Abstract
Objectives: To examine the association between vitamin E (VE) status and gestational diabetes mellitus (GDM). Methods: A retrospective cohort study was conducted by using data of 52,791 women at 137 hospitals across 22 provinces of China. A fasting plasma glucose (FPG) level of ≥5.1 mmol/L between the 24th and 40th weeks of gestation was used as the criteria for the diagnosis of GDM. Mean FPG level and GDM rate were calculated within each combination of the first-trimester VE concentration categories and gestational change categories. The associations of the first-trimester VE concentrations and gestational VE change with FPG and GDM were examined by employing generalized additive models (GAMs). Results: 7162 (13.57%) cases were diagnosed with GDM. The GDM rate was 22.44%, 11.50%, 13.41%, 12.87%, 13.17%, 13.44%, 12.64%, and 14.24% among women with the first-trimester VE concentrations of <7.2, 7.2–7.9, 8.0–9.3, 9.4–11.0, 11.1–13.2, 13.3–15.8, 15.9–17.7, and 17.8–35.9 mg/L, respectively. The GDM rate was 15.96%, 13.10%, 13.64%, and 12.87% among women with gestational VE change of <0, 0–0.19, 0.20–0.29, ≥0.30 mg/L per week, respectively. Multivariable adjusted GAM analyses found that the first-trimester VE concentration was associated with the FPG levels and GDM risk in an L-shaped pattern; the FPG levels and GDM risk decreased sharply to a threshold (around 7 mg/L), and then were keep flat. Gestational VE decreases when the first-trimester VE level was less than 11 mg/L were related to increased FPG levels and GDM risk. Conclusions: Both low first-trimester VE levels and subsequent gestational VE decrease were related with increased risk of GDM. The findings suggest the necessity of having VE-rich foods and appropriate VE supplementation to prevent GDM for pregnant women with low baseline VE levels.
1. Introduction
Gestational diabetes mellitus (GDM) is one of the most common complications in pregnancy. The prevalence of GDM is 1.8–25% in different countries and areas [1], and it was estimated to be 14.8% in China [2]. Women with GDM have higher risk of preeclampsia, cesarean section, type 2 diabetes after pregnancy, and fetal adverse outcomes, including macrosomia, shoulder dystocia, birth trauma, and neonatal hypoglycemia [3].
Oxidative stress may be implicated in the pathogenesis of diabetes mellitus and its complications [4,5,6,7,8]. The underlying pathophysiology is similar to type 2 diabetes in most instances, which is why hyperglycemia leads to excessive production of intracellular reactive oxygen species (ROS) with insufficient clearance, thus enhancing the oxidative stress level of the body, leading to adverse pregnancy outcomes [4,5,6,7]. Vitamin E (VE), a well documented antioxidant, can neutralize ROS, eliminate oxygen free radicals, and protect tissue from damage [9,10]. However, although a robust number of studies on the role of VE in the cause and prevention of diabetic complications [8,11,12,13,14,15], there are very few studies on its association with risk of diabetes mellitus, especially GDM [16,17]. The systematic review by Sharifipour et al. aimed to evaluate the association between VE and GDM, and this only included eleven relevant studies with a total of 596 participants published before December 2019, of which seven were the case-control studies, and the rest were cross-sectional [18]. Our further search of the literature identified very few relevant articles published since then. The biggest hurdle is the cost and labor involved in obtaining a large enough sample size to examine the quantitative association, given the low proportion of VE deficiency. More studies are evidently needed on this topic, especially those with longitudinal design.
In response to the lack of research, we did this retrospective large-sample cohort study to examine the association between VE and GDM.
2. Materials and Methods
2.1. Study Design and Population
An original prospective cohort study investigating VE concentrations was implemented at 180 hospitals across 23 provinces of China during 2015–2019. Pregnant women who planned to be registered for antenatal care in these hospitals were recruited to participate, and blood samples were collected during antenatal care visits.
In this present study, we did a retrospective analysis by using data from the prospective cohort. We included singleton pregnancies with fasting plasma glucose (FPG) test and available data of VE concentrations at gestational age of 4–13 and 24–40 weeks. Women with prepregnancy diabetes diagnosis or FPG of ≥7.0 mmol/L and those with age of less than 15 or more than 49 were excluded. Finally, we included 52,791 women at 137 hospitals across 22 provinces (Anhui, Beijing, Guangdong, Guangxi, Hebei, Henan, Heilongjiang, Hubei, Hunan, Jilin, Jiangsu, Liaoning, Inner Mongolia, Qinghai, Shandong, Shanxi, Shaanxi, Sichuan, Tianjin, Yunnan, Zhejiang, and Chongqing). All study process was reviewed and approved by the Peking University Third Hospital Medical Science Research Ethics Committee (IRB00006761–2015277).
2.2. Gestational VE Status
A fasting venous blood sample was collected by trained nurse technicians when the pregnant woman went on regular antenatal care. Laboratory staff centrifuged the samples within 1 h, extracted and aliquoted serum immediately under no-light condition, and then stored serum at −80 °C in a refrigerator. Subsequently, all serum samples were sent to the only collaborating laboratory on dry ice within 1 month and maintained at −80 °C until analyzed. After receiving samples, serum concentrations of VE (α-tocopherol) were quantified by high performance liquid chromatography (Prominence LC-20A, Shimadzu) in a dark room within 24 h. One-twentieth of each batch of samples were tested repeatedly, showing inter-assay coefficients of variation of <5%. The relative standard deviations were 3.86% and 4.52% within and between days, respectively.
In our analysis, we used the minimum VE concentration tested in the first trimester and the VE concentration at the time of FPG test with the highest FPG value in 24–40 weeks. We calculated gestational VE change by subtracting the first-trimester VE concentration from the VE concentration at the FPG test time and then dividing the results after subtraction by the interval of gestational weeks. This is a reasonable approach because there was no previously-published data of gestational VE gain reference charts for Chinese population to calculating gestational age-standardized z scores, and our data show a linear trend of VE gain during pregnancy.
2.3. Fasting Plasma Glucose Level and Diagnosis on Gestational Diabetes Mellitus
We extracted the highest fasting plasma glucose values at gestational age of 4–13 and 24–40 weeks from the original cohort data collected from electronic medical records. In pregnant women with no prepregnancy diabetes diagnosis and FPG of less than 7.0 mmol/L, a fasting plasma glucose level of ≥5.1 mmol/L between the 24th and 40th weeks of gestation was used as the criteria for the diagnosis of GDM [19].
2.4. Covariates
Information about sociodemographic characteristics and medical conditions was collected from medical records, including geographical region, ethnic origin, education, household registration (urban residents, rural residents, or rural-to-urban migrants), age, mode of conception, primigravida, pre-pregnancy body mass index (BMI), and hypertension.
2.5. Statistical Analysis
We calculated proportions to describe the distribution of geographical region, ethnic origin, education, household registration, age, mode of conception, primigravida, pre-pregnancy BMI group, and hypertension, according to the GDM status. Mean FPG level and GDM rate were calculated within each combination of the first-trimester VE concentration categories (<7.2, 7.2–7.9, 8.0–9.3, 9.4–11.0, 11.1–13.2, 13.3–15.8, 15.9–17.7, and 17.8–35.9 mg/L; divided according to the 5th, 10th, 25th, 50th, 75th, 90th, and 95th percentiles) and gestational VE change categories (<0, 0–0.19, 0.20–0.29, ≥0.30 mg/L per week; divided according to the 25th, 50th, and 75th percentiles).
The associations of the first-trimester VE concentration and the gestational VE change with FPG and GDM were identified by performing generalized additive models (GAMs) with smooth functions implemented in the Mgcv version 1.8 package in R statistical software. All models were adjusted for the nine covariates and FPG levels at gestational age of 4–13 weeks, and the confounding of the first-trimester VE concentration and the gestational VE change on each other. According to the models, predicted concentration of FPG and risk of GDM with 95% confidence intervals (CIs) were calculated with respect to the first-trimester VE concentration and the gestational VE change. Subsequently, we used the visreg 2.7.0.3 package for visualizing these regression models. Heatmaps were constructed to exhibit the differences based on combinations of the first-trimester VE concentration and the gestational VE change (red represents high FPG level or GDM incidence, blue represents low FPG level or GDM incidence).
Furthermore, we used a generalized linear model (GLM) to assess the association of the first-trimester VE concentration categories and gestational VE change categories with FPG and used robust Poisson regression to assess the associations with GDM. Sensitivity analyses were performed by adjusting for different covariates. Model A was adjusted for the nine covariates and FPG levels at a gestational age of 4–13 weeks. Model B was additionally adjusted for the confounding of the first-trimester VE concentration categories and the gestational VE change categories on each other. Model C was additionally adjusted for an interaction term of the first-trimester VE concentration categories and the gestational VE change categories based on Model B. We also performed a stratified analysis to examine the association of gestational VE change categories with FPG and GDM in each first-trimester VE concentration category, by using robust Poisson regression models that adjusted for the aforementioned nine covariates. Regression coefficient (β) or relative risks (RRs) with 95% CIs were calculated.
We performed statistical analyses with Statistical Package for the Social Sciences (SPSS) software 25.0 (SPSS, Inc., Chicago, IL, USA) and the R statistical software, version 3.6.2. A two-tailed p-value < 0.05 was considered statistically significant.
3. Results
A total of 7162 (13.57%) GDM cases were identified among 52,791 pregnant women (mean age [SD], 28.87 [4.33] years). The baseline characteristics of participants are detailed in Table 1.

Table 1.
Characteristics of participants.
Mean FPG level and GDM rate within the combination of the first-trimester VE concentration categories and gestational VE change categories are presented in Figure 1. The mean FPG concentrations were 4.78, 4.63, 4.62, 4.61, 4.60, 4.60, 4.60, and 4.65 mmol/L, and the GDM rates were 22.44%, 11.50%, 13.41%, 12.87%, 13.17%, 13.44%, 12.64%, and 14.24% among women with first-trimester VE concentrations of <7.2 (<5th), 7.2–7.9 (5th–9th), 8.0–9.3 (10th–24th), 9.4–11.0 (25th–49th),11.1–13.2 (50th–74th), 13.3–15.8 (75th–89th), 15.9–17.7 (90th–95th), and 17.8–35.9 (95th–100th) mg/L, respectively. The mean FPG concentrations were 4.70, 4.60, 4.61, and 4.61 mmol/L, and the GDM rates were 15.96%, 13.10%, 13.64%, and 12.87% among women with gestational VE change of <0 (<25th), 0–0.19 (25th–49th), 0.20–0.29 (50th–74th), ≥0.30 (75th–100th) mg/L per week, respectively. Higher FPG levels and GDM risks were showed in those combinations of the lower first-trimester VE concentration and gestational VE decrease categories.
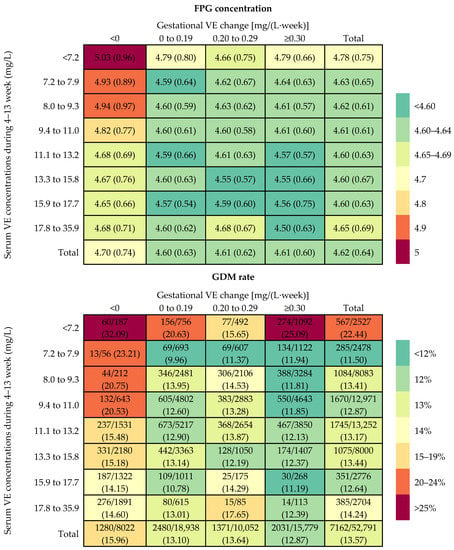
Figure 1.
Rate of GDM and concentration of FPG within each combination of the first-trimester vitamin E (VE) concentration categories and gestational VE change categories.
Multivariable adjusted GAM analyses showed that the first-trimester VE concentration was related to FPG levels (Figure 2a) and GDM risk (Figure 2b) in a L-shaped pattern; the FPG levels and GDM risk decreased sharply to a threshold (around 7 mg/L), and then they were kept flat. The association of gestational VE change to the FPG levels is presented as a reverse J-shaped curve, and the GDM risk is presented as a declining curve. Gestational VE decrease was associated with increased FPG levels and GDM risk (Figure 2c,d). Considerable differences in the FPG levels and GDM risk according to the combination of the first-trimester VE concentration and gestational VE change were identified (Figure 3). Higher FPG levels and GDM risks were found in women with lower first-trimester VE concentration and greater gestational VE decrease.
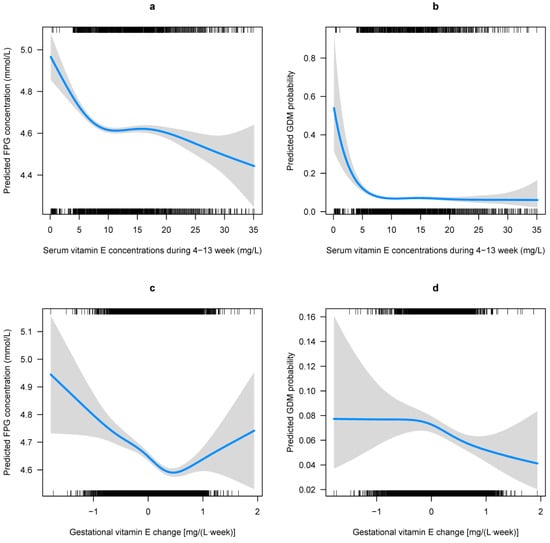
Figure 2.
Predicted concentration of FPG and risk of GDM with respect to gestational vitamin E (VE) status. Predicted concentration of FPG and the 95% CIs were calculated with respect to (a) the first-trimester VE concentrations and (c) the gestational VE change by employing generalized additive models. Predicted risk of GDM and the 95% CIs were also calculated with respect to (b) the first-trimester VE concentrations and (d) the gestational VE change by employing generalized additive models. All models were adjusted for region, household registration, education, ethnic origin, age, ART, primigravida, pre pregnancy BMI, hypertension, and FPG levels at gestational age of 4–13 weeks, as well as the confounding of the first-trimester VE concentrations and the gestational VE change on each other.
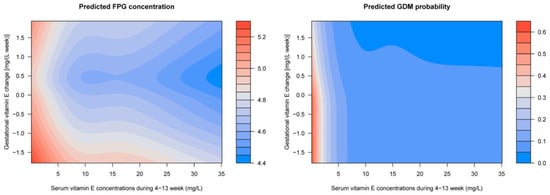
Figure 3.
Predicted concentration of FPG and risk of GDM with respect to the first-trimester VE concentrations and gestational VE change by employing generalized additive models. Models adjusted for region, household registration, education, ethnic origin, age, ART, primigravida, pre pregnancy BMI, hypertension, and FPG levels at gestational age of 4–13 weeks, as well as the confounding of the first-trimester VE concentrations and the gestational VE change on each other.
The results of multivariable adjusted robust Poisson regression on the association of gestational VE status with FPG and GDM was presented in Table 2. Compared to women with first-trimester VE concentrations of 11.1–13.2 (50th–74th) mg/L, those with first-trimester VE concentrations of <7.2 (<5th) mg/L had 0.123 (95% CI: 0.081, 0.166) increase in FPG and 1.36-fold (95% CI: 1.14, 1.62) increased risks for GDM. Gestational VE decrease was related with 0.050 (95% CI: 0.019, 0.082) increase in FPG, while gestational vitamin E gain of ≥0.30 mg/L per week was related to a −0.040 (95% CI: −0.063, −0.017) decrease in FPG. Sensitivity analyses showed that the estimates were robust. The stratified analysis showed that within the first-trimester VE concentration categories of less than 11 mg/L, gestational VE decreases were significantly associated with increased FPG levels and GDM risk (Table 3).

Table 2.
Adjusted FPG concentration differences and relative risks for GDM according to the first-trimester vitamin E (VE) concentration categories and gestational VE change categories.

Table 3.
Adjusted relative risks (95% CI) for GDM according to gestational vitamin E (VE) changes stratified by the first-trimester VE concentration categories.
4. Discussion
To our knowledge, this is the largest cohort study to date to investigate the association between VE and GDM. Several study characteristics, including unified and well established measures for gestational VE determination and use of FPG, the laboratory test indicator for assessing GDM, were beneficial for our analysis, as they minimized recall bias due to the retrospective nature. We found that the low first-trimester VE level and subsequent gestational VE decrease were related with increased risk of GDM, which strengthened the perception for occurrence of GDM and potential application value.
All available previous studies conducted the analyses based on the hypothesis that the association between VE concentration and GDM was linear [17]. The aforementioned systematic review by Sharifipour et al. concluded that the VE level was significantly lower in women with GDM compared to healthy pregnant women [18]. However, one large retrospective cohort study (n = 19,647) from China reported that maternal VE concentrations in the first and second trimesters were positively associated with GDM; this study did not exclude pregnant women with pre-gestational diabetes mellitus [16]. In contrast to previous findings, we found that the association of the first-trimester VE concentration with the GDM risk are presented as a L-shaped curve with an inflexion point around 7 mg/L. The threshold concentration identified far exceeded the clinical definition (5.0 mg/L) of VE deficiency, indicating the expansion of high-risk population in pregnancy. Large sample size, rigorous measurement, and the use of GAM models strongly contributed to the validity of our findings.
We found that maternal VE level rises in an almost linear trend during pregnancy, from 10 mg/L at 4th week to 16 mg/L at 40th week, with an average weekly increase of 0.16 mg/L. It was consistent with previous findings that VE levels increase with gestational age, which even exceed the normal level [20,21,22]. The variation may relate to the changes in lipid metabolism during pregnancy, since VE levels are highly correlated with overall fat content [22,23,24]. An animal experiment showed that dams with GDM had obvious changes in lipid metabolism, even earlier than changes in glucose [24]. In women with the first-trimester VE concentration of lower than 11 mg/L, we found that gestational VE decrease was related to increased GDM risk, whereas excessive gain showed no potential benefits in reducing the risk of GDM. These findings suggest the necessity of having VE-rich foods and appropriate VE supplementation to prevent GDM for pregnant women with low baseline vitamin E levels, although the idea needs further confirmation [25].
Our study has practical clinical application value. We recommend keeping a VE level of higher than 7 mg/L in the first trimester and avoiding gestational VE decrease when the first-trimester VE level is less than 11 mg/L to prevent GDM and other pregnancy complications, such as preeclampsia [26,27]. However, we could not recommend all pregnant women to test VE in the first trimester when the cost-effectiveness evaluation was insufficient. Therefore, development of the simple methods to identify women with low VE levels are warranted for future research. Considering the ethical issues of setting controls in RCTS, more real-world studies are needed to confirm the efficacy of VE supplementation among pregnant women with low VE levels in preventing GDM.
However, our research has several limitations. We diagnosed GDM by FPG in 24–40 weeks. It is well recognized that fasting hyperglycaemia represents predominantly hepatic insulin resistance, while post-prandial glucose excursions (and glucose excursions during OGTT) represent predominantly peripheral insulin resistance [28]. Hence, application of an OGTT for diagnosis of GDM, rather than just using FPG, might alter the result of the study, or at least affect some the calculated cut-off points for VE concentrations associated with an increased risk of GDM. However, our retrospective data were collected from 137 hospitals across 22 provinces of China, including low-resource settings where not all pregnant women had available access to OGTT. According to the IADPSG criteria, FPG of 4.8–5.0 mmol/L showed good test characteristics in screening for GDM [29]. Interestingly, another study found that mild hyperglycemia (FPG of 5.1–5.5 mmol/L) was more related with the increased risk of large for gestational age fetuses than GDM diagnosis by OGTT [30]. In addition, we failed to collect important confounders, such as dietary habits, nutritional supplements, and methods of glycemic control.
5. Conclusions
By the retrospective large-sample cohort, we found that the low first-trimester VE level of less than 7 mg/L and gestational VE decrease when the first-trimester VE level was less than 11 mg/L were related with increased FPG levels and GDM risk. These findings suggest the necessity of having VE-rich foods and appropriate VE supple-mentation to prevent GDM for pregnant women with low baseline VE levels, although the idea needs further confirmation. Further studies have to be conducted, including OGTT, a mandatory standard which has to be applied in all women, and it should also be used in lower standard hospitals in China in the future.
Author Contributions
Y.Z. and Y.W. (Yuan Wei) were responsible for the overall study design. H.S., X.G., Q.S., Y.W. (Ying Wang) and X.L. collected and cleared up data. H.S. and T.W. performed statistical analysis. H.S. and X.G. wrote the manuscript with inputs from the other co-authors. The corresponding authors had full access to the data in the study and take responsibility for the integrity of the data. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (82171661) and the National Key Research and Development Program of China (2021YFC2700700).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Peking University Third Hospital Medical Science Research Ethics Committee (IRB00006761–2015277).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors thank all the medical workers and pregnant women who participated in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhu, Y.; Zhang, C. Prevalence of Gestational Diabetes and Risk of Progression to Type 2 Diabetes: A Global Perspective. Curr. Diabetes Rep. 2016, 16, 7. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Sun, X.; Lu, L.; Liu, F.; Yuan, J. Prevalence of gestational diabetes mellitus in mainland China: A systematic review and meta-analysis. J. Diabetes Investig. 2019, 10, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstet. Gynecol. 2018, 131, e49–e64. [Google Scholar] [CrossRef]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef]
- Ornoy, A.; Becker, M.; Weinstein-Fudim, L.; Ergaz, Z. Diabetes during Pregnancy: A Maternal Disease Complicating the Course of Pregnancy with Long-Term Deleterious Effects on the Offspring. A Clinical Review. Int. J. Mol. Sci. 2021, 22, 2965. [Google Scholar] [CrossRef] [PubMed]
- Joo, E.H.; Kim, Y.R.; Kim, N.; Jung, J.E.; Han, S.H.; Cho, H.Y. Effect of Endogenic and Exogenic Oxidative Stress Triggers on Adverse Pregnancy Outcomes: Preeclampsia, Fetal Growth Restriction, Gestational Diabetes Mellitus and Preterm Birth. Int. J. Mol. Sci. 2021, 22, 10122. [Google Scholar] [CrossRef] [PubMed]
- Phoswa, W.N.; Khaliq, O.P. The Role of Oxidative Stress in Hypertensive Disorders of Pregnancy (Preeclampsia, Gestational Hypertension) and Metabolic Disorder of Pregnancy (Gestational Diabetes Mellitus). Oxid. Med. Cell. Longev. 2021, 2021, 5581570. [Google Scholar] [CrossRef]
- Pazdro, R.; Burgess, J.R. The role of vitamin E and oxidative stress in diabetes complications. Mech. Ageing Dev. 2010, 131, 276–286. [Google Scholar] [CrossRef]
- Erdemli, M.E.; Aksungur, Z.; Gul, M.; Yigitcan, B.; Bag, H.G.; Altinoz, E.; Turkoz, Y. The effects of acrylamide and vitamin E on kidneys in pregnancy: An experimental study. J. Matern.-Fetal Neonatal Med. 2019, 32, 3747–3756. [Google Scholar] [CrossRef]
- Traber, M.G. Vitamin E. Adv. Nutr. 2021, 12, 1047–1048. [Google Scholar] [CrossRef]
- Baburao Jain, A.; Anand Jain, V. Vitamin E, Its Beneficial Role in Diabetes Mellitus (DM) and Its Complications. J. Clin. Diagn. Res. 2012, 6, 1624–1628. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, A.; Falahi, E.; Barakatun-Nisak, M.Y.; Hanipah, Z.N.; Redzwan, S.M.; Yusof, L.M.; Gheitasvand, M.; Rezaie, F. Systematic review and meta-analyses of vitamin E (alpha-tocopherol) supplementation and blood lipid parameters in patients with diabetes mellitus. Diabetes Metab. Syndr. 2021, 15, 102158. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, M.; Jamilian, M.; Mazloomi, M.; Sanami, M.; Asemi, Z. A randomized-controlled clinical trial investigating the effect of omega-3 fatty acids and vitamin E co-supplementation on markers of insulin metabolism and lipid profiles in gestational diabetes. J. Clin. Lipidol. 2016, 10, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Chatzakis, C.; Sotiriadis, A.; Tsakmaki, E.; Papagianni, M.; Paltoglou, G.; Dinas, K.; Mastorakos, G. The Effect of Dietary Supplements on Oxidative Stress in Pregnant Women with Gestational Diabetes Mellitus: A Network Meta-Analysis. Nutrients 2021, 13, 2284. [Google Scholar] [CrossRef]
- Maktabi, M.; Jamilian, M.; Amirani, E.; Chamani, M.; Asemi, Z. The effects of magnesium and vitamin E co-supplementation on parameters of glucose homeostasis and lipid profiles in patients with gestational diabetes. Lipids Health Dis. 2018, 17, 163. [Google Scholar] [CrossRef]
- Zhou, Q.; Jiao, M.; Han, N.; Yang, W.; Bao, H.; Ren, Z. The Influence of Maternal Vitamin E Concentrations in Different Trimesters on Gestational Diabetes and Large-for-Gestational-Age: A Retrospective Study in China. Nutrients 2022, 14, 1629. [Google Scholar] [CrossRef]
- Lyu, Y.; Wang, G.; Sun, Z.; Cui, X.; Xiu, Q.; Wu, L. The association of maternal fat-soluble antioxidants in early pregnancy with gestational diabetes mellitus: A prospective cohort study. Nutr. Diabetes 2022, 12, 49. [Google Scholar] [CrossRef]
- Sharifipour, F.; Abedi, P.; Ciahkal, S.F.; Jahanfar, S.; Mohaghegh, Z.; Zahedian, M. Serum vitamin E level and gestational diabetes mellitus: A systematic review and meta-analysis. J. Diabetes Metab. Disord. 2020, 19, 1787–1795. [Google Scholar] [CrossRef]
- Chinese Society of Perinatal Medicine. Guidelines for the diagnosis and treatment of gestational hyperglycemia (2022) PART1. Chin. J. Obstet. Gynecol. 2022, 57, 3–12. [Google Scholar] [CrossRef]
- Xue, J.; Chen, Y.; Cui, L.; Peng, Z.; Li, J. Study on the relationship between vitamin A,D,E content in blood during pregnancy and pregnancy-related diseases. Chin. J. Fam. Plan. Gynecotokol. 2020, 12, 81–84. [Google Scholar] [CrossRef]
- Liang, L.; Ye, Y.; Yang, Z.; Huang, H.; Zheng, X. Association of serum vitamin A and vitamin E levels with high-risk pregnancy factors and age of pregnant women. J. Pract. Med. Tech. 2021, 28, 191–193. [Google Scholar] [CrossRef]
- Wang, P.; Tai, S.; Meng, X.; Niu, C.; Li, S.; Yuan, E. Analysis of serum vitamin E level in pregnant women. Chin. J. Lab. Diagn. 2022, 26, 229–231. [Google Scholar] [CrossRef]
- Conrad, M.; Kagan, V.E.; Bayir, H.; Pagnussat, G.C.; Head, B.; Traber, M.G.; Stockwell, B.R. Regulation of lipid peroxidation and ferroptosis in diverse species. Genes Dev. 2018, 32, 602–619. [Google Scholar] [CrossRef]
- Furse, S.; Fernandez-Twinn, D.S.; Chiarugi, D.; Koulman, A.; Ozanne, S.E. Lipid Metabolism Is Dysregulated before, during and after Pregnancy in a Mouse Model of Gestational Diabetes. Int. J. Mol. Sci. 2021, 22, 7452. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.; Holgan, N.; Craig, L.; Morgan, H.; Danielian, P.; Devereux, G. Development and piloting of a food-based intervention to increase vitamin E intake in pregnant women in a randomized controlled trial. Food Sci. Nutr. 2016, 4, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Jiang, Y.; Yuan, P.; Chen, L.; Gong, X.; Yang, Y.; Wang, Y.; Jiang, H.; Li, Y.; Sun, M.; et al. Association of Gestational Vitamin E Status With Pre-eclampsia: A Retrospective, Multicenter Cohort Study. Front. Nutr. 2022, 9, 911337. [Google Scholar] [CrossRef]
- Poston, L.; Briley, A.L.; Seed, P.T.; Kelly, F.J.; Shennan, A.H. Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): Randomised placebo-controlled trial. Lancet 2006, 367, 1145–1154. [Google Scholar] [CrossRef]
- Hoffman, R.P. Indices of insulin action calculated from fasting glucose and insulin reflect hepatic, not peripheral, insulin sensitivity in African-American and Caucasian adolescents. Pediatr. Diabetes 2008, 9, 57–61. [Google Scholar] [CrossRef]
- Saeedi, M.; Hanson, U.; Simmons, D.; Fadl, H. Characteristics of different risk factors and fasting plasma glucose for identifying GDM when using IADPSG criteria: A cross-sectional study. BMC Pregnancy Childbirth 2018, 18, 225. [Google Scholar] [CrossRef]
- Tennant, P.; Doxford-Hook, E.; Flynn, L.; Kershaw, K.; Goddard, J.; Stacey, T. Fasting plasma glucose, diagnosis of gestational diabetes and the risk of large for gestational age: A regression discontinuity analysis of routine data. BJOG 2022, 129, 82–89. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).