A Cross-Sectional Study Based on Forty Systematic Reviews of Foods with Function Claims (FFC) in Japan: Quality Assessment Using AMSTAR 2
Abstract
1. Introduction
2. Methods
2.1. Eligibility and Exclusion Criteria (Target Articles)
2.2. Data Source
2.3. Data Items and Evaluation of the Methodological Quality (AMSTAR 2)
2.4. Evidence Table
2.5. Statistical Analysis
2.6. Protocol Registration
3. Results
3.1. Study Selection and Characteristics
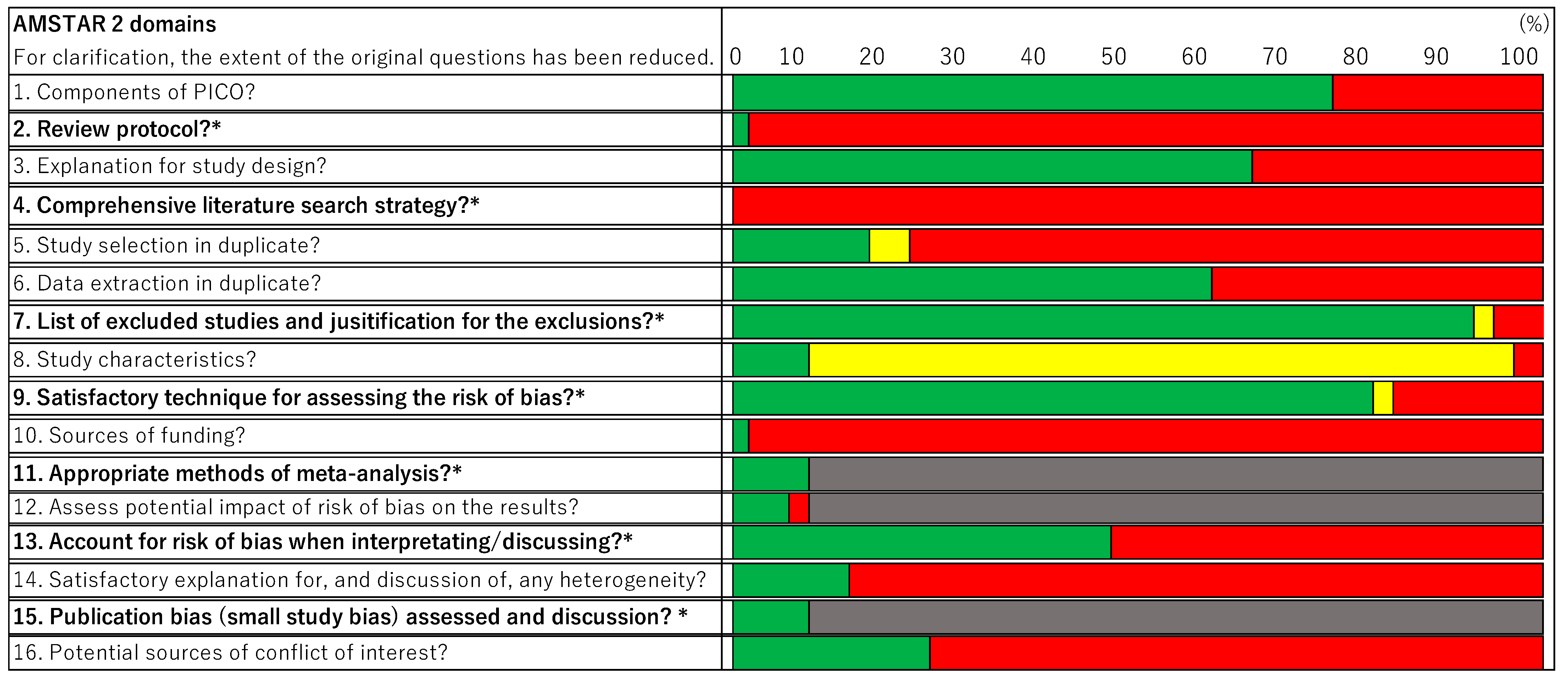
3.2. Quality Assessment
3.2.1. Critical Domains
3.2.2. Non-Critical Domains/Weaknesses
3.2.3. Common Critical Domains That Caused Degradation
4. Discussion
4.1. Main Findings
4.2. Findings in Context
4.3. Relevance to the GRADE Approach (Grading of Recommendations Assessment, Development, and Evaluation)
4.4. Challenges for Improving the Quality of SRs in the FFC
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Consumer Affairs Agency; Government of Japan. Introduction. 2015. Available online: https://www.caa.go.jp/policies/policy/food_labeling/information/pamphlets/pdf/151224_2.pdf (accessed on 1 March 2023).
- Consumer Affairs Agency; Government of Japan. Guideline (Updated March 2021). Available online: https://www.caa.go.jp/policies/policy/food_labeling/foods_with_function_claims/assets/foods_with_function_claims_210322_0002.pdf (accessed on 2 March 2023).
- Consumer Affairs Agency; Government of Japan. Verification of Scientific Evidence on “Foods with Function Claims”: Assessment of the Submitted Clinical Trials. Available online: https://www.caa.go.jp/policies/policy/food_labeling/foods_with_function_claims/pdf/foods_index_23_171025_0001.pdf (accessed on 3 March 2023).
- Tanemura, N.; Hamadate, N.; Urushibara, H. Evaluation of randomized controlled trials of foods with functional claims re-quest: The learning outcomes from studies in Japan. J. Funct. Foods 2018, 42, 248–253. [Google Scholar] [CrossRef]
- Kamioka, H.; Origasa, H.; Kitayuguchi, J.; Tsutani, K. Compliance of clinical trial protocols for Foods with Function Claims (FFC) in Japan: Consistency between clinical trial registrations and published reports. Nutrients 2022, 14, 81. [Google Scholar] [CrossRef] [PubMed]
- Kamioka, H.; Origasa, H.; Kitayuguchi, J.; Yoshizaki, T.; Shimada, M.; Wada, Y.; Takano-Ohmuro, H.; Tsutani, K. Risk of bias in clinical trials reported for Foods with Functional Claims in Japan: A cross-sectional study on research quality. J. Clin. Trials 2022, 12, 1000503. [Google Scholar]
- Consumer Affairs Agency; Government of Japan. Notification Information Search Site. Available online: https://www.fld.caa.go.jp/caaks/cssc01/ (accessed on 3 March 2023).
- Consumer Affairs Agency; Government of Japan. Verification of Scientific Evidence on Effectiveness of the System of “Foods with Function Claim”: Assessment of the Submitted Systematic Literature Reviews (Digest Edition). 2016. Available online: https://www.caa.go.jp/policies/policy/food_labeling/foods_with_function_claims/pdf/about_food_with_function_report_180416_0001.pdf (accessed on 3 March 2023).
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann. Intern. Med. 2009, 151, W65–W94. [Google Scholar] [CrossRef] [PubMed]
- Kamioka, H.; Tsutani, K.; Hideki, O.; Yoshizaki, T.; Kitayuguchi, J.; Shimada, M.; Tang, W.; Takano-Ohmuro, H. Quality of systematic reviews of the Foods with Function Claims registered at the Consumer Affairs Agency Web site in Japan: A prospective systematic review. Nutr. Res. 2017, 40, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.J.; Grimshaw, J.M.; Wells, G.A.; Boers, M.; Andersson, N.; Hamel, C.; Porter, A.C.; Tugwell, P.; Moher, D.; Bouter, L.M. Development of AMSTAR: A measurement tool to assess the methodological quality of systematic reviews. BMC Med. Res. Methodol. 2007, 7, 10. [Google Scholar] [CrossRef]
- Kamioka, H.; Tsutani, K.; Origasa, H.; Yoshizaki, T.; Kitayuguchi, J.; Shimada, M.; Wada, Y.; Takano-Ohmuro, H. Quality of systematic reviews of the Foods with Function Claims in Japan: Comparative before- and after-evaluation of verification reports by the Consumer Affairs Agency. Nutrients 2019, 11, 1583. [Google Scholar] [CrossRef]
- Niforatos, J.D.; Weaver, M.; Johansen, M.E. Assessment of publication trends of systematic reviews and randomized clinical trials, 1995 to 2017. JAMA Intern. Med. 2019, 179, 1593–1594. [Google Scholar] [CrossRef]
- Siontis, K.C.; Ioannidis, J.P.A. Replication, duplication, and waste in a quarter million systematic reviews and meta-analyses. Circ. Cardiovasc. Qual. Outcomes 2018, 11, e005212. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, V.; Kristjansson, E.; Henry, D.A. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomized or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, Y.; Kwong, J.S.; Zhang, C.; Li, S.; Sun, F.; Niu, Y.; Du, L. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: A systematic review. J. Evid. Base Med. 2015, 8, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Dai, F.; Xue, F.; Hu, G.; Zhang, Y.; Xu, X.; Wang, R.; Zhang, X.; Cong, D.; Wang, Y. Acupuncture for shoulder-hand syndrome after stroke: An overview of systematic reviews. Medicine 2022, 101, e31847. [Google Scholar] [CrossRef] [PubMed]
- Hasuike, A.; Ueno, D.; Nagashima, H.; Kubota, T.; Tsukune, N.; Watanabe, N.; Sato, S. Methodological quality and risk-of-bias assessments in systematic reviews of treatments for peri-implantitis. J. Periodontal. Res. 2019, 54, 374–387. [Google Scholar] [CrossRef]
- Kaplan, K.K.; Kaplan, L. Tai Chi and Parkinson’s disease (PD): A systematic overview of the scientific quality of the past systematic reviews. Complement. Ther. Med. 2019, 46, 144–152. [Google Scholar]
- Yang, G.; Hunter, J.; Bu, F.; Hao, W.; Zhang, H.; Wayne, P.M.; Liu, J.M. Determining the safety and effectiveness of Tai Chi: A critical overview of 210 systematic reviews of controlled clinical trials. Syst. Rev. 2022, 11, 260. [Google Scholar] [CrossRef]
- Yan, P.; Yao, L.; Li, H.; Zhang, M.; Xun, Y.; Li, M.; Cai, H.; Lu, C.; Hu, L.; Guo, T.; et al. The methodological quality of robotic surgical meta-analyses needed to be improved: A cross-sectional study. J. Clin. Epidemiol. 2019, 109, 20–29. [Google Scholar] [CrossRef]
- Liao, J.; Yin, Y.; Zhong, J.; Chen, Y.; Chen, Y.; Wen, Y.; Cai, Z. Bariatric surgery and health outcomes: An umbrella analysis. Front. Endocrinol. 2022, 13, 1016613. [Google Scholar] [CrossRef]
- Matthias, K.; Rissling, O.; Dawid Pieper, D.; Morche, J.; Nocon, M.; Jacobs, A.; Wegewitz, U.; Schirm, J.; Loren, R.C. The methodological quality of systematic reviews on the treatment of adult major depression needs improvement according to AMSTAR 2: A cross-sectional study. Heliyon 2020, 6, e04776. [Google Scholar] [CrossRef]
- Li, Y.; Ji, Z.; Yan Wang, Y.; Li, X.; Xie, Y. Breathing Exercises in the Treatment of COPD: An Overview of Systematic Reviews. Int. J. Chronic Obstr. Pulm. Dis. 2022, 17, 3075–3085. [Google Scholar] [CrossRef]
- Boini, S.; Bourgkard, E.; Ferrières, J.; Esquirol, Y. What do we know about the effect of night-shift work on cardiovascular risk factors? An umbrella review. Front. Public Health 2022, 23, 1034195. [Google Scholar] [CrossRef]
- den Braver, N.R.; Bengoechea, E.G.; Messing, S.; Kelly, L.; Schoonmade, L.J.; Volf, K.; Zukowska, J.; Gelius, P.; Forberger, S.; Woods, C.B.; et al. The impact of mass-media campaigns on physical activity: A review of reviews through a policy lens. Eur. J. Public Health 2022, 32 (Suppl. S4), iv71–iv83. [Google Scholar] [CrossRef] [PubMed]
- Gianfredi, V.; Ferrara, P.; Dinu, M.; Nardi, M.; Nucci, M. Diets, dietary patterns, single foods and pancreatic cancer risk: An umbrella review of meta-analyses. Int. J. Environ. Res. Public Health 2022, 19, 14787. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chen, J.; Wang, Y.; Yan, J.; Yan, X.; Wang, D.; Liu, Y. The role of Qishen Yiqi dripping pills in treating chronic heart failure: An overview of systematic reviews and meta-analyses. Front. Cardiovasc. Med. 2022, 24, 1001072. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, T.; Xu, Y.; Chen, Y.; Deng, L.; Wang, C.; Chen, J.; Tan, J. Effects of exercise interventions on cancer-related fatigue in breast cancer patients: An overview of systematic reviews. Support. Care Cancer 2022, 30, 10421–10440. [Google Scholar] [CrossRef]
- Zhong, C.C.W.; Zhao, J.; Wong, C.H.L.; Wu, I.X.Y.; Mao, C.; Yeung, J.W.F.; Chung, V.C.H. Methodological quality of systematic reviews on treatments for Alzheimer’s disease: A cross-sectional study. Alzheimers Res. Ther. 2022, 29, 159. [Google Scholar] [CrossRef]
- Martinez-Calderon, J.; de-la-Casa-Almeida, M.; Matias-Soto, J. The effects of mind-body exercises on chronic spinal pain outcomes: A synthesis based on 72 meta-analyses. Int. J. Environ. Res. Public Health 2022, 19, 12062. [Google Scholar] [CrossRef]
- Shen, Q.; Huang, Z.; Leng, H.; Luo, X.; Zheng, X. Efficacy and safety of non-pharmacological interventions for neonatal pain: An overview of systematic reviews. BMJ Open 2022, 12, e062296. [Google Scholar] [CrossRef]
- Webster, J.; Rycroft, C.E.; Greenwood, D.C.; Cade, J.E. Dietary risk factors for hip fracture in adults: An umbrella review of meta-analyses of prospective cohort studies. PLoS ONE 2021, 16, e0259144. [Google Scholar] [CrossRef]
- Axon, E.; Chalmers, J.R.; Santer, M.; Ridd, M.J.; Lawton, S.; Langan, S.M.; Grindlay, D.J.C.; Muller, I.; Roberts, A.; Ahmed, A.; et al. Safety of topical corticosteroids in atopic eczema: An umbrella review. BMJ Open 2021, 11, e046476. [Google Scholar] [CrossRef]
- Cruciani, M.; Pati, I.; Masiello, F.; Pupella, S.; Angelis, V.D. Corticosteroids use for COVID-19: An overview of systematic reviews. Infez. Med. 2022, 30, 469–479. [Google Scholar]
- International Prospective Register of Systematic Reviews. What Is Registration? Available online: https://www.crd.york.ac.uk/PROSPERO/#aboutpage (accessed on 3 March 2023).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Rethlefsen, M.L.; Kirtley, S.; Page, M.J.; Koffel, J.B.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; PRISMA-S Group. PRISMA-S: An extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomized trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Savović, J.; Page, M.J.; Jonathan, A.C.S.; on behalf of the RoB 2 Development Group. Revised Cochrane Risk-of-Bias Tool for Randomized Trials (RoB 2). Available online: https://drive.google.com/file/d/19R9savfPdCHC8XLz2iiMvL_71lPJERWK/view?pli=1 (accessed on 3 March 2023).
- GRADE Working Group. What Is GRADE? Available online: https://www.gradeworkinggroup.org/ (accessed on 3 March 2023).
- Uttley, L.; Quintana, D.S.; Montgomery, P.; Carroll, C.; Page, M.J.; Falzon, L.; Sutton, A.; Moher, D. The Problems with Systematic Reviews: A Living Systematic Review. J. Clin. Epidemiol. 2023, 156, 30–41. [Google Scholar] [CrossRef]
- Shamser, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocol (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 349, g7647. [Google Scholar] [CrossRef]
- Zeraatkar, D.; Bhasin, A.; Morassut, R.E.; Churchill, I.; Gupta, A.; Lawson, D.O.; Miroshnychenko, A.; Sirotich, E.; Aryal, K.; Mikhail, D.; et al. Characteristics and quality of systematic reviews and meta-analyses of observational nutritional epidemiology: A cross-sectional study. Am. J. Clin. Nutr. 2021, 113, 1578–1592. [Google Scholar] [CrossRef]
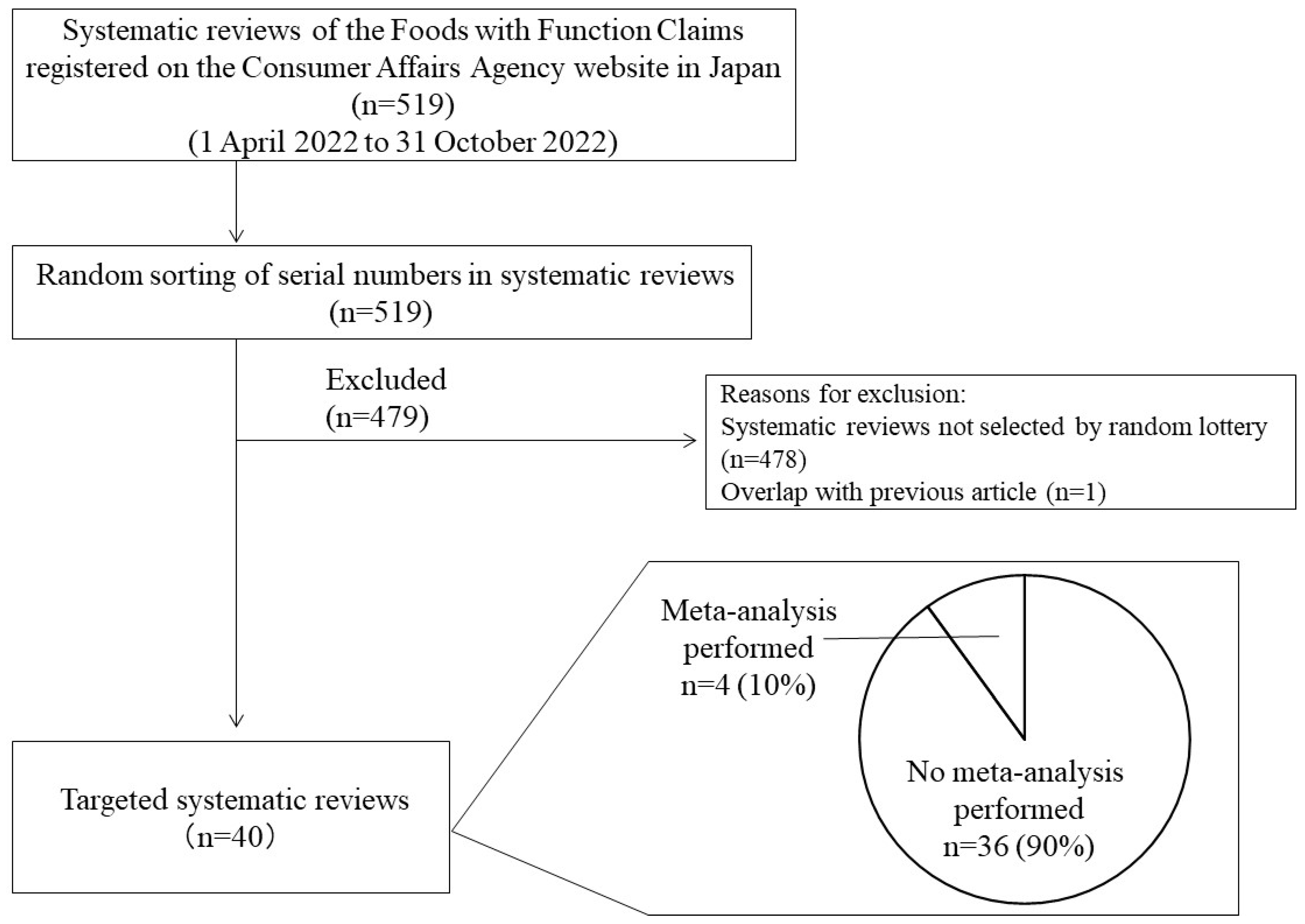


| No. | Product Name | Food Business Operator | Classification of Food 1 Supplement 2 Processed Food 3 Fresh Produce | Functional Substance |
|---|---|---|---|---|
| H488 | Kokunaisan Organic Kikuimo Tea | Aim Co., Ltd. | 2 | Inulin |
| H464 | Nisshin MCT Mayonnaise Sauce | The Nisshin OilliO Group, Ltd. | 2 | Medium chain triglyceride (octanoic acid, decanoic acid) |
| H339 | BIKOKUSAI chinese soup | BROOK’S Co., Ltd. | 2 | Indigestible dextrin (dietary fiber) |
| H24 | Pilkul miracle care | Nissin York Co., Ltd. | 2 | Lactic acid bacterium NY1301 strain |
| H1 | Terminalia slim +B | Aminocells Corp. | 1 | Gallic acid made from Terminalia berylica |
| H39 | Hapicolla Stick | Nitta Gelatin Inc. | 2 | Low-molecular-weight collagen peptide made from fish |
| H173 | DHA900 | Nakahara Co., Ltd. | 1 | DHA⋅EPA |
| H381 | Kekkan shinayaka elastin supplement | HAKUJU INSTITUTE FOR HEALTH SCIENCE Co., Ltd | 1 | Elastin peptide made from bonito |
| H414 | im Protein Resilie | Ortho corporation | 1 | Salmon nasal proteoglycan |
| H195 | Hisamitsu® metasapo® | HISAMITSU PHARMACEUTICAL CO.,INC. | 1 | Tea catechin, Epigallocatechin gallate (EGCg) |
| H337 | Swellieve | DIANA Co., Ltd. | 1 | Genquanine 5-O-β-primeveroside, Mangiferin, Piperines made from hihatsu |
| H236 | Psyllium fiber 100% | YAMAMOTO KANPOH PHARMACEUTICAL CO., LTD. | 1 | Dietary fiber made from psyllium seed coat |
| H318 | Shibo Chuiho EXb | MG PHARMA, Inc. | 1 | Valine-Valine-Tyrosine-Proline made from globin |
| H245 | YUTAKANI | SONOKO Co., Ltd. | 1 | Camellia saponin B2 |
| H15 | Rrefreshing life with lactobacillus | Willumina, Inc. | 1 | Bacillus coagulans SANK70258 |
| H438 | No response was received from the company. | Medione, Inc. | 1 | Elastin peptide made from bonito |
| H357 | Shokuji To Salacia | Asahi Bussan Co., Ltd. | 1 | Salacinol made from Salacia |
| H290 | MINUTE MAID PLASMA LACTO. IMMUNITY CARE | Coca-Cola (Japan) Company, Limited | 2 | L. lactis strain plasma |
| H120 | PURU-Kira Mamoru-kun | Nippi, Incorporated | 1 | Collagen peptide made from fish |
| H526 | Du-Zhong Tea | Kobayashi Pharmaceutical Co., Ltd. | 2 | Geniposidic acid |
| H358 | Umakara Kimuchi | PICKLES CORPORATION | 2 | Lb. plantarum PIC-NBN22, Fructo-oligosaccharide |
| H301 | Rosemary a | S&B FOODS, Inc. | 1 | Rosmarinic acid made from rosemary |
| H527 | CUTTO MAINTE | KARADA NI EIYO, Inc. | 1 | Piperines made from hihatsu |
| H53 | mimamoru a | Ogaland Co., Ltd. | 1 | Lutein |
| H456 | The product has not been named in English yet. | Pyuru Co., Ltd. | 1 | Lutein, Zeaxanthin |
| H91 | Nagarurumo | Ortho Corporation | 1 | Piperines made from hihatsu, Bacillus coagulans SANK70258 |
| H465 | Production and sales were discontinued. | YAMASA CORPORATION | 2 | Inulin |
| H155 | Eyebon supple a | Kobayashi Pharmaceutical Co., Ltd. | 1 | Lutein, Zeaxanthin |
| H149 | Mirasoup consomme flavor | PremiumCosme Inc. | 2 | Isoflavones made from kudzu (Tectorigenins), Glucosylceramide made from rice |
| H415 | SLIM BLOCK CHOKATSU PRO | Nihon Yakken Co., Ltd. | 1 | Gallic acid made from Terminalia berylica, Bacillus coagulans SANK70258 |
| H444 | Theracurmin 3E | Theravalues Corporation | 1 | Curcumin |
| H367 | Cirneco Rosso | Croix Co., Ltd. | 2 | GABA |
| H455 | SUYASUYANOTANE+ | Facelabo Co., Ltd. | 1 | GABA |
| H338 | The product has not been named in English yet. | Mikakuto Co., Ltd. | 1 | Plant lactobacillus K-1(L. casei 327) |
| H126 | Green juice for those worried about cholesterol or triglyceride | Taisho Pharmaceutical Co., Ltd. | 2 | Ellagic acid |
| H141 | ”KARADA-ni” Euglena Muscat & Herbal Flavor | Euglena Co., Ltd. | 2 | Paramylon made from Euglena Gracilis (β-1,3-glucan) |
| H29 | Joshu chicken (breast meat) | KURICHIKU, Inc. | 3 | Imidazole dipeptide |
| H428 | Slim apple | Setagaya Natural Foods Co., Ltd. | 1 | Procyanidins made from apple |
| H19 | Terminalia slim +A | Aminocells Corp. | 1 | Gallic acid made from Terminalia berylica |
| H360 | Oligosmart 100 milk chocolate | Meiji Co., Ltd. | 2 | Fructooligosaccharides |
| For Companies or Food Industry | |
|---|---|
| #1 | The applicants should conduct research based on AMSTAR 2, PRISMA 2020, PRISMA-S, PRISMA-P, and GRADE. |
| #2 | The applicants should receive guidance from searchers, biostatistics experts, and research methodologists on literature search methods, meta-analysis methods, and bias assessments, respectively. |
| For academia | |
| #3 | Academia researchers should conduct regular surveys and make proposals for improvement since the authorities in charge cannot evaluate the quality of individual SRs because of the notification system. |
| For the Consumer Affairs Agency in Japan | |
| #4 | The authority should be obliged in the guidelines to require companies to pre-register the protocol. |
| #5 | The authority should encourage companies to notify them if an SR is published as an article in an academic journal. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamioka, H.; Origasa, H.; Tsutani, K.; Kitayuguchi, J.; Yoshizaki, T.; Shimada, M.; Wada, Y.; Takano-Ohmuro, H. A Cross-Sectional Study Based on Forty Systematic Reviews of Foods with Function Claims (FFC) in Japan: Quality Assessment Using AMSTAR 2. Nutrients 2023, 15, 2047. https://doi.org/10.3390/nu15092047
Kamioka H, Origasa H, Tsutani K, Kitayuguchi J, Yoshizaki T, Shimada M, Wada Y, Takano-Ohmuro H. A Cross-Sectional Study Based on Forty Systematic Reviews of Foods with Function Claims (FFC) in Japan: Quality Assessment Using AMSTAR 2. Nutrients. 2023; 15(9):2047. https://doi.org/10.3390/nu15092047
Chicago/Turabian StyleKamioka, Hiroharu, Hideki Origasa, Kiichiro Tsutani, Jun Kitayuguchi, Takahiro Yoshizaki, Mikiko Shimada, Yasuyo Wada, and Hiromi Takano-Ohmuro. 2023. "A Cross-Sectional Study Based on Forty Systematic Reviews of Foods with Function Claims (FFC) in Japan: Quality Assessment Using AMSTAR 2" Nutrients 15, no. 9: 2047. https://doi.org/10.3390/nu15092047
APA StyleKamioka, H., Origasa, H., Tsutani, K., Kitayuguchi, J., Yoshizaki, T., Shimada, M., Wada, Y., & Takano-Ohmuro, H. (2023). A Cross-Sectional Study Based on Forty Systematic Reviews of Foods with Function Claims (FFC) in Japan: Quality Assessment Using AMSTAR 2. Nutrients, 15(9), 2047. https://doi.org/10.3390/nu15092047






