Interactions between Intestinal Homeostasis and NAD+ Biology in Regulating Incretin Production and Postprandial Glucose Metabolism
Abstract
1. Introduction
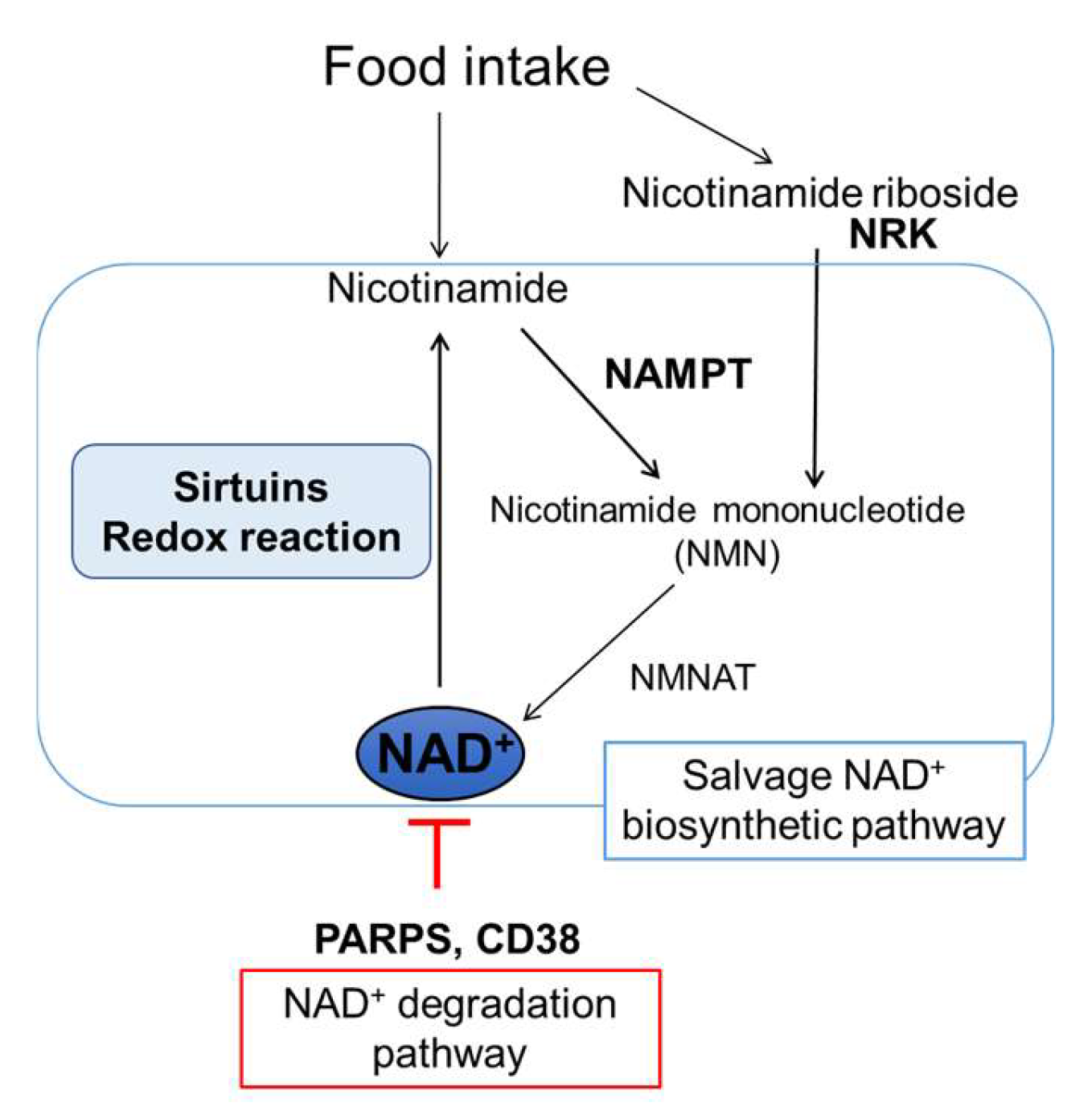
2. Impact of NAD+ Biology on Systemic Glucose Metabolism in an Organ-Specific Manner
2.1. Changes in NAD+ Biology with Aging and Obesity in Metabolic Organs
2.2. Impacts of Decrease in NAD+ Levels with Aging and Obesity on Metabolic Organ Function
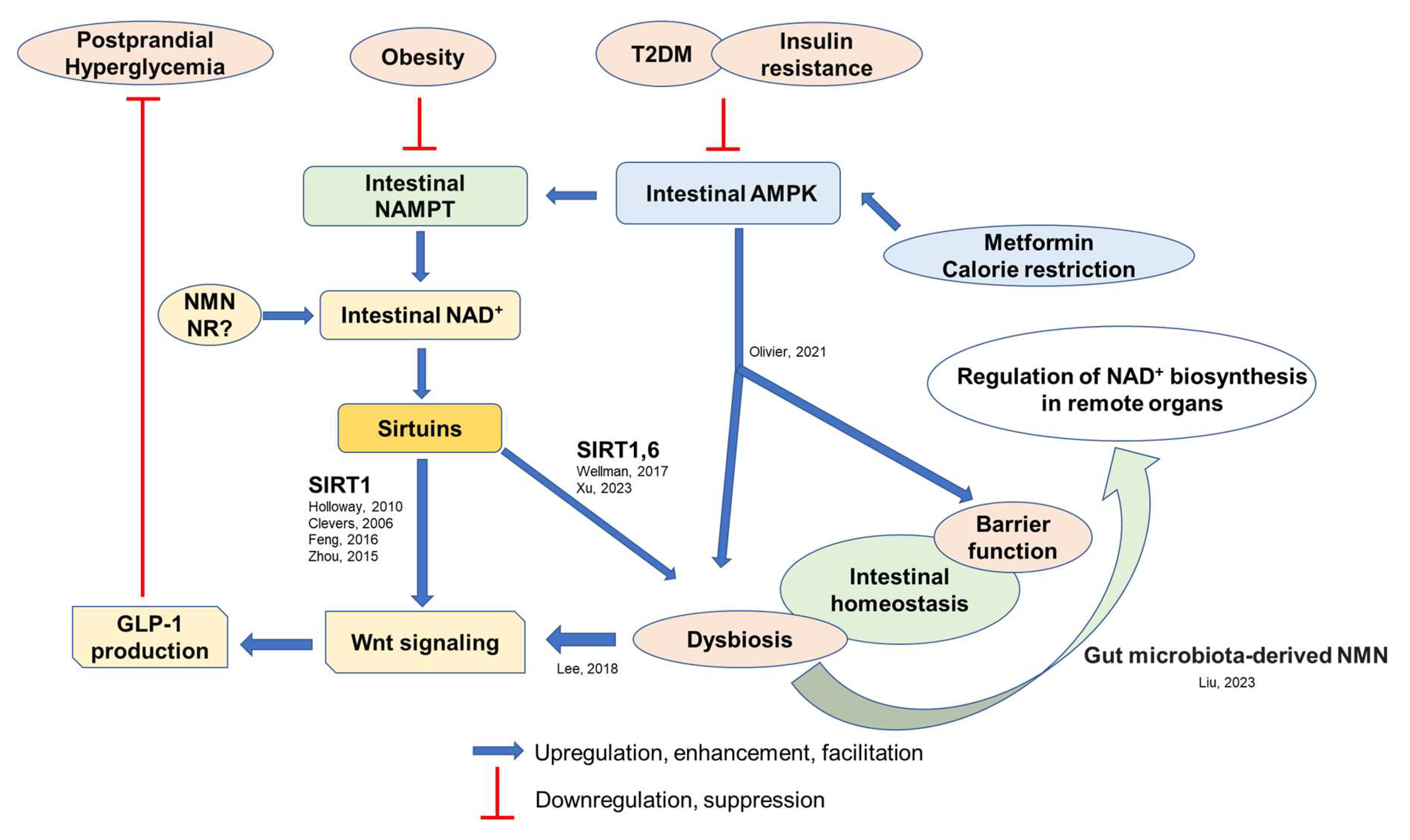
3. Regulators of Intestinal Homeostasis
3.1. Intestinal AMPK and NAD+ Biosynthesis
3.2. Intestinal Wnt Signaling
4. Pathophysiological Roles of Intestinal NAD+ Biosynthesis
5. Potential Effects of Dietary Habits and Its Associated Gut Environment on Intestinal Homeostasis and GLP-1 Secretion
6. Therapeutic Potential of NAD+ Intermediates as GLP-1 Stimulants
7. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tominaga, M.; Eguchi, H.; Manaka, H.; Igarashi, K.; Kato, T.; Sekikawa, A. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. The Funagata Diabetes Study. Diabetes Care 1999, 22, 920–924. [Google Scholar] [CrossRef]
- Abbott, R.D.; Donahue, R.P.; MacMahon, S.W.; Reed, D.M.; Yano, K. Diabetes and the risk of stroke. The Honolulu Heart Program. JAMA 1987, 257, 949–952. [Google Scholar] [CrossRef] [PubMed]
- DECODE Study Group, the European Diabetes Epidemiology Group. Glucose tolerance and cardiovascular mortality: Comparison of fasting and 2-h diagnostic criteria. Arch. Intern. Med. 2001, 161, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Hanefeld, M.; Fischer, S.; Julius, U.; Schulze, J.; Schwanebeck, U.; Schmechel, H.; Ziegelasch, H.J.; Lindner, J. Risk factors for myocardial infarction and death in newly detected NIDDM: The Diabetes Intervention Study, 11-year follow-up. Diabetologia 1996, 39, 1577–1583. [Google Scholar] [CrossRef] [PubMed]
- Nakagami, T.; DECODA Study Group. Hyperglycaemia and mortality from all causes and from cardiovascular disease in five populations of Asian origin. Diabetologia 2004, 47, 385–394. [Google Scholar] [CrossRef]
- Fujishima, M.; Kiyohara, Y.; Kato, I.; Ohmura, T.; Iwamoto, H.; Nakayama, K.; Ohmori, S.; Yoshitake, T. Diabetes and cardiovascular disease in a prospective population survey in Japan: The Hisayama Study. Diabetes 1996, 45 (Suppl. 3), S14–S16. [Google Scholar] [CrossRef]
- Oizumi, T.; Daimon, M.; Jimbu, Y.; Wada, K.; Kameda, W.; Susa, S.; Yamaguchi, H.; Ohnuma, H.; Tominaga, M.; Kato, T. Impaired glucose tolerance is a risk factor for stroke in a Japanese sample—The Funagata study. Metabolism 2008, 57, 333–338. [Google Scholar] [CrossRef]
- Blake, D.R.; Meigs, J.B.; Muller, D.C.; Najjar, S.S.; Andres, R.; Nathan, D.M. Impaired glucose tolerance, but not impaired fasting glucose, is associated with increased levels of coronary heart disease risk factors: Results from the Baltimore Longitudinal Study on Aging. Diabetes 2004, 53, 2095–2100. [Google Scholar] [CrossRef]
- Chiasson, J.L.; Josse, R.G.; Gomis, R.; Hanefeld, M.; Karasik, A.; Laakso, M.; STOP-NIDDM Trail Research Group. Acarbose for prevention of type 2 diabetes mellitus: The STOP-NIDDM randomised trial. Lancet 2002, 359, 2072–2077. [Google Scholar] [CrossRef]
- Chiasson, J.L.; Josse, R.G.; Gomis, R.; Hanefeld, M.; Karasik, A.; Laakso, M.; STOP-NIDDM Trial Research Group. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: The STOP-NIDDM trial. JAMA 2003, 290, 486–494. [Google Scholar] [CrossRef]
- Fujisawa, T.; Ikegami, H.; Inoue, K.; Kawabata, Y.; Ogihara, T. Effect of two α-glucosidase inhibitors, voglibose and acarbose, on postprandial hyperglycemia correlates with subjective abdominal symptoms. Metabolism 2005, 54, 387–390. [Google Scholar] [CrossRef]
- Wei, Y.; Lin, F.J.; Lin, S.Y.; Wang, C.C. Risk of hypoglycemia and concomitant use of repaglinide and clopidogrel: A population-based nested case-control Study. Clin. Pharmacol. Ther. 2019, 106, 1346–1352. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.J.; Nauck, M.A. Incretins and the development of type 2 diabetes. Curr. Diab. Rep. 2006, 6, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Todd, J.F.; Bloom, S.R. Incretins and other peptides in the treatment of diabetes. Diabet. Med. 2007, 24, 223–232. [Google Scholar] [CrossRef]
- Carr, R.D.; Larsen, M.O.; Winzell, M.S.; Jelic, K.; Lindgren, O.; Deacon, C.F.; Ahrén, B. Incretin and islet hormonal responses to fat and protein ingestion in healthy men. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E779–E784. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.M.; Morgan, L.M.; Tredger, J.A.; Deacon, S.; Wright, J.; Marks, V. Glucagon-like peptide-1 (7-36)amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: Acute post-prandial and 24-h secretion patterns. J. Endocrinol. 1993, 138, 159–166. [Google Scholar] [CrossRef]
- Lindgren, O.; Carr, R.D.; Holst, J.J.; Deacon, C.F.; Ahrén, B. Dissociated incretin hormone response to protein versus fat ingestion in obese subjects. Diabetes Obes. Metab. 2011, 13, 863–865. [Google Scholar] [CrossRef]
- Kuhre, R.E.; Holst, J.J.; Kappe, C. The regulation of function, growth and survival of GLP-1-producing L-cells. Clin. Sci. 2016, 130, 79–91. [Google Scholar] [CrossRef]
- Phillips, R. Incretin pathway regulates β-cell survival. Nat. Rev. Endocrinol. 2016, 12, 64. [Google Scholar] [CrossRef]
- Hira, T.; Pinyo, J.; Hara, H. What is GLP-1 really doing in obesity? Trends Endocrinol. Metab. 2020, 31, 71–80. [Google Scholar] [CrossRef]
- Færch, K.; Torekov, S.S.; Vistisen, D.; Johansen, N.B.; Witte, D.R.; Jonsson, A.; Pedersen, O.; Hansen, T.; Lauritzen, T.; Sandbæk, A.; et al. GLP-1 Response to oral glucose is reduced in prediabetes, screen-detected type 2 diabetes, and obesity and influenced by sex: The ADDITION-PRO study. Diabetes 2015, 64, 2513–2525. [Google Scholar] [CrossRef] [PubMed]
- Matikainen, N.; Bogl, L.H.; Hakkarainen, A.; Lundbom, J.; Lundbom, N.; Kaprio, J.; Rissanen, A.; Holst, J.J.; Pietiläinen, K.H. GLP-1 responses are heritable and blunted in acquired obesity with high liver fat and insulin resistance. Diabetes Care 2014, 37, 242–251. [Google Scholar] [CrossRef]
- Pham, H.; Marathe, C.S.; Phillips, L.K.; Trahair, L.G.; Hatzinikolas, S.; Huynh, L.; Wu, T.; Nauck, M.A.; Rayner, C.K.; Horowitz, M.; et al. Longitudinal changes in fasting and glucose-stimulated GLP-1 and GIP in healthy older subjects. J. Clin. Endocrinol. Metab. 2019, 104, 6201–6206. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef] [PubMed]
- Chimerel, C.; Emery, E.; Summers, D.K.; Keyser, U.; Gribble, F.M.; Reimann, F. Bacterial metabolite indole modulates incretin secretion from intestinal enteroendocrine L cells. Cell Rep. 2014, 9, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, J.; Igarashi, M.; Watanabe, K.; Karaki, S.I.; Mukouyama, H.; Kishino, S.; Li, X.; Ichimura, A.; Irie, J.; Sugimoto, Y.; et al. Gut microbiota confers host resistance to obesity by metabolizing dietary polyunsaturated fatty acids. Nat. Commun. 2019, 10, 4007. [Google Scholar] [CrossRef]
- Miyamoto, J.; Watanabe, K.; Taira, S.; Kasubuchi, M.; Li, X.; Irie, J.; Itoh, H.; Kimura, I. Barley β-glucan improves metabolic condition via short-chain fatty acids produced by gut microbial fermentation in high fat diet fed mice. PLoS ONE 2018, 13, e0196579. [Google Scholar] [CrossRef]
- Yoon, H.S.; Cho, C.H.; Yun, M.S.; Jang, S.J.; You, H.J.; Kim, J.H.; Han, D.; Cha, K.H.; Moon, S.H.; Lee, K.; et al. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat. Microbiol. 2021, 6, 563–573. [Google Scholar] [CrossRef]
- Wang, B.; Kong, Q.; Li, X.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. A high-fat diet increases gut microbiota biodiversity and energy expenditure due to nutrient difference. Nutrients 2020, 12, 3197. [Google Scholar] [CrossRef]
- Zheng, J.; Xiao, K.L.; Chen, L.; Wu, C.; Hu, X.; Zeng, T.; Chen, X.Q.; Li, W.J.; Deng, X.; Li, H.; et al. Insulin sensitizers improve the GLP-1 secretion and the amount of intestinal L cells on high-fat-diet-induced catch-up growth. Nutrition 2017, 39–40, 82–91. [Google Scholar] [CrossRef]
- Nagahisa, T.; Yamaguchi, S.; Kosugi, S.; Homma, K.; Miyashita, K.; Irie, J.; Yoshino, J.; Itoh, H. Intestinal epithelial NAD+ biosynthesis regulates GLP-1 production and postprandial glucose metabolism in mice. Endocrinology 2022, 163, bqac023. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, J.; Imai, S. Accurate measurement of nicotinamide adenine dinucleotide (NAD+) with high-performance liquid chromatography. Methods Mol. Biol. 2013, 1077, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Imai, S.; Yoshino, J. The importance of NAMPT/NAD/SIRT1 in the systemic regulation of metabolism and ageing. Diabetes Obes. Metab. 2013, 15 (Suppl. 3), 26–33. [Google Scholar] [CrossRef] [PubMed]
- Imai, S.; Guarente, L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014, 24, 464–471. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Yoshino, J. Adipose tissue NAD+ biology in obesity and insulin resistance: From mechanism to therapy. Bioessays 2017, 39, 1600227. [Google Scholar] [CrossRef]
- Kulkarni, C.A.; Brookes, P.S. Cellular compartmentation and the redox/nonredox functions of NAD+. Antioxid. Redox Signal. 2019, 31, 623–642. [Google Scholar] [CrossRef]
- Nakagawa, T.; Guarente, L. SnapShot: Sirtuins, NAD, and aging. Cell Metabol. 2014, 20, 192. [Google Scholar] [CrossRef]
- Ramsey, K.M.; Yoshino, J.; Brace, C.S.; Abrassart, D.; Kobayashi, Y.; Marcheva, B.; Hong, H.K.; Chong, J.L.; Buhr, E.D.; Lee, C.; et al. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science 2009, 324, 651–654. [Google Scholar] [CrossRef]
- Porter, L.C.; Franczyk, M.P.; Pietka, T.; Yamaguchi, S.; Lin, J.B.; Sasaki, Y.; Verdin, E.; Apte, R.S.; Yoshino, J. NAD+-dependent deacetylase SIRT3 in adipocytes is dispensable for maintaining normal adipose tissue mitochondrial function and whole body metabolism. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E520–E530. [Google Scholar] [CrossRef]
- Gomes, A.P.; Price, N.L.; Ling, A.J.; Moslehi, J.J.; Montgomery, M.K.; Rajman, L.; White, J.P.; Teodoro, J.S.; Wrann, C.D.; Hubbard, B.P.; et al. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 2013, 155, 1624–1638. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Pereira, J.; Tarrago, M.G.; Chini, C.C.S.; Nin, V.; Escande, C.; Warner, G.M.; Puranik, A.S.; Schoon, R.A.; Reid, J.M.; Galina, A.; et al. CD38 dictates age-related NAD decline and mitochondrial dysfunction through an SIRT3-dependent mechanism. Cell Metab. 2016, 23, 1127–1139. [Google Scholar] [CrossRef]
- Yoshino, J.; Mills, K.F.; Yoon, M.J.; Imai, S. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011, 14, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Frederick, D.W.; Loro, E.; Liu, L.; Davila, A., Jr.; Chellappa, K.; Silverman, I.M.; Quinn, W.J., 3rd; Gosai, S.J.; Tichy, E.D.; Davis, J.G.; et al. Loss of NAD homeostasis leads to progressive and reversible degeneration of skeletal muscle. Cell Metab. 2016, 24, 269–282. [Google Scholar] [CrossRef]
- Mouchiroud, L.; Houtkooper, R.H.; Moullan, N.; Katsyuba, E.; Ryu, D.; Canto, C.; Mottis, A.; Jo, Y.S.; Viswanathan, M.; Schoonjans, K.; et al. The NAD(+)/sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell 2013, 154, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.C.; Yang, X.; Hua, X.; Liu, J.; Fan, M.B.; Li, G.Q.; Song, J.; Xu, T.Y.; Li, Z.Y.; Guan, Y.F.; et al. Hepatic NAD(+) deficiency as a therapeutic target for non-alcoholic fatty liver disease in ageing. Br. J. Pharmacol. 2016, 173, 2352–2368. [Google Scholar] [CrossRef] [PubMed]
- Braidy, N.; Guillemin, G.J.; Mansour, H.; Chan-Ling, T.; Poljak, A.; Grant, R. Age related changes in NAD+ metabolism oxidative stress and Sirt1 activity in wistar rats. PLoS ONE 2011, 6, e19194. [Google Scholar] [CrossRef]
- Wei, X.; Jia, R.; Wang, G.; Hong, S.; Song, L.; Sun, B.; Chen, K.; Wang, N.; Wang, Q.; Luo, X.; et al. Depot-specific regulation of NAD+/SIRTs metabolism identified in adipose tissue of mice in response to high-fat diet feeding or calorie restriction. J. Nutr. Biochem. 2020, 80, 108377. [Google Scholar] [CrossRef]
- Stein, L.R.; Imai, S. Specific ablation of Nampt in adult neural stem cells recapitulates their functional defects during aging. EMBO J. 2014, 33, 1321–1340. [Google Scholar] [CrossRef]
- Massudi, H.; Grant, R.; Braidy, N.; Guest, J.; Farnsworth, B.; Guillemin, G.J. Age-associated changes in oxidative stress and NAD+ metabolism in human tissue. PLoS ONE 2012, 7, e42357. [Google Scholar] [CrossRef]
- Seyssel, K.; Alligier, M.; Meugnier, E.; Chanseaume, E.; Loizon, E.; Canto, C.; Disse, E.; Lambert-Porcheron, S.; Brozek, J.; Blond, E.; et al. Regulation of energy metabolism and mitochondrial function in skeletal muscle during lipid overfeeding in healthy men. J. Clin. Endocrinol. Metab. 2014, 99, E1254–E1262. [Google Scholar] [CrossRef]
- Zhu, X.H.; Lu, M.; Lee, B.Y.; Ugurbil, K.; Chen, W. In vivo NAD assay reveals the intracellular NAD contents and redox state in healthy human brain and their age dependences. Proc. Natl. Acad. Sci. USA 2015, 112, 2876–2881. [Google Scholar] [CrossRef]
- Bagga, P.; Hariharan, H.; Wilson, N.E.; Beer, J.C.; Shinohara, R.T.; Elliott, M.A.; Baur, J.A.; Marincola, F.M.; Witschey, W.R.; Haris, M.; et al. Single-Voxel 1 H MR spectroscopy of cerebral nicotinamide adenine dinucleotide (NAD+) in humans at 7T using a 32-channel volume coil. Magn. Reson. Med. 2020, 83, 806–814. [Google Scholar] [CrossRef]
- Clement, J.; Wong, M.; Poljak, A.; Sachdev, P.; Braidy, N. The plasma NAD+ metabolome is dysregulated in “normal” aging. Rejuvenation Res. 2019, 22, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Seyedsadjadi, N.; Berg, J.; Bilgin, A.A.; Braidy, N.; Salonikas, C.; Grant, R. High protein intake is associated with low plasma NAD+ levels in a healthy human cohort. PLoS ONE 2018, 13, e0201968. [Google Scholar] [CrossRef]
- Minhas, P.S.; Liu, L.; Moon, P.K.; Joshi, A.U.; Dove, C.; Mhatre, S.; Contrepois, K.; Wang, Q.; Lee, B.A.; Coronado, M.; et al. Macrophage de novo NAD+ synthesis specifies immune function in aging and inflammation. Nat. Immunol. 2019, 20, 50–63. [Google Scholar] [CrossRef]
- Wang, G.; Han, T.; Nijhawan, D.; Theodoropoulos, P.; Naidoo, J.; Yadavalli, S.; Mirzaei, H.; Pieper, A.A.; Ready, J.M.; McKnight, S.L. P7C3 neuroprotective chemicals function by activating the rate-limiting enzyme in NAD salvage. Cell 2014, 158, 1324–1334. [Google Scholar] [CrossRef] [PubMed]
- Bai, P.; Cantó, C.; Oudart, H.; Brunyánszki, A.; Cen, Y.; Thomas, C.; Yamamoto, H.; Huber, A.; Kiss, B.; Houtkooper, R.H.; et al. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 2011, 13, 461–468. [Google Scholar] [CrossRef]
- Jokinen, R.; Pirnes-Karhu, S.; Pietilainen, K.H.; Pirinen, E. Adipose tissue NAD+-homeostasis, sirtuins and poly(ADP-ribose) polymerases-important players in mitochondrial metabolism and metabolic health. Redox Biol. 2017, 12, 246–263. [Google Scholar] [CrossRef]
- Amici, S.A.; Young, N.A.; Narvaez-Miranda, J.; Jablonski, K.A.; Arcos, J.; Rosas, L.; Papenfuss, T.L.; Torrelles, J.B.; Jarjour, W.N.; Guerau-de-Arellano, M. CD38 is robustly induced in human macrophages and monocytes in inflammatory conditions. Front. Immunol. 2018, 9, 1593. [Google Scholar] [CrossRef] [PubMed]
- Matalonga, J.; Glaria, E.; Bresque, M.; Escande, C.; Carbó, J.M.; Kiefer, K.; Vicente, R.; León, T.E.; Beceiro, S.; Pascual-García, M.; et al. The nuclear receptor LXR limits bacterial infection of host macrophages through a mechanism that impacts cellular NAD metabolism. Cell Rep. 2017, 18, 1241–1255. [Google Scholar] [CrossRef]
- Barbosa, M.T.; Soares, S.M.; Novak, C.M.; Sinclair, D.; Levine, J.A.; Aksoy, P.; Chini, E.N. The enzyme CD38 (a NAD glycohydrolase, EC 3.2.2.5) is necessary for the development of diet-induced obesity. FASEB J. 2007, 21, 3629–3639. [Google Scholar] [CrossRef]
- McReynolds, M.R.; Chellappa, K.; Baur, J.A. Age-related NAD+ decline. Exp. Gerontol. 2020, 134, 110888. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Y.; Wang, F.; Zhang, X.Y.; Huang, P.; Lu, Y.B.; Wei, E.Q.; Zhang, W.P. Nicotinamide phosphoribosyltransferase may be involved in age-related brain diseases. PLoS ONE 2012, 7, e44933. [Google Scholar] [CrossRef] [PubMed]
- Jadeja, R.N.; Powell, F.L.; Jones, M.A.; Fuller, J.; Joseph, E.; Thounaojam, M.C.; Bartoli, M.; Martin, P.M. Loss of NAMPT in aging retinal pigment epithelium reduces NAD+ availability and promotes cellular senescence. Aging 2018, 10, 1306–1323. [Google Scholar] [CrossRef]
- de Guia, R.M.; Agerholm, M.; Nielsen, T.S.; Consitt, L.A.; Søgaard, D.; Helge, J.W.; Larsen, S.; Brandauer, J.; Houmard, J.A.; Treebak, J.T. Aerobic and resistance exercise training reverses age-dependent decline in NAD+ salvage capacity in human skeletal muscle. Physiol. Rep. 2019, 7, e14139. [Google Scholar] [CrossRef]
- Stein, L.R.; Wozniak, D.F.; Dearborn, J.T.; Kubota, S.; Apte, R.S.; Izumi, Y.; Zorumski, C.F.; Imai, S. Expression of Nampt in hippocampal and cortical excitatory neurons is critical for cognitive function. J. Neurosci. 2014, 34, 5800–5815. [Google Scholar] [CrossRef] [PubMed]
- Benzi, A.; Sturla, L.; Heine, M.; Fischer, A.W.; Spinelli, S.; Magnone, M.; Sociali, G.; Parodi, A.; Fenoglio, D.; Emionite, L.; et al. CD38 downregulation modulates NAD+ and NADP(H) levels in thermogenic adipose tissues. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158819. [Google Scholar] [CrossRef]
- Revollo, J.R.; Korner, A.; Mills, K.F.; Satoh, A.; Wang, T.; Garten, A.; Dasgupta, B.; Sasaki, Y.; Wolberger, C.; Townsend, R.R.; et al. Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab. 2007, 6, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.; Oka, S.I.; Imai, N.; Huang, C.Y.; Ralda, G.; Zhai, P.; Ikeda, Y.; Ikeda, S.; Sadoshima, J. Both gain and loss of Nampt function promote pressure overload-induced heart failure. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H711–H725. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Q.; Bao, R.; Zhang, N.; Wang, Y.; Polo-Parada, L.; Tarim, A.; Alemifar, A.; Han, X.; Wilkins, H.M.; et al. Deletion of Nampt in projection neurons of adult mice leads to motor dysfunction, neurodegeneration, and death. Cell Rep. 2017, 20, 2184–2200. [Google Scholar] [CrossRef]
- Stein, L.R.; Zorumski, C.F.; Imai, S.; Izumi, Y. Nampt is required for long-term depression and the function of GluN2B subunit-containing NMDA receptors. Brain Res. Bull. 2015, 119, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.; Wozniak, D.F.; Imai, S. CA1 Nampt knockdown recapitulates hippocampal cognitive phenotypes in old mice which nicotinamide mononucleotide improves. npj Aging Mech. Dis. 2018, 4, 10. [Google Scholar] [CrossRef]
- Lin, J.B.; Kubota, S.; Ban, N.; Yoshida, M.; Santeford, A.; Sene, A.; Nakamura, R.; Zapata, N.; Kubota, M.; Tsubota, K.; et al. NAMPT-mediated NAD(+) biosynthesis is essential for vision in mice. Cell Rep. 2016, 17, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Dall, M.; Hassing, A.S.; Niu, L.; Nielsen, T.S.; Ingerslev, L.R.; Sulek, K.; Trammell, S.A.J.; Gillum, M.P.; Barrès, R.; Larsen, S.; et al. Hepatocyte-specific perturbation of NAD+ biosynthetic pathways in mice induces reversible nonalcoholic steatohepatitis-like phenotypes. J. Biol. Chem. 2021, 297, 101388. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Chellappa, K.; Moffitt, A.; Ndungu, J.; Dellinger, R.W.; Davis, J.G.; Agarwal, B.; Baur, J.A. Nicotinamide adenine dinucleotide biosynthesis promotes liver regeneration. Hepatology 2017, 65, 616–630. [Google Scholar] [CrossRef]
- Muraoka, H.; Hasegawa, K.; Sakamaki, Y.; Minakuchi, H.; Kawaguchi, T.; Yasuda, I.; Kanda, T.; Tokuyama, H.; Wakino, S.; Itoh, H. Role of Nampt-Sirt6 axis in renal proximal tubules in extracellular matrix deposition in diabetic nephropathy. Cell Rep. 2019, 27, 199–212. [Google Scholar] [CrossRef]
- Watson, A.; Nong, Z.; Yin, H.; O’Neil, C.; Fox, S.; Balint, B.; Guo, L.; Leo, O.; Chu, M.W.A.; Gros, R.; et al. Nicotinamide phosphoribosyltransferase in smooth muscle cells maintains genome integrity, resists aortic medial degeneration, and is suppressed in human thoracic aortic aneurysm disease. Circ. Res. 2017, 120, 1889–1902. [Google Scholar] [CrossRef]
- Stromsdorfer, K.L.; Yamaguchi, S.; Yoon, M.J.; Moseley, A.C.; Franczyk, M.P.; Kelly, S.C.; Qi, N.; Imai, S.; Yoshino, J. NAMPT-mediated NAD(+) biosynthesis in adipocytes regulates adipose tissue function and multi-organ insulin sensitivity in mice. Cell Rep. 2016, 16, 1851–1860. [Google Scholar] [CrossRef]
- Franczyk, M.P.; Qi, N.; Stromsdorfer, K.L.; Li, C.; Yamaguchi, S.; Itoh, H.; Yoshino, M.; Sasaki, Y.; Brookheart, R.T.; Finck, B.N.; et al. Importance of adipose tissue NAD+ Biology in Regulating Metabolic Flexibility. Endocrinology 2021, 162, bqab006. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Franczyk, M.P.; Chondronikola, M.; Qi, N.; Gunawardana, S.C.; Stromsdorfer, K.L.; Porter, L.C.; Wozniak, D.F.; Sasaki, Y.; Rensing, N.; et al. Adipose tissue NAD+ biosynthesis is required for regulating adaptive thermogenesis and whole-body energy homeostasis in mice. Proc. Natl. Acad. Sci. USA 2019, 116, 23822–23828. [Google Scholar] [CrossRef]
- Yoon, M.J.; Yoshida, M.; Johnson, S.; Takikawa, A.; Usui, I.; Tobe, K.; Nakagawa, T.; Yoshino, J.; Imai, S. SIRT1-mediated eNAMPT secretion from adipose tissue regulates hypothalamic NAD+ and function in mice. Cell Metab. 2015, 21, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Sociali, G.; Grozio, A.; Caffa, I.; Schuster, S.; Becherini, P.; Damonte, P.; Sturla, L.; Fresia, C.; Passalacqua, M.; Mazzola, F.; et al. SIRT6 deacetylase activity regulates NAMPT activity and NAD(P)(H) pools in cancer cells. FASEB J. 2019, 33, 3704–3717. [Google Scholar] [CrossRef] [PubMed]
- Satoh, A.; Brace, C.S.; Rensing, N.; Cliften, P.; Wozniak, D.F.; Herzog, E.D.; Yamada, K.A.; Imai, S. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab. 2013, 18, 416–430. [Google Scholar] [CrossRef]
- Satoh, A.; Brace, C.S.; Rensing, N.; Imai, S. Deficiency of Prdm13, a dorsomedial hypothalamus-enriched gene, mimics age-associated changes in sleep quality and adiposity. Aging Cell 2015, 14, 209–218. [Google Scholar] [CrossRef]
- Yoshida, M.; Satoh, A.; Lin, J.B.; Mills, K.F.; Sasaki, Y.; Rensing, N.; Wong, M.; Apte, R.S.; Imai, S.I. Extracellular vesicle-contained eNAMPT delays aging and extends lifespan in mice. Cell Metab. 2019, 30, 329–342.e5. [Google Scholar] [CrossRef]
- Morató, L.; Astori, S.; Zalachoras, I.; Rodrigues, J.; Ghosal, S.; Huang, W.; Guillot de Suduiraut, I.; Grosse, J.; Zanoletti, O.; Cao, L.; et al. eNAMPT actions through nucleus accumbens NAD+/SIRT1 link increased adiposity with sociability deficits programmed by peripuberty stress. Sci. Adv. 2022, 8, eabj9109. [Google Scholar] [CrossRef]
- Zhang, L.Q.; Van Haandel, L.; Xiong, M.; Huang, P.; Heruth, D.P.; Bi, C.; Gaedigk, R.; Jiang, X.; Li, D.Y.; Wyckoff, G.; et al. Metabolic and molecular insights into an essential role of nicotinamide phosphoribosyltransferase. Cell Death Dis. 2017, 8, e2705. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Chen, H.; Xiao, P.; Huang, N.; Han, X.; Zhang, J.; Yang, Y.; Li, T.; Zhao, T.; Tai, H.; et al. miR-146a impedes the anti-aging effect of AMPK via NAMPT suppression and NAD+/SIRT inactivation. Signal Transduct. Target Ther. 2022, 7, 66. [Google Scholar] [CrossRef]
- Han, X.; Tai, H.; Wang, X.; Wang, Z.; Zhou, J.; Wei, X.; Ding, Y.; Gong, H.; Mo, C.; Zhang, J.; et al. AMPK activation protects cells from oxidative stress-induced senescence via autophagic flux restoration and intracellular NAD(+) elevation. Aging Cell 2016, 15, 416–427. [Google Scholar] [CrossRef]
- Cantó, C.; Jiang, L.Q.; Deshmukh, A.S.; Mataki, C.; Coste, A.; Lagouge, M.; Zierath, J.R.; Auwerx, J. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 2010, 11, 213–219. [Google Scholar] [CrossRef]
- Long, Y.C.; Zierath, J.R. AMP-activated protein kinase signaling in metabolic regulation. J. Clin. Investig. 2006, 116, 1776–1783. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.C.; Hardie, D.G. AMPK: Sensing Glucose as well as Cellular Energy Status. Cell Metab. 2018, 27, 299–313. [Google Scholar] [CrossRef]
- Harmel, E.; Grenier, E.; Bendjoudi Ouadda, A.; El Chebly, M.; Ziv, E.; Beaulieu, J.F.; Sané, A.; Spahis, S.; Laville, M.; Levy, E. AMPK in the small intestine in normal and pathophysiological conditions. Endocrinology 2014, 155, 873–888. [Google Scholar] [CrossRef]
- Igarashi, M.; Guarente, L. mTORC1 and SIRT1 Cooperate to foster expansion of gut adult stem cells during calorie restriction. Cell 2016, 166, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Ulgherait, M.; Rana, A.; Rera, M.; Graniel, J.; Walker, D.W. AMPK modulates tissue and organismal aging in a non-cell-autonomous manner. Cell Rep. 2014, 8, 1767–1780. [Google Scholar] [CrossRef]
- Sun, X.; Yang, Q.; Rogers, C.J.; Du, M.; Zhu, M.J. AMPK improves gut epithelial differentiation and barrier function via regulating Cdx2 expression. Cell Death Differ. 2017, 24, 819–831. [Google Scholar] [CrossRef]
- Zhang, E.; Jin, L.; Wang, Y.; Tu, J.; Zheng, R.; Ding, L.; Fang, Z.; Fan, M.; Al-Abdullah, I.; Natarajan, R.; et al. Intestinal AMPK modulation of microbiota mediates crosstalk with brown fat to control thermogenesis. Nat. Commun. 2022, 13, 1135. [Google Scholar] [CrossRef] [PubMed]
- Olivier, S.; Pochard, C.; Diounou, H.; Castillo, V.; Divoux, J.; Alcantara, J.; Leclerc, J.; Guilmeau, S.; Huet, C.; Charifi, W.; et al. Deletion of intestinal epithelial AMP-activated protein kinase alters distal colon permeability but not glucose homeostasis. Mol. Metab. 2021, 47, 101183. [Google Scholar] [CrossRef]
- Liao, X.; Huang, X.; Li, X.; Qiu, X.; Li, M.; Liu, R.; He, T.; Tang, Q. AMPK phosphorylates NAMPT to regulate NAD+ homeostasis under ionizing radiation. Open Biol. 2022, 12, 220213. [Google Scholar] [CrossRef]
- Zhu, X.; Shen, W.; Wang, Y.; Jaiswal, A.; Ju, Z.; Sheng, Q. Nicotinamide adenine dinucleotide replenishment rescues colon degeneration in aged mice. Signal Transduct. Target Ther. 2017, 2, 17017. [Google Scholar] [CrossRef]
- Wellman, A.S.; Metukuri, M.R.; Kazgan, N.; Xu, X.; Xu, Q.; Ren, N.S.X.; Czopik, A.; Shanahan, M.T.; Kang, A.; Chen, W.; et al. Intestinal epithelial Sirtuin 1 regulates intestinal inflammation during aging in mice by altering the intestinal microbiota. Gastroenterology 2017, 153, 772–786. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, D.; Clara, R.; Fedele, S.; Hu, J.; Lackzo, E.; Huang, J.Y.; Verdin, E.; Langhans, W.; Mansouri, A. Intestinal SIRT3 overexpression in mice improves whole body glucose homeostasis independent of body weight. Mol. Metab. 2017, 6, 1264–1273. [Google Scholar] [CrossRef]
- Xiong, X.; Yang, C.; He, W.Q.; Yu, J.; Xin, Y.; Zhang, X.; Huang, R.; Ma, H.; Xu, S.; Li, Z.; et al. Sirtuin 6 maintains epithelial STAT6 activity to support intestinal tuft cell development and type 2 immunity. Nat. Commun. 2022, 13, 5192. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.L.; Zhou, M.; Kang, C.; Lang, H.D.; Chen, M.T.; Hui, S.C.; Wang, B.; Mi, M.T. Crosstalk between gut microbiota and Sirtuin-3 in colonic inflammation and tumorigenesis. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Bu, H.F.; Geng, H.; De Plaen, I.G.; Gao, C.; Wang, P.; Wang, X.; Kurowski, J.A.; Yang, H.; Qian, J.; et al. Sirtuin-6 preserves R-spondin-1 expression and increases resistance of intestinal epithelium to injury in mice. Mol. Med. 2017, 23, 272–284. [Google Scholar] [CrossRef]
- Madiraju, A.K.; Erion, D.M.; Rahimi, Y.; Zhang, X.M.; Braddock, D.T.; Albright, R.A.; Prigaro, B.J.; Wood, J.L.; Bhanot, S.; MacDonald, M.J.; et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 2014, 510, 542–546. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, M.J.; Ansari, I.H.; Longacre, M.J.; Stoker, S.W. Metformin’s therapeutic efficacy in the treatment of diabetes does not involve inhibition of mitochondrial glycerol phosphate dehydrogenase. Diabetes 2021, 70, 1575–1580. [Google Scholar] [CrossRef] [PubMed]
- Alshawi, A.; Agius, L. Low metformin causes a more oxidized mitochondrial NADH/NAD redox state in hepatocytes and inhibits gluconeogenesis by a redox-independent mechanism. J. Biol. Chem. 2019, 294, 2839–2853. [Google Scholar] [CrossRef]
- Shin, N.R.; Lee, J.C.; Lee, H.Y.; Kim, M.S.; Whon, T.W.; Lee, M.S.; Bae, J.W. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 2014, 63, 727–735. [Google Scholar] [CrossRef]
- Lien, F.; Berthier, A.; Bouchaert, E.; Gheeraert, C.; Alexandre, J.; Porez, G.; Prawitt, J.; Dehondt, H.; Ploton, M.; Colin, S.; et al. Metformin interferes with bile acid homeostasis through AMPK-FXR crosstalk. J. Clin. Investig. 2014, 124, 1037–1051. [Google Scholar] [CrossRef]
- Thomas, C.; Gioiello, A.; Noriega, L.; Strehle, A.; Oury, J.; Rizzo, G.; Macchiarulo, A.; Yamamoto, H.; Mataki, C.; Pruzanski, M.; et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009, 10, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, A.; Miller, S.; Nicholls, A.W.; Baker, D.; Van Horn, S.; Thomas, E.; Rajpal, D.; Spivak, A.; Brown, J.R.; Nunez, D.J. Novel gut-based pharmacology of metformin in patients with type 2 diabetes mellitus. PLoS ONE 2014, 9, e100778. [Google Scholar] [CrossRef]
- Yi, F.; Sun, J.; Lim, G.E.; Fantus, I.G.; Brubaker, P.L.; Jin, T. Cross talk between the insulin and Wnt signaling pathways: Evidence from intestinal endocrine L cells. Endocrinology 2008, 149, 2341–2351. [Google Scholar] [CrossRef]
- Kim, M.H.; Jee, J.H.; Park, S.; Lee, M.S.; Kim, K.W.; Lee, M.K. Metformin enhances glucagon-like peptide 1 via cooperation between insulin and Wnt signaling. J. Endocrinol. 2014, 220, 117–128. [Google Scholar] [CrossRef]
- Holloway, K.R.; Calhoun, T.N.; Saxena, M.; Metoyer, C.F.; Kandler, E.F.; Rivera, C.A.; Pruitt, K. SIRT1 regulates Dishevelled proteins and promotes transient and constitutive Wnt signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 9216–9221. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. Wnt/beta-catenin signaling in development and disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef]
- Feng, G.; Zheng, K.; Song, D.; Xu, K.; Huang, D.; Zhang, Y.; Cao, P.; Shen, S.; Zhang, J.; Feng, X.; et al. SIRT1 was involved in TNF-α-promoted osteogenic differentiation of human DPSCs through Wnt/β-catenin signal. In Vitro Cell Dev. Biol. Anim. 2016, 52, 1001–1011. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, Z.; Zhang, W.; Hu, X.; Wei, H.; Peng, J.; Jiang, S. SIRT1 inhibits adipogenesis and promotes myogenic differentiation in C3H10T1/2 pluripotent cells by regulating Wnt signaling. Cell Biosci. 2015, 5, 61. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, T.Y.; Kim, Y.; Lee, S.H.; Kim, S.; Kang, S.W.; Yang, J.Y.; Baek, I.J.; Sung, Y.H.; Park, Y.Y.; et al. Microbiota-derived lactate accelerates intestinal stem-cell-mediated epithelial development. Cell Host Microbe 2018, 24, 833–846.e6. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.W.; Xie, Y.; Li, G.Q.; Zhang, T.; Sui, Y.H.; Zhao, Z.J.; Zhang, Y.Y.; Yang, W.B.; Geng, X.L.; Xue, D.B.; et al. Gut microbiota-derived nicotinamide mononucleotide alleviates acute pancreatitis by activating pancreatic SIRT3 signalling. Br. J. Pharmacol. 2023, 180, 647–666. [Google Scholar] [CrossRef]
- Xu, K.; Guo, Y.; Wang, Y.; Ren, Y.; Low, V.; Cho, S.; Ping, L.; Peng, K.; Li, X.; Qiu, Y.; et al. Decreased Enterobacteriaceae translocation due to gut microbiota remodeling mediates the alleviation of premature aging by a high-fat diet. Aging Cell 2023, 22, e13760. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Jin, D.; Huang, S.; Wu, J.; Xu, M.; Liu, T.; Dong, W.; Liu, X.; Wang, S.; Zhong, W.; et al. Clostridium butyricum, a butyrate-producing probiotic, inhibits intestinal tumor development through modulating Wnt signaling and gut microbiota. Cancer Lett. 2020, 469, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Swafford, D.; Shanmugam, A.; Ranganathan, P.; Hussein, M.S.; Koni, P.A.; Prasad, P.D.; Thangaraju, M.; Manicassamy, S. Canonical Wnt Signaling in CD11c(+) APCs Regulates Microbiota-Induced Inflammation and Immune Cell Homeostasis in the Colon. J. Immunol. 2018, 200, 3259–3268. [Google Scholar] [CrossRef] [PubMed]
- Novellasdemunt, L.; Antas, P.; Li, V.S. Targeting Wnt signaling in colorectal cancer. A review in the theme: Cell signaling: Proteins, pathways and mechanisms. Am. J. Physiol. Cell Physiol. 2015, 309, C511–C521. [Google Scholar] [CrossRef]
- Nalapareddy, K.; Nattamai, K.J.; Kumar, R.S.; Karns, R.; Wikenheiser-Brokamp, K.A.; Sampson, L.L.; Mahe, M.M.; Sundaram, N.; Yacyshyn, M.B.; Yacyshyn, B.; et al. Canonical Wnt signaling ameliorates aging of intestinal stem cells. Cell Rep. 2017, 18, 2608–2621. [Google Scholar] [CrossRef]
- Igarashi, M.; Miura, M.; Williams, E.; Jaksch, F.; Kadowaki, T.; Yamauchi, T.; Guarente, L. NAD+ supplementation rejuvenates aged gut adult stem cells. Aging Cell 2019, 18, e12935. [Google Scholar] [CrossRef]
- Li, X.; Shan, J.; Chang, W.; Kim, I.; Bao, J.; Lee, H.J.; Zhang, X.; Samuel, V.T.; Shulman, G.I.; Liu, D.; et al. Chemical and genetic evidence for the involvement of Wnt antagonist Dickkopf2 in regulation of glucose metabolism. Proc. Natl. Acad. Sci. USA 2012, 109, 11402–11407. [Google Scholar] [CrossRef]
- Kawano, Y.; Nakae, J.; Watanabe, N.; Kikuchi, T.; Tateya, S.; Tamori, Y.; Kaneko, M.; Abe, T.; Onodera, M.; Itoh, H. Colonic pro-inflammatory macrophages cause insulin resistance in an intestinal Ccl2/Ccr2-dependent manner. Cell Metab. 2016, 24, 295–310. [Google Scholar] [CrossRef]
- Luck, H.; Tsai, S.; Chung, J.; Clemente-Casares, X.; Ghazarian, M.; Revelo, X.S.; Lei, H.; Luk, C.T.; Shi, S.Y.; Surendra, A.; et al. Regulation of obesity-related insulin resistance with gut anti-inflammatory agents. Cell Metab. 2015, 21, 527–542. [Google Scholar] [CrossRef]
- Garidou, L.; Pomié, C.; Klopp, P.; Waget, A.; Charpentier, J.; Aloulou, M.; Giry, A.; Serino, M.; Stenman, L.; Lahtinen, S.; et al. The gut microbiota regulates intestinal CD4 T cells expressing RORγt and controls metabolic disease. Cell Metab. 2015, 22, 100–112. [Google Scholar] [CrossRef]
- Da Silva Xavier, G.; Loder, M.K.; McDonald, A.; Tarasov, A.I.; Carzaniga, R.; Kronenberger, K.; Barg, S.; Rutter, G.A. TCF7L2 regulates late events in insulin secretion from pancreatic islet beta-cells. Diabetes 2009, 58, 894–905. [Google Scholar] [CrossRef]
- Takamoto, I.; Kubota, N.; Nakaya, K.; Kumagai, K.; Hashimoto, S.; Kubota, T.; Inoue, M.; Kajiwara, E.; Katsuyama, H.; Obata, A.; et al. TCF7L2 in mouse pancreatic beta cells plays a crucial role in glucose homeostasis by regulating beta cell mass. Diabetologia 2014, 57, 542–553. [Google Scholar] [CrossRef] [PubMed]
- Laurenti, M.C.; Dalla Man, C.; Varghese, R.T.; Andrews, J.C.; Rizza, R.A.; Matveyenko, A.; De Nicolao, G.; Cobelli, C.; Vella, A. Diabetes-associated genetic variation in TCF7L2 alters pulsatile insulin secretion in humans. JCI Insight 2020, 5, e136136. [Google Scholar] [CrossRef]
- Grant, S.F.; Thorleifsson, G.; Reynisdottir, I.; Benediktsson, R.; Manolescu, A.; Sainz, J.; Helgason, A.; Stefansson, H.; Emilsson, V.; Helgadottir, A.; et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat. Genet. 2006, 38, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, S.A.; Tschritter, O.; Machicao, F.; Thamer, C.; Stefan, N.; Gallwitz, B.; Holst, J.J.; Dekker, J.M.; t’Hart, L.M.; Nijpels, G.; et al. Impaired glucagon-like peptide-1-induced insulin secretion in carriers of transcription factor 7-like 2 (TCF7L2) gene polymorphisms. Diabetologia 2007, 50, 2443–2450. [Google Scholar] [CrossRef]
- Shao, W.; Wang, D.; Chiang, Y.T.; Ip, W.; Zhu, L.; Xu, F.; Columbus, J.; Belsham, D.D.; Irwin, D.M.; Zhang, H.; et al. The Wnt signaling pathway effector TCF7L2 controls gut and brain proglucagon gene expression and glucose homeostasis. Diabetes 2013, 62, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Anini, Y.; Fang, X.; Mills, G.; Brubaker, P.L.; Jin, T. Transcriptional activation of the proglucagon gene by lithium and beta-catenin in intestinal endocrine L cells. J. Biol. Chem. 2003, 278, 1380–1387. [Google Scholar] [CrossRef]
- Daoudi, M.; Hennuyer, N.; Borland, M.G.; Touche, V.; Duhem, C.; Gross, B.; Caiazzo, R.; Kerr-Conte, J.; Pattou, F.; Peters, J.M.; et al. PPARβ/δ activation induces enteroendocrine L cell GLP-1 production. Gastroenterology 2011, 140, 1564–1574. [Google Scholar] [CrossRef]
- Hadjittofi, C.; Coran, A.G.; Mogilner, J.G.; Pollak, Y.; Matter, I.; Sukhotnik, I. Dietary supplementation with vitamin D stimulates intestinal epithelial cell turnover after massive small bowel resection in rats. Pediatr. Surg. Int. 2013, 29, 41–50. [Google Scholar] [CrossRef]
- Holick, M.F. Sunlight, ultraviolet radiation, vitamin D and skin cancer: How much sunlight do we need? Adv. Exp. Med. Biol. 2014, 810, 1–16. [Google Scholar]
- Newman, J.C.; Covarrubias, A.J.; Zhao, M.; Yu, X.; Gut, P.; Ng, C.P.; Huang, Y.; Haldar, S.; Verdin, E. Ketogenic diet reduces midlife mortality and improves memory in aging mice. Cell Metab. 2017, 26, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.W.; Biton, M.; Haber, A.L.; Gunduz, N.; Eng, G.; Gaynor, L.T.; Tripathi, S.; Calibasi-Kocal, G.; Rickelt, S.; Butty, V.L.; et al. Ketone body signaling mediates intestinal stem cell homeostasis and adaptation to diet. Cell 2019, 178, 1115–1131.e15. [Google Scholar] [CrossRef] [PubMed]
- Beyaz, S.; Mana, M.D.; Roper, J.; Kedrin, D.; Saadatpour, A.; Hong, S.J.; Bauer-Rowe, K.E.; Xifaras, M.E.; Akkad, A.; Arias, E.; et al. High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature 2016, 531, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, S.; Hata, K.; Hirose, K.; Okui, T.; Toyosawa, S.; Uzawa, N.; Nishimura, R.; Yoneda, T. The lactate sensor GPR81 regulates glycolysis and tumor growth of breast cancer. Sci. Rep. 2022, 12, 6261. [Google Scholar] [CrossRef]
- Dorton, H.M.; Luo, S.; Monterosso, J.R.; Page, K.A. Influences of dietary added sugar consumption on striatal food-cue reactivity and postprandial GLP-1 response. Front. Psychiatry 2018, 8, 297. [Google Scholar] [CrossRef]
- Hutchison, A.T.; Piscitelli, D.; Horowitz, M.; Jones, K.L.; Clifton, P.M.; Standfield, S.; Hausken, T.; Feinle-Bisset, C.; Luscombe-Marsh, N.D. Acute load-dependent effects of oral whey protein on gastric emptying, gut hormone release, glycemia, appetite, and energy intake in healthy men. Am. J. Clin. Nutr. 2015, 102, 1574–1584. [Google Scholar] [CrossRef]
- Giezenaar, C.; Hutchison, A.T.; Luscombe-Marsh, N.D.; Chapman, I.; Horowitz, M.; Soenen, S. Effect of age on blood glucose and plasma insulin, glucagon, ghrelin, CCK, GIP, and GLP-1 responses to whey protein ingestion. Nutrients 2017, 10, 2. [Google Scholar] [CrossRef]
- Di Francesco, V.; Barazzoni, R.; Bissoli, L.; Fantin, F.; Rizzotti, P.; Residori, L.; Antonioli, A.; Graziani, M.S.; Zanetti, M.; Bosello, O.; et al. The quantity of meal fat influences the profile of postprandial hormones as well as hunger sensation in healthy elderly people. J. Am. Med. Dir. Assoc. 2010, 11, 188–193. [Google Scholar] [CrossRef]
- Gentilcore, D.; Chaikomin, R.; Jones, K.L.; Russo, A.; Feinle-Bisset, C.; Wishart, J.M.; Rayner, C.K.; Horowitz, M. Effects of fat on gastric emptying of and the glycemic, insulin, and incretin responses to a carbohydrate meal in type 2 diabetes. J. Clin. Endocrinol. Metab. 2006, 91, 2062–2067. [Google Scholar] [CrossRef]
- Cunningham, K.M.; Read, N.W. The effect of incorporating fat into different components of a meal on gastric emptying and postprandial blood glucose and insulin responses. Br. J. Nutr. 1989, 61, 285–290. [Google Scholar] [CrossRef]
- Sun, L.; Goh, H.J.; Govindharajulu, P.; Leow, M.K.; Henry, C.J. Postprandial glucose, insulin and incretin responses differ by test meal macronutrient ingestion sequence (PATTERN study). Clin. Nutr. 2020, 39, 950–957. [Google Scholar] [CrossRef]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Zmora, N.; Adolph, T.E.; Elinav, E. The intestinal microbiota fuelling metabolic inflammation. Nat. Rev. Immunol. 2020, 20, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Le Roy, T.; Llopis, M.; Lepage, P.; Bruneau, A.; Rabot, S.; Bevilacqua, C.; Martin, P.; Philippe, C.; Walker, F.; Bado, A.; et al. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut 2013, 62, 1787–1794. [Google Scholar] [CrossRef]
- Scheithauer, T.P.M.; Rampanelli, E.; Nieuwdorp, M.; Vallance, B.A.; Verchere, C.B.; van Raalte, D.H.; Herrema, H. Gut microbiota as a trigger for metabolic inflammation in obesity and type 2 diabetes. Front. Immunol. 2020, 11, 571731. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C.; Bell, R.; Klag, K.A.; Lee, S.H.; Soto, R.; Ghazaryan, A.; Buhrke, K.; Ekiz, H.A.; Ost, K.S.; Boudina, S.; et al. T cell-mediated regulation of the microbiota protects against obesity. Science 2019, 365, eaat9351. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Winer, D.A.; Luck, H.; Tsai, S.; Winer, S. The intestinal immune system in obesity and insulin resistance. Cell Metab. 2016, 23, 413–426. [Google Scholar] [CrossRef]
- Borra, M.T.; Smith, B.C.; Denu, J.M. Mechanism of human SIRT1 activation by resveratrol. J. Biol. Chem. 2005, 280, 17187–17195. [Google Scholar] [CrossRef]
- Chaplin, A.; Carpéné, C.; Mercader, J. Resveratrol, metabolic syndrome, and gut microbiota. Nutrients 2018, 10, 1651. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Perino, A.; Huang, Q.; Von Alvensleben, G.V.G.; Banaei-Esfahani, A.; Velazquez-Villegas, L.A.; Gariani, K.; Korbelius, M.; Bou Sleiman, M.; Imbach, J.; et al. Integrative systems analysis identifies genetic and dietary modulators of bile acid homeostasis. Cell Metab. 2022, 34, 1594–1610. [Google Scholar] [CrossRef]
- Kusaczuk, M. Tauroursodeoxycholate-bile acid with chaperoning activity: Molecular and cellular effects and therapeutic perspectives. Cells 2019, 8, 1471. [Google Scholar] [CrossRef] [PubMed]
- Trabelsi, M.S.; Daoudi, M.; Prawitt, J.; Ducastel, S.; Touche, V.; Sayin, S.I.; Perino, A.; Brighton, C.A.; Sebti, Y.; Kluza, J.; et al. Farnesoid X receptor inhibits glucagon-like peptide-1 production by enteroendocrine L cells. Nat. Commun. 2015, 6, 7629. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.E.; Glicksman, C.; Alaghband-Zadeh, J.; Sherwood, R.A.; Akuji, N.; le Roux, C.W. The relationship between postprandial bile acid concentration, GLP-1, PYY and ghrelin. Clin. Endrocrinol. 2011, 74, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Ummarino, S.; Mozzon, M.; Zamporlini, F.; Amici, A.; Mazzola, F.; Orsomando, G.; Ruggieri, S.; Raffaelli, N. Simultaneous quantitation of nicotinamide riboside, nicotinamide mononucleotide and nicotinamide adenine dinucleotide in milk by a novel enzyme-coupled assay. Food Chem. 2017, 221, 161–168. [Google Scholar] [CrossRef]
- Mills, K.F.; Yoshida, S.; Stein, L.R.; Grozio, A.; Kubota, S.; Sasaki, Y.; Redpath, P.; Migaud, M.E.; Apte, R.S.; Uchida, K.; et al. Long-term administration of nicotinamide mononucleotide mitigates age-associated physiological decline in mice. Cell Metab. 2016, 24, 795–806. [Google Scholar] [CrossRef]
- Yoshino, J.; Baur, J.A.; Imai, S.I. NAD+ intermediates: The biology and therapeutic potential of NMN and NR. Cell Metab. 2018, 27, 513–528. [Google Scholar] [CrossRef]
- Rajman, L.; Chwalek, K.; Sinclair, D.A. Therapeutic potential of NAD-boosting molecules: The in vivo evidence. Cell Metab. 2018, 27, 529–547. [Google Scholar] [CrossRef]
- Johnson, S.; Imai, S.I. NAD+ biosynthesis, aging, and disease. F1000Res 2018, 7, 132. [Google Scholar] [CrossRef]
- Cantó, C.; Houtkooper, R.H.; Pirinen, E.; Youn, D.Y.; Oosterveer, M.H.; Cen, Y.; Fernandez-Marcos, P.J.; Yamamoto, H.; Andreux, P.A.; Cettour-Rose, P.; et al. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012, 15, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ryu, D.; Wu, Y.; Gariani, K.; Wang, X.; Luan, P.; D’Amico, D.; Ropelle, E.R.; Lutolf, M.P.; Aebersold, R.; et al. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science 2016, 352, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Su, X.; Quinn, W.J., 3rd; Hui, S.; Krukenberg, K.; Frederick, D.W.; Redpath, P.; Zhan, L.; Chellappa, K.; White, E.; et al. Quantitative analysis of NAD synthesis-breakdown fluxes. Cell Metab. 2018, 27, 1067–1080.e5. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, J.; Joffraud, M.; Trammell, S.A.; Ras, R.; Canela, N.; Boutant, M.; Kulkarni, S.S.; Rodrigues, M.; Redpath, P.; Migaud, M.E.; et al. NRK1 controls nicotinamide mononucleotide and nicotinamide riboside metabolism in mammalian cells. Nat. Commun. 2016, 7, 13103. [Google Scholar] [CrossRef]
- Grozio, A.; Mills, K.F.; Yoshino, J.; Bruzzone, S.; Sociali, G.; Tokizane, K.; Lei, H.C.; Cunningham, R.; Sasaki, Y.; Migaud, M.E.; et al. Slc12a8 is a nicotinamide mononucleotide transporter. Nat. Metab. 2019, 1, 47–57. [Google Scholar] [CrossRef]
- Li, W.; Zhou, Y.; Pang, N.; Hu, Q.; Li, Q.; Sun, Y.; Ding, Y.; Gu, Y.; Xiao, Y.; Gao, M.; et al. NAD supplement alleviates intestinal barrier injury induced by ethanol via protecting epithelial mitochondrial function. Nutrients 2022, 15, 174. [Google Scholar] [CrossRef]
- Trammell, S.A.; Schmidt, M.S.; Weidemann, B.J.; Redpath, P.; Jaksch, F.; Dellinger, R.W.; Li, Z.; Abel, E.D.; Migaud, M.E.; Brenner, C. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat. Commun. 2016, 7, 12948. [Google Scholar] [CrossRef]
- Airhart, S.E.; Shireman, L.M.; Risler, L.J.; Anderson, G.D.; Nagana Gowda, G.A.; Raftery, D.; Tian, R.; Shen, D.D.; O’Brien, K.D. An open-label, non-randomized study of the pharmacokinetics of the nutritional supplement nicotinamide riboside (NR) and its effects on blood NAD+ levels in healthy volunteers. PLoS ONE 2017, 12, e0186459. [Google Scholar] [CrossRef]
- Conze, D.; Brenner, C.; Kruger, C.L. Safety and metabolism of long-term administration of NIAGEN (nicotinamide riboside chloride) in a randomized, double-blind, placebo-controlled clinical trial of healthy overweight adults. Sci. Rep. 2019, 9, 9772. [Google Scholar] [CrossRef]
- Martens, C.R.; Denman, B.A.; Mazzo, M.R.; Armstrong, M.L.; Reisdorph, N.; McQueen, M.B.; Chonchol, M.; Seals, D.R. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD+ in healthy middle-aged and older adults. Nat. Commun. 2018, 9, 1286. [Google Scholar] [CrossRef]
- Dollerup, O.L.; Christensen, B.; Svart, M.; Schmidt, M.S.; Sulek, K.; Ringgaard, S.; Stødkilde-Jørgensen, H.; Møller, N.; Brenner, C.; Treebak, J.T.; et al. A randomized placebo-controlled clinical trial of nicotinamide riboside in obese men: Safety, insulin-sensitivity, and lipid-mobilizing effects. Am. J. Clin. Nutr. 2018, 108, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Remie, C.M.E.; Roumans, K.H.M.; Moonen, M.P.B.; Connell, N.J.; Havekes, B.; Mevenkamp, J.; Lindeboom, L.; de Wit, V.H.W.; van de Weijer, T.; Aarts, S.; et al. Nicotinamide riboside supplementation alters body composition and skeletal muscle acetylcarnitine concentrations in healthy obese humans. Am. J. Clin. Nutr. 2020, 112, 413–426. [Google Scholar] [CrossRef]
- Elhassan, Y.S.; Kluckova, K.; Fletcher, R.S.; Schmidt, M.S.; Garten, A.; Doig, C.L.; Cartwright, D.M.; Oakey, L.; Burley, C.V.; Jenkinson, N.; et al. Nicotinamide riboside augments the aged human skeletal muscle NAD(+) metabolome and induces transcriptomic and anti-inflammatory signatures. Cell Rep. 2019, 28, 1717–1728.e6. [Google Scholar] [CrossRef] [PubMed]
- Dollerup, O.L.; Trammell, S.A.J.; Hartmann, B.; Holst, J.J.; Christensen, B.; Møller, N.; Gillum, M.P.; Treebak, J.T.; Jessen, N. Effects of nicotinamide riboside on endocrine pancreatic function and incretin hormones in nondiabetic men with obesity. J. Clin. Endocrinol. Metab. 2019, 104, 5703–5714. [Google Scholar] [CrossRef]
- Irie, J.; Inagaki, E.; Fujita, M.; Nakaya, H.; Mitsuishi, M.; Yamaguchi, S.; Yamashita, K.; Shigaki, S.; Ono, T.; Yukioka, H.; et al. Effect of oral administration of nicotinamide mononucleotide on clinical parameters and nicotinamide metabolite levels in healthy Japanese men. Endocr. J. 2020, 67, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Okabe, K.; Yaku, K.; Uchida, Y.; Fukamizu, Y.; Sato, T.; Sakurai, T.; Tobe, K.; Nakagawa, T. Oral administration of nicotinamide mononucleotide is safe and efficiently increases blood nicotinamide adenine dinucleotide levels in healthy subjects. Front. Nutr. 2022, 9, 868640. [Google Scholar] [CrossRef]
- Fukamizu, Y.; Uchida, Y.; Shigekawa, A.; Sato, T.; Kosaka, H.; Sakurai, T. Safety evaluation of β-nicotinamide mononucleotide oral administration in healthy adult men and women. Sci. Rep. 2022, 12, 14442. [Google Scholar] [CrossRef]
- Akasaka, H.; Nakagami, H.; Sugimoto, K.; Yasunobe, Y.; Minami, T.; Fujimoto, T.; Yamamoto, K.; Hara, C.; Shiraki, A.; Nishida, K.; et al. Effects of nicotinamide mononucleotide on older patients with diabetes and impaired physical performance: A prospective, placebo-controlled, double-blind study. Geriatr. Gerontol. Int. 2023, 23, 38–43. [Google Scholar] [CrossRef]
- Igarashi, M.; Nakagawa-Nagahama, Y.; Miura, M.; Kashiwabara, K.; Yaku, K.; Sawada, M.; Sekine, R.; Fukamizu, Y.; Sato, T.; Sakurai, T.; et al. Chronic nicotinamide mononucleotide supplementation elevates blood nicotinamide adenine dinucleotide levels and alters muscle function in healthy older men. npj Aging 2022, 8, 5. [Google Scholar] [CrossRef]
- Kim, M.; Seol, J.; Sato, T.; Fukamizu, Y.; Sakurai, T.; Okura, T. Effect of 12-week intake of nicotinamide mononucleotide on sleep quality, fatigue, and physical performance in older japanese adults: A randomized, double-blind placebo-controlled study. Nutrients 2022, 14, 755. [Google Scholar] [CrossRef]
- Liao, B.; Zhao, Y.; Wang, D.; Zhang, X.; Hao, X.; Hu, M. Nicotinamide mononucleotide supplementation enhances aerobic capacity in amateur runners: A randomized, double-blind study. J. Int. Soc. Sports. Nutr. 2021, 18, 54. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Maier, A.B.; Tao, R.; Lin, Z.; Vaidya, A.; Pendse, S.; Thasma, S.; Andhalkar, N.; Avhad, G.; Kumbhar, V. The efficacy and safety of β-nicotinamide mononucleotide (NMN) supplementation in healthy middle-aged adults: A randomized, multicenter, double-blind, placebo-controlled, parallel-group, dose-dependent clinical trial. Geroscience 2023, 45, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Huang, H. A multicentre, randomised, double blind, parallel design, placebo controlled study to evaluate the efficacy and safety of uthever (NMN supplement), an orally administered supplementation in middle aged and older adults. Front. Aging 2022, 3, 851698. [Google Scholar] [CrossRef]
- Yoshino, M.; Yoshino, J.; Kayser, B.D.; Patti, G.J.; Franczyk, M.P.; Mills, K.F.; Sindelar, M.; Pietka, T.; Patterson, B.W.; Imai, S.I.; et al. Nicotinamide mononucleotide increases muscle insulin sensitivity in prediabetic women. Science 2021, 372, 1224–1229. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, C.; Qiang, L.; Liu, J.; Qiu, Z.; Zhang, Z.; Zhang, J.; Li, Y.; Zhang, M. Clinical observation of the effect of nicotinamide mononucleotide on the improvement of insomnia in middle-aged and old adults. Am. J. Transl. Res. 2022, 6, 167–176. [Google Scholar]
- Kimura, S.; Ichikawa, M.; Sugawara, S.; Katagiri, T.; Hirasawa, Y.; Ishikawa, T.; Matsunaga, W.; Gotoh, A. Nicotinamide mononucleotide is safely metabolized and significantly reduces blood triglyceride levels in healthy individuals. Cureus 2022, 14, e28812. [Google Scholar] [CrossRef]
- Sharma, D.; Verma, S.; Vaidya, S.; Kalia, K.; Tiwari, V. Recent updates on GLP-1 agonists: Current advancements & challenges. Biomed. Pharmacother. 2018, 108, 952–962. [Google Scholar] [CrossRef]
- Nakahata, Y.; Sahar, S.; Astarita, G.; Kaluzova, M.; Sassone-Corsi, P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science 2009, 324, 654–657. [Google Scholar] [CrossRef]
- Dantas Machado, A.C.; Brown, S.D.; Lingaraju, A.; Sivaganesh, V.; Martino, C.; Chaix, A.; Zhao, P.; Pinto, A.F.M.; Chang, M.W.; Richter, R.A.; et al. Diet and feeding pattern modulate diurnal dynamics of the ileal microbiome and transcriptome. Cell Rep. 2022, 40, 111008. [Google Scholar] [CrossRef]
- Shats, I.; Williams, J.G.; Liu, J.; Makarov, M.V.; Wu, X.; Lih, F.B.; Deterding, L.J.; Lim, C.; Xu, X.; Randall, T.A.; et al. Bacteria boost mammalian host NAD metabolism by engaging the deamidated biosynthesis pathway. Cell Metab. 2020, 31, 564–579.e7. [Google Scholar] [CrossRef]
- Yaku, K.; Palikhe, S.; Izumi, H.; Yoshida, T.; Hikosaka, K.; Hayat, F.; Karim, M.; Iqbal, T.; Nitta, Y.; Sato, A.; et al. BST1 regulates nicotinamide riboside metabolism via its glycohydrolase and base-exchange activities. Nat. Commun. 2021, 12, 6767. [Google Scholar] [CrossRef] [PubMed]

| Design | Subjects | Dose, Administration Route, and Form of NMN | Treatment Duration | Method for Validating Increased NAD+ Level | Findings | Publication Year | Ref. |
|---|---|---|---|---|---|---|---|
| Single-arm, nonrandomized, nonblinded study | Age, 40–60 years; healthy men (n = 10) | 100, 250, and 500 mg/day; oral administration; capsule | Single administration | NMN administration dose-dependently increased plasma concentrations of 2PY and 4PY. | Single oral administration of NMN did not cause significant changes in clinical symptoms, including vital signs, ophthalmic examination, sleep quality, and laboratory analysis results. | 2020 | [185] |
| Randomized, double-blind, placebo-controlled, parallel-group study | Age, 20–65 years; healthy males and females (NMN, n = 15; placebo, n = 15) | 250 mg/day; oral administration; tablet | 12 weeks | NMN administration increased NAD+ and NAMN levels in whole blood. | No obvious abnormalities in physiological and laboratory tests and no adverse effects were observed. | 2022 | [186] |
| Randomized, double-blind, placebo-controlled, parallel-group study | Age, 20–65 years; healthy males and females (NMN, n = 16; placebo, n = 15) | 1250 mg /day; oral administration; packaged powder dissolved in water (200 mL) | 4 weeks | N/A | Oral administration of NMN 1250 mg/day for 4 weeks did not cause significant abnormalities in anthropometry, hematological, biochemical, urine, and body composition analyses. | 2022 | [187] |
| Prospective, placebo-controlled, double-blind study | Age, over 65 years; elderly males with type 2 diabetes with reduced grip strength or walking speed (NMN, n = 6; placebo, n = 7) | 250 mg/day; oral administration; capsule | 24 weeks | N/A | Adverse events were not observed in the NMN group. NMN did not improve grip strength and walking speed. However, an improved prevalence of frailty and central retinal thickness was observed. | 2023 | [188] |
| Randomized, double-blind, placebo-controlled, parallel-group study | Age; over 65 years; elderly healthy males (NMN, n = 11; placebo, n = 11) | 250 mg/day; oral administration; pill | 12 weeks | NMN administration increased NAD+ and NAD+-related metabolites levels in whole blood, assessed by metabolomic analysis | NMN nominally but significantly improved gait speed and performance in the left grip tests without affecting body composition and glucose metabolism were observed. | 2022 | [189] |
| Randomized, double-blind, placebo-controlled study | Age, over 65 years; elderly males (NMN antemeridian, n = 27; post meridian, n = 27. Placebo antemeridian, n = 27; post meridian n = 27) | 250 mg/day; oral administration; tablet | 12 weeks | N/A | NMN intake in postmeridian improved lower limb function and drowsiness. | 2022 | [190] |
| Randomized, double-blind, placebo-controlled, four-arm clinical study | Age, 27–50 years; healthy recreationally trained runners (40 males and 8 females) | 300, 600, 1200 mg/day, oral administration; powder | 6 weeks | N/A | Exercise combined with 300, 600, and 1200 mg of daily NMN supplementation dose-dependently increased aerobic capacity. | 2021 | [191] |
| Randomized, double-blind, placebo-controlled, parallel-group study. Dose-dependent study | Age, 40–65 years; healthy males and females (NMN, 300, 600, and 900 mg; placebo, n = 20) | 300, 600, 900 mg/day; oral administration; capsule | 60 days | Blood NAD+ concentrations were increased compared to baseline in three NMN-treated groups (300, 600, 900 mg) on days 30 and 60. | NMN administration was safely tolerated. Walking distance during the six-minute walking test, the change of biological age, and SF-36 scores were improved in the NMN 300, 600, and 900 mg groups on day 60. | 2023 | [192] |
| Randomized, double-blind, placebo-controlled, parallel-group study | Age, 40–65 years; healthy males and females (NMN, n = 31; placebo, n = 35) | 300 mg/day; oral administration; capsule | 60 days | Serum NAD+/NADH levels were increased by 11.3% on day 30 and 38% on day 60 vs. baseline. | Walking endurance: SF-36 questionnaire score, a parameter for well-being, and HOMA-IR index improved with NMN administration for 60 days. | 2022 | [193] |
| Randomized, double-blind, placebo-controlled study | Age, 55–75 years; postmenopausal women with prediabetes (NMN, n = 13; placebo, n = 12) | 250 mg/day; oral administration; capsule | 10 weeks | Plasma concentrations of 2 PY and 4 PY and NAD+ contents in PBMCs increased after 10 weeks of NMN treatment. N-methyl-nicotinamide, 2PY, and 4PY increased in quadriceps muscle tissue samples obtained 1.5 h after the last dose of NMN | NMN increased muscle insulin sensitivity assessed using the hyperinsulinemic-euglycemic clamp. | 2021 | [194] |
| Single-blind study | Age, 45–75 years; males and females with sleep disturbance without primary conditions (NMN, n = 32; placebo, n = 31) | 300 mg/day; oral administration; capsule | 12 weeks | N/A | NMN improved sleep quality assessed using PSQI and smart bands sleep data | 2022 | [195] |
| Open-label, single-arm exploratory study | Age, 20–70 years; healthy males and females (n = 10) | 300 mg/day; intravenous administration; dissolved in saline (100 mL) | Single administration | Total amount of NAD+ levels in the blood was increased. | Intravenous NMN administration reduced blood triglyceride levels without affecting blood cells, electrocardiograms, pulse, blood pressure, and metabolic markers in the liver, heart, pancreas, and kidneys. | 2022 | [196] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagahisa, T.; Kosugi, S.; Yamaguchi, S. Interactions between Intestinal Homeostasis and NAD+ Biology in Regulating Incretin Production and Postprandial Glucose Metabolism. Nutrients 2023, 15, 1494. https://doi.org/10.3390/nu15061494
Nagahisa T, Kosugi S, Yamaguchi S. Interactions between Intestinal Homeostasis and NAD+ Biology in Regulating Incretin Production and Postprandial Glucose Metabolism. Nutrients. 2023; 15(6):1494. https://doi.org/10.3390/nu15061494
Chicago/Turabian StyleNagahisa, Taichi, Shotaro Kosugi, and Shintaro Yamaguchi. 2023. "Interactions between Intestinal Homeostasis and NAD+ Biology in Regulating Incretin Production and Postprandial Glucose Metabolism" Nutrients 15, no. 6: 1494. https://doi.org/10.3390/nu15061494
APA StyleNagahisa, T., Kosugi, S., & Yamaguchi, S. (2023). Interactions between Intestinal Homeostasis and NAD+ Biology in Regulating Incretin Production and Postprandial Glucose Metabolism. Nutrients, 15(6), 1494. https://doi.org/10.3390/nu15061494






