Abstract
This study examined the association between maternal serum vitamin B12 levels during early pregnancy and offspring autism spectrum disorders (ASD) and subtypes. Based on a Finnish national birth cohort, case offspring (n = 1558) born in 1987–2007 and diagnosed with ASD by 2015 were matched with one control on date of birth, sex and place of birth. Maternal vitamin B12 levels were measured during first and early second trimesters of pregnancy. High maternal vitamin B12 levels (≥81th percentile) was associated with increased risk for offspring childhood autism, adjusted odds ratio, 1.59, 95% confidence interval 1.06–2.41 (p = 0.026). No significant associations were observed between maternal vitamin B12 levels and offspring Asperger’s or pervasive developmental disorder/NOS.
1. Introduction
Autism Spectrum Disorder (ASD) is a heterogeneous group of neurodevelopmental conditions characterized by impairment in social communication and interaction, accompanied by restricted and repetitive patterns in behaviors, interests and activities [1]. There is a wide recognition that the etiology of ASD is multifactorial, involving the interplay of genetic and environmental factors [2]. Strong evidence has suggested that ASD is influenced by prenatal factors [3] including maternal nutrition [4]. Specific nutrients have been identified as potentially impacting the risk for ASD, for example, folate, vitamin D, iron and polyunsaturated fatty acids, but the findings are often mixed [5,6].
Vitamin B12 is essential for normal growth, development and physiological functions [7]. It plays an important role in neural myelination, synaptogenesis and neurotransmitter synthesis [8]. Since the fetus is completely dependent on maternal nutrients, inadequate vitamin B12 levels during pregnancy may impair these processes and cause neural damage or brain atrophy [9,10]. Experimental rodent studies have shown a link between maternal folate and vitamin B12 deficiency with structural brain abnormalities [11], alteration in gene expression in the cerebellum [12,13] and cognitive impairment in offspring [14,15]. In humans, folate and vitamin B12 deficiency have been associated with poor neurodevelopment [16] and negative effects on cognitive functions including memory, language and motor skills [17,18,19], preterm birth, low birth weight [20] and insulin resistance [21].
As shown in Table S1, prior studies on maternal vitamin B12 and offspring ASD were mainly focused on multivitamin or folate supplementation with inconsistent findings. Only one study specifically examined the association between maternal plasma vitamin B12 and offspring ASD [22]. The authors showed that high vitamin B12 levels (≥536.8 pmol/L) increased the risk for offspring ASD. Of note, the study included only 86 ASD cases and maternal blood samples were collected 24–72 h post-delivery. Four studies examined the association between maternal plasma folate and offspring ASD or autistic traits, but the findings were inconsistent [22,23,24,25]. Two of these studies, one from Sweden (N = 100) and the other from the USA (N = 86) showed high levels of maternal plasma folate associated with increased risk of ASD in offspring [22,24]. However, two other studies from the Netherlands (N = 3893) and the USA (N = 209) did not observe any associations between maternal folate concentration and child autistic traits [23,25]. The use of folate and/or multivitamins early or during pregnancy showed no associations, increased or decreased risk for ASD or ASD symptoms in offspring [26,27,28,29,30,31,32,33,34,35,36,37].
There is a lack of knowledge from the existing literature about the role of maternal vitamin B12 as a possible risk factor for offspring ASD. First, no previous study has examined maternal serum vitamin B12 levels during pregnancy and offspring risk for ASD diagnosis. Previous studies on maternal vitamin B12 and offspring ASD were based on multivitamin supplementation, and the composition of the multivitamins used during pregnancy was unclear [26,27,28,29,30,31,32,33,34,35,36,37]. Second, out of three studies examining associations between maternal folate concentrations during early pregnancy and offspring ASD [23,24,25], two studies were based on ASD symptoms [23,25] and one study was on ASD diagnosis [24].
The aim of this population-based study was to examine the association between maternal serum vitamin B12 levels in early pregnancy and the risk of ASD diagnosis in offspring. In addition, we investigated the association between maternal serum vitamin B12 levels in early pregnancy and specific ASD subtypes in the offspring. Based on the findings of maternal folate and vitamin B12 deficiency associated with impaired cognitive behavior in rodent offspring [14], we hypothesized that lower maternal vitamin B12 levels would be associated with an increased risk of offspring ASD. This would have important public health implications for preventative strategies. Since vitamin B12 is found in animal-sourced foods, vitamin B12 deficiency has been widely prevalent in those with poor intake of animal origin foods owing to the religious or cultural practice of vegetarianism [38]. This could be readily preventable by supplementation.
2. Materials and Methods
The Finnish Prenatal Study of Autism Spectrum Disorders (FIPS-A) is a nested case-control study including all singleton live births between 1987–2005 in Finland. Each child was followed for the diagnosis of ASD to the end of 2015. The data for this study were derived from three national registers: the Care Register for Health Care (CRHC), the Finnish Medical Birth Register (FMBR) and the Finnish Population Register Centre (FCPR). These registers are linked using personal identity codes that are issued at birth or on immigration to all Finnish residents since 1964.
2.1. Nationwide Registers
The CRHC includes all public and private inpatient diagnoses since 1967 and all outpatient diagnoses from specialized services since 1998. The diagnostic classification in Finland is based on the International Classification of Diseases (ICD): ICD-8 from 1969 to 1986, ICD-9 from 1987 to 1995 and ICD-10 since 1996. A previous diagnostic validation study of ASD has shown 96% specificity for childhood autism [39]. The FMBR contains nationwide comprehensive data on all live births during the neonatal period up to 7 days of age since 1987. The FCPR is a computerized national archive maintained by the Finnish population center and local register officers. It contains basic demographic information (for example, name, personal identity codes, address, citizenship and native language, family relations, date of birth and death (if applicable)) of current Finnish citizens and permanent residents in Finland dating back to 1969. The FCPR was used to identify the controls and to obtain information on the subjects’ parents and places of birth.
The study was conducted in accordance with the Declaration of Helsinki, and FIPS-A received ethical approval from the Ethics Committee of the Hospital District of Southwest Finland(Dnro number 24/007), the data protection authorities at the National Institute for Health and Welfare and the Institutional Review Board of the New York State Psychiatric Institute (5740). The study design and the request for biobank serum samples were approved by the responsible Biobank’s Scientific Committee (Biobank Borealis of Northern Finland, University of Oulu, Oulu, Finland) (BB_2017_1017).
2.2. Finnish Maternity Cohort
The Finnish Maternity Cohort of the Northern Finland Biobank Borealis (FMC) is a nationwide serum bank that consists of approximately 2 million serum samples collected during the first and early second trimesters of pregnancy (5th to 95th percentile: months 2–4 of pregnancy) from over 950,000 women. After informed consent, the remaining serum samples (one sample of 1–3 mL for each pregnancy) were stored at −25 °C in a protected biorepository at the Biobank Borealis in Oulu, Finland and were available for scientific research. All samples in the FMC were linked with offspring and other Finnish nationwide registers by a unique personal identification code that has been issued to each resident of Finland since 1971.
2.3. Information on Cases and Controls
The ASD cases were born in Finland between January 1987 and December 2007 and were registered in the CRHC with ICD-10 (F84x) and ICD-9 (299x) diagnoses by 2015. Controls were singleton offspring born in Finland and without a diagnosis of ASD or Intellectual disability (ID). Each case was matched with one control on date of birth (+/−30 days), sex and place of birth. The controls were alive at the time of the diagnosis of the matched cases. During 1987–2005, there were 4705 cases with diagnoses of childhood autism, Asperger’s and PDD/NOS. In total, 1558 cases and 1558 matched controls were included in the study. The subjects with ID were identified from CRHC with ICD codes: ICD-10 (F70, F71, F72, F73, F78, and F79) and ICD-9 (317, 318.0, 318.1, 318.2, and 319).
2.4. Maternal Vitamin B12 Measurement
To examine maternal vitamin B12 levels in the prenatal sera, we measured active B12 (HoloTC, Holotranscobalamin) using a chemiluminescence microparticle immunoassay on the Architect i2000SR automatic immunoassay analyzer (Abbott Diagnostics). The coefficient of variation (mean ± SD) derived from repeated quality control samples included in each set of daily assays was 4.7% in the control samples with high B12 levels (range 43.7–49.6 pmol/L) and 6.4% in those with low B12 levels (range 13.6–17.3 pmol/L).
2.5. Covariates
Potential confounders and mediators suggested to be associated with both maternal vitamin B12 and ASD were selected [40,41,42,43,44]. The FMBR was used to obtain information on the number of previous births, maternal socioeconomic status (SES), maternal age, maternal smoking during pregnancy, gestational age, Apgar score at 1 min and weight for gestational age. As smokers are known to have lower vitamin B12 levels [45,46], maternal smoking during pregnancy was included in the covariate testing. Information on maternal and paternal psychiatric diagnoses and maternal substance abuse diagnoses was obtained from the CRHC. Maternal immigrant status was obtained from the FCPR, while gestational week and season of blood draw were obtained from the FMC. The variables included in the analysis were categorized as follows: maternal smoking (yes, no), previous births (0, ≥1), history of maternal psychopathology (yes, no), history of paternal psychopathology (yes, no), maternal SES (upper white collar, lower white collar, blue collar, others, missing), history of maternal substance abuse (yes, no), gestational age (<37 years, ≥37 years), weight for gestational age (<−2 SD, −2SD to +2SD, > + 2SD), maternal immigration status (yes, no), season of blood collection (spring, summer, autumn, winter) and Apgar score (0–6, 7–8, 9–10). Detailed descriptions of covariates are given in Table 1 and Table 2.

Table 1.
Relationship between covariates and maternal vitamin B12 levels (≥/< median) among controls.

Table 2.
Relationship between covariates and ASD in case and control subjects.
2.6. Statistical Analysis
Maternal vitamin B12 levels were initially examined as a continuous variable. Since vitamin B12 levels had a skewed distribution, they were log-transformed before analysis. We also examined maternal vitamin B12 levels categorized into quintiles. The cut-off of the quintiles for case and control groups was based on the distribution of maternal vitamin B12 levels in the control group, with the third quintile as the reference group. Continuous potential confounders were tested with χ2, Student’s t and Fisher’s exact tests and/or Pearson chi-square tests for categorical variables for the association with log-transformed maternal vitamin B12 levels among controls. Potential confounders were then tested for association with ASD using conditional logistic regression models for the matched sets. The covariates were included in the adjusted models based on their association with both the exposure and the outcome at p < 0.1.
The point and interval estimate of odds ratios (OR) were obtained by fitting conditional logistic regression models for matched pairs. Unadjusted ORs and adjusted odds ratios (aORs) and 95% confidence intervals (CI) were calculated separately for ASD and 3 subgroups: childhood autism, Asperger’s and Pervasive Developmental Disorder (PDD)/PDD-not otherwise specified (NOS). A sensitivity analysis was performed adjusting for gestational age as it is a risk factor for ASD (Allen et al., 2020). Further sensitivity analysis was performed additionally adjusting for maternal psychopathology, substance abuse, and offspring gestational age to address prior evidence that maternal alcohol use affects vitamin B12 and folate levels (Laufer et al., 2004). Additional analyses were performed stratifying ASD with and without ID. Conditional logistic regression was used to test for interactions between continuous maternal vitamin B12 and ASD by subgroups of ASD, sex, timing of gestational week of blood draw and gestational age. Statistical significance was based on 2-sided p < 0.05. All statistical analyses were performed with SAS 9.4 software (SAS 9.4, SAS Institute, Cary, N.C., USA).
3. Results
The study included 1558 ASD case-controlled pairs. The mean age of ASD diagnosis for cases was 6.57 years (SD: 3.21, Range: 0–18 years). The median vitamin B12 level among cases was 114.40 pmol/L, and it was 116.80 pmol/L among controls. The mean gestational week of maternal blood draw was 10.79 weeks (SD 3.5) for cases and 10.10 weeks (SD 2.8) for controls. The gender distribution was 19.5% female and 80.5% male in cases and controls.
Among potential covariates in the study, gestational week of blood draw, maternal SES and maternal immigration status were associated with maternal vitamin B12 levels among controls with p < 0.1 (Table 1). Maternal age, gestational week of blood draw, previous births, maternal psychopathology, paternal psychopathology, maternal SES, maternal substance abuse, gestational age, weight for gestational age and Apgar score were associated with offspring ASD with p < 0.1 (Table 2). Maternal SES and gestational week of blood draw were associated with both maternal vitamin B12 levels and offspring ASD and were adjusted in multivariable models.
Table 3 shows the log-transformed maternal serum vitamin B12 levels and offspring ASD and their subcategories. Maternal serum vitamin B12 was not associated with offspring ASD in either unadjusted (OR 0.90, 95% CI 0.77–1.06, p = 0.209) or adjusted analyses (aOR 0.94, 95% CI 0.79–1.10, p = 0.441). In a subsequent analysis of ASD subtypes, no significant associations were observed between maternal serum vitamin B12 levels and ASD subtypes in unadjusted or adjusted analyses (Table 3).

Table 3.
Odds ratios and 95% CIs for the association between log-transformed maternal serum vitamin B12 (continuous) and offspring ASD and subtypes.
Table 4 shows the distribution of maternal vitamin B12 levels in quintiles by case-control status. A significant association was observed between maternal vitamin B12 levels in the highest quintile (≥81th percentile) and offspring childhood autism (aOR 1.59, 95% CI 1.06–2.41, p = 0.026) and the association was close to significance in the lowest quintile (aOR 1.49, 95% CI 0.98–2.26, p = 0.064), relative to the third quintile (Figure 1). No significant association was observed between maternal vitamin B12 levels and ASD, the Asperger’s subgroup or the PDD/PDD NOS subgroup.

Table 4.
Odds ratios and 95% CIs for the association between maternal serum vitamin B12 levels (in quintiles) and offspring ASD and subtypes.
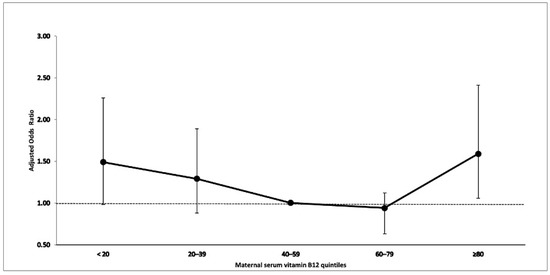
Figure 1.
Association between maternal serum vitamin B12 and offspring childhood autism.
In additional sensitivity analyses, we adjusted for offspring gestational age for the association between log-transformed maternal serum vitamin B12 levels or measured in quintiles, and offspring ASD and their subcategories. Furthermore, we additionally adjusted for maternal psychopathology (history of psychopathology and substance abuse) and gestational age (see Supplemental Tables S2 and S3). The findings were similar with significant association between childhood autism and maternal vitamin B12 in the highest quintile in model I (aOR 1.59, 95% CI 1.06–2.42, p = 0.027) and in model II (aOR 1.75, 95% CI 1.14–2.67, p = 0.010). No significant association was observed between maternal vitamin B12 levels and ASD, the Asperger’s subgroup or the PDD/PDD NOS subgroup.
In further additional analysis, we stratified the sample by ASD with and without ID and examined the association with maternal vitamin B12 levels. No significant association was observed between log-transformed maternal vitamin B12 levels and offspring ASD with ID (aOR 1.06, 95% CI 0.69–1.64, p = 0.796) or without ID (aOR 0.92, 95% CI 0.77–1.10, p = 0.369) (see Supplemental Table S4).
Testing for the sex and maternal vitamin B12 interaction did not reveal evidence for a modification effect by sex on the relationship between continuous maternal vitamin B12 and ASD (p = 0.492). We found no interaction between continuous maternal vitamin B12 and gestational week of blood draw (p = 0.437), and also no interaction was observed between continuous maternal vitamin B12 and gestational age (p = 0.334).
4. Discussion
This is the first population-based study examining maternal vitamin B12 levels in prenatal sera in relation to ASD. We found high levels of maternal vitamin B12 (≥81th percentile) during early pregnancy associated with offspring childhood autism. These findings are consistent with the study by Raghavan et al. [22] of 86 ASD cases that showed vitamin B12 in the highest decile (≥90th percentile) was associated with an increased risk of ASD in offspring. However, maternal blood samples were collected 24–72 h post-delivery. Furthermore, in the current study, low levels of maternal vitamin B12 (<20th percentile) during early pregnancy evidenced a statistical trend toward association with offspring childhood autism. While interesting, this result should be interpreted with caution. One possible explanation for this finding could be that vitamin B12 follows Bertrand’s rule, as do other micronutrients [47]. Bertrand’s rule describes the dose–response curve for many micronutrients as non-monotonic, with benefits increasing with intake at low levels, then plateauing with optimal concentrations and finally, toxicity occurring at higher levels. However, it is important to note that these findings were observed only in childhood autism and we do not know of a specific mechanism that would produce this pattern of association. More studies are needed to confirm these associations.
We did not find any associations between maternal vitamin B12 levels in early pregnancy and offspring Asperger’s syndrome or PDD/PDD-NOS. Since the etiology of ASD is multifactorial, different etiologic factors may have different levels of association with specific subgroups within the ASD spectrum. Of note, in a nested case-controlled study, maternal serum vitamin B12 levels in early pregnancy were not associated with offspring attention-deficit hyperactivity disorder [48]. In a study by Brown et al. [49], high homocysteine levels (which are inversely related to vitamin B12, as well as folate) were associated with an increased risk for schizophrenia among offspring.
Previous studies on humans and animal have shown that folic acid and vitamin B12 deficiency during pregnancy can cause persistent changes in the offspring’s genome, and resulting in brain abnormalities that are associated with ASD [12,13,50]. The key developmental genes involved in neural pathways showed altered expression and impairment in many of the pathways that are linked to ASD [51,52]. Vitamin B12 also plays an important role in deoxyribonucleic acid methylation, cellular growth and differentiation [53,54]. However, very little is known about the role of maternal vitamin B12 on human brain development. While our findings are observational and do not address the mechanisms behind the association, we believe that a potential role for high levels of maternal vitamin B12 may have some role in the etiology of childhood autism, the most severe phenotype of ASD, and this deserves future research.
The strengths of this study include the use of archived biospecimens for the measurement of maternal serum vitamin B12 during pregnancy, a large sample size and the capacity to adjust for a large number of confounders. There are several limitations that should be considered. First, ASD diagnoses included only children referred to specialized services and are likely to represent the most severe ASD cases. The findings of the study will not represent mild and moderate forms of ASD. Second, we did not measure neonatal vitamin B12 levels. However, the maternal and neonatal serum levels of vitamin B12 have been shown to significantly correlate with one another [55,56,57]. Third, maternal vitamin B12 levels in this study were collected in the first and early second trimesters of pregnancy. The findings thus cannot be generalized to B12 levels occurring in later pregnancy. Fourth, residual confounding by unmeasured factors (such as maternal body mass index, prenatal vitamin supplementation or maternal medications during pregnancy) could be possible.
5. Conclusions
The present study revealed high maternal vitamin B12 levels (≥81th percentile) during early pregnancy were associated with offspring childhood autism. The study raises questions on the impact of very extremely elevated levels of maternal vitamin B12 levels on early brain development. If these findings are confirmed in future studies, it would indicate that maternal vitamin B12 has specificity as an etiological factor for a severe form of ASD and high maternal vitamin B12 levels have toxic effects on offspring. Further studies may have potential for clinical relevance to identify optimal levels of maternal vitamin B12 supplementation during pregnancy.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/nu15082009/s1, Table S1: Review of previous studies on maternal vitamin B12 and multivitamin and autism spectrum disorders, by primary method of exposure assessment; Table S2. Odds ratios and 95% CIs for the association between log-transformed maternal serum vitamin B12 (continuous) and offspring ASD and subtypes adjusting for additional covariates; Table S3: Odds ratios and 95% CIs for the association between maternal serum vitamin B12 levels (in quintiles) and offspring ASD and subtypes adjusting for additional covariates; Table S4. Odds ratios and 95% CI of the association between log-transformed maternal serum vitamin B12 (continuous, quintiles) and offspring Autism with and without Intellectual Disability (ID).
Author Contributions
Conceptualization, A.S. and S.S.; methodology, S.S., S.H.-Y.-S., A.S.B., K.C.-P. and A.S.; validation, H.-M.S.; formal analysis, S.H.-Y.-S.; investigation, H.-M.S.; data curation, S.H.-Y.-S.; writing—original draft preparation, S.S. and A.S.; writing—review and editing, S.H.-Y.-S., A.S.B., K.C.-P., H.-M.S.; S.U. and I.W.M., visualization, S.S.; supervision, A.S.; project administration, A.S.; funding acquisition, A.S. and A.S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Institute of Environmental Health Sciences of the National Institutes of Health (Award Number R01ES028125), and the Academy of Finland INVEST Flagship (decision number: 320162) and the Academy of Finland Health from Cohorts and Biobanks Programme (decision number: 308552). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Institutional Review Board Statement
The FIPS-A received ethical approval from the Ethics Committee of the Hospital District of Southwest Finland, the data protection authorities at the National Institute for Health and Welfare and the Institutional Review Board of the New York State Psychiatric Institute. The study design and the request for biobank serum samples were approved by the responsible Biobank’s Scientific Committee (Biobank Borealis of Northern Finland, University of Oulu, Oulu, Finland).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Deidentified individual participant data will not be made available due to ethical restrictions. Summary level data can be obtained from the corresponding author upon reasonable request.
Acknowledgments
We thank the investigators and staff at the medical centers involved in this research.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar] [CrossRef]
- Gardener, H.; Spiegelman, D.; Buka, S.L. Prenatal Risk Factors for Autism: Comprehensive Meta-Analysis. Br. J. Psychiatry 2009, 195, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Lyall, K.; Croen, L.; Daniels, J.; Fallin, M.D.; Ladd-Acosta, C.; Lee, B.K.; Park, B.Y.; Snyder, N.W.; Schendel, D.; Volk, H.; et al. The Changing Epidemiology of Autism Spectrum Disorders. Annu. Rev. Public Health 2017, 38, 81–102. [Google Scholar] [CrossRef] [PubMed]
- Friel, C.; Leyland, A.H.; Anderson, J.J.; Havdahl, A.; Borge, T.; Shimonovich, M.; Dundas, R. Prenatal Vitamins and the Risk of Offspring Autism Spectrum Disorder: Systematic Review and Meta-Analysis. Nutrients 2021, 13, 2558. [Google Scholar] [CrossRef]
- Li, M.; Francis, E.; Hinkle, S.N.; Ajjarapu, A.S.; Zhang, C. Preconception and Prenatal Nutrition and Neurodevelopmental Disorders: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 1628. [Google Scholar] [CrossRef] [PubMed]
- Sourander, A.; Upadhyaya, S.; Surcel, H.-M.; Hinkka-Yli-Salomäki, S.; Cheslack-Postava, K.; Silwal, S.; Sucksdorff, M.; McKeague, I.W.; Brown, A.S. Maternal Vitamin D Levels During Pregnancy and Offspring Autism Spectrum Disorder. Biol. Psychiatry 2021, 90, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Pepper, M.R.; Black, M.M. B12 in Fetal Development. Semin. Cell Dev. Biol. 2011, 22, 619–623. [Google Scholar] [CrossRef]
- Scott, J.M.; Molloy, A.M. The Discovery of Vitamin B. Ann. Nutr. Metab. 2012, 61, 239–245. [Google Scholar] [CrossRef]
- King, J.C. A Summary of Pathways or Mechanisms Linking Preconception Maternal Nutrition with Birth Outcomes. J. Nutr. 2016, 146, 1437S–1444S. [Google Scholar] [CrossRef]
- Lövblad, K.-O.; Ramelli, G.; Remonda, L.; Nirkko, A.C.; Ozdoba, C.; Schroth, G. Retardation of Myelination Due to Dietary Vitamin B12 Deficiency: Cranial MRI Findings. Pediatr. Radiol. 1997, 27, 155–158. [Google Scholar] [CrossRef]
- Craciunescu, C.N.; Brown, E.C.; Mar, M.-H.; Albright, C.D.; Nadeau, M.R.; Zeisel, S.H. Folic Acid Deficiency During Late Gestation Decreases Progenitor Cell Proliferation and Increases Apoptosis in Fetal Mouse Brain. J. Nutr. 2004, 134, 162–166. [Google Scholar] [CrossRef]
- Barua, S.; Kuizon, S.; Brown, W.T.; Junaid, M.A. DNA Methylation Profiling at Single-Base Resolution Reveals Gestational Folic Acid Supplementation Influences the Epigenome of Mouse Offspring Cerebellum. Front. Neurosci. 2016, 10, 168. [Google Scholar] [CrossRef]
- Mahajan, A.; Sapehia, D.; Thakur, S.; Mohanraj, P.S.; Bagga, R.; Kaur, J. Effect of Imbalance in Folate and Vitamin B12 in Maternal/Parental Diet on Global Methylation and Regulatory MiRNAs. Sci. Rep. 2019, 9, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Mahajan, A.; Sapehia, D.; Kaur, J.; Sandhir, R. Effects of Altered Maternal Folate and Vitamin B12 on Neurobehavioral Outcomes in F1 Male Mice. Brain Res. Bull. 2019, 153, 93–101. [Google Scholar] [CrossRef]
- Troen, A.M.; Shea-Budgell, M.; Shukitt-Hale, B.; Smith, D.E.; Selhub, J.; Rosenberg, I.H. B-Vitamin Deficiency Causes Hyperhomocysteinemia and Vascular Cognitive Impairment in Mice. Proc. Natl. Acad. Sci. USA 2008, 105, 12474–12479. [Google Scholar] [CrossRef]
- del Río Garcia, C.; Torres-Sánchez, L.; Chen, J.; Schnaas, L.; Hernández, C.; Osorio, E.; Portillo, M.G.; López-Carrillo, L. Maternal MTHFR 677C>T Genotype and Dietary Intake of Folate and Vitamin B (12): Their Impact on Child Neurodevelopment. Nutr. Neurosci. 2009, 12, 13–20. [Google Scholar] [CrossRef]
- Bhate, V.; Deshpande, S.; Bhat, D.; Joshi, N.; Ladkat, R.; Watve, S.; Fall, C.; de Jager, C.A.; Refsum, H.; Yajnik, C. Vitamin B 12 Status of Pregnant Indian Women and Cognitive Function in Their 9-Year-Old Children. Food Nutr. Bull. 2008, 29, 249–254. [Google Scholar] [CrossRef]
- Lai, J.S.; Mohamad Ayob, M.N.; Cai, S.; Quah, P.L.; Gluckman, P.D.; Shek, L.P.; Yap, F.; Tan, K.H.; Chong, Y.S.; Godfrey, K.M.; et al. Maternal Plasma Vitamin B12 Concentrations during Pregnancy and Infant Cognitive Outcomes at 2 Years of Age. Br. J. Nutr. 2019, 121, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Torsvik, I.; Ueland, P.M.; Markestad, T.; Bjørke-Monsen, A.-L. Cobalamin Supplementation Improves Motor Development and Regurgitations in Infants: Results from a Randomized Intervention Study. Am. J. Clin. Nutr. 2013, 98, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Rogne, T.; Tielemans, M.J.; Chong, M.F.-F.; Yajnik, C.S.; Krishnaveni, G.V.; Poston, L.; Jaddoe, V.W.V.; Steegers, E.A.P.; Joshi, S.; Chong, Y.-S.; et al. Associations of Maternal Vitamin B12 Concentration in Pregnancy With the Risks of Preterm Birth and Low Birth Weight: A Systematic Review and Meta-Analysis of Individual Participant Data. Am. J. Epidemiol. 2017, 185, 212–223. [Google Scholar] [CrossRef]
- Yajnik, C.S.; Deshpande, S.S.; Jackson, A.A.; Refsum, H.; Rao, S.; Fisher, D.J.; Bhat, D.S.; Naik, S.S.; Coyaji, K.J.; Joglekar, C.V.; et al. Vitamin B12 and Folate Concentrations during Pregnancy and Insulin Resistance in the Offspring: The Pune Maternal Nutrition Study. Diabetologia 2008, 51, 29–38. [Google Scholar] [CrossRef]
- Raghavan, R.; Riley, A.W.; Volk, H.; Caruso, D.; Hironaka, L.; Sices, L.; Hong, X.; Wang, G.; Ji, Y.; Brucato, M.; et al. Maternal Multivitamin Intake, Plasma Folate and Vitamin B12 Levels and Autism Spectrum Disorder Risk in Offspring. Paediatr. Perinat. Epidemiol. 2018, 32, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.M.; Froehlich, T.; Kalkbrenner, A.; Pfeiffer, C.M.; Fazili, Z.; Yolton, K.; Lanphear, B.P. Are Autistic-Behaviors in Children Related to Prenatal Vitamin Use and Maternal Whole Blood Folate Concentrations? J. Autism. Dev. Disord. 2014, 44, 2602–2607. [Google Scholar] [CrossRef]
- Egorova, O.; Myte, R.; Schneede, J.; Hägglöf, B.; Bölte, S.; Domellöf, E.; Ivars A’Roch, B.; Elgh, F.; Ueland, P.M.; Silfverdal, S.-A. Maternal Blood Folate Status during Early Pregnancy and Occurrence of Autism Spectrum Disorder in Offspring: A Study of 62 Serum Biomarkers. Mol. Autism 2020, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Steenweg-de Graaff, J.; Ghassabian, A.; Jaddoe, V.W.V.; Tiemeier, H.; Roza, S.J.; Graaff, J.S.; Ghassabian, A.; Jaddoe, V.W.V.; Tiemeier, H.; Roza, S.J. Folate Concentrations during Pregnancy and Autistic Traits in the Offspring. The Generation R Study. Eur. J. Public Health 2015, 25, 431–433. [Google Scholar] [CrossRef]
- Moser, S.; Davidovitch, M.; Rotem, R.S.; Chodick, G.; Shalev, V.; Koren, G.; Sharman, M.S.; Davidovitch, M.; Rotem, R.; Chodick, G.; et al. High Dose Folic Acid during Pregnancy and the Risk of Autism; The Birth Order Bias: A Nested Case-Control Study. Reprod. Toxicol. 2019, 89, 173–177. [Google Scholar] [CrossRef]
- Levine, S.Z.; Kodesh, A.; Viktorin, A.; Smith, L.; Uher, R.; Reichenberg, A.; Sandin, S. Association of Maternal Use of Folic Acid and Multivitamin Supplements in the Periods Before and During Pregnancy With the Risk OfAutism Spectrum Disorder in Offspring. JAMA Psychiatry 2018, 75, 176–184. [Google Scholar] [CrossRef]
- Brieger, K.K.; Bakulski, K.M.; Pearce, C.L.; Baylin, A.; Dou, J.F.; Feinberg, J.I.; Croen, L.A.; Hertz-Picciotto, I.; Newschaffer, C.J.; Fallin, M.D.; et al. The Association of Prenatal Vitamins and Folic Acid Supplement Intake with Odds of Autism Spectrum Disorder in a High-Risk Sibling Cohort, the Early Autism Risk Longitudinal Investigation (EARLI). J. Autism Dev. Disord. 2021, 89, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Yang, T.; Zhu, J.; Li, Q.; Lai, X.; Li, Y.; Tang, T.; Chen, J.; Li, T. Maternal Folic Acid and Micronutrient Supplementation Is Associated with Vitamin Levels and Symptoms in Children with Autism Spectrum Disorders. Reprod. Toxicol. 2020, 91, 109–115. [Google Scholar] [CrossRef]
- Devilbiss, E.A.; Magnusson, C.; Gardner, R.M.; Rai, D.; Newschaffer, C.J.; Lyall, K.; Dalman, C.; Lee, B.K. Antenatal Nutritional Supplementation and Autism Spectrum Disorders in the Stockholm Youth Cohort: Population Based Cohort Study. BMJ 2017, 359, j4273. [Google Scholar] [CrossRef]
- Surén, P.; Roth, C.; Bresnahan, M.; Haugen, M.; Hornig, M.; Hirtz, D.; Lie, K.K.; Lipkin, W.I.; Magnus, P.; Reichborn-Kjennerud, T.; et al. Association between Maternal Use of Folic Acid Supplements and Risk of Autism Spectrum Disorders in Children. JAMA 2013, 309, 570–577. [Google Scholar] [CrossRef]
- Nilsen, R.M.; Surén, P.; Gunnes, N.; Alsaker, E.R.; Bresnahan, M.; Hirtz, D.; Hornig, M.; Lie, K.K.; Lipkin, W.I.; Reichborn-Kjennerud, T.; et al. Analysis of Self-Selection Bias in a Population-Based Cohort Study of Autism Spectrum Disorders. Paediatr. Perinat. Epidemiol. 2013, 27, 553–563. [Google Scholar] [CrossRef]
- Schmidt, R.J.; Iosif, A.M.; Guerrero Angel, E.; Ozonoff, S. Association of Maternal Prenatal Vitamin Use with Risk for Autism Spectrum Disorder Recurrence in Young Siblings. JAMA Psychiatry 2019, 76, 391–398. [Google Scholar] [CrossRef]
- Li, Y.; Shen, Y.; Li, Y.; Xun, G.; Liu, H.; Wu, R.; Xia, K.; Zhao, J.; Ou, J. Maternal Dietary Patterns, Supplements Intake and Autism Spectrum Disorders A Preliminary Case-Control Study. Medicine 2018, 97, e13902. [Google Scholar] [CrossRef]
- Strøm, M.; Granström, C.; Lyall, K.; Ascherio, A.; Olsen, S.F. Research Letter: Folic Acid Supplementation and Intake of Folate in Pregnancy in Relation to Offspring Risk of Autism Spectrum Disorder. Psychol. Med. 2018, 48, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Virk, J.; Liew, Z.; Olsen, J.; Nohr, E.A.; Catov, J.M.; Ritz, B. Preconceptional and Prenatal Supplementary Folic Acid and Multivitamin Intake and Autism Spectrum Disorders. Autism 2016, 20, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.J.; Tancredi, D.J.; Ozonoff, S.; Hansen, R.L.; Hartiala, J.; Allayee, H.; Schmidt, L.C.; Tassone, F.; Hertz-Picciotto, I. Maternal Periconceptional Folic Acid Intake and Risk of Autism Spectrum Disorders and Developmental Delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) Case-Control Study. Am. J. Clin. Nutr. 2012, 96, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Gammon, C.S.; von Hurst, P.R.; Coad, J.; Kruger, R.; Stonehouse, W. Vegetarianism, Vitamin B12 Status, and Insulin Resistance in a Group of Predominantly Overweight/Obese South Asian Women. Nutrition 2012, 28, 20–24. [Google Scholar] [CrossRef]
- Lampi, K.M.; Sourander, A.; Gissler, M.; Niemelä, S.; Rehnström, K.; Pulkkinen, E.; Peltonen, L.; Von Wendt, L. Brief Report: Validity of Finnish Registry-Based Diagnoses of Autism with the ADI-R. Acta Paediatr. 2010, 99, 1425–1428. [Google Scholar] [CrossRef]
- Behere, R.V.; Deshmukh, A.S.; Otiv, S.; Gupte, M.D.; Yajnik, C.S. Maternal Vitamin B12 Status During Pregnancy and Its Association With Outcomes of Pregnancy and Health of the Offspring: A Systematic Review and Implications for Policy in India. Front. Endocrinol. 2021, 12, 288. [Google Scholar] [CrossRef] [PubMed]
- Jokiranta, E.; Brown, A.S.; Heinimaa, M.; Cheslack-Postava, K.; Suominen, A.; Sourander, A. Parental Psychiatric Disorders and Autism Spectrum Disorders. Psychiatry Res. 2013, 207, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Lampi, K.M.; Hinkka-Yli-Salomäki, S.; Lehti, V.; Helenius, H.; Gissler, M.; Brown, A.S.; Sourander, A. Parental Age and Risk of Autism Spectrum Disorders in a Finnish National Birth Cohort. J. Autism Dev. Disord. 2013, 43, 2526–2535. [Google Scholar] [CrossRef]
- Polo-Kantola, P.; Lampi, K.M.; Hinkka-Yli-Salomäki, S.; Gissler, M.; Brown, A.S.; Sourander, A. Obstetric Risk Factors and Autism Spectrum Disorders in Finland. J. Pediatr. 2014, 164, 358–365. [Google Scholar] [CrossRef]
- Tran, P.L.; Lehti, V.; Lampi, K.M.; Helenius, H.; Suominen, A.; Gissler, M.; Brown, A.S.; Sourander, A. Smoking during Pregnancy and Risk of Autism Spectrum Disorder in a Finnish National Birth Cohort. Paediatr. Perinat. Epidemiol. 2013, 27, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Bergen, N.; Jaddoe, V.; Timmermans, S.; Hofman, A.; Lindemans, J.; Russcher, H.; Raat, H.; Steegers-Theunissen, R.; Steegers, E. Homocysteine and Folate Concentrations in Early Pregnancy and the Risk of Adverse Pregnancy Outcomes: The Generation R Study. BJOG: Int. J. Obstet. Gynaecol. 2012, 119, 739–751. [Google Scholar] [CrossRef]
- Adaikalakoteswari, A.; Vatish, M.; Lawson, A.; Wood, C.; Sivakumar, K.; McTernan, P.G.; Webster, C.; Anderson, N.; Yajnik, C.S.; Tripathi, G.; et al. Low Maternal Vitamin B12 Status Is Associated with Lower Cord Blood HDL Cholesterol in White Caucasians Living in the UK. Nutrients 2015, 7, 2401–2414. [Google Scholar] [CrossRef]
- Raubenheimer, D.; Lee, K.P.; Simpson, S.J. Does Bertrand’s Rule Apply to Macronutrients? Proc. R. Soc. B Biol. Sci. 2005, 272, 2429–2434. [Google Scholar] [CrossRef] [PubMed]
- Sourander, A.; Silwal, S.; Upadhyaya, S.; Surcel, H.-M.; Hinkka-Yli-Salomäki, S.; McKeague, I.W.; Cheslack-Postava, K.; Brown, A.S. Maternal Serum Vitamin B12 and Offspring Attention-Deficit/Hyperactivity Disorder (Adhd). Eur. Child Adolesc. Psychiatry 2020, 30, 1449–1462. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.S.; Bottiglieri, T.; Schaefer, C.A.; Quesenberry, C.P.; Liu, L.; Bresnahan, M.; Susser, E.S. Elevated Prenatal Homocysteine Levels as a Risk Factor for Schizophrenia. Arch. Gen. Psychiatry 2007, 64, 31–39. [Google Scholar] [CrossRef]
- Choi, S.-Y. Synaptic and Circuit Development of the Primary Sensory Cortex. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef]
- Bourgeron, T. From the Genetic Architecture to Synaptic Plasticity in Autism Spectrum Disorder. Nat. Rev. Neurosci. 2015, 16, 551–563. [Google Scholar] [CrossRef]
- Rodriguez-Gomez, D.A.; Garcia-Guaqueta, D.P.; Charry-Sánchez, J.D.; Sarquis-Buitrago, E.; Blanco, M.; Velez-van-Meerbeke, A.; Talero-Gutiérrez, C. A Systematic Review of Common Genetic Variation and Biological Pathways in Autism Spectrum Disorder. BMC Neurosci. 2021, 22, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Black, M.M. Effects of Vitamin B12 and Folate Deficiency on Brain Development in Children. Food Nutr. Bull. 2008, 29, S126–S131. [Google Scholar] [CrossRef] [PubMed]
- McCullough, L.E.; Miller, E.E.; Mendez, M.A.; Murtha, A.P.; Murphy, S.K.; Hoyo, C. Maternal B Vitamins: Effects on Offspring Weight and DNA Methylation at Genomically Imprinted Domains. Clin. Epigenetics 2016, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Peker, E.; Demir, N.; Tuncer, O.; Üstyol, L.; Balahoroğlu, R.; Kaba, S.; Karaman, K. The Levels of Vitamin B 12, Folate and Homocysteine in Mothers and Their Babies with Neural Tube Defects. J. Matern. -Fetal Neonatal Med. 2016, 29, 2944–2948. [Google Scholar] [CrossRef] [PubMed]
- Reischl-Hajiabadi, A.T.; Garbade, S.F.; Feyh, P.; Weiss, K.H.; Mütze, U.; Kölker, S.; Hoffmann, G.F.; Gramer, G. Maternal Vitamin B12 Deficiency Detected by Newborn Screening-Evaluation of Causes and Characteristics. Nutrients 2022, 14, 3767. [Google Scholar] [CrossRef]
- Ünsür, E.; Kınaş, B.; Kutlusoy, F.; Aksoy, Ü.; Şahin, K.; Ünsür, T.; Aksoy, H.; Tekkeşin, N. The Relationship between Maternal and Neonatal Vitamin B12 and Folate Levels, Anthropometric Measurements, and Metabolic Indicators. J. Surg. Med. 2020, 4, 43–47. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).