Dietary Folate Deficiency Promotes Lactate Metabolic Disorders to Sensitize Lung Cancer Metastasis through MTOR-Signaling-Mediated Druggable Oncotargets
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Culture and Treatment
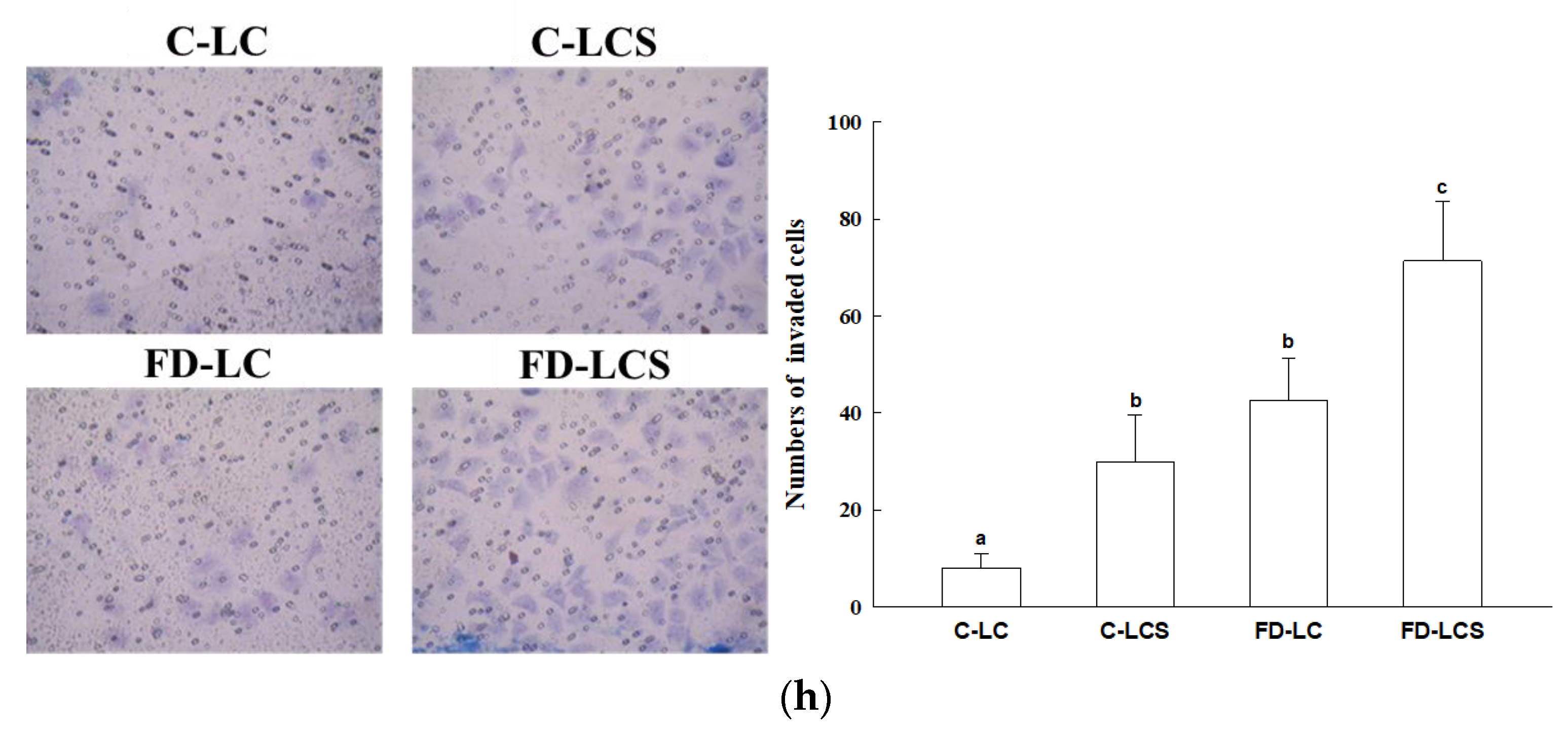
2.3. In Vitro Transwell Invasion Assay
2.4. Wound Healing Assay
2.5. Spheroid Formation Assay
2.6. Animal Study and Drug Treatment
2.7. Western Blotting
- normalization factor = total volume (Intensity) reference lane/total lane stain-free volume (Intensity) of each lane.
- normalized volume = normalization factor × volume (Intensity)
2.8. Biochemical Assay
2.9. Microbiological Assay for Folate Levels
2.10. Statistical Analysis
3. Results
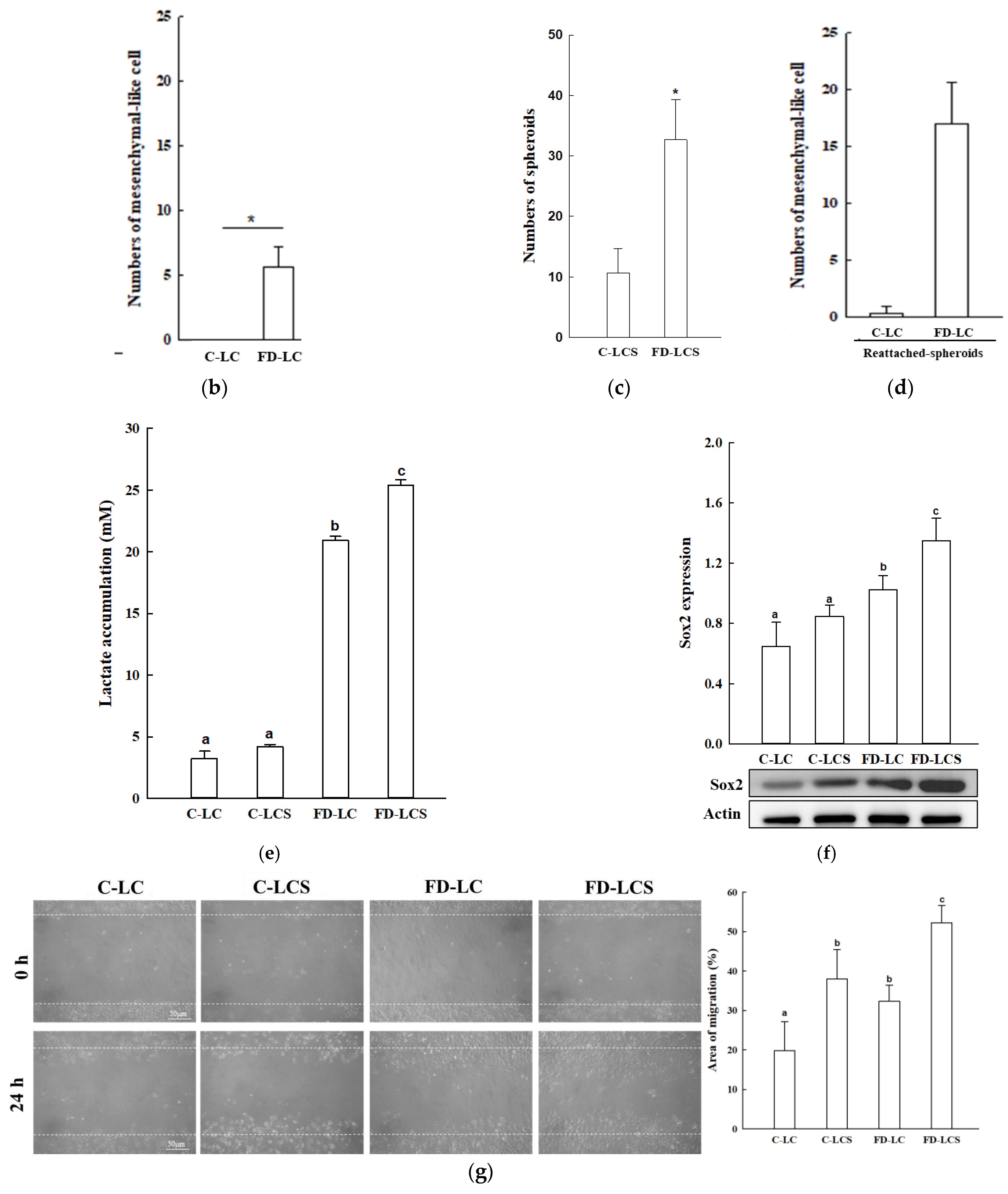
3.1. FD Effect on Lactate Production and Metastatic Potential of LC Growing at Tumor Malignancy Stages
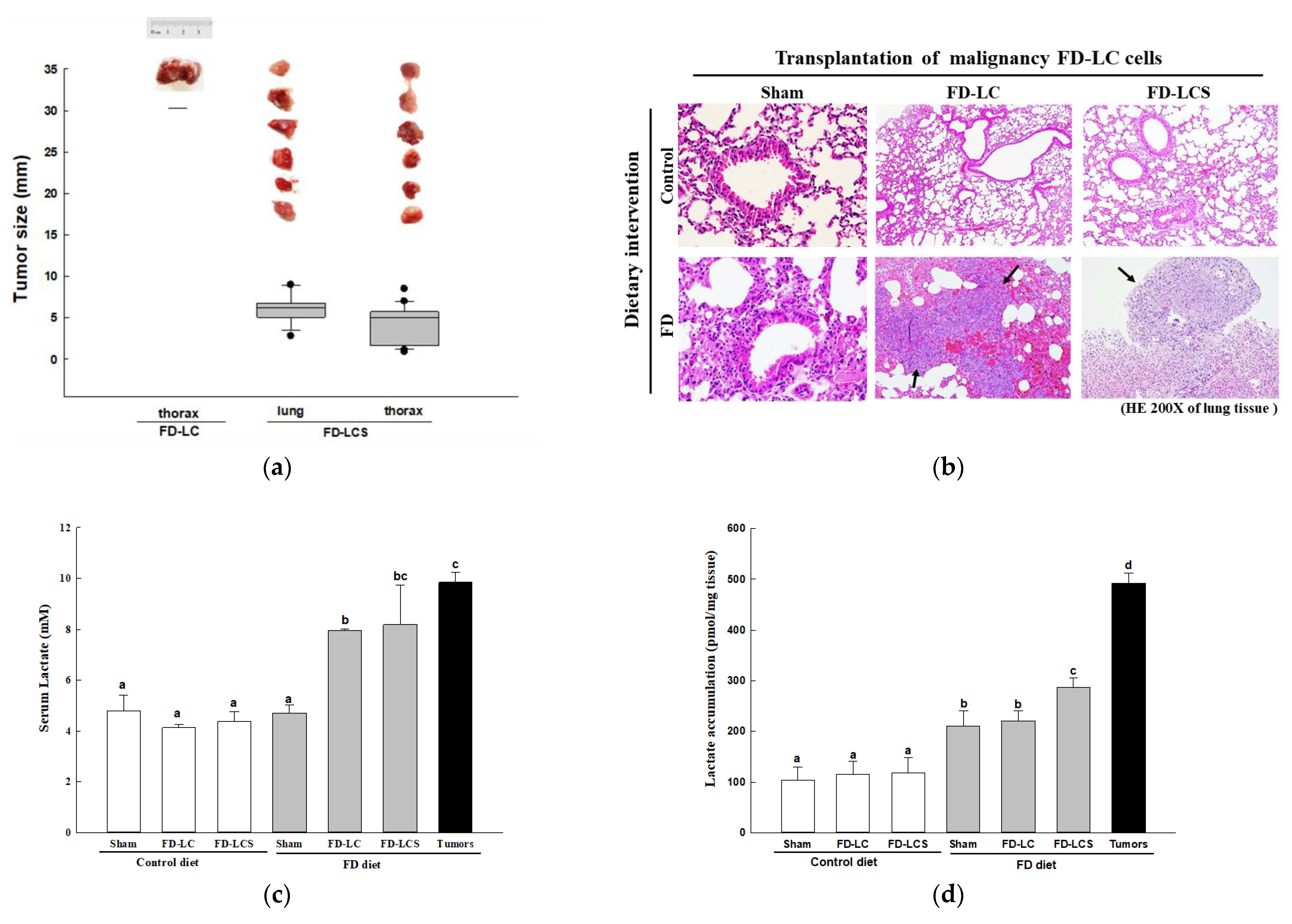
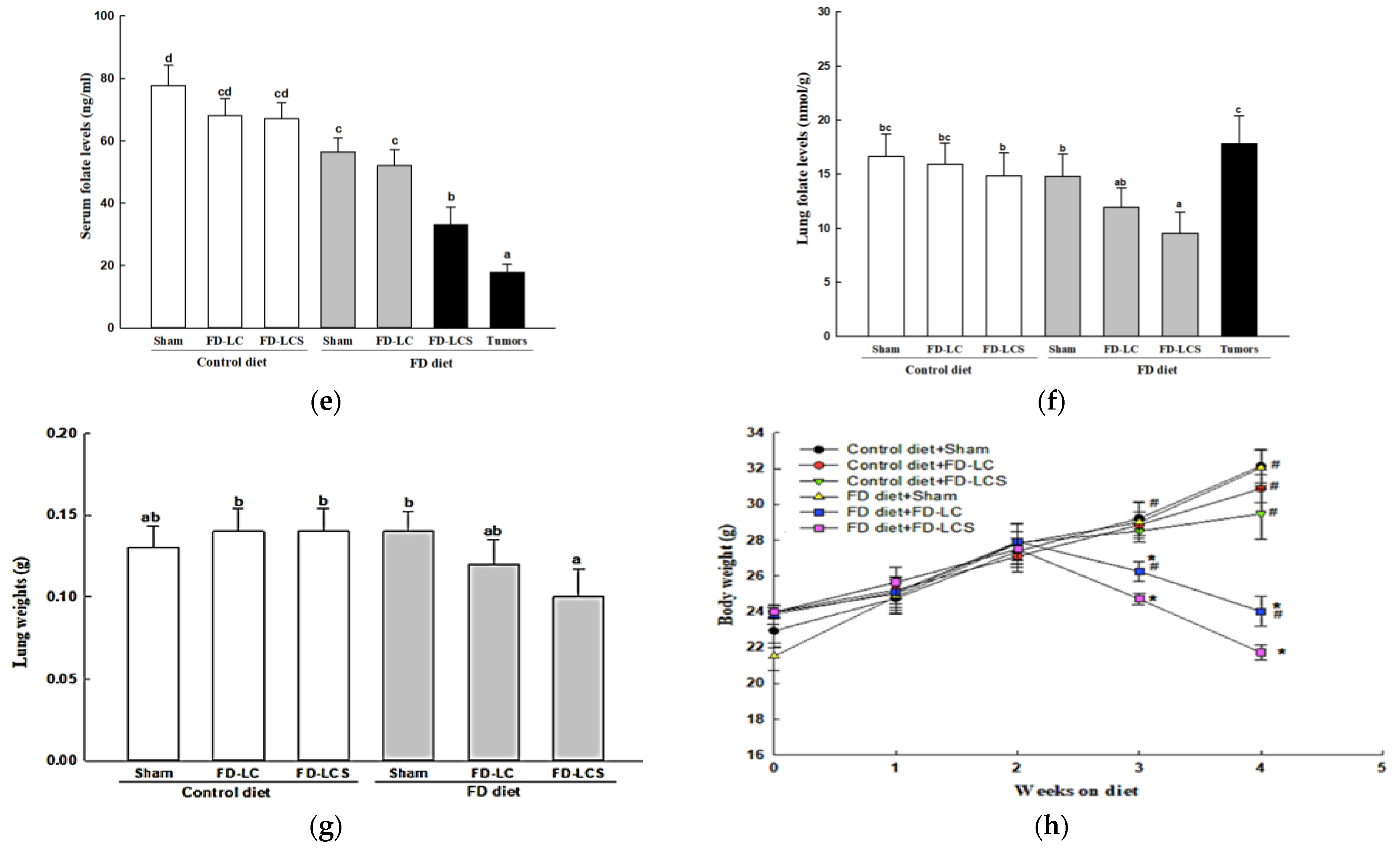
3.2. Effect of Dietary FD Intervention on Cancer Metastasis Efficiency and Lactate Metabolic Disorders in the LC-Implanted Mouse Model
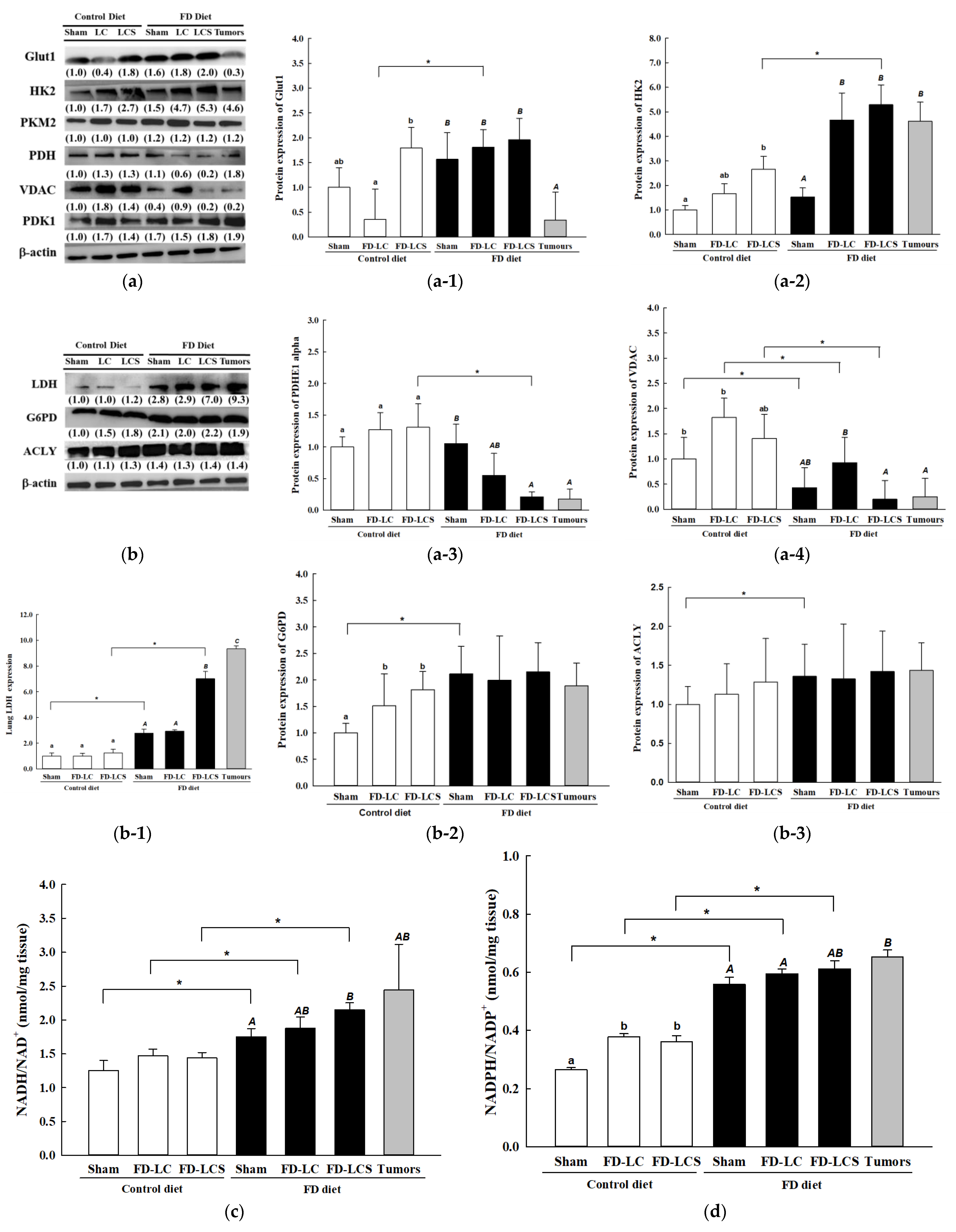
3.3. Dietary FD and LC Invasion Modified Lactate Metabolic Targets in Lung and/or Metastatic Tumors of the Experimental Mice
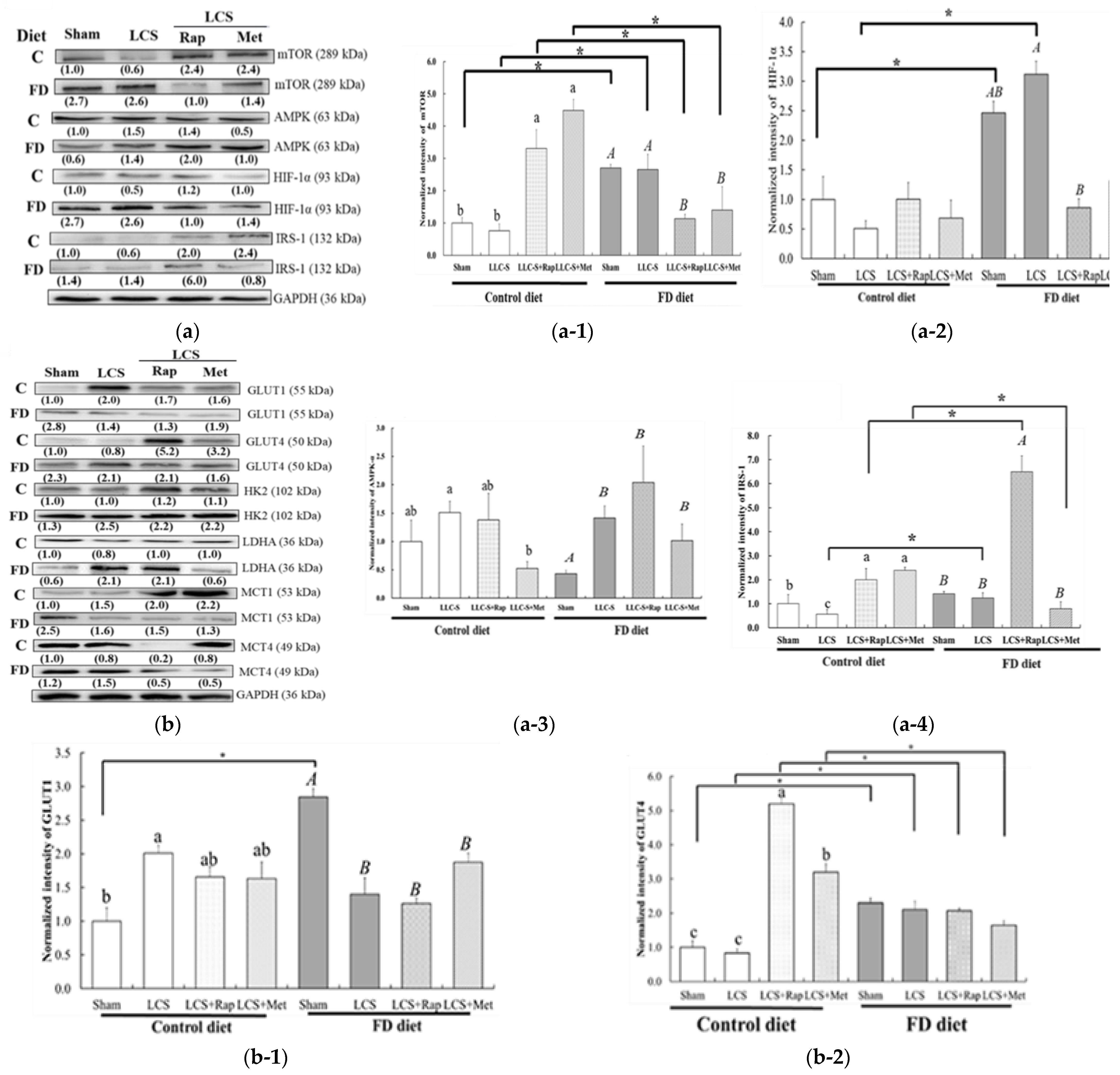
3.4. Dietary FD and LC Invasion Activated the Metabolic Stress–mTOR-Signaling Pathways
3.5. Efficiency of Anti-Neoplastic Drug Treatments Rapamycin (Rap) and Metformin (Met) on FD-LCS-Induced LC Metastasis
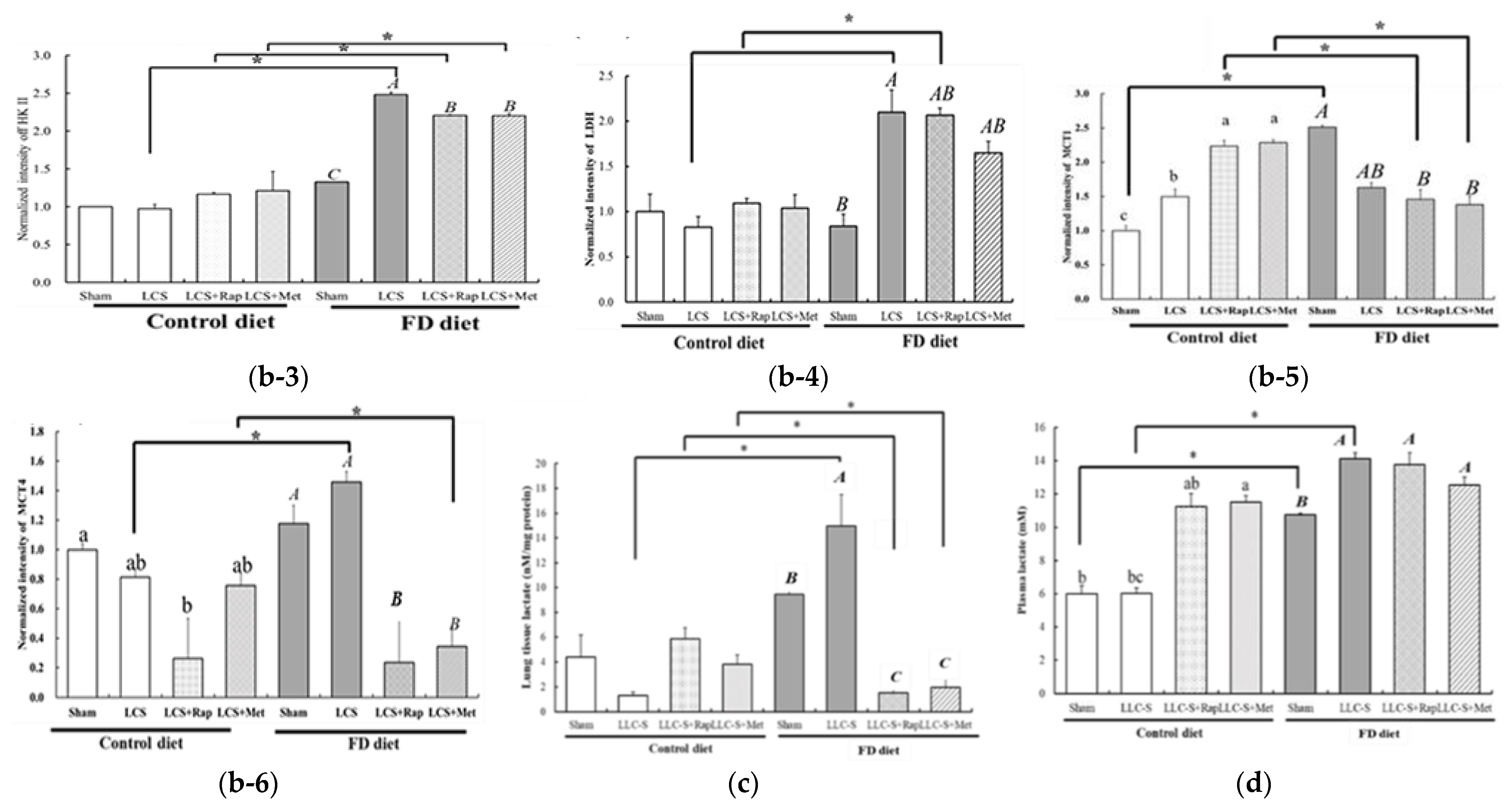
3.6. Druggable Protein Targets of Rap and Met Treatment in FD/LCS-Activated mTORC1 Signaling and Lactate Metabolic Disorder
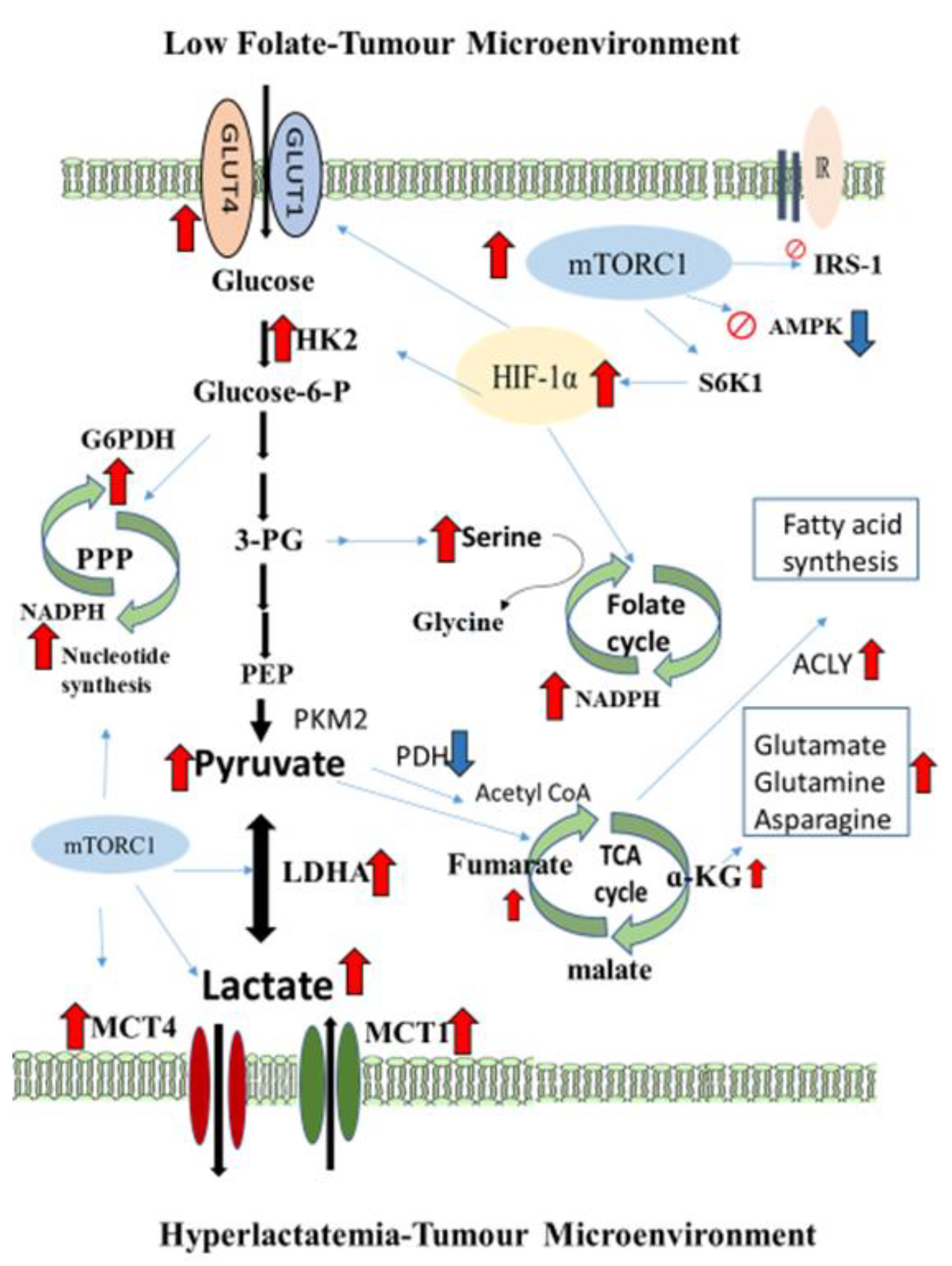
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Handbook of Vitamins. Chapter 11: Folate, 5th ed.; Janos, Z., John, W.S., Jesse, F.G., III, Patrick, J.S., Eds.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Boca Raton, FL, USA, 2014; pp. 421–446. [Google Scholar]
- Huang, R.F.S.; Hsu, Y.C.; Lin, H.L.; Yang, F.L. Folate depletion and elevated plasma homocysteine promote oxidative stress in rat livers. J. Nutr. 2001, 131, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.S.; Lin, C.Y.; Wu, M.Y.; Lu, C.L.; Huang, R.F.S. Relationship between folate status and tumour progression in patients with hepatocellular carcinoma. Br. J. Nutr. 2008, 100, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.S.; Huang, C.Y.; Tao, K.H.; Cheng, C.P.; Chen, C.H.; Lu, C.L.; Yang, F.L.; Syu Huang, R.-F. Interrelationships among genetic C677T polymorphism of 5,10-methylenetetrahydrofolate reductase, biochemical folate status and lymphocytic p53 oxidative damage in association with tumour malignancy and survivals of patients with hepatocellular carcinoma. Mol. Nutr. Food Res. 2014, 58, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.F.S.; Ho, Y.H.; Lin, H.L.; Wei, J.S.; Liu, T.Z. Folate deficiency induces a cell cycle-specific apoptosis in HepG2 cells. J. Nutr. 1999, 129, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Oleinik, N.V.; Helke, K.L.; Kistner-Griffin, E.; Krupenko, N.I.; Krupenko, S.A. Rho GTPases RhoA and Rac1 mediate effects of dietary folate on metastatic potential of A549 cancer cells through the control of cofilin phosphorylation. J. Biol. Chem. 2014, 289, 26383–26394. [Google Scholar] [CrossRef]
- Feng, H.C.; Lin, J.Y.; Hsu, S.H.; Lan, W.Y.; Kuo, C.S.; Tian, Y.F.; Sun, D.-P.; Syu Huang, R.-F. Low folate metabolic stress reprograms DNA methylation-activated sonic hedgehog signaling to mediate cancer stem cell-like signatures and invasive tumour stage-specific malignancy of human colorectal cancers. Int. J. Cancer 2017, 141, 2537–2550. [Google Scholar] [CrossRef]
- Chen, W.J.; Huang, R.F.S. Low-folate stress reprograms cancer stem cell-like potentials and bioenergetics metabolism through activation of mTOR signaling pathway to promote in vitro invasion and in vivo tumorigenicity of lung cancers. J. Nutr. Biochem. 2018, 53, 28–38. [Google Scholar] [CrossRef]
- Lee, T.-Y.; Chiang, E.-P.; Shih, Y.-T.; Lane, H.-Y.; Lin, J.-T.; Wu, C.-Y. Lower serum folate is associated with development and invasiveness of gastric cancer. World J. Gastroenterol. 2014, 20, 11313–11320. [Google Scholar] [CrossRef]
- Su, Y.H.; Huang, W.C.; Huang, T.H.; Huang, Y.J.; Sue, Y.K.; Huynh, T.T.; Hsiao, M.; Liu, T.-Z.; Wu, A.T.H.; Lin, C.M. Folate deficient tumor microenvironment promotes epithelial-to- mesenchymal transition and cancer stem-like phenotypes. Oncotarget 2016, 22, 33246–33256. [Google Scholar] [CrossRef]
- Wang, T.P.; Shu, C.H.; Fang, S.C.; Huang, R.F.S. Folate deprivation enhanced invasiveness of human colon cancer cells mediated by activation of sonic hedgehog signaling through its promoter hypomethylation and cross-action with transcription nuclear factor-kappa B pathway. Carcinogenesis 2012, 33, 1158–1168. [Google Scholar] [CrossRef]
- Chou, Y.F.; Yu, C.C.; Huang, R.F.S. Changes in mitochondrial (mt) DNA in folate-deficient tissues of young rats depend on mt folate, mt biogenesis, and oxidative DNA injuries. J. Nutr. 2007, 137, 2036–2042. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.M.; Yu, C.C.; Lu, H.T.; Chou, Y.F.; Huang, R.F.S. Folate deprivation promotes mitochondrial oxidative decay: DNA large deletions, cytochrome c oxidase dysfunction, membrane depolarization and superoxide overproduction in rat liver. Br. J. Nutr. 2007, 97, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.F.; Huang, R.F.S. Mitochondrial DNA deletions of blood lymphocytes as genetic markers of low folate-related mitochondrial genotoxicity in periphery tissues. Eur. J. Nutr. 2009, 48, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Hirschhaeuser, F.; Sattler, U.G.A.; Mueller-Klieser, W. Lactate: A metabolic key player in cancer. Cancer Res. 2011, 71, 6921–6925. [Google Scholar] [CrossRef] [PubMed]
- Danhier, P.; Bański, P.; Payen, V.L.; Grasso, D.; Ippolito, L.; Sonveaux, P.; Porporato, P.E. Cancer metabolism in space and time: Beyond the Warburg effect. Biochim. Biophys. Acta 2017, 1858, 556–572. [Google Scholar] [CrossRef]
- Wang, Y.P.; Li, J.T.; Qu, J.; Yin, M.; Lei, Q.Y. Metabolite sensing and signaling in cancer. J. Biol. Chem. 2020, 295, 11938–11946. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, S.; Yin, S.; Niu, W.; Xiong, W.; Tan, M.; Li, G.; Zhou, M. The reverse Warburg effect is likely to be an Achilles’ heel of cancer that can be exploited for cancer therapy. Oncotarget. 2017, 8, 57813–57825. [Google Scholar] [CrossRef]
- Vlachostergios, P.J.; Oikonomou, K.G.; Gibilaro, E.; Apergis, G. Elevated lactic acid is a negative prognostic factor in metastatic lung cancer. Cancer Biomarkers 2015, 15, 725–734. [Google Scholar] [CrossRef]
- Walenta, S.; Wetterling, M.; Lehrke, M.; Schwickert, G.; Sundfer, K.; Rofstad, E.K.; Mueller-Klieser, W. High lactate levels predict likelihood of metastases, tumour recurrence, and restricted patients survival in human cervical cancers. Cancer Res. 2000, 60, 916–921. [Google Scholar]
- Brizel, D.M.; Clough, R.W.; Dewhirst, M.W.; Schroeder, T.; Walenta, S.; Mueller-Klieser, W. Elevated tumour lactate concentrations predict for an increased risk of metastases in head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 349–353. [Google Scholar] [CrossRef]
- Cruz-López, K.G.d.; Castro-Muñoz, L.J.; Reyes-Hernández, D.O.; García-Carrancá, A.; Manzo-Merino, J. Lactate in the regulation of tumor microenvironment and therapeutic approaches. Front. Oncol. 2019, 9, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Outschoorn, U.E.; Peiris-Pagés, M.; Pestell, R.G.; Sotgia, F.; Michael, P.; Lisanti, M.P. Cancer metabolism: A therapeutic perspective. Nat. Rev. Clin. Oncol. 2017, 14, 11–31. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Faubert, B.; Li, K.Y.; Cai, L.; Hensley, C.T.; Kim, J.; Zacharias, L.G.; Yang, C.; Do, Q.N.; Doucette, S.; Burguete, D.; et al. Lactate metabolism in human lung tumors. Cell 2017, 171, 358–371. [Google Scholar] [CrossRef]
- Shen, H.; Wei, Q.; Pillow, P.C.; Amos, C.I.; Hong, W.K.; Spitz, M.R. Dietary folate intake and lung cancer risk in former smokers: A case-control analysis. Cancer Epidemiol Biomarkers Prev. 2003, 12, 980–986. [Google Scholar] [PubMed]
- E Voorrips, L.; A Goldbohm, R.; A Brants, H.; A Van Poppel, G.; Sturmans, F.; Hermus, R.J.; Brandt, P.A.V.D. A prospective cohort study on antioxidant and folate intake and male lung cancer risk. Cancer Epidemiol Biomarkers Prev. 2000, 9, 357–365. [Google Scholar]
- Zhang, Y.-F.; Zhou, L.; Zhang, H.-W.; Hou, A.-J.; Gao, H.-F.; Zhou, Y.-H. Association between folate intake and the risk of lung cancer: A dose-response meta-analysis of prospective studies. PLoS ONE 2014, 9, e93465. [Google Scholar] [CrossRef]
- Durda, K.; Kąklewski, K.; Gupta, S.; Szydłowski, M.; Baszuk, P.; Jaworska-Bieniek, K.; Sukiennicki, G.; Kaczmarek, K.; Waloszczyk, P.; Narod, S.; et al. Serum folate concentration and the incidence of lung cancer. PLoS ONE 2017, 12, e0177441. [Google Scholar] [CrossRef]
- Horne, D.W.; Patterson, D. Lactobacillus Casei microbiological assay of folic acid derivations in 96-well microtiter plates. Clin. Chem. 1988, 34, 2357–2359. [Google Scholar] [CrossRef]
- Patra, K.C.; Wang, Q.; Bhaskar, P.T.; Miller, L.; Wang, Z.; Wheaton, W.; Chandel, N.; Laakso, M.; Muller, W.J.; Allen, E.L.; et al. Hexokinase 2 is required for tumour initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell 2013, 24, 213–228. [Google Scholar] [CrossRef]
- Xie, H.; Hanai, J.I.; Ren, J.G.; Lev Kats, L.; Burgess, K.; Bhargava, P.; Signoretti, S.; Billiard, J.; Duffy, K.J.; Grant, A.; et al. Targeting lactate dehydrogenase-A inhibits tumorigenesis and tumour progression in mouse models of lung cancer and impacts tumour initiating cells. Cell Metab. 2014, 19, 795–809. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, S.L.; Hu, X.; Tam, K.Y. Targeting tumor metabolism for cancer treatment: Is pyruvate dehydrogenase kinases (PDKs) a viable anticancer target? Int. J. Biol. Sci. 2015, 11, 1390–1400. [Google Scholar] [CrossRef]
- Koukourakis, M.I.; Giatromanolaki, A.; Sivridis, E.; Gatter, K.C.; Harris, A.L.; Tumor and Angiogenesis Research Group. Pyruvate dehydrogenase and pyruvate dehydrogenase kinas expression in non-small cell lung cancer and tumour-assocciated stroma. Neoplasia 2005, 7, 1–6. [Google Scholar] [CrossRef]
- Hensley, C.T.; Faubert, B.; Yuan, Q.; Lev-Cohain, N.; Jin, E.; Kim, J.; Jiang, L.; Ko, B.; Skelton, R.; Loudat, L.; et al. Metabolic Heterogeneity in Human Lung Tumors. Cell 2016, 164, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Cornu, M.; Albert, V.; Hall, M.N. mTOR in aging, metabolism, and cancer. Curr. Opin. Genet. Dev. 2013, 23, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Denko, N.C. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat. Rev. Cancer Rev. 2008, 8, 705–713. [Google Scholar] [CrossRef]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Terentiev, A.A. Metabolic heterogeneity of cancer cells: An interplay between HIF-1, GLUTs, and AMPK. Cancers 2020, 12, 862–893. [Google Scholar] [CrossRef]
- Gremke, N.; Polo, P.; Dort, A.; Schneikert, J.; Elmshäuser, S.; Brehm, C.; Klingmüller, U.; Schmitt, A.; Reinhardt, H.C.; Timofeev, O.; et al. mTOR-mediated cancer drug resistance suppresses autophagy and generates a druggable metabolic vulnerability. Nat. Commun. 2020, 11, 4684. [Google Scholar] [CrossRef]
- Ben-Sahra, I.; Hoxhaj, G.; Ricoult, S.J.H.; Asara, J.M.; Manning, B.D. mTORC1 Induces purine synthesis through control of the mitochondrial tetrahydrofolate cycle. Science 2016, 351, 728–733. [Google Scholar] [CrossRef]
- Fan, J.; Ye, J.; Kamphorst, J.J.; Shlomi, T.; Thompson, C.B.; Rabinowitz, J.D. Quantitative flux analysis reveals folate-dependent NADPH production. Nature 2014, 510, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, C.; Longatto-Filho, A.; Azevedo-Silva, J.; Casal, M.; Schmitt, F.C.; Baltazar, F. Role of monocarboxylate transporters in human cancers. State of the art. J. Bioenerg. Biomembr. 2012, 44, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, A.M.; Lone, A.; Betts, D.H.; Cumming, R.C. Lactate preconditioning promotes a HIF-1alpha-mediated metabolic shift from OXPHOS to glycolysis in normal human diploid fibroblasts. Sci. Rep. 2020, 10, 8388–8404. [Google Scholar] [CrossRef] [PubMed]
- Izumi, H.; Takahashi, M.; Uramoto, H.; Nakayama, Y.; Oyama, T.; Wang, K.-Y.; Sasaguri, Y.; Nishizawa, S.; Kohno, K. Monocarboxylate transporters 1 and 4 are involved in the invasion activity of human lung cancer cells. Cancer Sci. 2011, 102, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Meijer, T.W.; Schuurbiers, O.C.; Kaanders, J.H.; Looijen-Salamon, M.G.; de Geus-Oei, L.-F.; Verhagen, A.F.; Lok, J.; van der Heijden, H.F.; Rademakers, S.E.; Span, P.N.; et al. Differences in metabolism between adeno- and squamous cell non-small cell lung carcinomas: Spatial distribution and prognostic value of GLUT1 and MCT4. Lung Cancer 2012, 76, 316–323. [Google Scholar] [CrossRef]
- Bovenzi, C.D.; Hamilton, J.; Tassone, P.; Johnson, J.; Cognetti, D.M.; Luginbuhl, A.; Keane, W.M.; Zhan, T.; Tuluc, M.; Bar-Ad, V.; et al. Prognostic indications of elevated MCT4 and CD147 across cancer types: A Meta-Analysis. Biomed Res. Int. 2015, 5, 242437. [Google Scholar] [CrossRef] [PubMed]
- Yousef, M.; Tsiani, E. Metformin in lung cancer: Review of in vitro and in vivo animal studies. Cancers 2017, 9, 45. [Google Scholar] [CrossRef]
- Yao, L.; Liu, M.; Huang, Y.; Wu, K.; Huang, X.; Zhao, Y.; He, W.; Zhang, R. Metformin use and lung cancer risk in diabetic patients: A systematic review and meta-analysis. Dis. Markers 2019, 2019, 6230162. [Google Scholar] [CrossRef]
- Salani, B.; Maffioli, S.; Hamoudane, M.; Parodi, A.; Ravera, S.; Passalacqua, M.; Alama, A.; Nhiri, M.; Cordera, R.; Maggi, D. Caveolin-1 is essential for metformin inhibitory effect on IGF1 action in non-small-cell lung cancer cells. FASEB J. 2012, 26, 788–798. [Google Scholar] [CrossRef]
- Ma, Y.; Guo, F.C.; Wang, W.; Shi, H.S.; Li, D.; Wang, Y.S. K-ras gene mutation as a predictor of cancer cell responsiveness to metformin. Mol. Med. Rep. 2013, 8, 763–765. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, Z.; Jiang, L.; Liu, M.; Ma, J.; Yang, C.; Han, L.; Nan, K.; Liang, X. Metformin inhibits growth of human non-small cell lung cancer cells via liver kinase B-1-independent activation of adenosine monophosphate-activated protein kinas. Mol. Med. Rep. 2016, 12, 2590–2596. [Google Scholar] [CrossRef] [PubMed]
- Groenendijk, F.H.; Mellema, W.W.; Van Der Burg, E.; Schut, E.; Hauptmann, M.; Horlings, H.M.; Willems, S.M.; Van Den Heuvel, M.M.; Jonkers, J.; Smit, E.F.; et al. Sorafenib synergizes with metformin in NSCLC through AMPK pathway activation. Int. J. Cancer 2015, 136, 1434–1444. [Google Scholar] [CrossRef] [PubMed]



| Control Diet | FD Diet | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sham | FD-LC | FD-LCS | C-LCS | Sham | FD-LC | FD-LCS | C-LCS | ||
| Colonization rate (%) | 0/4 (0) | 0/6 (0) | 0/8 (0) | 0/4 (0) | 0/6(0) | 1/8 (12.5) * | 8/8 (100) † | 0/5 (0) | |
| Tumor burden | Thorax | Lung | Thorax | ||||||
| Total multiplicity, n | 0 | 0 | 0 | 0 | 0 | 1 | 12 | 22 | 0 |
| weight (g) | 0 | 0 | 0 | 0 | 0 | 3.47 | 0.06 ± 0.04 | 0.27 ± 0.80 | 0 |
| size (mm) | 0 | 0 | 0 | 0 | 0 | 30.24 | 6.09 ± 1.68 | 4.00 ± 5.95 | 0 |
| size > 5 mm, n | 0 | 0 | 0 | 0 | 0 | 1 | 11 | 12 | 0 |
| Dietary Intervention | FD-LCS Transplantation | Metastatic Tumor Colonization, Rate (%) | Metastatic Tumor Site | |
|---|---|---|---|---|
| Lung (n/Total) | Thorax (n/Total) | |||
| Sham | 0/4 (0) | 0 | 0 | |
| Control | LCS | 0/6 (0) | 0 | 0 |
| LCS + Rap | 0/5 (0) | 0 | 0 | |
| LCS + Met | 0/3 (0) | 0 | 0 | |
| Sham | 0/6 (0) | 0 | 0 | |
| FD | LCS | 6/6 (100) | 12/27 | 15/27 |
| LCS + Rap | 0/6 (0) | 0 | 0 | |
| LCS + Met | 0/6 (0) | 0 | 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.-J.; Huang, S.-Y.; Chen, Y.-W.; Liu, Y.-F.; Huang, R.-F.S. Dietary Folate Deficiency Promotes Lactate Metabolic Disorders to Sensitize Lung Cancer Metastasis through MTOR-Signaling-Mediated Druggable Oncotargets. Nutrients 2023, 15, 1514. https://doi.org/10.3390/nu15061514
Chen W-J, Huang S-Y, Chen Y-W, Liu Y-F, Huang R-FS. Dietary Folate Deficiency Promotes Lactate Metabolic Disorders to Sensitize Lung Cancer Metastasis through MTOR-Signaling-Mediated Druggable Oncotargets. Nutrients. 2023; 15(6):1514. https://doi.org/10.3390/nu15061514
Chicago/Turabian StyleChen, Wan-Jing, Su-Yu Huang, Yi-Wen Chen, Yi-Fang Liu, and Rwei-Fen S. Huang. 2023. "Dietary Folate Deficiency Promotes Lactate Metabolic Disorders to Sensitize Lung Cancer Metastasis through MTOR-Signaling-Mediated Druggable Oncotargets" Nutrients 15, no. 6: 1514. https://doi.org/10.3390/nu15061514
APA StyleChen, W.-J., Huang, S.-Y., Chen, Y.-W., Liu, Y.-F., & Huang, R.-F. S. (2023). Dietary Folate Deficiency Promotes Lactate Metabolic Disorders to Sensitize Lung Cancer Metastasis through MTOR-Signaling-Mediated Druggable Oncotargets. Nutrients, 15(6), 1514. https://doi.org/10.3390/nu15061514






