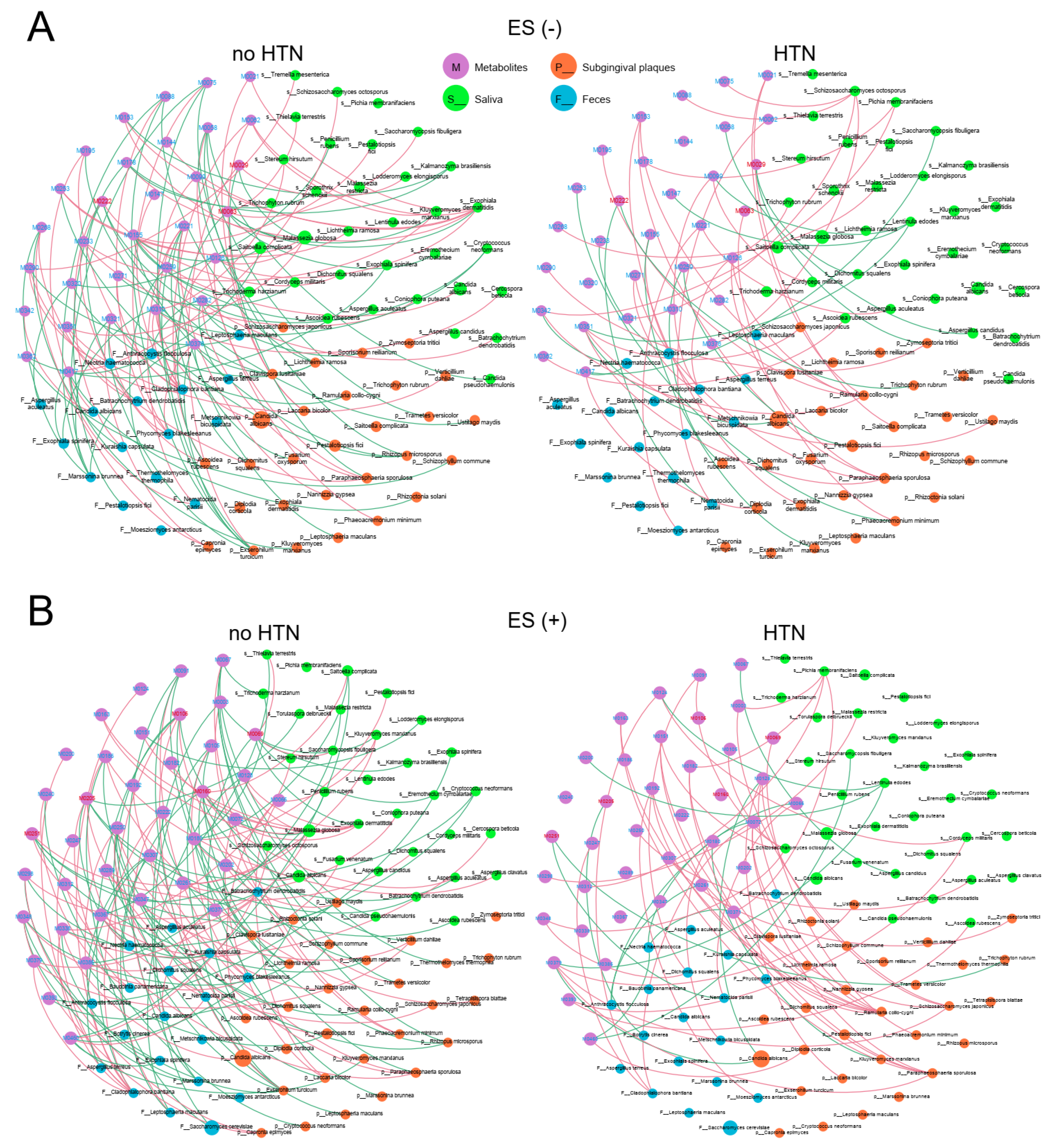
2.3. Associations between Plasma Metabolites and Community Composition of Oral and Gut Microbes
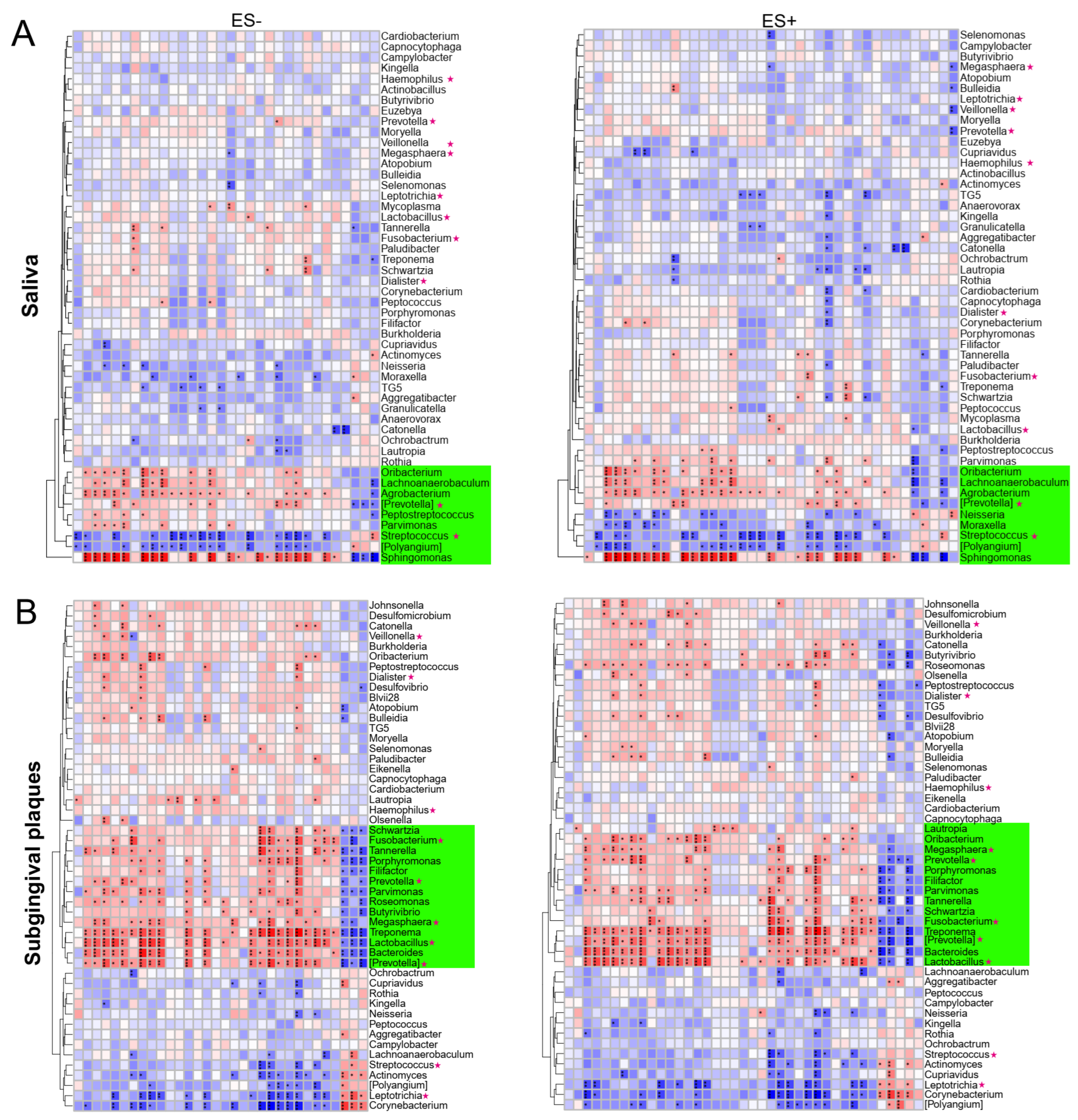
2.4. Associations between Plasma Metabolites and Oral/Gut Bacteria at Genus Level
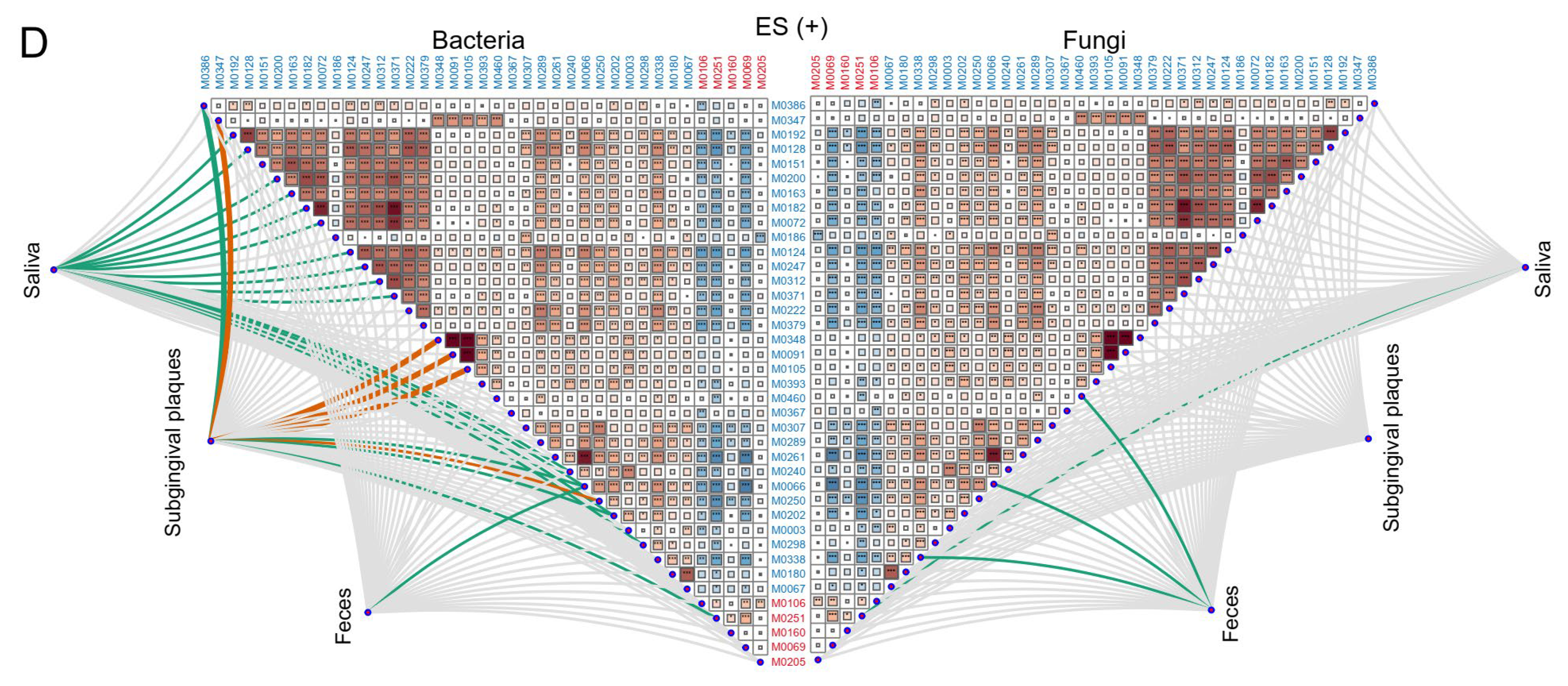
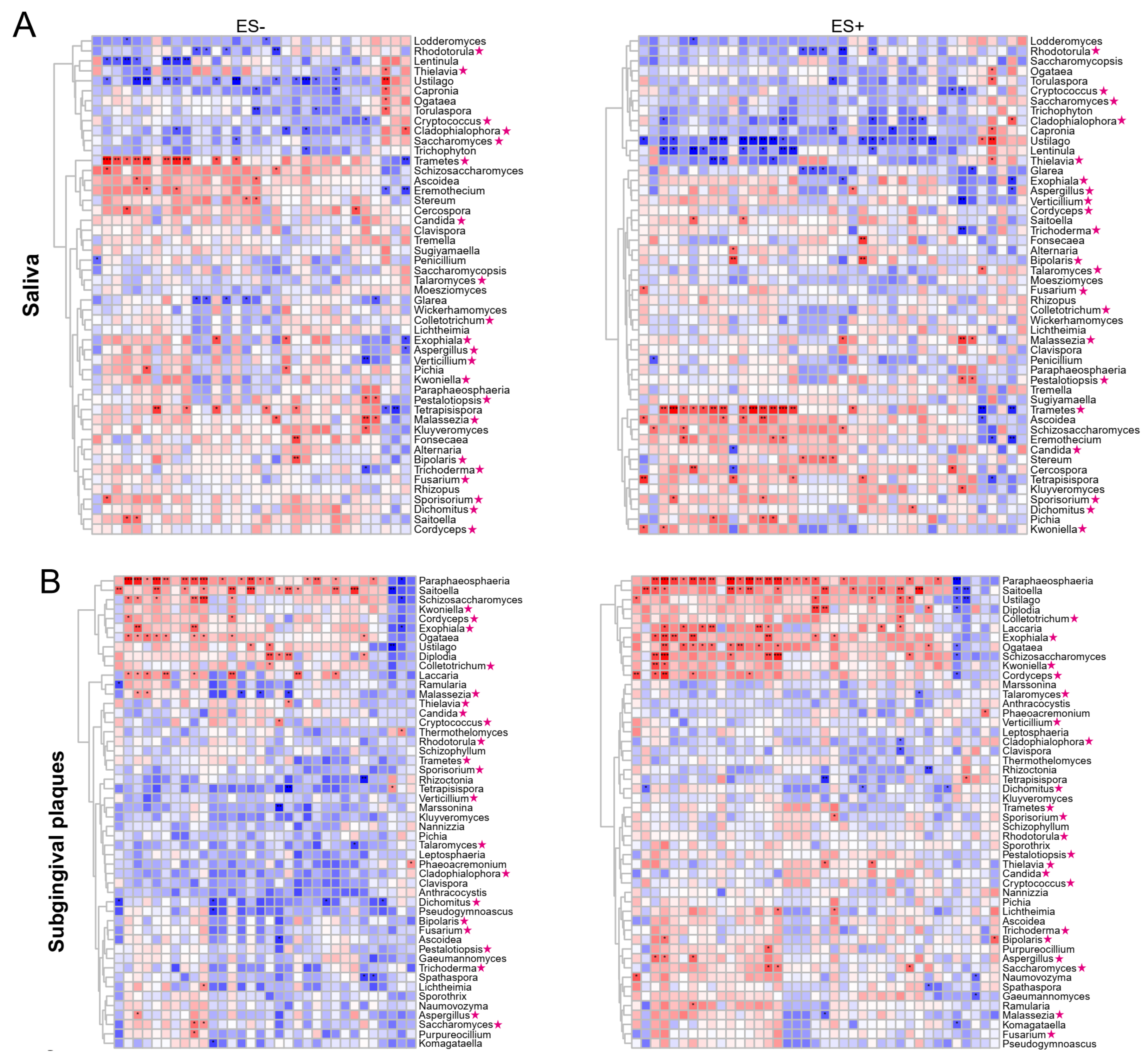
2.5. Associations between Plasma Metabolites and Oral/Gut Fungi at Genus Level
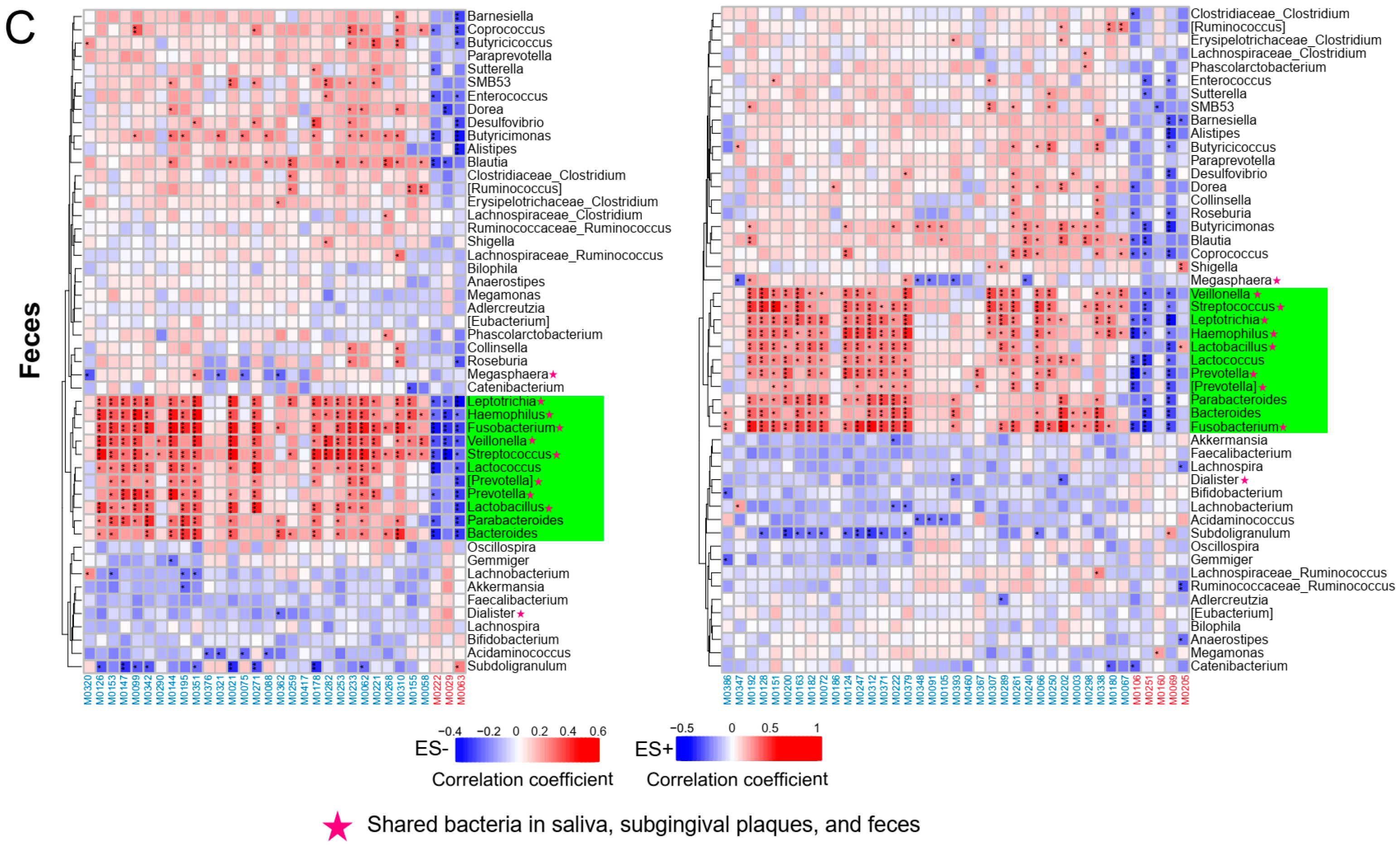
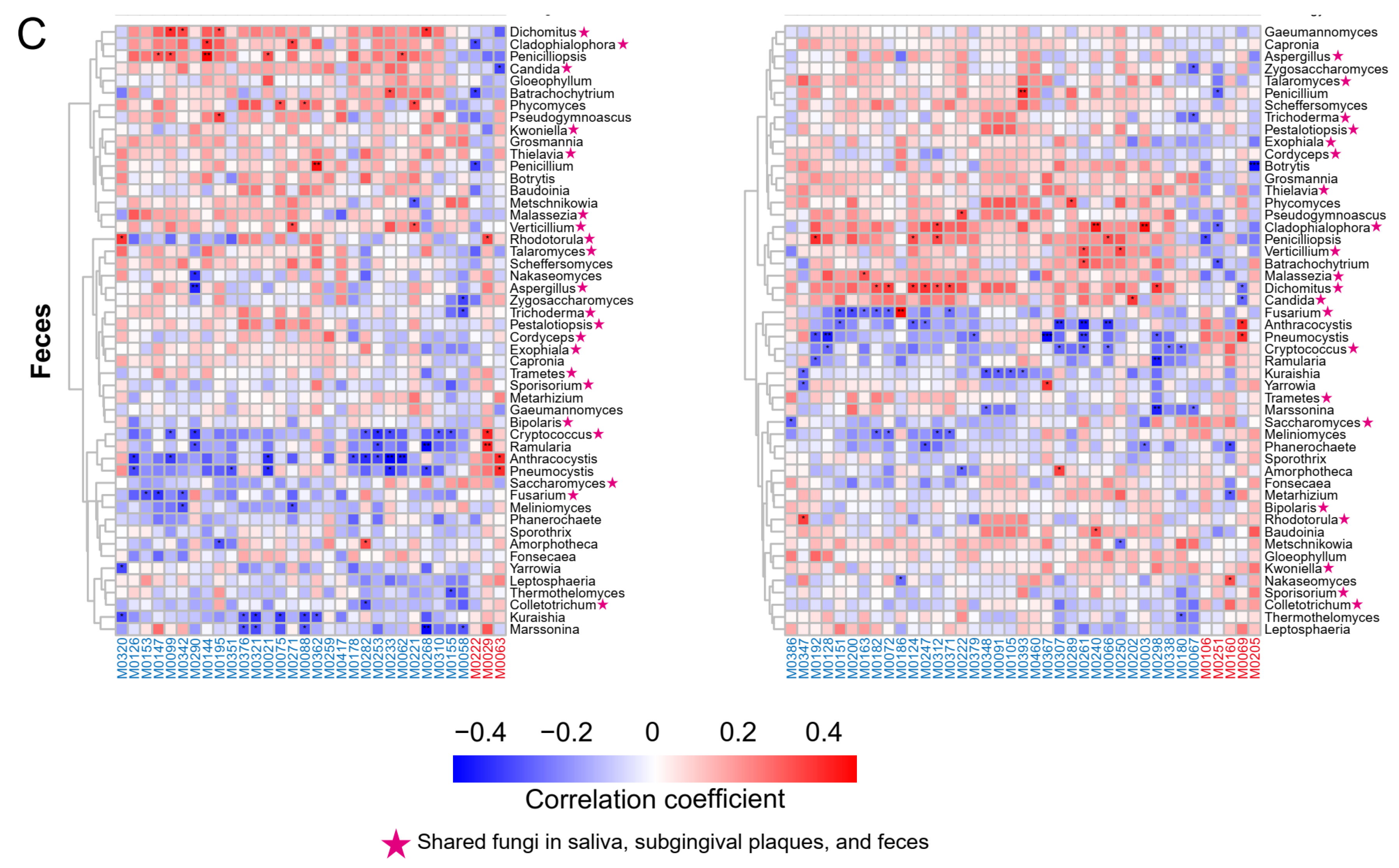
2.6. Associations between Plasma Metabolites and Oral/Gut Microbiota at the Species Level
3. Discussion
4. Materials and Methods
4.1. Human Cohort and Sample Collection
4.2. DNA Extraction
4.3. 16S rRNA Gene Sequencing and Metagenomic Sequencing
4.4. Untargeted Metabolomics Analysis
5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Zhao, F.; Wang, Y.; Chen, J.; Tao, J.; Tian, G.; Wu, S.; Liu, W.; Cui, Q.; Geng, B.; et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 2017, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Richards, E.M.; Li, J.; Stevens, B.R.; Pepine, C.J.; Raizada, M.K. Gut Microbiome and Neuroinflammation in Hypertension. Circ. Res. 2022, 130, 401–417. [Google Scholar] [CrossRef] [PubMed]
- Holmes, E.; Li, J.V.; Marchesi, J.R.; Nicholson, J.K. Gut Microbiota Composition and Activity in Relation to Host Metabolic Phenotype and Disease Risk. Cell Metab. 2012, 16, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Marques, F.Z.; Nelson, E.; Chu, P.-Y.; Horlock, D.; Fiedler, A.; Ziemann, M.; Tan, J.K.; Kuruppu, S.; Rajapakse, N.W.; El-Osta, A.; et al. High-Fiber Diet and Acetate Supplementation Change the Gut Microbiota and Prevent the Development of Hypertension and Heart Failure in Hypertensive Mice. Circulation 2017, 135, 964–977. [Google Scholar] [CrossRef]
- Yan, X.; Jin, J.; Su, X.; Yin, X.; Gao, J.; Wang, X.; Zhang, S.; Bu, P.; Wang, M.; Zhang, Y.; et al. Intestinal Flora Modulates Blood Pressure by Regulating the Synthesis of Intestinal-Derived Corticosterone in High Salt-Induced Hypertension. Circ. Res. 2020, 126, 839–853. [Google Scholar] [CrossRef]
- Zhao, M.; Zhao, L.; Xiong, X.; He, Y.; Huang, W.; Liu, Z.; Ji, L.; Pan, B.; Guo, X.; Wang, L.; et al. TMAVA, a Metabolite of Intestinal Microbes, Is Increased in Plasma from Patients with Liver Steatosis, Inhibits γ-Butyrobetaine Hydroxylase, and Exacerbates Fatty Liver in Mice. Gastroenterology 2020, 158, 2266–2281.e27. [Google Scholar] [CrossRef]
- Souto-Carneiro, M.; Tóth, L.; Behnisch, R.; Urbach, K.; Klika, K.D.; Carvalho, R.; Lorenz, H.-M. Differences in the serum metabolome and lipidome identify potential biomarkers for seronegative rheumatoid arthritis versus psoriatic arthritis. Ann. Rheum. Dis. 2020, 79, 499–506. [Google Scholar] [CrossRef]
- Chen, D.-Q.; Cao, G.; Chen, H.; Argyopoulos, C.P.; Yu, H.; Su, W.; Chen, L.; Samuels, D.C.; Zhuang, S.; Bayliss, G.P.; et al. Identification of serum metabolites associating with chronic kidney disease progression and anti-fibrotic effect of 5-methoxytryptophan. Nat. Commun. 2019, 10, 1476. [Google Scholar] [CrossRef]
- Varma, V.R.; Oommen, A.M.; Varma, S.; Casanova, R.; An, Y.; Andrews, R.M.; O’Brien, R.; Pletnikova, O.; Troncoso, J.C.; Toledo, J.; et al. Thambisetty, Brain and blood metabolite signatures of pathology and progression in Alzheimer disease: A targeted metabo-lomics study. PLoS Med. 2018, 15, e1002482. [Google Scholar]
- Ke, C.; Zhu, X.; Zhang, Y.; Shen, Y. Metabolomic characterization of hypertension and dyslipidemia. Metabolomics 2018, 14, 117. [Google Scholar] [CrossRef]
- Ahn, Y.; Nam, M.H.; Kim, E. Relationship Between the Gastrointestinal Side Effects of an Anti-Hypertensive Medication and Changes in the Serum Lipid Metabolome. Nutrients 2020, 12, 205. [Google Scholar] [CrossRef] [PubMed]
- Walejko, J.M.; Kim, S.; Goel, R.; Handberg, E.M.; Richards, E.M.; Pepine, C.J.; Raizada, M.K. Gut microbiota and serum metabolite differences in African Americans and White Americans with high blood pressure. Int. J. Cardiol. 2018, 271, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Rebholz, C.M.; Lichtenstein, A.H.; Zheng, Z.; Appel, L.J.; Coresh, J. Serum untargeted metabolomic profile of the Dietary Ap-proaches to Stop Hypertension (DASH) dietary pattern. Am. J. Clin. Nutr. 2018, 108, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Barbour, A.; Elebyary, O.; Fine, N.; Oveisi, M.; Glogauer, M. Metabolites of the oral microbiome: Important mediators of multi-kingdom interactions. FEMS Microbiol. Rev. 2022, 46, fuab039. [Google Scholar] [CrossRef]
- Wikoff, W.R.; Anfora, A.T.; Liu, J.; Schultz, P.G.; Lesley, S.A.; Peters, E.C.; Siuzdak, G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. USA 2009, 106, 3698–3703. [Google Scholar] [CrossRef]
- Bartolomaeus, H.; Balogh, A.; Yakoub, M.; Homann, S.; Markó, L.; Höges, S.; Tsvetkov, D.; Krannich, A.; Wundersitz, S.; Avery, E.G.; et al. Short-Chain Fatty Acid Propionate Protects from Hypertensive Cardiovascular Damage. Circulation 2019, 139, 1407–1421. [Google Scholar] [CrossRef]
- Jiang, S.; Shui, Y.; Cui, Y.; Tang, C.; Wang, X.; Qiu, X.; Hu, W.; Fei, L.; Li, Y.; Zhang, S.; et al. Gut microbiota dependent trimethylamine N-oxide aggravates angiotensin II–induced hypertension. Redox Biol. 2021, 46, 102115. [Google Scholar] [CrossRef]
- Vanhatalo, A.; Blackwell, J.R.; L’heureux, J.E.; Williams, D.W.; Smith, A.; van der Giezen, M.; Winyard, P.G.; Kelly, J.; Jones, A.M. Nitrate-responsive oral microbiome modulates nitric oxide homeostasis and blood pressure in humans. Free. Radic. Biol. Med. 2018, 124, 21–30. [Google Scholar] [CrossRef]
- Carlström, M.; Persson, A.E.G.; Larsson, E.; Hezel, M.; Scheffer, P.G.; Teerlink, T.; Weitzberg, E.; Lundberg, J.O. Dietary nitrate attenuates oxidative stress, prevents cardiac and renal injuries, and reduces blood pressure in salt-induced hypertension. Cardiovasc. Res. 2010, 89, 574–585. [Google Scholar] [CrossRef]
- Santisteban, M.; Qi, Y.; Zubcevic, J.; Kim, S.; Yang, T.; Shenoy, V.; Cole-Jeffrey, C.T.; Lobaton, G.; Stewart, D.C.; Rubiano, A.; et al. Hypertension-Linked Pathophysiological Alterations in the Gut. Circ. Res. 2017, 120, 312–323. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Chavakis, T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat. Rev. Immunol. 2021, 21, 426–440. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Reddy, K.R.; O’Leary, J.G.; Vargas, H.E.; Lai, J.C.; Kamath, P.S.; Tandon, P.; Wong, F.; Subramanian, R.M.; Thuluvath, P.; et al. Serum Levels of Metabolites Produced by Intestinal Microbes and Lipid Moieties Independently Associated with Acute-on-Chronic Liver Failure and Death in Patients with Cirrhosis. Gastroenterology 2020, 159, 1715–1730.e12. [Google Scholar] [CrossRef] [PubMed]
- Tomita, Y.; Sakata, S.; Arima, H.; Yamato, I.; Ibaraki, A.; Ohtsubo, T.; Matsumura, K.; Fukuhara, M.; Goto, K.; Kitazono, T. Rela-tionship between casual serum triglyceride levels and the development of hypertension in Japanese. J. Hypertens. 2021, 39, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Guan, L.; Zhao, Y.; Liu, Y.; Sun, X.; Li, H.; Yin, Z.; Li, L.; Ren, Y.; Wang, B.; et al. Association of triglycerides to high-density lipoprotein-cholesterol ratio with risk of incident hypertension. Hypertens. Res. 2020, 43, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Heresi, G.A.; Aytekin, M.; Newman, J.; DiDonato, J.; Dweik, R.A. Plasma levels of high-density lipoprotein cholesterol and out-comes in pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2010, 182, 661–668. [Google Scholar] [CrossRef]
- Neal, B.; Wu, Y.; Feng, X.; Zhang, R.; Zhang, Y.; Shi, J.; Zhang, J.; Tian, M.; Huang, L.; Li, Z.; et al. Effect of Salt Substitution on Cardiovascular Events and Death. N. Engl. J. Med. 2021, 385, 1067–1077. [Google Scholar] [CrossRef]
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef]
- Linderman, G.C.; Lu, J.; Lu, Y.; Sun, X.; Xu, W.; Nasir, K.; Schulz, W.; Jiang, L.; Krumholz, H.M. Association of Body Mass Index with Blood Pressure Among 1.7 Million Chinese Adults. JAMA Netw. Open 2018, 1, e181271. [Google Scholar] [CrossRef]
- Tesfaye, F.; Nawi, N.G.; Van Minh, H.; Byass, P.; Berhane, Y.; Bonita, R.; Wall, S. Association between body mass index and blood pressure across three populations in Africa and Asia. J. Hum. Hypertens. 2007, 21, 28–37. [Google Scholar] [CrossRef]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.H.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef]
- Yang, T.; Richards, E.M.; Pepine, C.J.; Raizada, M.K. The gut microbiota and the brain–gut–kidney axis in hypertension and chronic kidney disease. Nat. Rev. Nephrol. 2018, 14, 442–456. [Google Scholar] [CrossRef] [PubMed]
- Muralitharan, R.R.; Jama, H.A.; Xie, L.; Peh, A.; Snelson, M.; Marques, F.Z. Microbial Peer Pressure: The Role of the Gut Microbiota in Hypertension and Its Complications. Hypertension 2020, 76, 1674–1687. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-Y.; Lin, W.-Z.; Li, Y.-L.; Bi, C.; Du, L.-J.; Liu, Y.; Zhou, L.-J.; Liu, T.; Xu, S.; Shi, C.-J.; et al. Roles of oral microbiota and oral-gut microbial transmission in hypertension. J. Adv. Res. 2022, 43, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-Y.; Lin, W.-Z.; Li, Y.-L.; Bi, C.; Du, L.-J.; Liu, Y.; Zhou, L.-J.; Liu, T.; Xu, S.; Shi, C.-J.; et al. Characteristics and Correlations of the Oral and Gut Fungal Microbiome with Hypertension. Microbiol. Spectr. 2022, 11, e01956-22. [Google Scholar] [CrossRef]
- Intapad, S. Sphingosine-1-phosphate signaling in blood pressure regulation. Am. J. Physiol. Physiol. 2019, 317, F638–F640. [Google Scholar] [CrossRef]
- Cantalupo, A.; Di Lorenzo, A. S1P Signaling and De Novo Biosynthesis in Blood Pressure Homeostasis. Experiment 2016, 358, 359–370. [Google Scholar] [CrossRef]
- Senchenkova, E.Y.; Russell, J.; Yildirim, A.; Granger, D.N.; Gavins, F.N.E. Novel Role of T Cells and IL-6 (Interleukin-6) in Angi-otensin II-Induced Microvascular Dysfunction. Hypertension 2019, 73, 829–838. [Google Scholar] [CrossRef]
- Nishida, K.; Otsu, K. Inflammation and metabolic cardiomyopathy. Cardiovasc. Res. 2017, 113, 389–398. [Google Scholar] [CrossRef]
- Mills, E.; O’neill, L.A. Succinate: A metabolic signal in inflammation. Trends Cell Biol. 2014, 24, 313–320. [Google Scholar] [CrossRef]
- Chimenti, M.S.; Triggianese, P.; Conigliaro, P.; Candi, E.; Melino, G.; Perricone, R. The interplay between inflammation and me-tabolism in rheumatoid arthritis. Cell Death Dis. 2015, 6, e1887. [Google Scholar] [CrossRef]
- Greig, F.H.; Nather, K.; Ballantyne, M.D.; Kazi, Z.H.; Alganga, H.; Ewart, M.-A.; Zaborska, K.E.; Fertig, B.; Pyne, N.J.; Pyne, S.; et al. Requirement for sphingosine kinase 1 in mediating phase 1 of the hypotensive response to anandamide in the anaesthetised mouse. Eur. J. Pharmacol. 2018, 842, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, J.; Michel, T. Sphingosine-1-phosphate and modulation of vascular tone. Cardiovasc. Res. 2009, 82, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Murga, M.L.; Olivares, M.; Sanz, Y. Bifidobacterium pseudocatenulatum CECT 7765 reverses the adverse effects of diet-induced obesity through the gut-bone axis. Bone 2020, 141, 115580. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, B.; Stanton, C.; Ross, R.P.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacterium pseudocatenulatum Ameliorates DSS-Induced Colitis by Maintaining Intestinal Mechanical Barrier, Blocking Proinflammatory Cytokines, Inhibiting TLR4/NF-κB Signaling, and Altering Gut Microbiota. J. Agric. Food Chem. 2021, 69, 1496–1512. [Google Scholar] [CrossRef] [PubMed]
- Moratalla, A.; Caparrós, E.; Juanola, O.; Portune, K.; Puig-Kröger, A.; Estrada-Capetillo, L.; Bellot, P.; Gómez-Hurtado, I.; Piñero, P.; Zapater, P.; et al. Bifidobacterium pseudocatenulatum CECT7765 induces an M2 anti-inflammatory transition in macrophages from patients with cirrhosis. J. Hepatol. 2016, 64, 135–145. [Google Scholar] [CrossRef]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2′s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Li, D.; Liu, C.-M.; Luo, R.; Sadakane, K.; Lam, T.-W. MEGAHIT: An ultra-fast single-node solution for large and complex meta-genomics assembly via succinct de Bruijn graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar] [CrossRef]
- Wood, D.E.; Lu, J.; Langmead, B. Improved metagenomic analysis with Kraken 2. Genome Biol. 2019, 20, 257. [Google Scholar] [CrossRef]
- Menzel, P.; Ng, K.L.; Krogh, A. Fast and sensitive taxonomic classification for metagenomics with Kaiju. Nat. Commun. 2016, 7, 11257. [Google Scholar] [CrossRef]

| Characteristics | No HTN | HTN | p Values (No HTN vs. HTN) |
|---|---|---|---|
| Sex | 15 women; 9 men | 30 women; 22 men | 0.69 |
| Age (years) | 66.29 ± 1.42 | 69.21 ± 0.69 | 0.073 |
| Height (cm) | 163.40 ± 1.99 | 164.10 ± 1.02 | 0.77 |
| Weight (kg) | 64.93 ± 2.16 | 65.35 ± 1.37 | 0.87 |
| BMI (kg/m2;) | 24.56 ± 0.82 | 24.33 ± 0.44 | 0.79 |
| SBP (mmHg) | 120.6 ± 3.31 | 133.10 ± 2.01 | 0.0025 |
| DBP (mmHg) | 76.58 ± 1.27 | 79.10 ± 1.23 | 0.16 |
| Drinkers, n (%) | 1 (4.17%) | 2 (3.84%) | 0.31 |
| Diabetes mellitus, n (%) | 0 (0.00%) | 6 (11.54%) | NA |
| Antihypertensive treatment, n (%) | 0 (0.00%) | 40 (76.92%) | NA |
| NaÏve to antihypertensive treatment, n (%) | NA | 12 (23.08%) | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, B.-Y.; Li, Y.-L.; Lin, W.-Z.; Bi, C.; Du, L.-J.; Liu, Y.; Zhou, L.-J.; Liu, T.; Xu, S.; Zhang, J.; et al. Integrated Omic Analysis of Human Plasma Metabolites and Microbiota in a Hypertension Cohort. Nutrients 2023, 15, 2074. https://doi.org/10.3390/nu15092074
Chen B-Y, Li Y-L, Lin W-Z, Bi C, Du L-J, Liu Y, Zhou L-J, Liu T, Xu S, Zhang J, et al. Integrated Omic Analysis of Human Plasma Metabolites and Microbiota in a Hypertension Cohort. Nutrients. 2023; 15(9):2074. https://doi.org/10.3390/nu15092074
Chicago/Turabian StyleChen, Bo-Yan, Yu-Lin Li, Wen-Zhen Lin, Chao Bi, Lin-Juan Du, Yuan Liu, Lu-Jun Zhou, Ting Liu, Shuo Xu, Jun Zhang, and et al. 2023. "Integrated Omic Analysis of Human Plasma Metabolites and Microbiota in a Hypertension Cohort" Nutrients 15, no. 9: 2074. https://doi.org/10.3390/nu15092074
APA StyleChen, B.-Y., Li, Y.-L., Lin, W.-Z., Bi, C., Du, L.-J., Liu, Y., Zhou, L.-J., Liu, T., Xu, S., Zhang, J., Liu, Y., Zhu, H., Zhang, W.-C., Zhang, Z.-Y., & Duan, S.-Z. (2023). Integrated Omic Analysis of Human Plasma Metabolites and Microbiota in a Hypertension Cohort. Nutrients, 15(9), 2074. https://doi.org/10.3390/nu15092074






