Individual, Family, and Social Factors Associated with Gestational Weight Gain in Adolescents: A Scoping Review
Abstract
1. Introduction
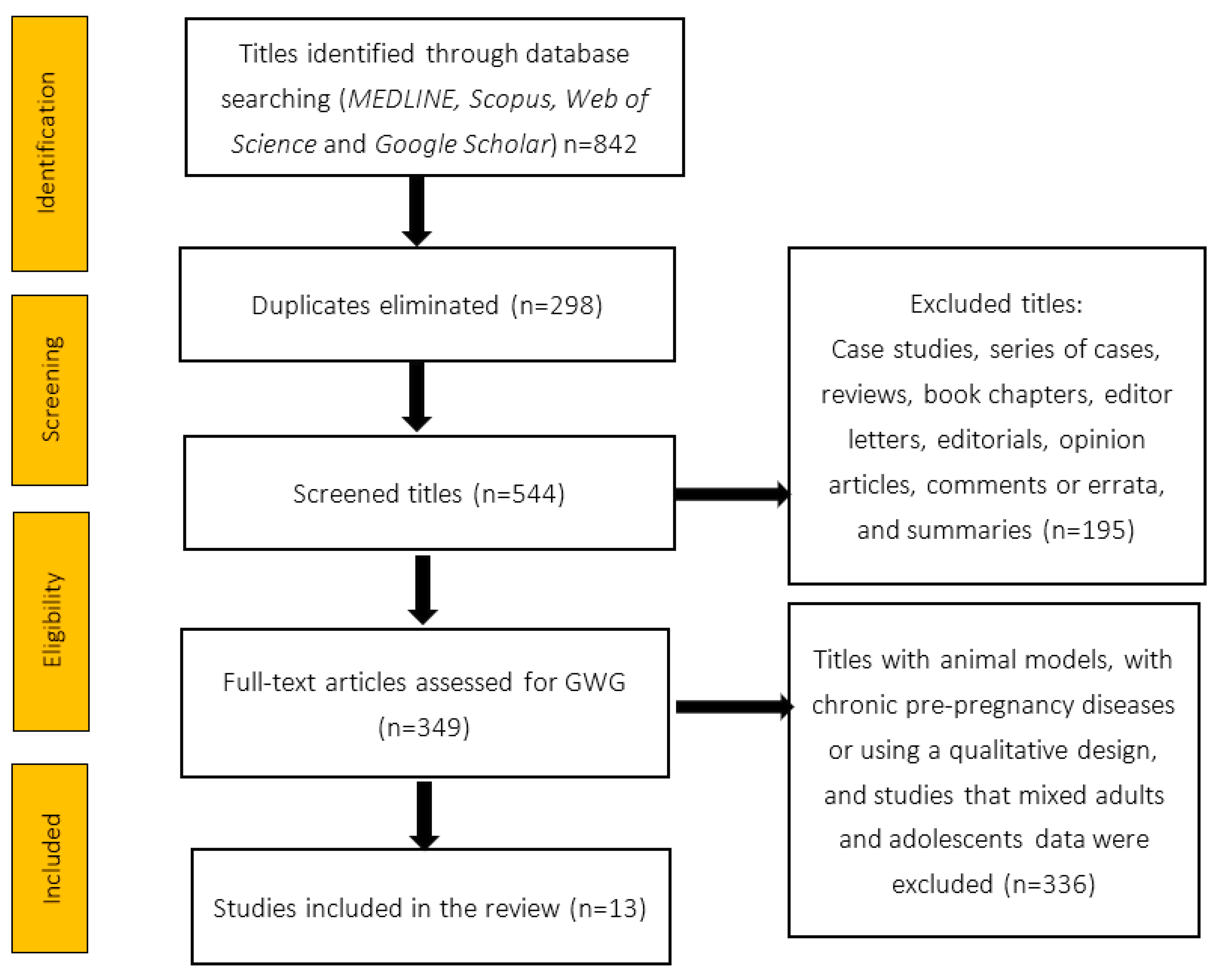
2. Material and Methods
2.1. Design
2.2. Inclusion Criteria and Selected Studies
2.3. Data Sources
2.4. Organization of the Information
2.5. Assessment of the Quality
3. Results
3.1. General Data
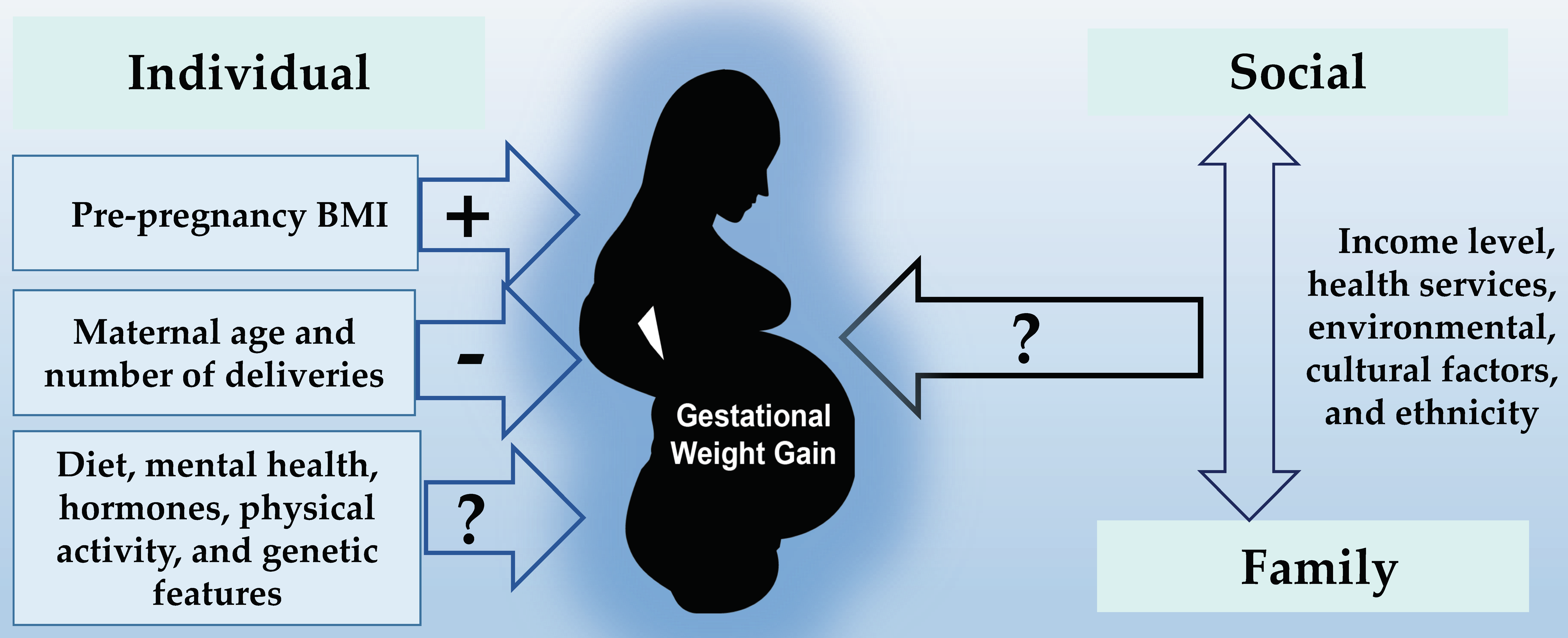
3.2. Individual Factors
3.3. Family Factors
3.4. Sociocultural Factors
4. Discussion
4.1. Findings
4.2. Individual Factors
4.3. Family and Sociocultural Factors
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- World Health Organization. Genova. Swiss. Adolescent Pregnancy. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/adolescent-pregnancy (accessed on 18 January 2023).
- Ogawa, K.; Matsushima, S.; Urayama, K.Y.; Kikuchi, N.; Nakamura, N.; Tanigaki, S.; Sago, H.; Satoh, S.; Saito, S.; Morisaki, N. Association between Adolescent Pregnancy and Adverse Birth Outcomes, a Multicenter Cross-Sectional Japanese Study. Sci. Rep. 2019, 9, 2365. [Google Scholar] [CrossRef] [PubMed]
- Macedo, T.C.C.; Montagna, E.; Trevisan, C.M.; Zaia, V.; de Oliveira, R.; Barbosa, C.P.; Laganà, A.S.; Bianco, B. Prevalence of Preeclampsia and Eclampsia in Adolescent Pregnancy: A Systematic Review and Meta-Analysis of 291,247 Adolescents Worldwide since 1969. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 248, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Lundeen, E.A.; Norris, S.A.; Martorell, R.; Suchdev, P.S.; Mehta, N.K.; Richter, L.M.; Stein, A.D. Adolescent Pregnancy and Attained Height among Black South African Girls: Matched-Pair Prospective Study. PLoS ONE 2016, 11, e0147861. [Google Scholar] [CrossRef]
- Sámano, R.; Martínez-Rojano, H.; Chico-Barba, G.; Hernández-Trejo, M.; Guzmán, R.; Arteaga-Troncoso, G.; Figueroa-Pérez, M.A.; Morales, R.M.; Martínez, G. Associations between Prenatal Serum Levels of Leptin, IGF-I, and Estradiol and Adolescent Mothers’ Height Gain during and after Pregnancy. PLoS ONE 2020, 15, e0228706. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Trumpff, C.; Genkinger, J.; Davis, A.; Spann, M.; Werner, E.; Monk, C. Micronutrient Dietary Intake in Latina Pregnant Adolescents and Its Association with Level of Depression, Stress, and Social Support. Nutrients 2017, 9, 1212. [Google Scholar] [CrossRef]
- Marvin-Dowle, K.; Burley, V.J.; Soltani, H. Nutrient Intakes and Nutritional Biomarkers in Pregnant Adolescents: A Systematic Review of Studies in Developed Countries. BMC Pregnancy Childbirth 2016, 16, 268. [Google Scholar] [CrossRef]
- Nguyen, P.H.; Scott, S.; Neupane, S.; Tran, L.M.; Menon, P. Social, Biological, and Programmatic Factors Linking Adolescent Pregnancy and Early Childhood Undernutrition: A Path Analysis of India’s 2016 National Family and Health Survey. Lancet Child Adolesc. Health 2019, 3, 463–473. [Google Scholar] [CrossRef]
- Elchert, J.; Beaudrot, M.; DeFranco, E. Gestational Weight Gain in Adolescent Compared with Adult Pregnancies: An Age-Specific Body Mass Index Approach. J. Pediatr. 2015, 167, 579–585.e2. [Google Scholar] [CrossRef]
- MacSween, K.; Whelan, E.; Woolcott, C.G. Gestational Weight Gain and Perinatal Outcomes in Adolescent Mothers: A Retrospective Cohort Study. J. Obstet. Gynaecol. Can. 2016, 38, 338–345. [Google Scholar] [CrossRef]
- Whelan, E.; Armson, B.A.; Ashley-Martin, J.; MacSween, K.; Woolcott, C. Gestational Weight Gain and Interpregnancy Weight Change in Adolescent Mothers. J. Pediatr. Adolesc. Gynecol. 2017, 30, 356–361. [Google Scholar] [CrossRef]
- Chavira-Suárez, E.; Ramírez-Mendieta, A.J.; Martínez-Gutiérrez, S.; Zárate-Segura, P.; Beltrán-Montoya, J.; Espinosa-Maldonado, N.C.; de la Cerda-Ángeles, J.C.; Vadillo-Ortega, F. Influence of Pre-Pregnancy Body Mass Index (p-BMI) and Gestational Weight Gain (GWG) on DNA Methylation and Protein Expression of Obesogenic Genes in Umbilical Vein. PLoS ONE 2019, 14, e0226010. [Google Scholar] [CrossRef] [PubMed]
- Houghton, L.C.; Ester, W.A.; Lumey, L.H.; Michels, K.B.; Wei, Y.; Cohn, B.A.; Susser, E.; Terry, M.B. Maternal Weight Gain in Excess of Pregnancy Guidelines Is Related to Daughters Being Overweight 40 Years Later. Am. J. Obstet. Gynecol. 2016, 215, e246.e1–e246.e8. [Google Scholar] [CrossRef]
- Groth, S.W.; Holland, M.L.; Kitzman, H.; Meng, Y. Gestational Weight Gain of Pregnant African American Adolescents Affects Body Mass Index 18 Years Later. J. Obstet. Gynecol. Neonatal Nurs. 2013, 42, 541–550. [Google Scholar] [CrossRef] [PubMed]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [PubMed]
- Rivera, J.; de Cossío, T.G.; Pedraza, L.S.; Aburto, T.C.; Sánchez, T.G.; Martorell, R. Childhood and adolescent overweight and obesity in Latin America: A systematic review. Lancet Diabetes Endocrinol. 2014, 2, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Shamah-Levy, T.; Cuevas-Nasu, L.; Gaona-Pineda, E.B.; Gómez-Acosta, L.M.; Morales-Rúan, M.D.C.; Hernández-Ávila, M.; Rivera-Dommarco, J.Á. Overweight and obesity in children and adolescents, 2016 Halfway National Health and Nutrition Survey update. Salud Publica Mex. 2018, 60, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Elsamadony, A.; Yates, K.F.; Sweat, V.; Yau, P.L.; Mangone, A.; Joseph, A.; Fierman, A.; Convit, A. Asian Adolescents with Excess Weight Are at Higher Risk for Insulin Resistance than Non-Asian Peers. Obesity 2017, 25, 1974–1979. [Google Scholar] [CrossRef]
- Ogden, C.L.; Carroll, M.D.; Curtin, L.R.; Lamb, M.M.; Flegal, K.M. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA 2010, 303, 242–249. [Google Scholar] [CrossRef]
- Templin, T.; Cravo Oliveira Hashiguchi, T.; Thomson, B.; Dieleman, J.; Bendavid, E. The Overweight and Obesity Transition from the Wealthy to the Poor in Low- and Middle-Income Countries: A Survey of Household Data from 103 Countries. PLoS Med. 2019, 16, e1002968. [Google Scholar] [CrossRef]
- Rasmussen, K.M.; Yaktine, A.L. Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines Weight Gain During Pregnancy: Reexamining the Guidelines; National Academies Press: Washington, DC, USA, 2009; ISBN 9780309131131. [Google Scholar]
- Kominiarek, M.A.; Peaceman, A.M. Gestational weight gain. Am. J. Obstet. Gynecol. 2017, 217, 642–651. [Google Scholar] [CrossRef]
- Goldstein, R.F.; Abell, S.K.; Ranasinha, S.; Misso, M.L.; Boyle, J.A.; Harrison, C.L.; Black, M.H.; Li, N.; Hu, G.; Corrado, F.; et al. Gestational weight gain across continents and ethnicity: Systematic review and meta-analysis of maternal and infant outcomes in more than one million women. BMC Med. 2018, 16, 153. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Gestational Weight Gain—MeSH—NCBI. Available online: https://www.ncbi.nlm.nih.gov/mesh/?term=gestational+weight+gain (accessed on 22 January 2023).
- National Health and Medical Research Council. How to Use the Evidence: Assessment and Application of Scientific Evidence; Handbook Series on Preparing Clinical Practice Guidelines. Table 1.3: Designation of Levels of Evidence 8; National Health and Medical Research Council: Canberra, Australia, 2000.
- Egger, M.; Higgins, J.P.T.; Smith, G.D. Systematic Reviews in Health Research: Meta-Analysis in Context; John Wiley & Sons: Hoboken, NJ, USA, 2022; ISBN 9781119099383. [Google Scholar]
- Eden, J.; Levit, L.; Berg, A.; Morton, S. Institute of Medicine (US) Committee on Standards for Systematic Reviews of Comparative Effectiveness Research Finding What Works in Health Care: Standards for Systematic Reviews; National Academies Press: Washington, DC, USA, 2011; ISBN 9780309164252. [Google Scholar]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. Ann. Intern. Med. 2009, 151, W65–W94. [Google Scholar] [CrossRef]
- Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. NIH. 2018. Available online: https://bmjopen.bmj.com/content/bmjopen/9/6/e023937/DC2/embed/inline (accessed on 15 June 2020).
- O’Connor, S.R.; Tully, M.A.; Ryan, B.; Bradley, J.M.; Baxter, G.D.; McDonough, S.M. Failure of a Numerical Quality Assessment Scale to Identify Potential Risk of Bias in a Systematic Review: A Comparison Study. BMC Res. Notes 2015, 8, 224. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, S.D.; Mokshagundam, S.; Chai, H.; Lewis, J.B.; Levine, J.; Tobin, J.N.; Ickovics, J.R. Postpartum Depressive Symptoms: Gestational Weight Gain as a Risk Factor for Adolescents Who Are Overweight or Obese. J. Midwifery Women’s Health 2018, 63, 178–184. [Google Scholar] [CrossRef]
- Danilack, V.A.; Brousseau, E.C.; Phipps, M.G. The Effect of Gestational Weight Gain on Persistent Increase in Body Mass Index in Adolescents: A Longitudinal Study. J. Women’s Health 2018, 27, 1456–1458. [Google Scholar] [CrossRef]
- Groth, S.W. The Long-Term Impact of Adolescent Gestational Weight Gain. Res. Nurs. Health 2008, 31, 108–118. [Google Scholar] [CrossRef]
- Ekambaram, M.; Irigoyen, M.; DeFreitas, J.; Rajbhandari, S.; Geaney, J.L.; Braitman, L.E. Gestational Weight Gain among Minority Adolescents Predicts Term Birth Weight. World J. Pediatr. 2018, 14, 357–363. [Google Scholar] [CrossRef]
- Joseph, N.P.; Hunkali, K.B.; Wilson, B.; Morgan, E.; Cross, M.; Freund, K.M. Pre-Pregnancy Body Mass Index among Pregnant Adolescents: Gestational Weight Gain and Long-Term Post Partum Weight Retention. J. Pediatr. Adolesc. Gynecol. 2008, 21, 195–200. [Google Scholar] [CrossRef]
- Timur, H.; Kokanalı, M.K.; Topçu, H.O.; Topçu, S.; Erkılınç, S.; Uygur, D.; Yakut, H.İ. Factors That Affect Perinatal Outcomes of the Second Pregnancy of Adolescents. J. Pediatr. Adolesc. Gynecol. 2016, 29, 18–21. [Google Scholar] [CrossRef]
- Groth, S.W.; Holland, M.L.; Smith, J.A.; Meng, Y.; Kitzman, H. Effect of Gestational Weight Gain and Prepregnancy Body Mass Index in Adolescent Mothers on Weight and Body Mass Index of Adolescent Offspring. J. Adolesc. Health 2017, 61, 626–633. [Google Scholar] [CrossRef]
- Noreña, I.; Pardo, M.P.; Mockus, I. Serum Adipokine Levels and Insulin Resistance in the First Trimester of Pregnancy in Adolescents and Their Relationship with Neonatal Weight. Biomedica 2018, 38, 427–436. [Google Scholar] [CrossRef]
- Sámano, R.; Martínez-Rojano, H.; Chico-Barba, G.; Godinez-Martínez, E.; Sánchez-Jiménez, B.; Montiel-Ojeda, D.; Tolentino, M. Serum Concentration of Leptin in Pregnant Adolescents Correlated with Gestational Weight Gain, Postpartum Weight Retention and Newborn Weight/Length. Nutrients 2017, 9, 1067. [Google Scholar] [CrossRef] [PubMed]
- Sam-Soto, S.; Sámano, R.; Flores-Ramos, M.; Rodríguez-Bosch, M.; García-Salazar, D.; Hernández-Mohar, G.; García-Espinosa, V. Weight gain during pregnancy and perinatal outcomes in pregnant adolescents with a history of sexual abuse. Nutr. Hosp. 2015, 32, 1075–1081. [Google Scholar] [CrossRef]
- Sámano, R.; Martínez-Rojano, H.; Chico-Barba, G.; Sánchez-Jiménez, B.; Illescas-Zarate, D.; Rodríguez-Ventura, A.L. Characteristics of the Family Support Network of Pregnant Adolescents and Its Association with Gestational Weight Gain and Birth Weight of Newborns. Int. J. Environ. Res. Public Health 2019, 16, 1222. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.Y.; Callaghan, W.M.; Bish, C.L.; D’Angelo, D. Gestational Weight Gain by Body Mass Index among US Women Delivering Live Births, 2004–2005: Fueling Future Obesity. Am. J. Obstet. Gynecol. 2009, 200, 271.e1–271.e7. [Google Scholar] [CrossRef]
- Scholl, T.O.; Hediger, M.L.; Schall, J.I.; Ances, I.G.; Smith, W.K. Gestational Weight Gain, Pregnancy Outcome, and Postpartum Weight Retention. Obstet. Gynecol. 1995, 86, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Thame, M.; Trotman, H.; Osmond, C.; Fletcher, H.; Antoine, M. Body Composition in Pregnancies of Adolescents and Mature Women and the Relationship to Birth Anthropometry. Eur. J. Clin. Nutr. 2007, 61, 47–53. [Google Scholar] [CrossRef]
- Gunderson, E.P.; Striegel-Moore, R.; Schreiber, G.; Hudes, M.; Biro, F.; Daniels, S.; Crawford, P.B. Longitudinal Study of Growth and Adiposity in Parous Compared with Nulligravid Adolescents. Arch. Pediatr. Adolesc. Med. 2009, 163, 349–356. [Google Scholar] [CrossRef]
- Gunderson, E.P.; Schreiber, G.; Striegel-Moore, R.; Hudes, M.; Daniels, S.; Biro, F.M.; Crawford, P.B. Pregnancy during Adolescence Has Lasting Adverse Effects on Blood Lipids: A 10-Year Longitudinal Study of Black and White Females. J. Clin. Lipidol. 2012, 6, 139–149. [Google Scholar] [CrossRef]
- Rondó, P.H.C.; Souza, M.R.; Moraes, F.; Nogueira, F. Relationship between Nutritional and Psychological Status of Pregnant Adolescents and Non-Adolescents in Brazil. J. Health Popul. Nutr. 2004, 22, 34–45. [Google Scholar] [PubMed]
- Chang, T.; Moniz, M.H.; Plegue, M.A.; Sen, A.; Davis, M.M.; Villamor, E.; Richardson, C.R. Characteristics of Women Age 15-24 at Risk for Excess Weight Gain during Pregnancy. PLoS ONE 2017, 12, e0173790. [Google Scholar] [CrossRef] [PubMed]
- Buschman, N.A.; Foster, G.; Vickers, P. Adolescent Girls and Their Babies: Achieving Optimal Birthweight. Gestational Weight Gain and Pregnancy Outcome in Terms of Gestation at Delivery and Infant Birth Weight: A Comparison between Adolescents under 16 and Adult Women. Child Care Health Dev. 2001, 27, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Minjares-Granillo, R.O.; Reza-López, S.A.; Caballero-Valdez, S.; Levario-Carrillo, M.; Chávez-Corral, D.V. Maternal and Perinatal Outcomes Among Adolescents and Mature Women: A Hospital-Based Study in the North of Mexico. J. Pediatr. Adolesc. Gynecol. 2016, 29, 304–311. [Google Scholar] [CrossRef]
- Marković, S.; Cerovac, A.; Cerovac, E.; Marković, D.; Bogdanović, G.; Kunosić, S. Antenatal Care and Weight Gain in Adolescent Compared to Adult Pregnancy. Int. J. Prev. Med. 2020, 11, 115. [Google Scholar] [CrossRef] [PubMed]
- Sámano, R.S.; Casanueva, E.; Vergara, A.; Pizano-Zárate, M.L.; Jiménez, D.; Godinez, E. Adolescents increase to more gestational weight and fat than adults according to BMI pre-gestational. Rev. Investig. Clin. 2011, 63, 500–508. [Google Scholar]
- Hill, B.; Bergmeier, H.; McPhie, S.; Fuller-Tyszkiewicz, M.; Teede, H.; Forster, D.; Spiliotis, B.E.; Hills, A.P.; Skouteris, H. Is Parity a Risk Factor for Excessive Weight Gain during Pregnancy and Postpartum Weight Retention? A Systematic Review and Meta-Analysis. Obes. Rev. 2017, 18, 755–764. [Google Scholar] [CrossRef]
- Sha, T.; Cheng, G.; Li, C.; Gao, X.; Li, L.; Chen, C.; Yan, Y. Patterns of Women’s Postpartum Weight Retention and Its Associations with Maternal Obesity-Related Factors and Parity. Int. J. Environ. Res. Public Health 2019, 16, 4510. [Google Scholar] [CrossRef]
- Jebeile, H.; Mijatovic, J.; Louie, J.C.Y.; Prvan, T.; Brand-Miller, J.C. A Systematic Review and Metaanalysis of Energy Intake and Weight Gain in Pregnancy. Am. J. Obstet. Gynecol. 2016, 214, 465–483. [Google Scholar] [CrossRef]
- Ratsavong, K.; van Elsacker, T.; Doungvichit, D.; Siengsounthone, L.; Kounnavong, S.; Essink, D. Are Dietary Intake and Nutritional Status Influenced by Gender? The Pattern of Dietary Intake in Lao PDR: A Developing Country. Nutr. J. 2020, 19, 31. [Google Scholar] [CrossRef]
- Rah, J.H.; Christian, P.; Shamim, A.A.; Arju, U.T.; Labrique, A.B.; Rashid, M. Pregnancy and Lactation Hinder Growth and Nutritional Status of Adolescent Girls in Rural Bangladesh. J. Nutr. 2008, 138, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- Stevens-Simon, C.; McAnarney, E.R. Determinants of Weight Gain in Pregnant Adolescents. J. Adolesc. Health 1991, 12, 174. [Google Scholar] [CrossRef]
- Trujillo, J.; Vieira, M.C.; Lepsch, J.; Rebelo, F.; Poston, L.; Pasupathy, D.; Kac, G. A Systematic Review of the Associations between Maternal Nutritional Biomarkers and Depression And/or Anxiety during Pregnancy and Postpartum. J. Affect. Disord. 2018, 232, 185–203. [Google Scholar] [CrossRef]
- Kapadia, M.Z.; Gaston, A.; Van Blyderveen, S.; Schmidt, L.; Beyene, J.; McDonald, H.; McDonald, S.D. Psychological Antecedents of Excess Gestational Weight Gain: A Systematic Review. BMC Pregnancy Childbirth 2015, 15, 107. [Google Scholar] [CrossRef]
- Casanueva, E.; Labastida, J.; Sanz, C.; Morales-Carmona, F. Depression and Body Fat Deposition in Mexican Pregnant Adolescents. Arch. Med. Res. 2000, 31, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Heery, E.; Kelleher, C.C.; Wall, P.G.; McAuliffe, F.M. Prediction of Gestational Weight Gain—A Biopsychosocial Model. Public Health Nutr. 2015, 18, 1488–1498. [Google Scholar] [CrossRef]
- Jiménez-González, A.; Granados-Cosme, J.A.; Rosales-Flores, R.A. Adolescense pregnancy in a marginalized rural community in Mexico. Salud Publica Mex. 2017, 59, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.D.C.A.N.; Silva, R.M.D.; Queiroz, M.V.O.; Jorge, H.M.F.; Brilhante, A.V.M. Realities and Perspectives of Adolescent Mothers in Their First Pregnancy. Rev. Bras. Enferm. 2018, 71, 65–72. [Google Scholar] [CrossRef]
- Thomas, J.L.; Lewis, J.B.; Martinez, I.; Cunningham, S.D.; Siddique, M.; Tobin, J.N.; Ickovics, J.R. Associations between Intimate Partner Violence Profiles and Mental Health among Low-Income, Urban Pregnant Adolescents. BMC Pregnancy Childbirth 2019, 19, 120. [Google Scholar] [CrossRef]
- Amjad, S.; MacDonald, I.; Chambers, T.; Osornio-Vargas, A.; Chandra, S.; Voaklander, D.; Ospina, M.B. Social Determinants of Health and Adverse Maternal and Birth Outcomes in Adolescent Pregnancies: A Systematic Review and Meta-Analysis. Paediatr. Perinat. Epidemiol. 2019, 33, 88–99. [Google Scholar] [CrossRef]
- Guimarães, A.M.D.N.; Bettiol, H.; Souza, L.D.; Gurgel, R.Q.; Almeida, M.L.D.; Ribeiro, E.R.D.O.; Goldaniv, M.Z.; Barbieri, M.A. Is Adolescent Pregnancy a Risk Factor for Low Birth Weight? Rev. Saude Publica 2013, 47, 11–19. [Google Scholar] [CrossRef]
- Rickert, V.I.; Wiemann, C.M.; Goodrum, L.A.; Berenson, A.B. Employment and Health-Risk Behaviors among Pregnant Adolescents. J. Pediatr. Adolesc. Gynecol. 1998, 11, 79–84. [Google Scholar] [CrossRef]
- Opiyo, C.O.; Okeyo, D.O.; Gumo, S.; Munde, E.O.; Omungo, Z.O.; Olyaro, M.; Ndirangu, R.K.; Ogbureke, N.; Efange, S.; Ouma, C. Power Dynamics as a Determinant of Access and Utilization of Nutrition Services by Pregnant and Lactating Adolescent Girls in Trans-Mara East Sub-County, Narok County, Kenya. BMC Public Health 2020, 20, 537. [Google Scholar] [CrossRef]
- Osok, J.; Kigamwa, P.; Huang, K.-Y.; Grote, N.; Kumar, M. Adversities and Mental Health Needs of Pregnant Adolescents in Kenya: Identifying Interpersonal, Practical, and Cultural Barriers to Care. BMC Women’s Health 2018, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- Sámano, R.; Lara-Cervantes, C.; Martínez-Rojano, H.; Chico-Barba, G.; Sánchez-Jiménez, B.; Lokier, O.; Hernández-Trejo, M.; Grosso, J.M.; Heller, S. Dietary Knowledge and Myths Vary by Age and Years of Schooling in Pregnant Mexico City Residents. Nutrients 2020, 12, 362. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, A.C.; Machado, M.M.T.; Wallington, S.F.; Greaney, M.L. Sociocultural and Interpersonal Influences on Latina Women’s Beliefs, Attitudes, and Experiences with Gestational Weight Gain. PLoS ONE 2019, 14, e0219371. [Google Scholar] [CrossRef]
- Orloff, N.C.; Hormes, J.M. Pickles and Ice Cream! Food Cravings in Pregnancy: Hypotheses, Preliminary Evidence, and Directions for Future Research. Front. Psychol. 2014, 5, 1076. [Google Scholar] [CrossRef]
- Blau, L.E.; Orloff, N.C.; Flammer, A.; Slatch, C.; Hormes, J.M. Food Craving Frequency Mediates the Relationship between Emotional Eating and Excess Weight Gain in Pregnancy. Eat. Behav. 2018, 31, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Cypher, R.L. Collaborative Approaches to Prenatal Care: Strategies of Successful Adolescent Programs. J. Perinat. Neonatal Nurs. 2013, 27, 134–144. [Google Scholar] [CrossRef]
- Warrington, N.M.; Richmond, R.; Fenstra, B.; Myhre, R.; Gaillard, R.; Paternoster, L.; Wang, C.A.; Beaumont, R.N.; Das, S.; Murcia, M.; et al. Maternal and Fetal Genetic Contribution to Gestational Weight Gain. Int. J. Obes. 2018, 42, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Groth, S.W.; LaLonde, A.; Wu, T.; Fernandez, I.D. Obesity Candidate Genes, Gestational Weight Gain, and Body Weight Changes in Pregnant Women. Nutrition 2018, 48, 61–66. [Google Scholar] [CrossRef]
- Meng, Y.; Groth, S.W.; Li, D. The Association between Obesity-Risk Genes and Gestational Weight Gain Is Modified by Dietary Intake in African American Women. J. Nutr. Metab. 2018, 2018, 5080492. [Google Scholar] [CrossRef] [PubMed]
- Sonestedt, E.; Roos, C.; Gullberg, B.; Ericson, U.; Wirfält, E.; Orho-Melander, M. Fat and Carbohydrate Intake Modify the Association between Genetic Variation in the FTO Genotype and Obesity. Am. J. Clin. Nutr. 2009, 90, 1418–1425. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, I.D.; Olson, C.M.; De Ver Dye, T. Discordance in the Assessment of Prepregnancy Weight Status of Adolescents: A Comparison between the Centers for Disease Control and Prevention Sex- and Age-Specific Body Mass Index Classification and the Institute of Medicine-Based Classification Used for Maternal Weight Gain Guidelines. J. Am. Diet. Assoc. 2008, 108, 998–1002. [Google Scholar] [CrossRef] [PubMed]
- Sámano, R.; Chico-Barba, G.; Martínez-Rojano, H.; Godinez, E.; Rodríguez-Ventura, A.L.; Ávila-Koury, G.; Aguilar-Sánchez, K. Pre-Pregnancy Body Mass Index Classification and Gestational Weight Gain on Neonatal Outcomes in Adolescent Mothers: A Follow-up Study. PLoS ONE 2018, 13, e0200361. [Google Scholar] [CrossRef]
- WHO Multicentre Growth Reference Study Group; de Onis, M. WHO Child Growth Standards Based on Length/height, Weight and Age. Acta Paediatr. 2007, 95, 76–85. [Google Scholar]
- Kuczmarski, R.J.; Ogden, C.L.; Guo, S.S.; Grummer-Strawn, L.M.; Flegal, K.M.; Mei, Z.; Wei, R.; Curtin, L.R.; Roche, A.F.; Johnson, C.L. 2000 CDC Growth Charts for the United States: Methods and Development. Vital Health Stat. 2002, 11, 1–190. [Google Scholar]
| Author, Year, Study Design | Participants | Exposure | GWG | Findings | Quality |
|---|---|---|---|---|---|
| Pre-pregnancy Body Mass Index (pBMI) | |||||
| Elchert et al. [9]. 2015 Retrospective cohort, 2006–2012 | n = 326,368. Pregnant women stratified by maternal age: 0.3%, <15 years; 7.0%, 15–17 years; 14.8%, 18–19 years; 77.9%, 20–34 years. Representative sample United States | Distribution of pBMI (in %) <15 years old: 23.2 15–17 years old: 23.5 18–19 years old: 24.5 WHO age- and sex-specific BMI | Excessive GWG, in % <15 years: 59.8 15–17 years: 59.9 18–19 years: 62.6 IOM definition, 2009 | pBMI was associated positively with GWG A large proportion of pregnancies had excessive GWG. The teens least likely to gain an adequate amount of weight were those who had a pBMI indicating obesity. | High, 12 |
| Cunningham et al. [32]. 2018 Retrospective cohort, 2008–2012 | n = 505 Adolescents aged 15–21 years Mean = 18.6 years Low income and from minorities in New York City United States | Distribution of pBMI, n (%) Underweight: 54 (10.7) Normal: 265 (52.5) Overweight: 96 (19) Obesity: 90 (17.8) IOM 2009 | Excessive GWG in 50% of participants IOM 2009 | pBMI was associated positively with GWG Association between pBMI and excessive GWG. | Good, 9 |
| Danilack et al. [33]. 2018 Retrospective cohort, 2007–2008 | n = 91 Adolescents aged ≤17 years Mean = 16.5 years, Different ethnicities United States | Distribution of pBMI, n (%) Low weight: 3 (3.3) Normal: 55 (60.4) Overweight: 22 (24.4) Obesity; 11 (12.1) CDC 2007 | GWG in kg: Mean: 15.5 ± 6.3 Low weight: 13 Normal: 15 Overweight: 17 Obesity: 12 (p = 0.171). | pBMI was not associated with GWG There was no linear trend in the averages of GWG kg per pBMI. | Good, 11 |
| Ekambaram et al. [35]. 2018 Retrospective cohort, 2012–2013 | n = 411 Adolescents aged 13–18 years Low-income urban residents, 90% Afro-American United States | Distribution of pBMI in % Underweight 5.6 Normal: 58.2 Overweight: 21.1 Obesity: 15.1 WHO. | GWG in % Excessive: 53 Adequate: 29 Below: 17.8 GWG: 15.6 ± 6 kg. GWG general (%) Inadequate: 17.8 Adequate: 29.3 Excessive: 53 Excessive GWG according to pBMI Underweight: 33 Normal: 42.6 Overweight: 67.9 Obesity: 77.8, p < 0.001 IOM 2009 | pBMI was associated positively when analyzed by categories, but not with GWG when analyzed overall. | High, 13 |
| Joseph et al. [36]. 2008 Retrospective cohort, 2002–2005 | n = 102 Adolescents aged 15–21 years; average, 15 years Vulnerable urban zones, different ethnicities United States | Distribution of pBMI in % Low weight: 19 (20%) Normal: 52 (54%) Overweight: 18 (18.6%) Obesity: 8 (8.2%) IOM 1990 | Total GWG in % Excessive: 36 Adequate: 30 Below: 34 Excessive GWG according to pBMI (%) Underweight: 26.5 Normal: 36.5 Overweight: 66.5 Obesity: 25 Inadequate or low GWG according to pBMI (%) Underweight: 31.6 Normal: 34.6 Overweight: 16 Obesity: 25 IOM 1990 | pBMI was associated positively by with GWG categories, particularly among those who were overweight. | High, 13 |
| Chu et al. [43]. 2009 Retrospective cohort, 2004–2005 | n = 5861 Adolescents aged 14–19 years A representative sample of pregnant women United States | Distribution of pBMI, in % Underweight: 9.9 ± 0.6 Normal: 59.3 ± 1.0 Overweight: 19 ± 0.8 Obese: 11.7 ± 0.7 NHLBI 1998 | GWG in pounds in the adolescent group Mean GWG: 16.1 Those with an overweight pBMI had a higher frequency (60%) of excessive GWG than those of normal 40% | pBMI was associated positively by with GWG categories, particularlly among those overweight, regardless of their chronological age. | High, 12 |
| Groth et al. [38]. 2017 Prospective cohort, 2009–2014 | n = 360 Adolescents aged ≤20 years Low income, African American, and primiparous United States | Distribution of pBMI, (%) Underweight: 1.7 Healthy weight: 75.5 Overweight: 15.3 Obesity: 7.5% CDC 2007 | GWG in % Excessive: 38 Adequate: 29 Below: 33 GWG in kg: 13.7 ± 7 GWG in kg by pBMI Underweight: 13.5 Normal: 13.8 Overweight: 14 Obesity: 13 IOM 2009 | pBMI was not associated with absolute GWG. | Good, 9 |
| Sámano et al. [40]. 2017 Prospective cohort, 2009–2016 | n = 168 Adolescents aged 12–17 years Low income, without public medical services, government support, or health insurance Mexico | Distribution of pBMI, in % Underweight: 4 Normal: 75 Overweight: 17 Obesity: 4 IOM 2009 | Distribution of GWG Median of GWG (kg) Underweight: 13 Normal: 12 Overweight: 11 Obesity: 4 Excessive GWG (%): Underweight: 0 Normal: 25 Overweight: 42 Obesity: 33 Insufficient GWG, Underweight: 0 Normal: 35 Overweight: 25 Obesity: 67 IOM 2009 | pBMI was associated positively with GWG by categories. | High, 14 |
| Maternal age | |||||
| Elchert et al. [9]. 2015 Retrospective cohort, 2006–2012 | n = 72,126 Pregnant women stratified by maternal age: n = 979: <15 years n = 22,845: 15–17 years n = 48,302: 18–19 years Representative sample United States | Distribution of pBMI in % <15 years old: 23.2 15–17 years old: 23.5 18–19 years old: 24.5 WHO age- and sex-specific BMI | GWG in % Excessive GWG <15 years: 59.8 15–17 years: 59.9 18–19 years: 62.6 GWG in kg <15 years: 14.9 15–17 years: 15.8 18–19 years: 16.3 Definition IOM 2009 | Risk (aOR) of low GWG <15 years: 1.12 (95% CI: 1.01–1.51) 15 to 17 years: 1.33 (95% CI: 1.27–1.40) 18 to 19 years: 1.26 (95% CI: 1.21–1.30). All compared with adult mothers, p < 0.001 | High, 12 |
| Groth et al. [34]. 2008 Retrospective cohort, 1990 | n = 330 Adolescents aged 12–19 years. African Americans, low-income, primiparous United States | Age in three categories: <16 years n = 106, 16–17 years, n = 146; 18–19 years, n = 78 | GWG in kg by age <16 years: 13.7 16–17 years: 14.1 18–19 years: 13.8 There were no differences in the mean GWG (kg) by age | Chronological age was not associated with GWG. | High 12 |
| Number of deliveries (parity) | |||||
| Chu et al. [43] 2009 Retrospective cohort, 2004–2005 | n = 5861 Adolescents aged 14–19 years Representative sample United States | Three groups for the number of pregnancies: Primiparous (0), 1–2 deliveries, ≥3 deliveries | GWG in kg Excessive GWG % Primiparous: 20.1 1–2 births: 12.7 ≥3 births: 10.8 (p < 0.001). Below GWG % Primiparous: 23.2 1–2 deliveries: 16.8 ≥3 deliveries: 11.5 (p < 0.001) | Number of deliveries was associated with GWG categories. Association between excessive GWG and parity: 1–2 births β = −3.15, SE = 0.20 p < 0.001; ≥3 births β = −4.27, SE = 0.35, p < 0.001. Excessive GWG was considered if above pBMI Normal gain: >15.75 Overweight gain: >11.25 kg | High, 12 |
| Timur et al. [37]. 2016 Retrospective cohort, 2010–2014 | n = 66 Adolescents aged 16–19 years Turkey | Maternal BMI (kg/m2) by parity: second pregnancy, 25 (20–37)/ first pregnancy, 23 (19–35) p = 0.672 | GWG (kg) showed no difference between the second pregnancy (11.5 ± 5.8) and first pregnancy (12.4 ± 5.2), p = 0.462 | First and second pregnancy and GWG were not associated. | Good, 11 |
| Diet | |||||
| Sámano et al. [40]. 2017 Prospective cohort, 2009–2016 | n = 168 Adolescents aged 12–17 years Low income, without public medical services, government support, or health insurance Mexico | Adequacy of energy, as a percentage | Distribution of GWG Median of GWG (kg) Underweight: 13 Normal: 12 Overweight: 11 Obesity: 4 Excessive GWG (%): Underweight: 0 Normal: 25 Overweight: 42 Obesity: 33 Insufficient GWG, Underweight: 0 Normal: 35 Overweight: 25 Obesity: 67 IOM 2009 | Percentage of energy adequacy was not associated with GWG. The percentage of energy adequacy, serum leptin, and pregestational weight explained the GWG. R2 = 0.192, SE = 3.99 (95% CI 14.89–30.890, p = 0.001) for the difference between pre-pregnancy and maximum gestational weight in kg. | High, 14 |
| Variables related to cardio-metabolic risk | |||||
| Noreña et al. [39]. 2018 Prospective cohort, 2009–2016 | n = 40 Adolescents aged 14–17 years Primiparous, low income Colombia | Leptin, insulin, and adiponectin in the second trimester determined by ELISA (ng/mL) | Leptin, insulin, and HOMA-IR were associated with pBMI, but not with GWG | A positive correlation (p < 0.001) was found between leptin levels and pBMI (r = 0.839). A positive correlation was observed between pBMI and insulin levels (r = 0.56; p ≤ 0.001), and between the HOMA-IR index and pBMI (r = 0.54; p = 0.0003). | Regular, 7 |
| Sámano et al. [40]. 2017 Prospective cohort, 2009–2016 | n = 168 Adolescents aged 12–17 years Low income, without public medical services, government support, or health insurance Mexico | Serum leptin in the last trimester determined by ELISA (ng/mL) | Distribution of GWG Median of GWG (kg) Underweight: 13 Normal: 12 Overweight: 11 Obesity: 4 Excessive GWG (%): Underweight: 0 Normal: 25 Overweight: 42 Obesity: 33 Insufficient GWG, Underweight: 0 Normal: 35 Overweight: 25 Obesity: 67 IOM 2009 | Leptin from the last trimester was associated positively with GWG. Higher leptin concentrations in the last trimester of gestation were associated with higher GWG (R2 = 0.177, p < 0.001) Leptin explained GWG SE = 0.03 (95% CI 0.100–0.248). GWG (%) was determined by the difference between maximum gestational and pre-pregnancy weight in kg. IOM 2009. | High, 14 |
| Author, Year, Study Design | Participants | Exposure | GWG | Findings | Quality |
|---|---|---|---|---|---|
| Sam-Soto et al. [41]. 2015 Cross-sectional, 2010–2013 | n = 165 Adolescents aged 10 to 16 years With and without a history of sexual abuse. from vulnerable urban zones Mexico | Adolescents with or without a history of sexual abuse Adolescents who lived or did not live with their father | Mean GWG (kg) Sexually abused: 7.5 Non-abused adolescents: 12.5, p = 0.005. | History of sexual abuse was related to low GWG. The adolescents who were most likely to have been sexually abused had lower socioeconomic status and did not live with their father. | Good, 11 |
| Sámano et al. [42]. 2019. Cross-sectional, 2008–2014 | n = 352 Adolescents aged 12 to 18 years From low resources, without public medical services, government support, or health insurance Mexico | Family support network, divided in quartiles (Q) according to the support network size Energy intake (kcal) | GWG (%) by IOM 2009 Excessive: 23.5 Adequate: 38 Below: 37.5 GWG (%) by IOM 2009 in quartile (p = 0.003) Q I: Excessive: 26 Adequate: 43 Below: 34 Q II: Excessive: 30 Adequate: 37 Below: 33, Q III: Excessive: 20 Adequate: 34 Below: 46 Q IV: Excessive: 25 Adequate: 38 Below: 37 | Family support by quartile was associated with GWG but showed a non-linear trend. The type of members in each quartile was uncertain. | High, 13 |
| Danilack et al. [33]. 2018 Retrospective cohort, 2007–2008 | n = 91 Adolescents aged 17 years, mean age: 16.5 years United States | Race/ethnicity (%) Latina: 55 African American: 18.7 White: 14.3 Other: 12.1 | GWG kg: 15.5 ± 6.3 Low weight: 13 Normal: 15 Overweight: 17 Obesity: 12 (p = 0.171) | There was no average GWG (in kg) per maternal race/ethnicity. BMI was similar in all racial/ethnic groups. | Good, 11 |
| Cunningham et al. [32]. 2018 Retrospective cohort, 2008–2012. | n = 505 Adolescents aged 15–21 years, mean age: 18.62 years. Low income and minorities in New York City United States | Race/ethnicity (%) Latina: 266 (52.7%) Black, non-Latina: 196 (38.8%) Other: 43 (8.5%) | Excessive GWG was present in n = 255 (50%) By race/ethnicity Latina: 135 (52.9%) Black-non-Latina: 100 (39.2%) Other: 20 (7.8%) Overweight: β 2.41, SE 1.06 p < 0.05 Obese: β 2.58, SE 1.08 p < 0.05 IOM 2009 | Race/ethnicity was associated with excessive GWG (Latina group), maybe due to pBMI. | Good, 9 |
| Joseph et al. [36]. 2008. Retrospective cohort, 2002–2005. | n = 102 Adolescents aged 15–21 years, mean age: 15 years. Vulnerable urban zones United States | Race/ethnicity (%) African-American: 84 Latina: 12 Non-Hispanic white: 1% Other ethnicities: 3% | GWG in % Excessive: 36 Adequate: 30 Below: 34 IOM 1990 | There was no average GWG (in kg) per maternal race/ethnicity. | High, 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sámano, R.; Martínez-Rojano, H.; Ortiz-Hernández, L.; Nájera-Medina, O.; Chico-Barba, G.; Gamboa, R.; Mendoza-Flores, M.E. Individual, Family, and Social Factors Associated with Gestational Weight Gain in Adolescents: A Scoping Review. Nutrients 2023, 15, 1530. https://doi.org/10.3390/nu15061530
Sámano R, Martínez-Rojano H, Ortiz-Hernández L, Nájera-Medina O, Chico-Barba G, Gamboa R, Mendoza-Flores ME. Individual, Family, and Social Factors Associated with Gestational Weight Gain in Adolescents: A Scoping Review. Nutrients. 2023; 15(6):1530. https://doi.org/10.3390/nu15061530
Chicago/Turabian StyleSámano, Reyna, Hugo Martínez-Rojano, Luis Ortiz-Hernández, Oralia Nájera-Medina, Gabriela Chico-Barba, Ricardo Gamboa, and María Eugenia Mendoza-Flores. 2023. "Individual, Family, and Social Factors Associated with Gestational Weight Gain in Adolescents: A Scoping Review" Nutrients 15, no. 6: 1530. https://doi.org/10.3390/nu15061530
APA StyleSámano, R., Martínez-Rojano, H., Ortiz-Hernández, L., Nájera-Medina, O., Chico-Barba, G., Gamboa, R., & Mendoza-Flores, M. E. (2023). Individual, Family, and Social Factors Associated with Gestational Weight Gain in Adolescents: A Scoping Review. Nutrients, 15(6), 1530. https://doi.org/10.3390/nu15061530






