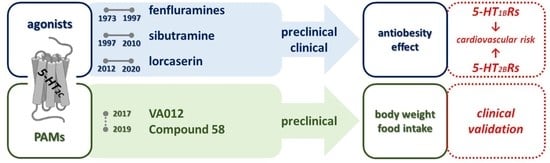
5-HT2C Receptor Stimulation in Obesity Treatment: Orthosteric Agonists vs. Allosteric Modulators
Abstract
1. Introduction
2. 5-HT2C Receptor Action in Food Intake Regulation
3. 5-HT2C Receptor Agonists in Control over Food Intake Preclinical Research
3.1. Piperazine Derivatives
3.2. Fenfluramines
3.3. Sibutramine
3.4. Lorcaserin
4. Clinical Effects of 5-HT2C Receptor Agonist Drugs
4.1. Fenfluramines
4.2. Sibutramine
4.3. Lorcaserin
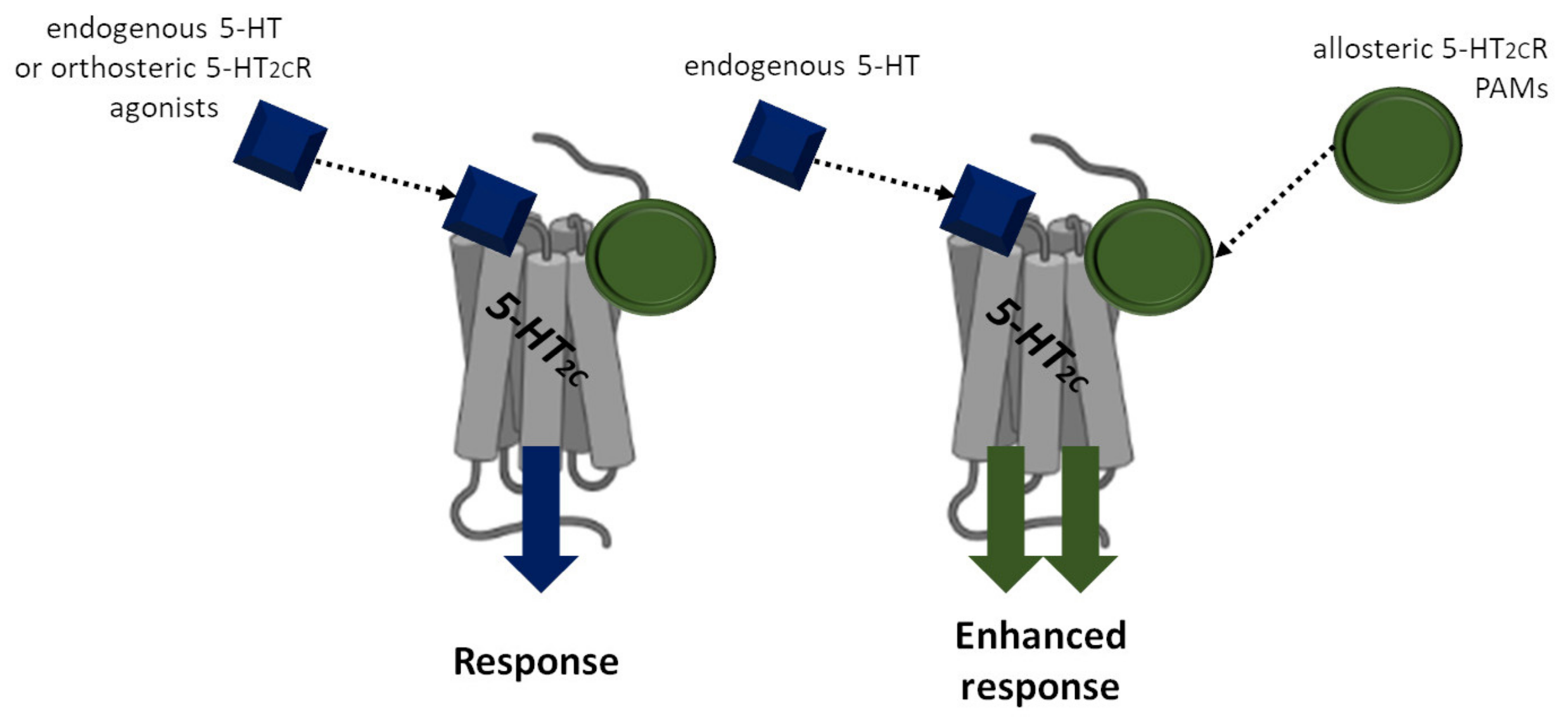
5. Positive-Allosteric Modulators of the 5-HT2CR
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hurt, R.T.; Mundi, M.S.; Ebbert, J.O. Challenging Obesity, Diabetes, and Addiction: The Potential of Lorcaserin Extended Release. Diabetes Metab. Syndr. Obes. 2018, 11, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Dutton, A.C.; Barnes, N.M. Anti-Obesity Pharmacotherapy: Future Perspectives Utilising 5-HT2C Receptor Agonists. Drug Discov. Today Ther. Strat. 2006, 3, 577–583. [Google Scholar] [CrossRef]
- Saller, C.F.; Stricker, E.M. Hyperphagia and Increased Growth in Rats After Intraventricular Injection of 5,7-Dihydroxytryptamine. Science 1976, 191, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.E.; Leshem, M.B. The Effect of 5-Hydroxytryptophan on Food Intake and on the Anorexic Action of Amphetamine and Fenfluramine. J Pharm. Pharmacol. 1975, 27, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Duhault, J.; Malen, C.; Boulanger, M.; Voisin, C.; Beregi, L.; Schmitt, H. Fenfluramine and 5 Hydroxytryptamine. I: Is Fenfluramine or Norfenfluramine Involved in the Decrease of Brain 5 Hydroxytryptamine? Arzneim.-Forsch./Drug Res. 1975, 25, 1755–1758. [Google Scholar]
- Masson, J.; Emerit, M.B.; Hamon, M.; Darmon, M. Serotonergic Signaling: Multiple Effectors and Pleiotropic Effects. Wiley Interdiscip. Rev. Membr. Transp. Signal. 2012, 1, 685–713. [Google Scholar] [CrossRef]
- Tecott, L.H.; Sun, L.M.; Akana, S.F.; Strack, A.M.; Lowenstein, D.H.; Dallman, M.F.; Julius, D. Eating Disorder and Epilepsy in Mice Lacking 5-HT2C Serotonin Receptors. Nature 1995, 374, 542–546. [Google Scholar] [CrossRef]
- Nonogaki, K.; Nozue, K.; Oka, Y. Hyperphagia Alters Expression of Hypothalamic 5-HT2C and 5-HT1B Receptor Genes and Plasma Des-Acyl Ghrelin Levels in Ay Mice. Endocrinology 2006, 147, 5893–5900. [Google Scholar] [CrossRef][Green Version]
- Bello, N.T.; Liang, N.C. The Use of Serotonergic Drugs to Treat Obesity—Is There Any Hope? Drug Des. Dev. Ther. 2011, 5, 95–109. [Google Scholar] [CrossRef][Green Version]
- Schuhler, S.; Clark, A.; Joseph, W.; Patel, A.; Lehnen, K.; Stratford, E.; Horan, T.L.; Fone, K.C.F.; Ebling, F.J.P.; Schuhler, A. Involvement of 5-HT Receptors in the Regulation of Food Intake in Siberian Hamsters. J. Neuroendocrinol. 2005, 17, 276–285. [Google Scholar] [CrossRef]
- Bonhaus, D.W.; Weinhardt, K.K.; Taylor, M.; Desouza, A.; Mcneeley, P.M.; Szczepanski, K.; Fontana, D.J.; Trinh, J.; Rocha, C.L.; Dawson, M.W.; et al. RS-102221: A Novel High Affinity and Selective, 5-HT2C Receptor Antagonist. Neuropharmacology 1997, 36, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Wold, E.A.; Wild, C.T.; Cunningham, K.A.; Zhou, J. Targeting the 5-HT2C Receptor in Biological Context and the Current State of 5-HT2C Receptor Ligand Development. Curr. Top. Med. Chem. 2019, 19, 1381–1398. [Google Scholar] [CrossRef] [PubMed]
- Myers, M.G.; Olson, D.P. Central Nervous System Control of Metabolism. Nature 2012, 491, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Gropp, E.; Shanabrough, M.; Borok, E.; Xu, A.W.; Janoschek, R.; Buch, T.; Plum, L.; Balthasar, N.; Hampel, B.; Waisman, A.; et al. Agouti-Related Peptide–Expressing Neurons Are Mandatory for Feeding. Nat. Neurosci. 2005, 8, 1289–1291. [Google Scholar] [CrossRef]
- Balthasar, N.; Dalgaard, L.T.; Lee, C.E.; Yu, J.; Funahashi, H.; Williams, T.; Ferreira, M.; Tang, V.; McGovern, R.A.; Kenny, C.D.; et al. Divergence of Melanocortin Pathways in the Control of Food Intake and Energy Expenditure. Cell 2005, 123, 493–505. [Google Scholar] [CrossRef]
- Van Galen, K.A.; Ter Horst, K.W.; Serlie, M.J. Serotonin, Food Intake, and Obesity. Obes. Rev. 2021, 22, e13210. [Google Scholar] [CrossRef]
- Doslikova, B.; Garfield, A.S.; Shaw, J.; Evans, M.L.; Burdakov, D.; Billups, B.; Heisler, L.K. 5-HT2C Receptor agonist Anorectic Efficacy Potentiated by 5-HT1B Receptor agonist Coapplication: An Effect Mediated via Increased Proportion of Pro-Opiomelanocortin Neurons Activated. J. Neurosci. 2013, 33, 9800–9804. [Google Scholar] [CrossRef]
- Berglund, E.D.; Liu, C.; Sohn, J.W.; Liu, T.; Kim, M.H.; Lee, C.E.; Vianna, C.R.; Williams, K.W.; Xu, Y.; Elmquist, J.K. Serotonin 2C Receptors in Pro-Opiomelanocortin Neurons Regulate Energy and Glucose Homeostasis. J. Clin. Investig. 2013, 123, 5061–5070. [Google Scholar] [CrossRef]
- Wyler, S.C.; Lord, C.C.; Lee, S.; Elmquist, J.K.; Liu, C. Serotonergic Control of Metabolic Homeostasis. Front. Cell. Neurosci. 2017, 11, 277. [Google Scholar] [CrossRef]
- Roepke, T.A.; Smith, A.W.; Rønnekleiv, O.K.; Kelly, M.J. Serotonin 5-HT2C Receptor-Mediated Inhibition of the M-Current in Hypothalamic POMC Neurons. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E1399–E1406. [Google Scholar] [CrossRef]
- Xu, Y.; Jones, J.E.; Kohno, D.; Williams, K.W.; Lee, C.E.; Choi, M.J.; Anderson, J.G.; Heisler, L.K.; Zigman, J.M.; Lowell, B.B.; et al. 5-HT2CRs Expressed by Pro-Opiomelanocortin Neurons Regulate Energy Homeostasis. Neuron 2008, 60, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Alonso, M.; Woods, S.C.; Pelchat, M.; Grigson, P.S.; Stice, E.; Farooqi, S.; Khoo, C.S.; Mattes, R.D.; Beauchamp, G.K. Food Reward System: Current Perspectives and Future Research Needs. Nutr. Rev. 2015, 73, 296–307. [Google Scholar] [CrossRef]
- Leenaerts, N.; Jongen, D.; Ceccarini, J.; van Oudenhove, L.; Vrieze, E. The Neurobiological Reward System and Binge Eating: A Critical Systematic Review of Neuroimaging Studies. Int. J. Eat. Disord. 2022, 55, 1421–1458. [Google Scholar] [CrossRef] [PubMed]
- Amianto, F.; Ottone, L.; Abbate Daga, G.; Fassino, S. Binge-Eating Disorder Diagnosis and Treatment: A Recap in Front of DSM-5. BMC Psychiatry 2015, 15, 70. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, K.A.; Fox, R.G.; Anastasio, N.C.; Bubar, M.J.; Stutz, S.J.; Moeller, F.G.; Gilbertson, S.R.; Rosenzweig-Lipson, S. Selective Serotonin 5-HT(2C) Receptor Activation Suppresses the Reinforcing Efficacy of Cocaine and Sucrose but Differentially Affects the Incentive-Salience Value of Cocaine- vs. Sucrose-Associated Cues. Neuropharmacology 2011, 61, 513–523. [Google Scholar] [CrossRef]
- Price, A.E.; Anastasio, N.C.; Stutz, S.J.; Hommel, J.D.; Cunningham, K.A. Serotonin 5-HT2C Receptor Activation Suppresses Binge Intake and the Reinforcing and Motivational Properties of High-Fat Food. Front. Pharmacol. 2018, 9, 821. [Google Scholar] [CrossRef]
- Xu, P.; He, Y.; Cao, X.; Valencia-Torres, L.; Yan, X.; Saito, K.; Wang, C.; Yang, Y.; Hinton, A.; Zhu, L.; et al. Activation of Serotonin 2C Receptors in Dopamine Neurons Inhibits Binge-like Eating in Mice. Biol. Psychiatry 2017, 81, 737–747. [Google Scholar] [CrossRef]
- Bubar, M.J.; Cunningham, K.A. Distribution of Serotonin 5-HT2C Receptors in the Ventral Tegmental Area. Neuroscience 2007, 146, 286–297. [Google Scholar] [CrossRef]
- Bubar, M.J.; Stutz, S.J.; Cunningham, K.A. 5-HT2C Receptors Localize to Dopamine and GABA Neurons in the Rat Mesoaccumbens Pathway. PLoS ONE 2011, 6, e20508. [Google Scholar] [CrossRef]
- De Deurwaerdère, P.; Navailles, S.; Berg, K.A.; Clarke, W.P.; Spampinato, U. Constitutive Activity of the Serotonin2C Receptor Inhibits In Vivo Dopamine Release in the Rat Striatum and Nucleus Accumbens. J. Neurosci. 2004, 24, 3235. [Google Scholar] [CrossRef] [PubMed]
- Hayes, D.J.; Mosher, T.M.; Greenshaw, A.J. Differential Effects of 5-HT2C Receptor Activation by WAY 161503 on Nicotine-Induced Place Conditioning and Locomotor Activity in Rats. Behav. Brain Res. 2009, 197, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Torres, L.; Olarte-Sánchez, C.M.; Lyons, D.J.; Georgescu, T.; Greenwald-Yarnell, M.; Myers, M.G.; Bradshaw, C.M.; Heisler, L.K. Activation of Ventral Tegmental Area 5-HT2C Receptors Reduces Incentive Motivation. Neuropsychopharmacology 2017, 42, 1511–1521. [Google Scholar] [CrossRef] [PubMed]
- Rothman, R.B.; Baumann, M.H.; Savage, J.E.; Rauser, L.; McBride, A.; Hufeisen, S.J.; Roth, B.L. Evidence for Possible Involvement of 5-HT2B Receptors in the Cardiac Valvulopathy Associated With Fenfluramine and Other Serotonergic Medications. Circulation 2000, 102, 2836–2841. [Google Scholar] [CrossRef]
- Setola, V.; Dukat, M.; Glennon, R.A.; Roth, B.L. Molecular Determinants for the Interaction of the Valvulopathic Anorexigen Norfenfluramine with the 5-HT2B Receptor. Mol. Pharmacol. 2005, 68, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Frassetto, S.S.; Delia Santa Rubio, Â.; Lopes, J.J.; Pereira, P.; Brum, C.; Khazzaka, M.; Vinagre, A.S. Locomotor and Peripheral Effects of Sibutramine Modulated by 5-HT2 Receptors. Can. J. Physiol. Pharmacol. 2006, 84, 1239–1244. [Google Scholar] [CrossRef]
- Thomsen, W.J.; Grottick, A.J.; Menzaghi, F.; Reyes-Saldana, H.; Espitia, S.; Yuskin, D.; Whelan, K.; Martin, M.; Morgan, M.; Chen, W.; et al. Lorcaserin, a Novel Selective Human 5-Hydroxytryptamine2C Agonist: In Vitro and in Vivo Pharmacological Characterization. J. Pharmacol. Exp. Ther. 2008, 325, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Bickerdike, M. 5-HT2C Receptor agonists as Potential Drugs for the Treatment of Obesity. Curr. Top. Med. Chem. 2003, 3, 885–897. [Google Scholar] [CrossRef]
- Georgescu, T.; Lyons, D.; Heisler, L.K. Role of Serotonin in Body Weight, Insulin Secretion and Glycaemic Control. J. Neuroendocrinol. 2021, 33, e12960. [Google Scholar] [CrossRef]
- Hayashi, A.; Suzuki, M.; Sasamata, M.; Miyata, K. Agonist diversity in 5-HT2C receptor-mediated weight control in rats. Psychopharmacology 2005, 178, 241–249. [Google Scholar] [CrossRef]
- Xu, Y.; Jones, J.E.; Lauzon, D.A.; Anderson, J.G.; Balthasar, N.; Heisler, L.K.; Zinn, A.R.; Lowell, B.B.; Elmquist, J.K. A Serotonin and Melanocortin Circuit Mediates D-Fenfluramine Anorexia. J. Neurosci. 2010, 30, 14630–14634. [Google Scholar] [CrossRef]
- Martin, J.R.; Bös, M.; Jenck, F.; Moreau, J.L.; Mutel, V.; Sleight, A.J.; Wichmann, J.; Andrews, J.S.; Berendsen, H.H.G.; Broekkamp, C.L.E.; et al. 5-HT2C Receptor Agonists: Pharmacological Characteristics and Therapeutic Potential. J. Pharmacol. Exp. Ther. 1998, 286, 913–924. [Google Scholar] [PubMed]
- Kimura, Y.; Hatanaka, K.I.; Naitou, Y.; Maeno, K.; Shimada, I.; Koakutsu, A.; Wanibuchi, F.; Yamaguchi, T. Pharmacological Profile of YM348, a Novel, Potent and Orally Active 5-HT2C Receptor Agonist. Eur. J. Pharmacol. 2004, 483, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Vickers, S.P.; Benwell, K.R.; Porter, R.H.; Bickerdike, M.J.; Kennett, G.A.; Dourish, C.T. Comparative Effects of Continuous Infusion of MCPP, Ro 60-0175 and d-Fenfluramine on Food Intake, Water Intake, Body Weight and Locomotor Activity in Rats. Br. J. Pharmacol. 2000, 130, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, J.; Sabb, A.L.; Mazandarani, H.; Zhang, J.; Kalgaonker, S.; Shukhina, E.; Sukoff, S.; Vogel, R.L.; Stack, G.; Schechter, L.; et al. WAY-163909 [(7bR, 10aR)-1,2,3,4,8,9,10,10a-Octahydro-7bH-Cyclopenta-[b][1,4]Diazepino[6,7,1hi]Indole], a Novel 5-Hydroxytryptamine 2C Receptor-Selective Agonist with Anorectic Activity. J. Pharmacol. Exp. Ther. 2005, 313, 862–869. [Google Scholar] [CrossRef]
- Vickers, S.P.; Clifton, P.G.; Dourish, C.T.; Tecott, L.H. Reduced Satiating Effect of D-Fenfluramine in Serotonin 5-HT(2C) Receptor Mutant Mice. Psychopharmacology 1999, 143, 309–314. [Google Scholar] [CrossRef]
- Vickers, S.P.; Dourish, C.T.; Kennett, G.A. Evidence That Hypophagia Induced by D-Fenfluramine and d-Norfenfluramine in the Rat Is Mediated by 5-HT2C Receptors. Neuropharmacology 2001, 41, 200–209. [Google Scholar] [CrossRef]
- Fisler, J.S.; Underberger, S.J.; York, D.A.; Bray, G.A. D-Fenfluramine in a Rat Model of Dietary Fat-Induced Obesity. Pharmacol. Biochem. Behav. 1993, 45, 487–493. [Google Scholar] [CrossRef]
- Clifton, P.G.; Lee, M.D.; Dourish, C.T. Similarities in the Action of Ro 60-0175, a 5-HT2C Receptor Agonist, and d-Fenfluramine on Feeding Patterns in the Rat. Psychopharmacology 2000, 152, 256–267. [Google Scholar] [CrossRef]
- Brindley, D.N.; Hales, P.; Al-Sieni, A.I.I.; Russell, J.C. Sustained Decreases in Weight and Serum Insulin, Glucose, Triacylglycerol and Cholesterol in JCR:LA-Corpulent Rats Treated with D-Fenfluramine. Br. J. Pharmacol. 1992, 105, 679–685. [Google Scholar] [CrossRef]
- Pratt, W.E.; Ford, R.T. Systemic Treatment with D-Fenfluramine, but Not Sibutramine, Blocks Cue-Induced Reinstatement of Food-Seeking Behavior in the Rat. Neurosci. Lett. 2013, 556, 232–237. [Google Scholar] [CrossRef]
- Burke, L.K.; Doslikova, B.; D’Agostino, G.; Garfield, A.S.; Farooq, G.; Burdakov, D.; Low, M.J.; Rubinstein, M.; Evans, M.L.; Billups, B.; et al. 5-HT Obesity Medication Efficacy via POMC Activation Is Maintained During Aging. Endocrinology 2014, 155, 3732–3738. [Google Scholar] [CrossRef] [PubMed]
- Higgs, S.; Cooper, A.J.; Barnes, N.M. Reversal of Sibutramine-Induced Anorexia with a Selective 5-HT(2C) Receptor Antagonist. Psychopharmacology 2011, 214, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Levin, B.E.; Dunn-Meynell, A.A. Sibutramine Alters the Central Mechanisms Regulating the Defended Body Weight in Diet-Induced Obese Rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R2222–R2228. [Google Scholar] [CrossRef]
- Hansen, H.H.; Hansen, G.; Tang-Christensen, M.; Larsen, P.J.; Axel, A.M.D.; Raben, A.; Mikkelsen, J.D. The Novel Triple Monoamine Reuptake Inhibitor Tesofensine Induces Sustained Weight Loss and Improves Glycemic Control in the Diet-Induced Obese Rat: Comparison to Sibutramine and Rimonabant. Eur. J. Pharmacol. 2010, 636, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Madsen, A.N.; Hansen, G.; Paulsen, S.J.; Lykkegaard, K.; Tang-Christensen, M.; Hansen, H.S.; Levin, B.E.; Larsen, P.J.; Knudsen, L.B.; Fosgerau, K.; et al. Long-Term Characterization of the Diet-Induced Obese and Diet-Resistant Rat Model: A Polygenetic Rat Model Mimicking the Human Obesity Syndrome. J. Endocrinol. 2010, 206, 287–296. [Google Scholar] [CrossRef]
- Hansen, G.; Jelsing, J.; Vrang, N. Effects of Liraglutide and Sibutramine on Food Intake, Palatability, Body Weight and Glucose Tolerance in the Gubra DIO-Rats. Acta Pharmacol. Sin. 2012, 33, 194–200. [Google Scholar] [CrossRef]
- Casado, A.; Rodríguez, V.M.; Portillo, M.P.; Macarulla, M.T.; Abecia, L.C.; Echevarría, E.; Casis, L. Sibutramine Decreases Body Weight Gain and Increases Energy Expenditure in Obese Zucker Rats without Changes in NPY and Orexins. Nutr. Neurosci. 2003, 6, 102–111. [Google Scholar] [CrossRef]
- Jackson, H.C.; Needham, A.M.; Hutchins, L.J.; Mazurkiewicz, S.E.; Heal, D.J. Comparison of the Effects of Sibutramine and Other Monoamine Reuptake Inhibitors on Food Intake in the Rat. Br. J. Pharmacol. 1997, 121, 1758–1762. [Google Scholar] [CrossRef]
- Pratt, W.E.; Connolly, M.E. Contrasting Effects of Systemic and Central Sibutramine Administration on the Intake of a Palatable Diet in the Rat. Neurosci. Lett. 2010, 484, 30–34. [Google Scholar] [CrossRef]
- Smith, B.M.; Smith, J.M.; Tsai, J.H.; Schultz, J.A.; Gilson, C.A.; Estrada, S.A.; Chen, R.R.; Park, D.M.; Prieto, E.B.; Gallardo, C.S.; et al. Discovery and Structure-Activity Relationship of (1R)-8-Chloro-2,3,4,5-Tetrahydro-1-Methyl-1H-3-Benzazepine (Lorcaserin), a Selective Serotonin 5-HT2C Receptor Agonist for the Treatment of Obesity. J. Med. Chem. 2008, 51, 305–313. [Google Scholar] [CrossRef]
- Higgs, S.; Cooper, A.J.; Barnes, N.M. The 5-HT2C Receptor Agonist, Lorcaserin, and the 5-HT6 Receptor Antagonist, SB-742457, Promote Satiety; A Microstructural Analysis of Feeding Behaviour. Psychopharmacology 2016, 233, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Higgins, G.A.; Desnoyer, J.; van Niekerk, A.; Silenieks, L.B.; Lau, W.; Thevarkunnel, S.; Izhakova, J.; Delannoy, I.A.M.; Fletcher, P.J.; Delay, J.; et al. Characterization of the 5-HT2C Receptor Agonist Lorcaserin on Efficacy and Safety Measures in a Rat Model of Diet-Induced Obesity. Pharmacol. Res. Perspect. 2015, 3, e00084. [Google Scholar] [CrossRef] [PubMed]
- Price, A.E.; Brehm, V.D.; Hommel, J.D.; Anastasio, N.C.; Cunningham, K.A. Pimavanserin and Lorcaserin Attenuate Measures of Binge Eating in Male Sprague-Dawley Rats. Front. Pharmacol. 2018, 9, 1424. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, S.A.; Pratt, W.E. D-Fenfluramine and Lorcaserin Inhibit the Binge-like Feeding Induced by μ-Opioid Receptor Stimulation of the Nucleus Accumbens in the Rat. Neurosci. Lett. 2018, 687, 43–48. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, G.; Lyons, D.; Cristiano, C.; Lettieri, M.; Olarte-Sanchez, C.; Burke, L.K.; Greenwald-Yarnell, M.; Cansell, C.; Doslikova, B.; Georgescu, T.; et al. Nucleus of the Solitary Tract Serotonin 5-HT2C Receptors Modulate Food Intake. Cell Metab. 2018, 28, 619. [Google Scholar] [CrossRef] [PubMed]
- Zhan, C.; Zhou, J.; Feng, Q.; Zhang, J.E.; Lin, S.; Bao, J.; Wu, P.; Luo, M. Acute and Long-Term Suppression of Feeding Behavior by POMC Neurons in the Brainstem and Hypothalamus, Respectively. J. Neurosci. 2013, 33, 3624. [Google Scholar] [CrossRef]
- Kwon, E.; Jo, Y.H. Activation of the ARCPOMC→MeA Projection Reduces Food Intake. Front. Neural Circuits 2020, 14, 595783. [Google Scholar] [CrossRef]
- Higgins, G.A.; Zeeb, F.D.; Fletcher, P.J. Role of Impulsivity and Reward in the Anti-Obesity Actions of 5-HT 2C Receptor Agonists. J. Psychopharmacol. 2017, 31, 1403–1418. [Google Scholar] [CrossRef]
- Burke, L.K.; Ogunnowo-Bada, E.; Georgescu, T.; Cristiano, C.; de Morentin, P.B.M.; Valencia Torres, L.; D’Agostino, G.; Riches, C.; Heeley, N.; Ruan, Y.; et al. Lorcaserin Improves Glycemic Control via a Melanocortin Neurocircuit. Mol. Metab. 2017, 6, 1092–1102. [Google Scholar] [CrossRef]
- Marbury, T.C.; Angelo, J.E.; Michael Gulley, R.; Krosnick, A.; Sugimoto, D.H.; Zellner, S.R. A Placebo-Controlled, Dose-Response Study of Dexfenfluramine in the Treatment of Obese Patients. Curr. Ther. Res. 1996, 57, 663–674. [Google Scholar] [CrossRef]
- Lucas, C.P.; Sandage, B.W. Treatment of Obese Patients with Dexfenfluramine: A Multicenter, Placebo-Controlled Study. Am. J. Ther. 1995, 2, 962–967. [Google Scholar] [CrossRef] [PubMed]
- Drent, M.L.; Adèr, H.J.; van der Veen, E.A. The Influence of Chronic Administration of the Serotonin Agonist Dexfenfluramine on Responsiveness to Corticotropin Releasing Hormone and Growth Hormone-Releasing Hormone in Moderately Obese People. J. Endocrinol. Investig. 1995, 18, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Guy-Grand, B.; Crepaldi, G.; Vre, P.L.; Apfelbaum, M.; Gries, A.; Turner, P. International Trial of Long-Term Dexfenfluramine in Obesity. Lancet 1989, 2, 1142–1145. [Google Scholar] [CrossRef] [PubMed]
- Lafreniere, F.; Lambert, L.J.; Rasio, E.; Serri, O. Effects of Dexfenfluramine Treatment on Body Weight and Postprandial Thermogenesis in Obese Subjects. A Double-Blind Placebo-Controlled Study. Int. J. Obes. Relat. Metab. Disord. 1993, 17, 25–30. [Google Scholar] [PubMed]
- Mathus-Vliegen, E.M.H.; van de Voorde, K.; Kok, A.M.E.; Res, A.M.A. Dexfenfluramine in the Treatment of Severe Obesity: A Placebo-Controlled Investigation of the Effects on Weight Loss, Cardiovascular Risk Factors, Food Intake and Eating Behaviour. J. Intern. Med. 1992, 232, 119–127. [Google Scholar] [CrossRef]
- Weintraub, M.; Rubio, A.; Golik, A.; Byrne, L.; Scheinbaum, M.L. Sibutramine in Weight Control: A Dose-Ranging, Efficacy Study. Clin. Pharmacol. Ther. 1991, 50, 330–337. [Google Scholar] [CrossRef]
- Hanotin, C.; Thomas, F.; Jones, S.P.; Leutenegger, E.; Drouin, P. Efficacy and Tolerability of Sibutramine in Obese Patients: A Dose-Ranging Study. Int. J. Obes. 1997, 22, 32–38. [Google Scholar] [CrossRef][Green Version]
- Bray, G.A.; Blackburn, G.L.; Ferguson, J.M.; Greenway, F.L.; Jain, A.K.; Mendel, C.M.; Mendels, J.; Ryan, D.H.; Schwartz, S.L.; Scheinbaum, M.L.; et al. Sibutramine Produces Dose-Related Weight Loss. Obes. Res. 1999, 7, 189–198. [Google Scholar] [CrossRef]
- Di Francesco, V.; Sacco, T.; Zamboni, M.; Bissoli, L.; Zoico, E.; Mazzali, G.; Minniti, A.; Salanitri, T.; Cancelli, F.; Bosello, O. Weight Loss and Quality of Life Improvement in Obese Subjects Treated with Sibutramine: A Double-Blind Randomized Multicenter Study. Ann. Nutr. Metab. 2007, 51, 75–81. [Google Scholar] [CrossRef]
- James, W.P.T.; Caterson, I.D.; Coutinho, W.; Finer, N.; van Gaal, L.F.; Maggioni, A.P.; Torp-Pedersen, C.; Sharma, A.M.; Shepherd, G.M.; Rode, R.A.; et al. Effect of Sibutramine on Cardiovascular Outcomes in Overweight and Obese Subjects. N. Engl. J. Med. 2010, 363, 905–917. [Google Scholar] [CrossRef]
- Berkowitz, R.I.; Fujioka, K.; Daniels, S.R.; Hoppin, A.G.; Owen, S.; Perry, A.C.; Sothern, M.S.; Renz, C.L.; Pirner, M.A.; Walch, J.K.; et al. Effects of Sibutramine Treatment in Obese Adolescents: A Randomized Trial. Ann. Intern. Med. 2006, 145, 81–90. [Google Scholar] [CrossRef] [PubMed]
- McNulty, S.J.; Ur, E.; Williams, G. A Randomized Trial of Sibutramine in the Management of Obese Type 2 Diabetic Patients Treated With Metformin. Diabetes Care 2003, 26, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Hauner, H.; Meier, M.; Wendland, G.; Kurscheid, T.; Lauterbach, K. Weight Reduction by Sibutramine in Obese Subjects in Primary Care Medicine: The S.A.T. Study. Exp. Clin. Endocrinol. Diabetes 2004, 112, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Dujovne, C.A.; Zavoral, J.H.; Rowe, E.; Mendel, C.M. Effects of Sibutramine on Body Weight and Serum Lipids: A Double-Blind, Randomized, Placebo-Controlled Study in 322 Overweight and Obese Patients with Dyslipidemia. Am. Heart J. 2001, 142, 489–497. [Google Scholar] [CrossRef][Green Version]
- Martin, C.K.; Redman, L.M.; Zhang, J.; Sanchez, M.; Anderson, C.M.; Smith, S.R.; Ravussin, E. Lorcaserin, a 5-HT(2C) Receptor Agonist, Reduces Body Weight by Decreasing Energy Intake without Influencing Energy Expenditure. J. Clin. Endocrinol. Metab. 2011, 96, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.R.; Prosser, W.A.; Donahue, D.J.; Morgan, M.E.; Anderson, C.M.; Shanahan, W.R. Lorcaserin (APD356), a Selective 5-HT2C Agonist, Reduces Body Weight in Obese Men and Women. Obesity 2009, 17, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Shaw Tronieri, J.; Wadden, T.A.; Berkowitz, R.I.; Chao, A.M.; Pearl, R.L.; Alamuddin, N.; Leonard, S.M.; Carvajal, R.; Bakizada, Z.M.; Pinkasavage, E.; et al. A Randomized Trial of Lorcaserin and Lifestyle Counseling for Maintaining Weight Loss Achieved with a Low-Calorie Diet. Obesity 2018, 26, 299–309. [Google Scholar] [CrossRef]
- Bohula, E.A.; Wiviott, S.D.; McGuire, D.K.; Inzucchi, S.E.; Kuder, J.; Im, K.; Fanola, C.L.; Qamar, A.; Brown, C.; Budaj, A.; et al. Cardiovascular Safety of Lorcaserin in Overweight or Obese Patients. N. Engl. J. Med. 2018, 379, 1107–1117. [Google Scholar] [CrossRef]
- O’Neil, P.M.; Smith, S.R.; Weissman, N.J.; Fidler, M.C.; Sanchez, M.; Zhang, J.; Raether, B.; Anderson, C.M.; Shanahan, W.R. Randomized Placebo-Controlled Clinical Trial of Lorcaserin for Weight Loss in Type 2 Diabetes Mellitus: The BLOOM-DM Study. Obesity 2012, 20, 1426–1436. [Google Scholar] [CrossRef]
- Smith, S.R.; Weissman, N.J.; Anderson, C.M.; Sanchez, M.; Chuang, E.; Stubbe, S.; Bays, H.; Shanahan, W.R. Multicenter, Placebo-Controlled Trial of Lorcaserin for Weight Management. N. Engl. J. Med. 2010, 363, 245–256. [Google Scholar] [CrossRef]
- Aronne, L.; Shanahan, W.; Fain, R.; Glicklich, A.; Soliman, W.; Li, Y.; Smith, S. Safety and Efficacy of Lorcaserin: A Combined Analysis of the BLOOM and BLOSSOM Trials. Postgrad. Med. 2014, 126, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Connolly, H.M.; Crary, J.L.; McGoon, M.D.; Hensrud, D.D.; Edwards, B.S.; Edwards, W.D.; Schaff, H.V. Valvular Heart Disease Associated with Fenfluramine-Phentermine. N. Engl. J. Med. 1997, 337, 141. [Google Scholar] [CrossRef]
- Khan, M.A.; Herzog, C.A.; Peter, J.V.S.; Hartley, G.G.; Madlon-Kay, R.; Dick, C.D.; Asinger, R.W.; Vessey, J.T. The Prevalence of Cardiac Valvular Insufficiency Assessed by Transthoracic Echocardiography in Obese Patients Treated with Appetite-Suppressant Drugs. N. Engl. J. Med. 1998, 339, 713–718. [Google Scholar] [CrossRef]
- Kancherla, M.K.; Salti, H.I.; Mulderink, T.A.; Parker, M.; Bonow, R.O.; Mehlman, D.J. Echocardiographic Prevalence of Mitral and/or Aortic Regurgitation in Patients Exposed to Either Fenfluramine-Phentermine Combination or to Dexfenfluramine. Am. J. Cardiol. 1999, 84, 1335–1338. [Google Scholar] [CrossRef] [PubMed]
- Surapaneni, P.; Vinales, K.L.; Najib, M.Q.; Chaliki, H.P. Valvular Heart Disease with the Use of Fenfluramine-Phentermine. Tex. Heart Inst. J. 2011, 38, 581. [Google Scholar]
- McMurray, C.; Bloomfield, P.; Miller, H.C. Irreversible Pulmonary Hypertension after Treatment with Fenfluramine. Br. Med. J. 1986, 292, 239. [Google Scholar] [CrossRef] [PubMed]
- Pouwels, H.; Smeets, J.; Cheriex, E.; Wouters, E. Pulmonary Hypertension and Fenfluramine. Eur. Respir. J. 1990, 3, 606–607. [Google Scholar] [CrossRef] [PubMed]
- Bang, W.D.; Kim, J.Y.; Yu, H.T.; Cho, S.S.; Jang, J.Y.; Oh, C.M.; Joung, B.; Chang, H.J. Pulmonary Hypertension Associated with Use of Phentermine. Yonsei Med. J. 2010, 51, 971–973. [Google Scholar] [CrossRef][Green Version]
- Schoonjans, A.S.; Marchau, F.; Paelinck, B.P.; Lagae, L.; Gammaitoni, A.; Pringsheim, M.; Keane, M.G.; Ceulemans, B. Cardiovascular Safety of Low-Dose Fenfluramine in Dravet Syndrome: A Review of Its Benefit-Risk Profile in a New Patient Population. Curr. Med. Res. Opin. 2017, 33, 1773–1781. [Google Scholar] [CrossRef]
- Davis, R.; Faulds, D. Dexfenfluramine. An Updated Review of Its Therapeutic Use in the Management of Obesity. Drugs 1996, 52, 696–724. [Google Scholar] [CrossRef]
- Fitzgerald, L.W.; Burn, T.C.; Brown, B.S.; Patterson, J.P.; Corjay, M.H.; Valentine, P.A.; Sun, J.H.; Link, J.R.; Abbaszade, I.; Hollis, J.M.; et al. Possible Role of Valvular Serotonin 5-HT(2B) Receptors in the Cardiopathy Associated with Fenfluramine. Mol. Pharmacol. 2000, 57, 75–81. [Google Scholar] [PubMed]
- Seimon, R.V.; Espinoza, D.; Ivers, L.; Gebski, V.; Finer, N.; Legler, U.F.; Sharma, A.M.; James, W.P.T.; Coutinho, W.; Caterson, I.D. Changes in Body Weight and Blood Pressure: Paradoxical Outcome Events in Overweight and Obese Subjects with Cardiovascular Disease. Int. J. Obes. 2014, 38, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Daniels, S.R.; Long, B.; Crow, S.; Styne, D.; Sothern, M.; Vargas-Rodriguez, I.; Harris, L.; Walch, J.; Jasinsky, O.; Cwik, K.; et al. Cardiovascular Effects of Sibutramine in the Treatment of Obese Adolescents: Results of a Randomized, Double-Blind, Placebo-Controlled Study. Pediatrics 2007, 120, e147–e157. [Google Scholar] [CrossRef] [PubMed]
- Torp-Pedersen, C.; Caterson, I.; Coutinho, W.; Finer, N.; van Gaal, L.; Maggioni, A.; Sharma, A.; Brisco, W.; Deaton, R.; Shepherd, G.; et al. Cardiovascular Responses to Weight Management and Sibutramine in High-Risk Subjects: An Analysis from the SCOUT Trial. Eur. Heart J. 2007, 28, 2915–2923. [Google Scholar] [CrossRef] [PubMed]
- Zannad, F.; Gille, B.; Grentzinger, A.; Bruntz, J.F.; Hammadi, M.; Boivin, J.M.; Hanotin, C.; Igau, B.; Drouin, P. Effects of Sibutramine on Ventricular Dimensions and Heart Valves in Obese Patients during Weight Reduction. Am. Heart J. 2002, 144, 508–515. [Google Scholar] [CrossRef]
- Gürsoy, A.; Erdoǧan, M.F.; Cin, M.Ö.; Cesur, M.; Başkal, N. Effect of Sibutramine on Blood Pressure in Patients with Obesity and Well-Controlled Hypertension or Normotension. Endocr. Pract. 2005, 11, 308–312. [Google Scholar] [CrossRef]
- De Simone, G.; Romano, C.; de Caprio, C.; Contaldo, F.; Salanitri, T.; di Luzio Paparatti, U.; Pasanisi, F. Effects of Sibutramine-Induced Weight Loss on Cardiovascular System in Obese Subjects. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 24–30. [Google Scholar] [CrossRef]
- Heusser, K.; Engeli, S.; Tank, J.; Diedrich, A.; Wiesner, S.; Janke, J.; Luft, F.C.; Jordan, J. Sympathetic Vasomotor Tone Determines Blood Pressure Response to Long-Term Sibutramine Treatment. J. Clin. Endocrinol. Metab. 2007, 92, 1560–1563. [Google Scholar] [CrossRef][Green Version]
- Merenich, J.A. The Long-Term Outcomes of Sibutramine Effectiveness on Weight (LOSE Weight) Study: Evaluating the Role of Drug Therapy Within a Weight Management Program in a Group-Model Health Maintenance Organizati. Am. J. Manag. Care 2004, 10, 369–376. [Google Scholar] [CrossRef]
- Fanghänel, G.; Cortinas, L.; Sánchez-Reyes, L.; Berber, A. A Clinical Trial of the Use of Sibutramine for the Treatment of Patients Suffering Essential Obesity. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 144–150. [Google Scholar] [CrossRef][Green Version]
- Guven, A.; Koksal, N.; Cetinkaya, A.; Sokmen, G.; Ozdemir, R. Effects of the Sibutramine Therapy on Pulmonary Artery Pressure in Obese Patients. Diabetes Obes. Metab. 2004, 6, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Saraç, S.; Saraç, F. Cardiac Valve Evaluation and Adipokine Levels in Obese Women Treated with Sibutramine. Anadolu Kardiyol. Derg. 2010, 10, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Caterson, I.D.; Finer, N.; Coutinho, W.; van Gaal, L.F.; Maggioni, A.P.; Torp-Pedersen, C.; Sharma, A.M.; Legler, U.F.; Shepherd, G.M.; Rode, R.A.; et al. Maintained Intentional Weight Loss Reduces Cardiovascular Outcomes: Results from the Sibutramine Cardiovascular OUTcomes (SCOUT) Trial. Diabetes Obes. Metab. 2012, 14, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Maggioni, A.P.; Caterson, I.; Coutinho, W.; Finer, N.; van Gaal, L.; Sharma, A.M.; Torp-Pedersen, C.; Bacher, P.; Shepherd, G.; Sun, R.; et al. Tolerability of Sibutramine during a 6-Week Treatment Period in High-Risk Patients with Cardiovascular Disease and/or Diabetes: A Preliminary Analysis of the Sibutramine Cardiovascular Outcomes (SCOUT) Trial. J. Cardiovasc. Pharmacol. 2008, 52, 393–402. [Google Scholar] [CrossRef]
- Weeke, P.; Andersson, C.; Fosbøl, E.L.; Brendorp, B.; Køber, L.; Sharma, A.M.; Finer, N.; James, P.T.; Caterson, I.D.; Rode, R.A.; et al. The Weight Lowering Effect of Sibutramine and Its Impact on Serum Lipids in Cardiovascular High Risk Patients with and without Type 2 Diabetes Mellitus—An Analysis from the SCOUT Lead-in Period. BMC Endocr. Disord. 2010, 10, 3. [Google Scholar] [CrossRef]
- Nisoli, E.; Carruba, M.O. An Assessment of the Safety and Efficacy of Sibutramine, an Anti-Obesity Drug with a Novel Mechanism of Action. Obes. Rev. 2000, 1, 127–139. [Google Scholar] [CrossRef]
- Tuccinardi, D.; Farr, O.M.; Upadhyay, J.; Oussaada, S.M.; Mathew, H.; Paschou, S.A.; Perakakis, N.; Koniaris, A.; Kelesidis, T.; Mantzoros, C.S. Lorcaserin Treatment Decreases Body Weight and Reduces Cardiometabolic Risk Factors in Obese Adults: A Six-Month, Randomized, Placebo-Controlled, Double-Blind Clinical Trial. Diabetes Obes. Metab. 2019, 21, 1487–1492. [Google Scholar] [CrossRef]
- Pi-Sunyer, X.; Shanahan, W.; Fain, R.; Ma, T.; Garvey, W.T. Impact of Lorcaserin on Glycemic Control in Overweight and Obese Patients with Type 2 Diabetes: Analysis of Week 52 Responders and Nonresponders. Postgrad. Med. 2016, 128, 591–597. [Google Scholar] [CrossRef]
- Bohula, E.A.; Scirica, B.M.; Inzucchi, S.E.; McGuire, D.K.; Keech, A.C.; Smith, S.R.; Kanevsky, E.; Murphy, S.A.; Leiter, L.A.; Dwyer, J.P.; et al. Effect of Lorcaserin on Prevention and Remission of Type 2 Diabetes in Overweight and Obese Patients (CAMELLIA-TIMI 61): A Randomised, Placebo-Controlled Trial. Lancet 2018, 392, 2269–2279. [Google Scholar] [CrossRef]
- Fidler, M.C.; Sanchez, M.; Raether, B.; Weissman, N.J.; Smith, S.R.; Shanahan, W.R.; Anderson, C.M. A One-Year Randomized Trial of Lorcaserin for Weight Loss in Obese and Overweight Adults: The BLOSSOM Trial. J. Clin. Endocrinol. Metab. 2011, 96, 3067–3077. [Google Scholar] [CrossRef]
- Weissman, N.J.; Sanchez, M.; Koch, G.G.; Smith, S.R.; Shanahan, W.R.; Anderson, C.M. Echocardiographic Assessment of Cardiac Valvular Regurgitation with Lorcaserin from Analysis of 3 Phase 3 Clinical Trials. Circ. Cardiovasc. Imaging 2013, 6, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Greenway, F.L.; Shanahan, W.; Fain, R.; Ma, T.; Rubino, D. Safety and Tolerability Review of Lorcaserin in Clinical Trials. Clin. Obes. 2016, 6, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Gorelik, E.; Gorelik, B.; Masarwa, R.; Perlman, A.; Hirsh-Raccah, B.; Matok, I. The Cardiovascular Safety of Antiobesity Drugs-Analysis of Signals in the FDA Adverse Event Report System Database. Int. J. Obes. 2020, 44, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Scirica, B.M.; Bohula, E.A.; Dwyer, J.P.; Qamar, A.; Inzucchi, S.E.; McGuire, D.K.; Keech, A.C.; Smith, S.R.; Murphy, S.A.; Im, K.; et al. Lorcaserin and Renal Outcomes in Obese and Overweight Patients in the CAMELLIA-TIMI 61 Trial. Circulation 2019, 139, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A. Medical Treatment of Obesity: The Past, the Present and the Future. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 665–684. [Google Scholar] [CrossRef] [PubMed]
- De Andrade Mesquita, L.; Fagundes Piccoli, G.; Richter da Natividade, G.; Frison Spiazzi, B.; Colpani, V.; Gerchman, F. Is Lorcaserin Really Associated with Increased Risk of Cancer? A Systematic Review and Meta-Analysis. Obes. Rev. 2021, 22, e13170. [Google Scholar] [CrossRef] [PubMed]
- Sharretts, J.; Galescu, O.; Gomatam, S.; Andraca-Carrera, E.; Hampp, C.; Yanoff, L. Cancer Risk Associated with Lorcaserin—The FDA’s Review of the CAMELLIA-TIMI 61 Trial. N. Engl. J. Med. 2020, 383, 1000–1002. [Google Scholar] [CrossRef]
- Jeffrey Conn, P.; Christopoulos, A.; Lindsley, C.W. Allosteric Modulators of GPCRs: A Novel Approach for the Treatment of CNS Disorders. Nat. Rev. Drug Discov. 2009, 8, 41–54. [Google Scholar] [CrossRef]
- García-Cárceles, J.; Decara, J.M.; Vázquez-Villa, H.; Rodríguez, R.; Codesido, E.; Cruces, J.; Brea, J.; Loza, M.I.; Alén, F.; Botta, J.; et al. A Positive Allosteric Modulator of the Serotonin 5-HT 2C Receptor for Obesity. J. Med. Chem. 2017, 60, 9575–9584. [Google Scholar] [CrossRef]
- Singh, K.; Sona, C.; Ojha, V.; Singh, M.; Mishra, A.; Kumar, A.; Siddiqi, M.I.; Tripathi, R.P.; Yadav, P.N. Identification of Dual Role of Piperazine-Linked Phenyl Cyclopropyl Methanone as Positive Allosteric Modulator of 5-HT2C and Negative Allosteric Modulator of 5-HT2B Receptors. Eur. J. Med. Chem. 2019, 164, 499–516. [Google Scholar] [CrossRef]
- Cifuentes, L.; Eckel-Passow, J.; Acosta, A. Precision Medicine for Obesity. Dig. Dis. Interv. 2021, 5, 239. [Google Scholar] [CrossRef] [PubMed]
- Severin, R.; Sabbahi, A.; Mahmoud, A.M.; Arena, R.; Phillips, S.A. Precision Medicine in Weight Loss and Healthy Living. Prog. Cardiovasc. Dis. 2019, 62, 15. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, A.M.D.; Acosta, A. Precision Medicine and Obesity. Gastroenterol. Clin. N. Am. 2021, 50, 127. [Google Scholar] [CrossRef] [PubMed]
- Griebsch, N.I.; Kern, J.; Hansen, J.; Rullmann, M.; Luthardt, J.; Helfmeyer, S.; Dekorsy, F.J.; Soeder, M.; Hankir, M.K.; Zientek, F.; et al. Central Serotonin/Noradrenaline Transporter Availability and Treatment Success in Patients with Obesity. Brain Sci. 2022, 12, 1437. [Google Scholar] [CrossRef]
- He, Y.; Brouwers, B.; Liu, H.; Liu, H.; Lawler, K.; Mendes de Oliveira, E.; Lee, D.K.; Yang, Y.; Cox, A.R.; Keogh, J.M.; et al. Human Loss-of-Function Variants in the Serotonin 2C Receptor Associated with Obesity and Maladaptive Behavior. Nat. Med. 2022, 28, 2537. [Google Scholar] [CrossRef]
- Wold, E.A.; Garcia, E.J.; Wild, C.T.; Miszkiel, J.M.; Soto, C.A.; Chen, J.; Pazdrak, K.; Fox, R.G.; Anastasio, N.C.; Cunningham, K.A.; et al. Discovery of 4-Phenylpiperidine-2-Carboxamide Analogues as Serotonin 5-HT2C Receptor-Positive Allosteric Modulators with Enhanced Drug-like Properties. J. Med. Chem. 2020, 63, 7529. [Google Scholar] [CrossRef]
| Drug | Affinity to 5-HT1-2R Subtypes * | Selectivity to 5-HT2CR | Mechanism of 5-HT2CR Activation |
|---|---|---|---|
| piperazine derivatives (e.g., m-chlorophenyl piperazine) | 5-HT2B ≥ 5-HT2C > 5-HT2A > 5-HT1A ≥ 5-HT1B [33] | nonselective ligand | indirect agonism (affinity to 5-HT transporter and followed by 5-HT reuptake inhibition or 5-HT release enhancement) |
| fenfluramines | 5-HT2B ≥ 5-HT2C > 5-HT2A [34] | nonselective ligand | indirect agonism (affinity to 5-HT transporter and followed by 5-HT reuptake inhibition) |
| sibutramine | 5-HT1A = 5-HT1B = 5-HT2A = 5-HT2C [35] | nonselective ligand | indirect agonism (affinity to 5-HT transporter and followed by 5-HT reuptake inhibition) |
| lorcaserin | 5-HT2C > 5-HT2A > 5-HT2B [36] | selective ligand | direct agonism |
| Drug | Dosage (mg) | Duration (Months) | Patients Completed Study D|P | Key Inclusion Criteria (BMI > 25 kg/m2 or IBW 120–180%) | Mean Weight Loss D vs. P (kg or %) | Ref. |
|---|---|---|---|---|---|---|
| d-fenfluramine | 10 BID | 3 | 85|85 * | 120–180% | 2.79 vs. 2.83 kg | [70] |
| 15 BID | 168|169 | 120–180% | 5.84 vs. 1.85 kg | [71] | ||
| 12|12 | 28–35 kg/m2 | 3.1 ± 2.3 vs. 0.1 ± 1.2 kg | [72] | |||
| 12 | 404|418 * | ≥120% | 9.82 vs. 7.15 kg | [73] | ||
| 30 QD or BID | 3 | 82|85 * | 120–180% | 5.63 vs. 2.83 kg | [70] | |
| 30|30 | 120–180% | 4.6 ± 1.6 kg vs. no changed | [74] | |||
| 12 | 36|39 | ≥135% | 12.8 vs. 8.6 kg | [75] | ||
| 60 BID | 3 | 87|85 * | 120–180% | 7.23 vs. 2.83 kg | [70] | |
| Sibutramine | 5 QD | 2 | 19|20 | 130–180% | 2.9 ± 2.3 vs. 1.4 ± 2.1 kg | [76] |
| 3 | 56|59 * | 27–40 kg/m2 | 2.4 ± 0.5 vs. 1.4 ± 0.5 kg | [77] | ||
| 6 | 107|87 * | 30–40 kg/m2 | 3.7 vs. 1.3 kg | [78] | ||
| 10 QD | 3 | 59|59 * | 27–40 kg/m2 | 5.1 ± 0.5 vs. 1.4 ± 0.5 kg | [77] | |
| 6 | 99|87 * | 30–40 kg/m2 | 5.7 vs. 1.3 kg | [78] | ||
| 104|94 * | ≥30 and ≤40 kg/m2 | 8.2 vs. 3.9 kg | [79] | |||
| 40 | 2933|2825 * | 25–45 kg/m2 | 1.7 vs. +0.7 kg | [80] | ||
| 15 QD | 3 | 62|59 * | 27–40 kg/m2 | 2 4.9 ± 0.5 vs. 1.4 ± 0.5 kg | [77] | |
| 6 | 98|87 * | 30–40 kg/m2 | 7.0 vs. 1.3 kg | [78] | ||
| 12 | 281|80 | ±44 kg/m2 | 6.5 ± 0.31 vs. 1.9 ± 0.56 kg | [81] | ||
| 68|64 * | >27 kg/m2 | 5.5 ± 0.6 vs. 0.2 ± 0.5 kg | [82] | |||
| ~14 | 114|103 * | ≥30 and <40 kg/m2 | 8.1 ± 8.2 vs. 5.1 ± 6.5 kg | [83] | ||
| 20 QD | 2 | 21|20 | 130–180% | 5.0 ± 2.7 vs. 1.4 ± 2.1 | [76] | |
| 6 | 96|87 * | 30–40 kg/m2 | 8.2 vs. 1.3 kg | [78] | ||
| 151|152 * | ≥27 kg/m2 | 4.9 vs. 0.6 kg | [84] | |||
| 12 | 62|64 * | >27 kg/m2 | 8.0 ± 0.9 vs. 0.2 ± 0.5 kg | [82] | ||
| 30 QD | 6 | 101|87 * | 30–40 kg/m2 | 9.0 vs. 1.3 kg | [78] | |
| Lorcaserin | 10 QD or 10 BID | ~2 | 29|28 | 27–45 kg/m2 | 3.8 ± 0.4 vs. 2.2 ± 0.5 kg | [85] |
| 3 | 86 or 77|88 * | 30–45 kg/m2 | 1.8 or 3.6 vs. 0.3 kg | [86] | ||
| 6 | 59|53 * | ≥33 and ≤55 kg/m2 | 2.4 ± 0.8 vs. +0.6 ± 0.8 kg | [87] | ||
| 12 | 748|243 * | ≥27 kg/m2 | 4.2 vs. 1.4 kg | [88] | ||
| 12 | 75 or 169|157 * | 27–45 kg/m2 | 44.7 or 37.5 vs. 16.1% | [89] | ||
| 275|684 * | 27–45 kg/m2 | 5.81 ± 0.16 vs. 2.16 ± 0.14% | [90] | |||
| 13 | 1800|1550 | 30–45 kg/m2 | 47.1 vs. 22.6% | [91] | ||
| 24 | 564|684 * | 27–45 kg/m2 | 7.0 ± 0.2 vs. 3.0 ± 0.2% | [90] | ||
| 40 | 748|243 * | ≥27 kg/m2 | 4.0 kg vs. 2.1 kg | [88] | ||
| 15 QD | 3 | 82|88 * | 30–45 kg/m2 | 2.6 vs. 0.3 kg | [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przegaliński, E.; Witek, K.; Wydra, K.; Kotlińska, J.H.; Filip, M. 5-HT2C Receptor Stimulation in Obesity Treatment: Orthosteric Agonists vs. Allosteric Modulators. Nutrients 2023, 15, 1449. https://doi.org/10.3390/nu15061449
Przegaliński E, Witek K, Wydra K, Kotlińska JH, Filip M. 5-HT2C Receptor Stimulation in Obesity Treatment: Orthosteric Agonists vs. Allosteric Modulators. Nutrients. 2023; 15(6):1449. https://doi.org/10.3390/nu15061449
Chicago/Turabian StylePrzegaliński, Edmund, Kacper Witek, Karolina Wydra, Jolanta H. Kotlińska, and Małgorzata Filip. 2023. "5-HT2C Receptor Stimulation in Obesity Treatment: Orthosteric Agonists vs. Allosteric Modulators" Nutrients 15, no. 6: 1449. https://doi.org/10.3390/nu15061449
APA StylePrzegaliński, E., Witek, K., Wydra, K., Kotlińska, J. H., & Filip, M. (2023). 5-HT2C Receptor Stimulation in Obesity Treatment: Orthosteric Agonists vs. Allosteric Modulators. Nutrients, 15(6), 1449. https://doi.org/10.3390/nu15061449