Factors Affecting BMI Changes in Mothers during the First Year Postpartum
Abstract
1. Introduction
2. Materials and Methods
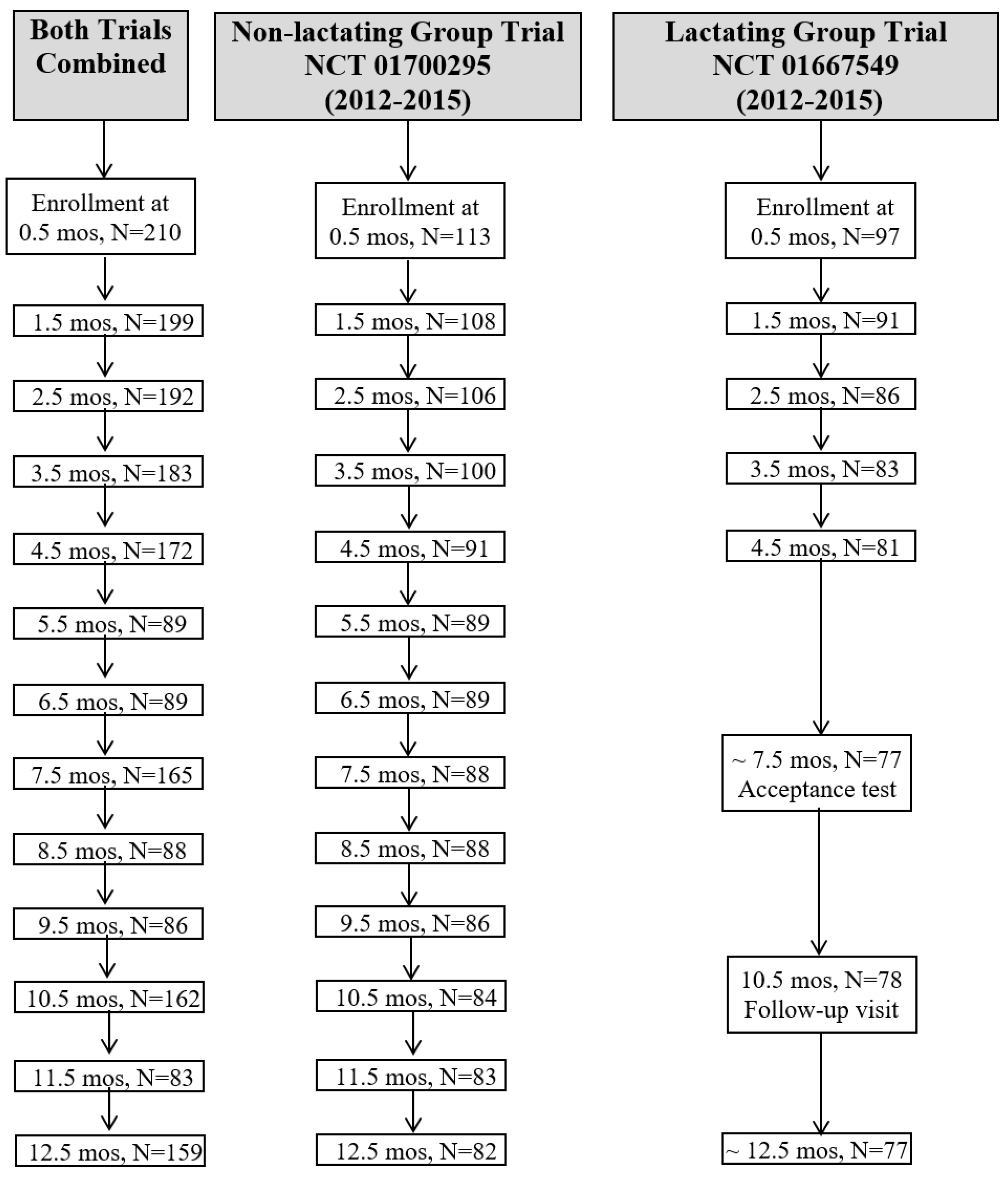
2.1. Participants and Trial Designs
2.2. Prepregnancy BMI Category Groupings
2.3. Anthropometry
2.4. Psychological Eating Behavior Traits
2.5. Statistical Analyses
3. Results
3.1. Participant Characteristics
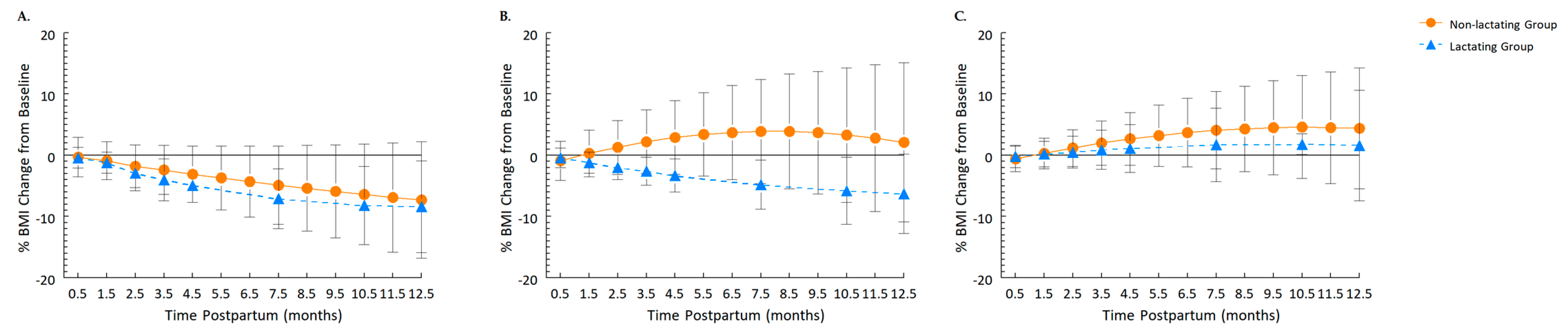
3.2. Independent and Interactive Effects of Infant Feeding Modality and Prepregnancy BMI
3.3. BMI at 1 year Postpartum Relative to Prepregnancy
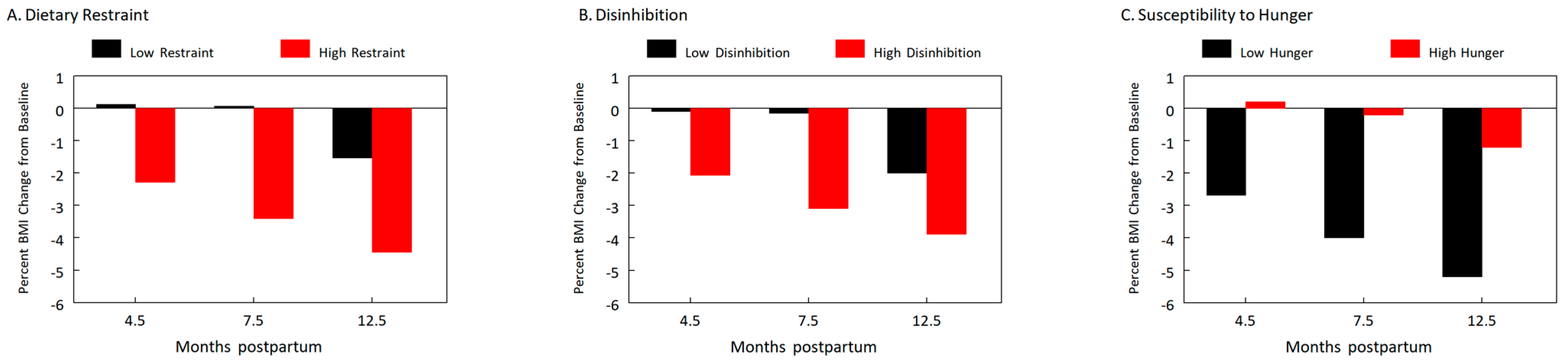
3.4. The Effects of the Psychological Eating Behavior Traits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Olson, C.M.; Strawderman, M.S.; Dennison, B.A. Maternal weight gain during pregnancy and child weight at age 3 years. Matern. Child Health J. 2009, 13, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.A.; Rasmussen, K.M.; King, J.C.; Abrams, B. Trajectories of maternal weight from before pregnancy through postpartum and associations with childhood obesity. Am. J. Clin. Nutr. 2017, 106, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Butte, N.F.; Hopkinson, J.M.; Mehta, N.; Moon, J.K.; Smith, E.O. Adjustments in energy expenditure and substrate utilization during late pregnancy and lactation. Am. J. Clin. Nutr. 1999, 69, 299–307. [Google Scholar] [CrossRef]
- Institute of Medicine; National Research Council. Weight Gain during Pregnancy: Reexamining the Guidelines; National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- Dewey, K.G. Energy and protein requirements during lactation. Annu. Rev. Nutr. 1997, 17, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Kirkegaard, H.; Stovring, H.; Rasmussen, K.M.; Abrams, B.; Sorensen, T.I.; Nohr, E.A. How do pregnancy-related weight changes and breastfeeding relate to maternal weight and BMI-adjusted waist circumference 7 year after delivery? Results from a path analysis. Am. J. Clin. Nutr. 2014, 99, 312–319. [Google Scholar] [CrossRef]
- Waits, A.; Guo, C.Y.; Chang, Y.S.; Chien, L.Y. Dose-response relationships between breastfeeding and postpartum weight retention differ by pre-pregnancy body-mass index in Taiwanese women. Nutrients 2020, 12, 1065. [Google Scholar] [CrossRef]
- Endres, L.K.; Straub, H.; McKinney, C.; Plunkett, B.; Minkovitz, C.S.; Schetter, C.D.; Ramey, S.; Wang, C.; Hobel, C.; Raju, T.; et al. Postpartum weight retention risk factors and relationship to obesity at 1 year. Obstet. Gynecol. 2015, 125, 144–152. [Google Scholar] [CrossRef]
- Vinter, C.A.; Jensen, D.M.; Ovesen, P.; Beck-Nielsen, H.; Tanvig, M.; Lamont, R.F.; Jorgensen, J.S. Postpartum weight retention and breastfeeding among obese women from the randomized controlled Lifestyle in Pregnancy (LiP) trial. Acta Obstet. Gynecol. Scand. 2014, 93, 794–801. [Google Scholar] [CrossRef]
- Neville, C.E.; McKinley, M.C.; Holmes, V.A.; Spence, D.; Woodside, J.V. The relationship between breastfeeding and postpartum weight change--a systematic review and critical evaluation. Int. J. Obes. 2014, 38, 577–590. [Google Scholar] [CrossRef]
- Nguyen, B.; Jin, K.; Ding, D. Breastfeeding and maternal cardiovascular risk factors and outcomes: A systematic review. PLoS ONE 2017, 12, e0187923. [Google Scholar] [CrossRef]
- Chowdhury, R.; Sinha, B.; Sankar, M.J.; Taneja, S.; Bhandari, N.; Rollins, N.; Bahl, R.; Martines, J. Breastfeeding and maternal health outcomes: A systematic review and meta-analysis. Acta Paediatr. 2015, 104, 96–113. [Google Scholar] [CrossRef]
- Stunkard, A.J.; Messick, S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J. Psychosom. Res. 1985, 29, 71–83. [Google Scholar] [CrossRef] [PubMed]
- James, B.L.; Roe, L.S.; Loken, E.; Rolls, B.J. Early predictors of weight loss in a 1-year behavioural weight-loss programme. Obes. Sci. Pract. 2018, 4, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Mennella, J.A.; Inamdar, L.; Pressman, N.; Schall, J.; Papas, M.A.; Schoeller, D.; Stallings, V.A.; Trabulsi, J.C. Type of infant formula increases early weight gain and impacts energy balance: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 1015–1025. [Google Scholar] [CrossRef]
- Mennella, J.A.; Daniels, L.M.; Reiter, A.R. Learning to like vegetables during breastfeeding: A randomized clinical trial of lactating mothers and infants. Am. J. Clin. Nutr. 2017, 106, 67–76. [Google Scholar] [CrossRef]
- Palad, C.J.; Stanford, F.C. Use of people-first language with regard to obesity. Am. J. Clin. Nutr. 2018, 108, 201–203. [Google Scholar] [CrossRef]
- Siega-Riz, A.M.; Bodnar, L.M.; Stotland, N.E.; Stang, J. The current understanding of gestational weight gain among women with obesity and the need for future research. NAM Perspect. 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Olson, C.M.; Strawderman, M.S.; Hinton, P.S.; Pearson, T.A. Gestational weight gain and postpartum behaviors associated with weight change from early pregnancy to 1 year postpartum. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 117–127. [Google Scholar] [CrossRef]
- Gunderson, E.P.; Abrams, B. Epidemiology of gestational weight gain and body weight changes after pregnancy. Epidemiol. Rev. 1999, 21, 261–275. [Google Scholar] [CrossRef]
- Hall, K.D.; Sacks, G.; Chandramohan, D.; Chow, C.C.; Wang, Y.C.; Gortmaker, S.L.; Swinburn, B.A. Quantification of the effect of energy imbalance on bodyweight. Lancet 2011, 378, 826–837. [Google Scholar] [CrossRef]
- Belle, S.H.; Berk, P.D.; Courcoulas, A.P.; Engel, S.; Flum, D.R.; Gourash, W.; Horlick, M.; Hsu, J.Y.; Khandelwal, S.; Mitchell, J.E.; et al. Reporting weight change: Standardized reporting accounting for baseline weight. Surg. Obes. Relat. Dis. 2013, 9, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Savage, J.S.; Hohman, E.E.; McNitt, K.M.; Pauley, A.M.; Leonard, K.S.; Turner, T.; Pauli, J.M.; Gernand, A.D.; Rivera, D.E.; Symons Downs, D. Uncontrolled Eating during Pregnancy Predicts Fetal Growth: The Healthy Mom Zone Trial. Nutrients 2019, 11, 899. [Google Scholar] [CrossRef]
- Pew Charitable Trust. Philadelphia 2022: The State of the City Report. Available online: https://www.thedevelopmentworkshop.org/single-post/pew-reports-philadelphia-2022-the-state-of-the-city (accessed on 7 March 2023).
- Bogaerts, A.; De Baetselier, E.; Ameye, L.; Dilles, T.; Van Rompaey, B.; Devlieger, R. Postpartum weight trajectories in overweight and lean women. Midwifery 2017, 49, 134–141. [Google Scholar] [CrossRef]
- Dewey, K.G.; Heinig, M.J.; Nommsen, L.A. Maternal weight-loss patterns during prolonged lactation. Am. J. Clin. Nutr. 1993, 58, 162–166. [Google Scholar] [CrossRef]
- Villamor, E.; Cnattingius, S. Interpregnancy weight change and risk of adverse pregnancy outcomes: A population-based study. Lancet 2006, 368, 1164–1170. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; The National Academies Press: Washington, DC, USA, 2006. [Google Scholar] [CrossRef]
- Rasmussen, K.M. Association of maternal obesity before conception with poor lactation performance. Annu. Rev. Nutr. 2007, 27, 103–121. [Google Scholar] [CrossRef]
- Kitsantas, P.; Pawloski, L.R. Maternal obesity, health status during pregnancy, and breastfeeding initiation and duration. J. Matern.-Fetal Neonatal Med. 2010, 23, 135–141. [Google Scholar] [CrossRef]
- Butte, N.F.; Hopkinson, J.M. Body composition changes during lactation are highly variable among women. J. Nutr. 1998, 128, 381S–385S. [Google Scholar] [CrossRef] [PubMed]
- Butte, N.F.; Ellis, K.J.; Wong, W.W.; Hopkinson, J.M.; Smith, E.O. Composition of gestational weight gain impacts maternal fat retention and infant birth weight. Am. J. Obstet. Gynecol. 2003, 189, 1423–1432. [Google Scholar] [CrossRef]
- Nunnery, D.; Ammerman, A.; Dharod, J. Predictors and outcomes of excess gestational weight gain among low-income pregnant women. Health Care Women Int. 2018, 39, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Voerman, E.; Santos, S.; Inskip, H.; Amiano, P.; Barros, H.; Charles, M.A.; Chatzi, L.; Chrousos, G.P.; Corpeleijn, E.; Crozier, S.; et al. Association of gestational weight gain with adverse maternal and infant outcomes. JAMA 2019, 321, 1702–1715. [Google Scholar] [CrossRef]
- Hays, N.P.; Bathalon, G.P.; McCrory, M.A.; Roubenoff, R.; Lipman, R.; Roberts, S.B. Eating behavior correlates of adult weight gain and obesity in healthy women aged 55–65 y. Am. J. Clin. Nutr. 2002, 75, 476–483. [Google Scholar] [CrossRef]
- Bellisle, F.; Clément, K.; Le Barzic, M.; Le Gall, A.; Guy-Grand, B.; Basdevant, A. The Eating Inventory and body adiposity from leanness to massive obesity: A study of 2509 adults. Obes. Res. 2004, 12, 2023–2030. [Google Scholar] [CrossRef]
- Hays, N.P.; Roberts, S.B. Aspects of eating behaviors “disinhibition” and “restraint” are related to weight gain and BMI in women. Obesity 2008, 16, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Bryant, E.J.; Rehman, J.; Pepper, L.B.; Walters, E.R. Obesity and eating disturbance: The role of TFEQ restraint and disinhibition. Curr. Obes. Rep. 2019, 8, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Stinson, E.J.; Graham, A.L.; Thearle, M.S.; Gluck, M.E.; Krakoff, J.; Piaggi, P. Cognitive dietary restraint, disinhibition, and hunger are associated with 24-h energy expenditure. Int. J. Obes. 2019, 43, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Horta, B.L.; Bahl, R.; Martinés, J.C.; Victora, C.G. Evidence on the Long-Term Effects of Breastfeeding: Systematic Review and Meta-Analyses; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Phelan, S.; Phipps, M.G.; Abrams, B.; Darroch, F.; Grantham, K.; Schaffner, A.; Wing, R.R. Does behavioral intervention in pregnancy reduce postpartum weight retention? Twelve-month outcomes of the Fit for Delivery randomized trial. Am. J. Clin. Nutr. 2014, 99, 302–311. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Infant Feeding Modality Groups 1 | p-Value 2 | |
|---|---|---|---|
| Lactating (n = 96) | Non-Lactating (n = 112) | ||
| Age, years | 30.8 ± 5.3 | 27.0 ± 5.6 | <0.001 |
| Self-reported race | <0.001 | ||
| Asian | 2.0% (2) | 0% (0) | |
| Black | 36.5% (35) a | 67.9% (76) b | |
| More than one | 5.2% (5) | 3.5% (4) | |
| White | 56.3% (54) a | 28.6% (32) b | |
| Household income 3 | <0.001 | ||
| <USD 35,000 | 40.6% (39) a | 73.6% (81) b | |
| USD 35,000–USD 75,000 | 21.9% (21) | 10.9% (12) | |
| >USD 75,000 | 37.5% (36) a | 15.5% (17) b | |
| Education | <0.001 | ||
| Primary school | 4.2% (4) | 15.2% (17) | |
| High/technical school | 33.3% (32) a | 60.7% (68) b | |
| College degree or higher | 62.5% (60) a | 24.1% (27) b | |
| Parity, primiparous | 34.4% (33) | 19.6% (22) | 0.02 |
| Prepregnancy weight (kg) | 70.9 ± 18.9 | 78.9 ± 25.8 | 0.01 |
| Prepregnancy BMI | 26.0 ± 6.8 | 29.2 ± 8.4 | 0.003 |
| Prepregnancy BMI groups | 0.005 | ||
| Healthy weight | 60.4% (58) a | 38.4% (43) b | |
| Overweight | 15.6% (15) | 20.5% (23) | |
| Obesity | 24.0% (23) | 41.1% (46) | |
| GWG, kg | 14.1 ± 5.7 | 13.2 ± 9.2 | 0.42 |
| GWG relative to IOM/NRC recommendations | 0.006 | ||
| Below | 14.6% (14) | 22.3% (25) | |
| Within | 40.6% (39) a | 20.5% (23) b | |
| Exceeded | 44.8% (43) | 57.1% (64) | |
| Characteristics | Coefficient (95% CI) 1 |
|---|---|
| Prepregnancy Healthy Weight group | |
| Age | −0.01 (−0.14, 0.12) |
| Income | −0.25 (−1.04, 0.54) |
| GWG | −0.14 (−0.24, −0.04) |
| Time | −1.37 (−1.67, −1.07) |
| Time2 | 0.06 (0.04, 0.08) |
| Infant feeding group | |
| Non-lactating group | −0.17 (−1.47, 1.14) |
| Infant feeding modality group × time | |
| Non-lactating group | 0.63 (0.19, 1.06) |
| Infant feeding modality group × time2 | |
| Non-lactating group | −0.05 (−0.07, −0.02) |
| Prepregnancy Overweight group | |
| Age | −0.07 (−0.22, 0.09) |
| Income | −1.56 (−3.0, −0.16) |
| GWG | −0.04 (−0.20, 0.12) |
| Time | −0.81 (−1.56, −0.07) |
| Time2 | 0.03 (−0.02, 0.08) |
| Infant feeding modality group | |
| Non-lactating group | −1.27 (−3.66, 1.12) |
| Infant feeding modality group × time | |
| Non-lactating group | 2.10 (1.16, 3.03) |
| Infant feeding modality group × time2 | |
| Non-lactating group | −0.11 (−0.17, −0.06) |
| Prepregnancy Obesity group | |
| Age | 0.06 (−0.09, 0.21) |
| Income | −1.03 (−2.11, 0.05) |
| GWG | −0.07 (−0.13, −0.01) |
| Time | 0.43 (−0.11, 0.97) |
| Time2 | −0.02 (−0.06, 0.01) |
| Infant feeding modality | |
| Non-lactating group | −0.58 (−2.01, 0.86) |
| Infant feeding modality group × time | |
| Non-lactating group | 0.60 (−0.03, 1.23) |
| Infant feeding modality group × time2 | |
| Non-lactating group | −0.03 (−0.06, 0.01) |
| Characteristics | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| Coefficient (95% CI) | Coefficient (95% CI) | Coefficient (95% CI) | |
| Age | 0.01 (−0.08, 0.09) | 0.01 (−0.08, 0.09) | 0.01 (−0.08, 0.09) |
| Income | −0.64 (−1.25, −0.03) | −0.64 (−1.25, −0.03) | −0.64 (−1.25, −0.03) |
| GWG | −0.08 (−0.12, −0.03) | −0.08 (−0.13, −0.03) | −0.08 (−0.13, −0.02) |
| Infant feeding modality group | 0.38 (−0.50, 1.27) | 0.38 (−0.50, 1.27) | 0.38 (−0.51, 1.26) |
| Prepregnancy BMI group | 0.18 (−0.38, 0.71) | 0.17 (−0.38, 0.71) | 0.17 (−0.37, 0.72) |
| Dietary restraint | −0.05 (−0.18, 0.09) | −0.05 (−0.18, 0.09) | 0.10 (−0.05, 0.24) |
| Disinhibition | −0.06 (−0.20, 0.09) | −0.06 (−0.21, 0.09) | 0.18 (0.01, 0.33) |
| Susceptibility to hunger | 0.10 (−0.15, 0.34) | 0.09 (−0.15, 0.34) | −0.18 (−0.45, 0.08) |
| Time | −0.12 (−0.29, 0.06) | 0.06 (−0.24, 0.36) | 0.73 (0.37, 1.09) |
| Time2 | −0.01 (−0.02, 0.00) | −0.01 (−0.02, 0.00) | −0.07 (−0.09, −0.05) |
| Dietary restraint × time | −0.04 (−0.09, −0.00) | −0.14 (−0.19, −0.08) | |
| Disinhibition × time | −0.05 (−0.10, −0.00) | −0.20 (−0.26, −0.14) | |
| Susceptibility to hunger × time | 0.10 (0.02, 0.18) | 0.28 (0.18, 0.38) | |
| Dietary restraint × time2 | 0.01 (0.00, 0.01) | ||
| Disinhibition × time2 | 0.01 (0.01, 0.02) | ||
| Susceptibility to hunger × time2 | −0.02 (−0.02, −0.01) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smethers, A.D.; Trabulsi, J.C.; Stallings, V.A.; Papas, M.A.; Mennella, J.A. Factors Affecting BMI Changes in Mothers during the First Year Postpartum. Nutrients 2023, 15, 1364. https://doi.org/10.3390/nu15061364
Smethers AD, Trabulsi JC, Stallings VA, Papas MA, Mennella JA. Factors Affecting BMI Changes in Mothers during the First Year Postpartum. Nutrients. 2023; 15(6):1364. https://doi.org/10.3390/nu15061364
Chicago/Turabian StyleSmethers, Alissa D., Jillian C. Trabulsi, Virginia A. Stallings, Mia A. Papas, and Julie A. Mennella. 2023. "Factors Affecting BMI Changes in Mothers during the First Year Postpartum" Nutrients 15, no. 6: 1364. https://doi.org/10.3390/nu15061364
APA StyleSmethers, A. D., Trabulsi, J. C., Stallings, V. A., Papas, M. A., & Mennella, J. A. (2023). Factors Affecting BMI Changes in Mothers during the First Year Postpartum. Nutrients, 15(6), 1364. https://doi.org/10.3390/nu15061364







