Optimal Serum 25(OH)D Levels and Vitamin D Intake in Korean Postmenopausal Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. General Characteristics, Lifestyle, and Outdoor Time
2.3. Dietary Assessment
2.4. Anthropometry, Biochemical Indices, and Bone Mineral Density
2.5. Statistical Analysis
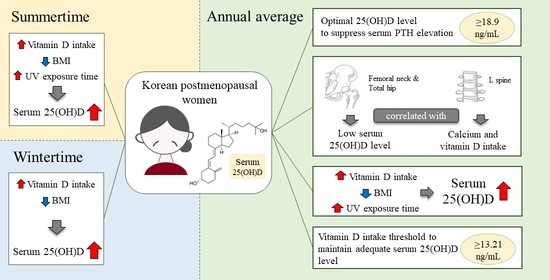
3. Results
3.1. General Characteristics and Lifestyle of the Participants
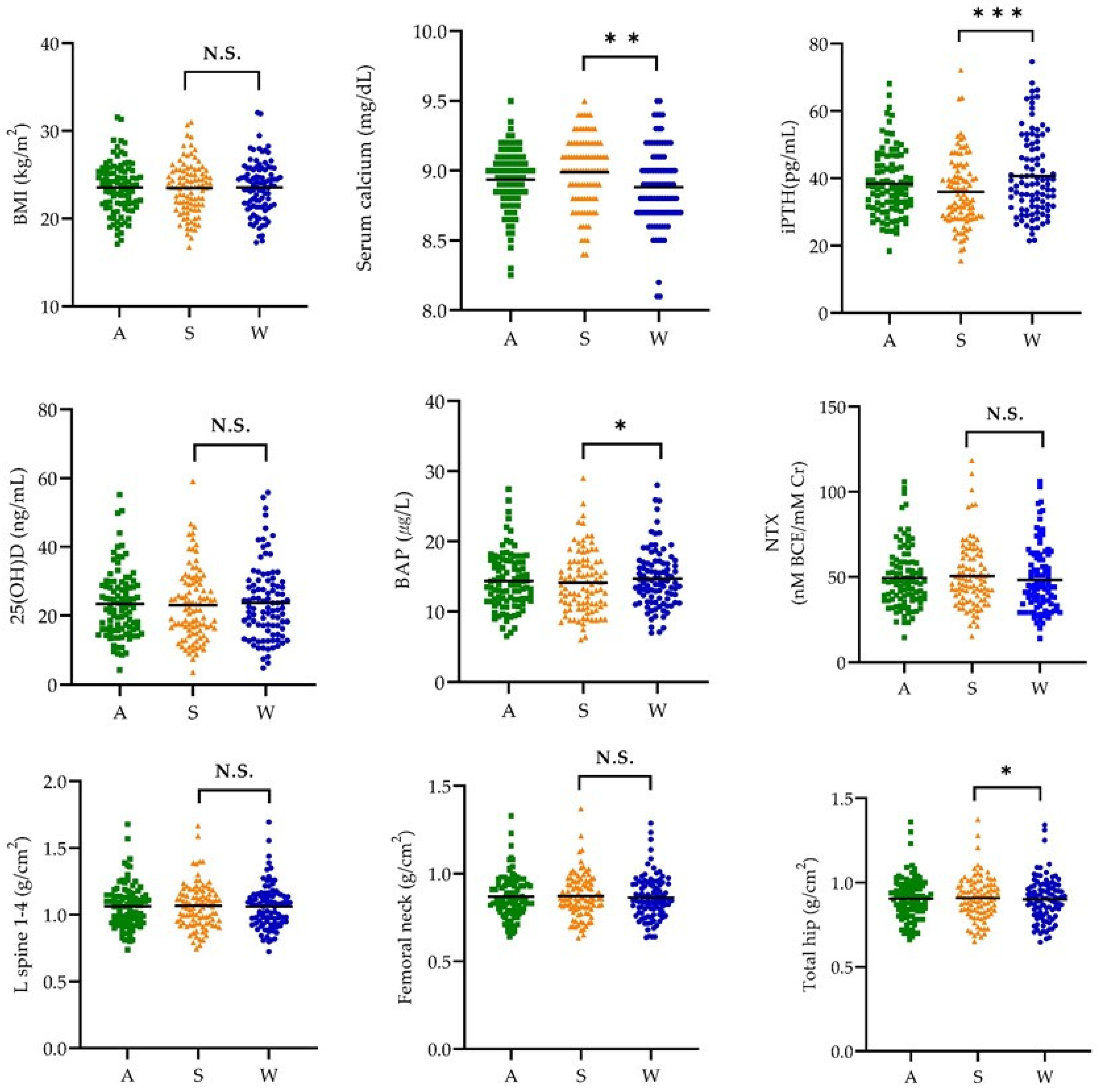
3.2. Body Mass Index, Biochemical Indices, and Bone Mineral Density by Season
3.3. Correlation Coefficient of Body Mass Index, Biochemical Indices, and Bone Mineral Density
3.4. Nutrient Intake
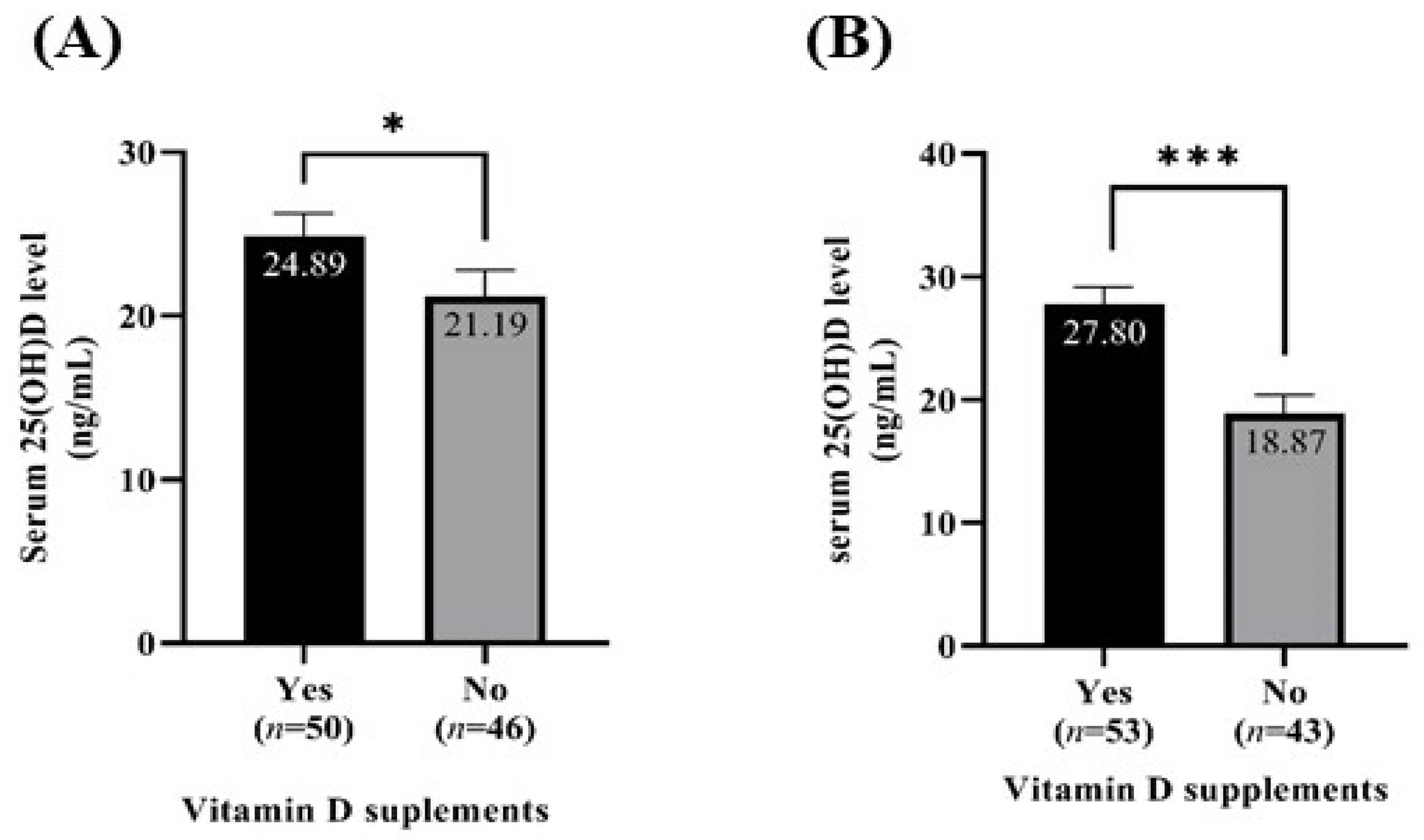
3.5. Serum 25(OH)D Level According to Vitamin D Supplementation
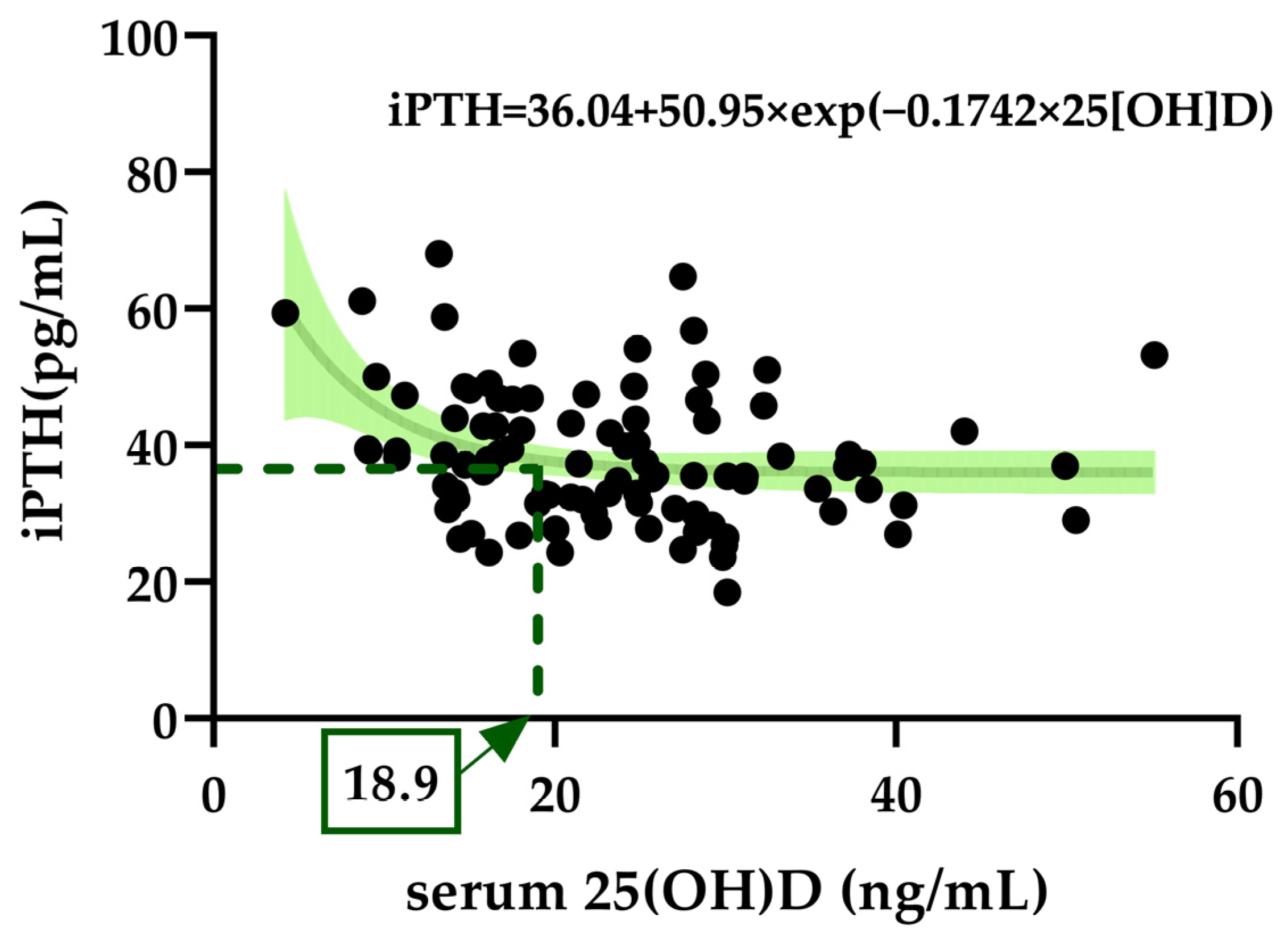
3.6. Exponential Decay for Optimal Serum 25(OH)D to Suppress iPTH Elevation
3.7. Relationship between Bone Mineral Density and Serum 25(OH)D Levels
3.8. L-Spine Bone Mineral Density According to Calcium and Vitamin D Intake
3.9. Multivariate Linear Regression Analyzing the Factors Influencing the Serum 25(OH)D Level by Season
3.10. ROC Analysis for Optimal Vitamin D Intake at Serum 25(OH)D ≥20 and ≥18.9 ng/mL
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- López-Baena, M.T.; Pérez-Roncero, G.R.; Pérez-López, F.R.; Mezones-Holguín, E.; Chedraui, P. Vitamin D, menopause, and aging: Quo vadis? Climacteric 2020, 23, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Gil, A.; Plaza-Diaz, J.; Mesa, M.D. Vitamin D: Classic and novel actions. Ann. Nutr. Metab. 2018, 72, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Moulas, A.N.; Vaiou, M. Vitamin D fortification of foods and prospective health outcomes. J. Biotechnol. 2018, 285, 91–101. [Google Scholar] [CrossRef]
- Haq, A.; Hasnain, S.E.; Razzaque, M.S. Diverse functions of vitamin D in health and disease. J. Steroid Biochem. Mol. Biol. 2020, 200, 105668. [Google Scholar] [CrossRef] [PubMed]
- Sizar, O.; Khare, S.; Goyal, A.; Givler, A. Vitamin D deficiency. In Statpearls [Internet]; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Sözen, T.; Özışık, L.; Başaran, N.Ç. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef]
- Ceglia, L. Vitamin D and skeletal muscle tissue and function. Mol. Aspects Med. 2008, 29, 407–414. [Google Scholar] [CrossRef]
- Muir, S.W.; Montero-Odasso, M. Effect of vitamin D supplementation on muscle strength, gait and balance in older adults: A systematic review and meta-analysis. J. Am. Geriatr. Soc. 2011, 59, 2291–2300. [Google Scholar] [CrossRef]
- Sipos, W.; Pietschmann, P.; Rauner, M.; Kerschan-Schindl, K.; Patsch, J. Pathophysiology of osteoporosis. Wien. Med. Wochenschr. 2009, 159, 230–234. [Google Scholar] [CrossRef]
- Laird, E.; Ward, M.; McSorley, E.; Strain, J.J.; Wallace, J. Vitamin D and bone health: Potential mechanisms. Nutrients 2010, 2, 693–724. [Google Scholar] [CrossRef]
- Lips, P.; Hosking, D.; Lippuner, K.; Norquist, J.M.; Wehren, L.; Maalouf, G.; Ragi-Eis, S.; Chandler, J. The prevalence of vitamin D inadequacy amongst women with osteoporosis: An international epidemiological investigation. J. Intern. Med. 2006, 260, 245–254. [Google Scholar] [CrossRef]
- Nimitphong, H.; Holick, M.F. Vitamin D status and sun exposure in Southeast Asia. Dermato-Endocrinology 2013, 5, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.R.; Park, H.J.; Song, S.; Ly, S.Y. Dietary vitamin D intake in low ultraviolet irradiation seasons is associated with a better nutritional status of vitamin D in Korean adults according to the 2013–2014 national health and nutrition examination survey. Nutr. Res. 2022, 105, 53–65. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Shin, H.R.; Song, S.; Ly, S.Y. Vitamin D intake and bone mineral density in Korean adults: Analysis of the 2009–2011 Korea National Health and Nutrition Examination Survey. Nutr. Res. Pract. 2021, 16, e19. [Google Scholar] [CrossRef]
- Yoo, K.; Cho, J.; Ly, S. Vitamin D intake and serum 25-hydroxyvitamin D levels in Korean adults: Analysis of the 2009 Korea National Health and Nutrition Examination Survey (KNHANES IV-3) using a newly established vitamin D database. Nutrients 2016, 8, 610. [Google Scholar] [CrossRef] [PubMed]
- Chailurkit, L.-O.; Aekplakorn, W.; Ongphiphadhanakul, B. Regional variation and determinants of vitamin D status in sunshine-abundant thailand. BMC Public Health 2011, 11, 853. [Google Scholar] [CrossRef]
- Nakamura, K.; Kitamura, K.; Takachi, R.; Saito, T.; Kobayashi, R.; Oshiki, R.; Watanabe, Y.; Tsugane, S.; Sasaki, A.; Yamazaki, O. Impact of demographic, environmental, and lifestyle factors on vitamin D sufficiency in 9084 Japanese adults. Bone 2015, 74, 10–17. [Google Scholar] [CrossRef]
- Choi, H.S.; Oh, H.J.; Choi, H.; Choi, W.H.; Kim, J.G.; Kim, K.M.; Kim, K.J.; Rhee, Y.; Lim, S.K. Vitamin D insufficiency in Korea--a greater threat to younger generation: The Korea National Health and Nutrition Examination Survey (KNHANES) 2008. J. Clin. Endocrinol. Metab. 2011, 96, 643–651. [Google Scholar] [CrossRef]
- Chalcraft, J.R.; Cardinal, L.M.; Wechsler, P.J.; Hollis, B.W.; Gerow, K.G.; Alexander, B.M.; Keith, J.F.; Larson-Meyer, D.E. Vitamin D synthesis following a single bout of sun exposure in older and younger men and women. Nutrients 2020, 12, 2237. [Google Scholar] [CrossRef]
- Holick, M.F.; MacLaughlin, J.A.; Clark, M.B.; Holick, S.A.; Potts, J.T.; Anderson, R.R.; Blank, I.H.; Parrish, J.A.; Elias, P. Photosynthesis of previtamin D3 in human skin and the physiologic consequences. Science. 1980, 210, 203–205. [Google Scholar] [CrossRef]
- Dharmshaktu, P.; Saha, S.; Kar, P.; Sreenivas, V.; Ramakrishnan, L.; Goswami, R. Absence of vitamin D deficiency among common outdoor workers in Delhi. Clin. Endocrinol. Oxf. 2019, 91, 356–362. [Google Scholar] [CrossRef]
- Holick, M.F.; Chen, T.C.; Lu, Z.; Sauter, E. Vitamin D and skin physiology: A d-lightful story. J. Bone Miner. Res. 2007, 22, V28–V33. [Google Scholar] [CrossRef] [PubMed]
- The Korean Nutrition Society. Dietary Reference Intakes for Koreans 2020; Ministry of Health and Welfare: Sejong, Republic of Korea; The Korean Nutrition Society: Seoul, Republic of Korea, 2020. [Google Scholar]
- Oh, J.Y.; Yang, Y.J.; Kim, B.S.; Kang, J.H. Validity and reliability of Korean version of international physical activity questionnaire (IPAQ) short form. Korean J. Fam. Med. 2007, 28, 532–541. [Google Scholar]
- Computer Aided Nutritional Analysis Program; Can-Pro 5.0 (Web Ver.). Available online: http://canpro5.kns.or.kr/ (accessed on 25 August 2022).
- Chen, Y.; Kinney, L.; Božović, A.; Smith, H.; Tarr, H.; Diamandis, E.P.; LeBlanc, A. Performance evaluation of siemens advia centaur and roche modular analytics E170 Total 25-OH vitamin D assays. Clin. Biochem. 2012, 45, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, H.B.; Yaktine, A.L.; Taylor, C.L.; Ross, A.C. Institute of medicine (US) committee to review dietary reference intakes for vitamin D and calcium. In Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Guillemant, J.; Taupin, P.; Le, H.T.; Taright, N.; Allemandou, A.; Pérès, G.; Guillemant, S. Vitamin D status during puberty in French healthy male adolescents. Osteoporos. Int. 1999, 10, 222–225. [Google Scholar] [CrossRef]
- Chapuy, M.C.; Preziosi, P.; Maamer, M.; Arnaud, S.; Galan, P.; Hercberg, S.; Meunier, P.J. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos. Int. 1997, 7, 439–443. [Google Scholar] [CrossRef]
- Bone metabolism. In Encyclopedic Reference of Molecular Pharmacology; Springer: Berlin/Heidelberg, Germany, 2004; pp. 190–197.
- Analytical Note for 25-Hydroxyvitamin D Data Analysis Using NHANES III (1988–1994), NHANES 2001–2006, and NHANES 2007–2010 (October 2015). Available online: https://wwwn.cdc.gov/nchs/nhanes/vitamind/analyticalnote.aspx (accessed on 14 February 2023).
- EFSA Panel on Dietetic Products, Nutrition and Allergies. Dietary reference values for vitamin D. EFSA J. 2016, 14, e04547. [Google Scholar] [CrossRef]
- Shin, H.R.; Park, H.J.; Ly, S.Y. Optimal serum 25(OH)D level and vitamin D intake in young Korean women. Nutrients 2022, 14, 4845. [Google Scholar] [CrossRef]
- Saneei, P.; Salehi-Abargouei, A.; Esmaillzadeh, A. Serum 25-hydroxy vitamin D levels in relation to body mass index: A systematic review and meta-analysis. Obes. Rev. 2013, 14, 393–404. [Google Scholar] [CrossRef]
- Yildiz, Z.; Hürmeydan, Ö.; Madenci, Ö.Ç.; Orçun, A.; Yücel, N. Age, gender and season dependent 25(OH)D levels in children and adults living in Istanbul. Turkish J. Biochem. 2020, 45, 533–541. [Google Scholar] [CrossRef]
- Lee, M.-J.; Hsu, H.-J.; Wu, I.W.; Sun, C.-Y.; Ting, M.-K.; Lee, C.-C. Vitamin D deficiency in Northern Taiwan: A community-based cohort study. BMC Public Health 2019, 19, 337. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.P.J.; Greene-Finestone, L.; Whiting, S.; Fioletov, V.E.; Laffey, P.; Petronella, N. An analysis of factors associated with 25-hydroxyvitamin D levels in white and non-white Canadians. J. AOAC Int. 2019, 100, 1345–1354. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, L.; De Lorenzo, R.; Giustina, A.; Rovere-Querini, P.; Conte, C. Vitamin D in osteosarcopenic obesity. Nutrients 2022, 14, 1816. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.Y.; Lee, J.Y.; Bae, J.M.; Lee, C.; Hong, S.N.; Kim, A.; Kim, H.Y. 25-hydroxyvitamin D levels and body mass index in healthy postmenopausal women. Korean J. Obstet. Gynecol. 2012, 55, 378–383. [Google Scholar] [CrossRef]
- Deng, W.-M.; Wei, Q.-S.; Tan, X.; Shao, Y.; Chen, X.-H.; Sun, W.-S. Relation of serum 25 hydroxyvitamin D levels to bone mineral density in Southern Chinese postmenopausal women: A preliminary study. Indian J. Med. Res. 2015, 142, 430–437. [Google Scholar] [PubMed]
- Yamanaka, Y.; Menuki, K.; Zenke, Y.; Ikeda, S.; Hatakeyama, E.; Kawano, K.; Nishida, S.; Tanaka, H.; Yumisashi, K.; Sakai, A. Serum 25-hydroxyvitamin D concentrations in Japanese postmenopausal women with osteoporotic fractures. Osteoporos Sarcopenia 2019, 5, 116–121. [Google Scholar] [CrossRef]
- Osteoporosis Markers. Available online: https://www.ncbi.nlm.nih.gov/books/NBK559306/ (accessed on 19 October 2022).
- Hlaing, T.T.; Compston, J.E. Biochemical markers of bone turnover—Uses and limitations. Ann. Clin. Biochem. 2014, 51, 189–202. [Google Scholar] [CrossRef]
- Seong-Woo, C.; Sun-Seog, K.; Jin-Su, C.; Jung-Ae, R.; Young-Hoon, L.; Hae-Sung, N.; Seul-Ki, J.; Park, K.S.; So-Yeon, R.; Hye-Rim, S.; et al. Estimation of the cutoff value of vitamin D: The Dong-gu study. J. Physiol. Anthropol. 2015, 34, 10. [Google Scholar]
- Lee, D.Y.; Jee, J.H.; Cho, Y.Y.; Jang, J.Y.; Yu, T.Y.; Kim, T.H.; Hong, Y.J.; Hong, W.J.; Jin, S.M.; Hur, K.Y.; et al. Serum 25-hydroxyvitamin D cutoffs for functional bone measures in postmenopausal osteoporosis. Osteoporos. Int. 2017, 28, 1377–1384. [Google Scholar] [CrossRef]
- Xie, Z.; Xia, W.; Zhang, Z.; Wu, W.; Lu, C.; Tao, S.; Wu, L.; Gu, J.; Chandler, J.; Peter, S.; et al. Prevalence of vitamin D inadequacy among Chinese postmenopausal women: A nationwide, multicenter, cross-sectional study. Front. Endocrinol. Lausanne 2018, 9, 782. [Google Scholar] [CrossRef]
- Hwang, Y.-C.; Ahn, H.-Y.; Jeong, I.-K.; Ahn, K.J.; Chung, H.Y. Optimal serum concentration of 25-hydroxyvitamin D for bone health in older Korean adults. Calcif. Tissue Int. 2013, 92, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A.; Kiel, D.P.; Dawson-Hughes, B.; Orav, J.E.; Li, R.; Spiegelman, D.; Dietrich, T.; Willett, W.C. Dietary calcium and serum 25-hydroxyvitamin D status in relation to BMD among U.S. Adults. J. Bone Miner. Res. 2009, 24, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Kuchuk, N.O.; Pluijm, S.M.; van Schoor, N.M.; Looman, C.W.; Smit, J.H.; Lips, P. Relationships of serum 25-hydroxyvitamin D to bone mineral density and serum parathyroid hormone and markers of bone turnover in older persons. J. Clin. Endocrinol. Metab. 2009, 94, 1244–1250. [Google Scholar] [CrossRef] [PubMed]
- Lowe, N.M.; Mitra, S.R.; Foster, P.C.; Bhojani, I.; McCann, J.F. Vitamin D status and markers of bone turnover in Caucasian and South Asian postmenopausal women living in the UK. Br. J. Nutr. 2010, 103, 1706–1710. [Google Scholar] [CrossRef]
- Chandran, M.; Hoeck, H.C.; Wong, H.C.; Zhang, R.F.; Dimai, H.P. Vitamin D status and its relationship with bone mineral density and parathyroid hormone in Southeast Asian adults with low bone density. Endocr. Pract. 2011, 17, 226–234. [Google Scholar] [CrossRef]
- Shin, S.; Joung, H. A dairy and fruit dietary pattern is associated with a reduced likelihood of osteoporosis in Korean postmenopausal women. Br. J. Nutr. 2013, 110, 1926–1933. [Google Scholar] [CrossRef]
- Bæksgaard, L.; Andersen, K.P.; Hyldstrup, L. Calcium and vitamin D supplementation increases spinal BMD in healthy, postmenopausal women. Osteoporos. Int. 1998, 8, 255–260. [Google Scholar] [CrossRef]
- Matikainen, N.; Pekkarinen, T.; Ryhänen, E.M.; Schalin-Jäntti, C. Physiology of calcium homeostasis: An overview. Endocrinol. Metab. Clin. N. Am. 2021, 50, 575–590. [Google Scholar] [CrossRef]
- National Nutrition Statistics. Available online: https://www.khidi.or.kr/kps/dhraStat/result5?menuId=MENU01656&gubun=age3&year=2020 (accessed on 26 August 2022).
- Spiro, A.; Buttriss, J. Vitamin D: An overview of vitamin D status and intake in Europe. Nutr. Bull. 2014, 39, 322–350. [Google Scholar] [CrossRef]
- Bouillon, R. Comparative analysis of nutritional guidelines for vitamin D. Nat. Rev. Endocrinol. 2017, 13, 466–479. [Google Scholar] [CrossRef]
- Kim, S.; Park, S. Association between age at natural menopause and prevalence of obesity, hypertension, diabetes, and hypercholesterolemia. J. Korean Public Health Assoc. 2021, 47, 1–9. [Google Scholar]


| Variables | Mean ± S.E or N (%) | |
|---|---|---|
| N | 96 (100.0) | |
| Age (years) | 57.08 ± 0.55 | |
| YSM (years) | 7.27 ± 0.57 | |
| Height (cm) | 158.2 ± 0.51 | |
| Weight (kg) | 58.42 ± 0.85 | |
| Number of ways to avoid sun exposure | 0 | 9 (9.4) |
| 1 | 9 (9.4) | |
| 2 | 20 (20.8) | |
| ≥3 | 58 (60.4) | |
| Subtotal | 96 (100.0) | |
| Alcohol consumption | Yes | 34 (35.4) |
| No | 62 (64.6) | |
| Subtotal | 96 (100.0) | |
| IPAQ | High | 6 (6.3) |
| Moderate | 73 (76.0) | |
| Low | 17 (17.7) | |
| Subtotal | 96 (100.0) | |
| Taking vitamin D supplements | Summertime | 50 (52.1) |
| Wintertime | 53 (55.2) | |
| Taking calcium supplements | Summertime | 25 (26.0) |
| Wintertime | 34 (35.4) | |
| sun exposure time (min) | Summertime | 35.95 ± 2.59 |
| Wintertime | 38.23 ± 2.91 N.S. | |
| Annual Average | Summertime | Wintertime | |||||||
|---|---|---|---|---|---|---|---|---|---|
| L-Spine 1–4 | Femoral Neck | Total Hip | L-Spine 1–4 | Femoral Neck | Total Hip | L-Spine 1–4 | Femoral Neck | Total Hip | |
| BMI | 0.245 * | 0.306 ** | 0.398 *** | 0.213 * | 0.262 * | 0.379 *** | 0.255 * | 0.323 ** | 0.397 *** |
| iPTH | −0.088 | −0.161 | −0.144 | −0.120 | −0.147 | −0.176 | −0.041 | −0.138 | −0.100 |
| BAP | −0.174 | −0.180 | −0.196 | −0.139 | −0.143 | −0.155 | −0.198 | −0.214 * | −0.233 ** |
| NTX | −0.205 * | −0.150 | −0.197 | −0.153 | −0.114 | −0.123 | −0.236 * | −0.152 | −0.230 * |
| 25(OH)D | −0.090 | −0.131 | −0.154 | −0.112 | −0.124 | −0.150 | −0.057 | −0.091 | −0.129 |
| serumcalcium | 0.139 | −0.011 | 0.001 | 0.123 | −0.035 | −0.007 | 0.337 | 0.879 | 0.882 |
| Nutrients per 1000 kcal (n = 96) | |||||
|---|---|---|---|---|---|
| Variables | Summertime | Wintertime | Annual Average | p-Value | |
| Energy (kcal/day) | 1623.38 ± 36.54 | 1736.27 ± 49.10 | 1679.83 ± 35.64 | 0.024 | |
| Carbohydrate (g/1000 kcal/day) | 135.40 ± 1.74 | 136.11 ± 1.93 | 135.75 ± 1.48 | 0.872 | |
| Protein (g/1000 kcal/day) | 44.36 ± 0.68 | 43.11 ± 0.55 | 43.73 ± 0.45 | 0.135 | |
| Fat (g/1000 kcal/day) | 31.41 ± 0.54 | 31.27 ± 0.66 | 31.34 ± 0.48 | 0.530 | |
| Calcium total (mg/1000 kcal/day) † | 372.04 ± 13.40 | 397.98 ± 16.39 | 385.01 ± 12.50 | 0.136 | |
| With supplements | 529.61 ± 22.43 | 549.89 ± 28.00 | |||
| Without supplements | 316.56 ± 9.99 | 314.67 ± 9.74 | |||
| p-value | <0.001 | <0.001 | |||
| Vitamin D total (µg/1000 kcal/day) ‡ | 10.07 ± 1.31 | 11.51 ± 1.48 | 10.79 ± 1.07 | 0.371 | |
| With supplements | 16.52 ± 2.13 | 18.79 ± 2.23 | |||
| Without supplements | 3.06 ± 0.24 | 2.53 ± 0.25 | |||
| p-value | <0.001 | <0.001 | |||
| Skeletal Site | Serum 25(OH)D Level | β | 95% CI | p-Value |
|---|---|---|---|---|
| L-spine 1–4 | <20 ng/mL † (n = 39) | 0.004 | −0.013, 0.021 | 0.639 |
| ≥20 ng/mL (n = 57) | −0.002 | −0.007, 0.004 | 0.595 | |
| Femoral neck | <20 ng/mL (n = 39) | 0.012 | 0.001, 0.022 | 0.038 |
| ≥20 ng/mL (n = 57) | −0.001 | −0.005, 0.002 | 0.449 | |
| Total hip | <20 ng/mL (n = 39) | 0.013 | 0.000, 0.026 | 0.050 |
| ≥20 ng/mL (n = 57) | −0.001 | −0.005, 0.002 | 0.378 | |
| L-spine 1–4 | <18.9 ng/mL ‡ (n = 36) | 0.006 | −0.013, 0.024 | 0.532 |
| ≥18.9 ng/mL (n = 60) | −0.001 | −0.006, 0.004 | 0.673 | |
| Femoral neck | <18.9 ng/mL (n = 36) | 0.015 | 0.004, 0.027 | 0.010 |
| ≥18.9 ng/mL (n = 60) | 0.000 | −0.004, 0.003 | 0.784 | |
| Total hip | <18.9 ng/mL (n = 36) | 0.018 | 0.005, 0.031 | 0.007 |
| ≥18.9 ng/mL (n = 60) | −0.001 | −0.004, 0.002 | 0.647 |
| Seasons | Variables | β | 95% CI | p-Value |
|---|---|---|---|---|
| Annual average | Vitamin D intake (µg/1000 kcal) | 0.370 | 0.204, 0.536 | <0.001 |
| BMI (kg/m2) | −0.928 | −1.522, −0.334 | 0.003 | |
| sun exposure time (min) | 0.105 | 0.014, 0.195 | 0.024 | |
| Summertime | Vitamin D intake (µg/1000 kcal) | 0.226 | 0.071, 0.381 | 0.005 |
| sun exposure time (min) | 0.100 | 0.022, 0.178 | 0.013 | |
| BMI (kg/m2) | −0.674 | −1.318, −0.030 | 0.040 | |
| Wintertime | Vitamin D intake (µg/1000 kcal) | 0.314 | 0.182, 0.446 | <0.001 |
| BMI (kg/m2) | −0.985 | −1.637, −0.334 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, H.R.; Lee, Y.J.; Ly, S.Y. Optimal Serum 25(OH)D Levels and Vitamin D Intake in Korean Postmenopausal Women. Nutrients 2023, 15, 1856. https://doi.org/10.3390/nu15081856
Shin HR, Lee YJ, Ly SY. Optimal Serum 25(OH)D Levels and Vitamin D Intake in Korean Postmenopausal Women. Nutrients. 2023; 15(8):1856. https://doi.org/10.3390/nu15081856
Chicago/Turabian StyleShin, Hye Ran, Ye Jin Lee, and Sun Yung Ly. 2023. "Optimal Serum 25(OH)D Levels and Vitamin D Intake in Korean Postmenopausal Women" Nutrients 15, no. 8: 1856. https://doi.org/10.3390/nu15081856
APA StyleShin, H. R., Lee, Y. J., & Ly, S. Y. (2023). Optimal Serum 25(OH)D Levels and Vitamin D Intake in Korean Postmenopausal Women. Nutrients, 15(8), 1856. https://doi.org/10.3390/nu15081856