Vegan Diet Is Associated with a Lower Risk of Chronic Kidney Disease in Patients with Hyperuricemia
Abstract
1. Introduction
2. Materials and Methods
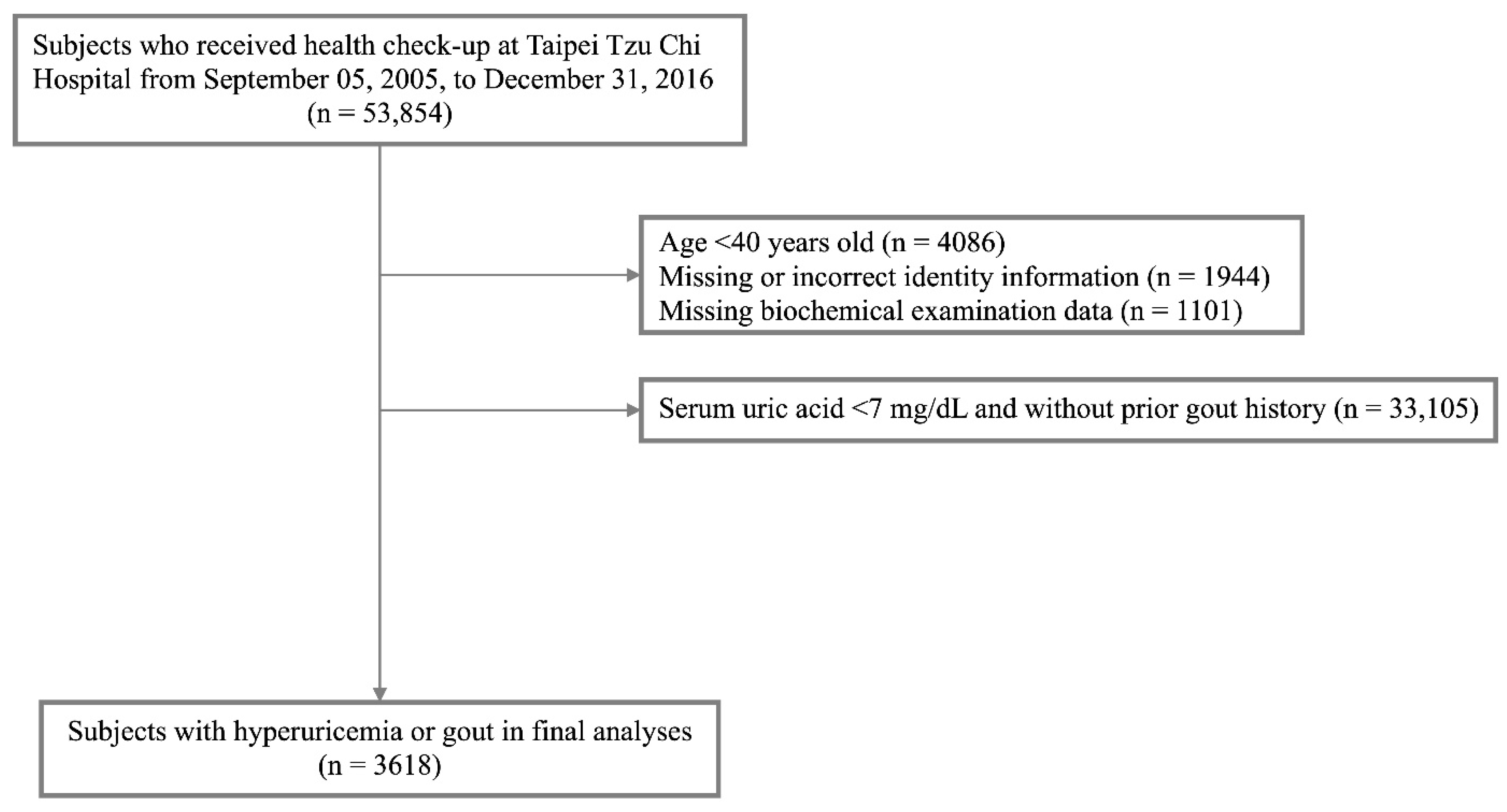
2.1. Participants
2.2. Outcome Measures
2.3. Clinical and Biochemical Measurements
2.4. Statistical Analyses
3. Results
3.1. Patient Characteristics
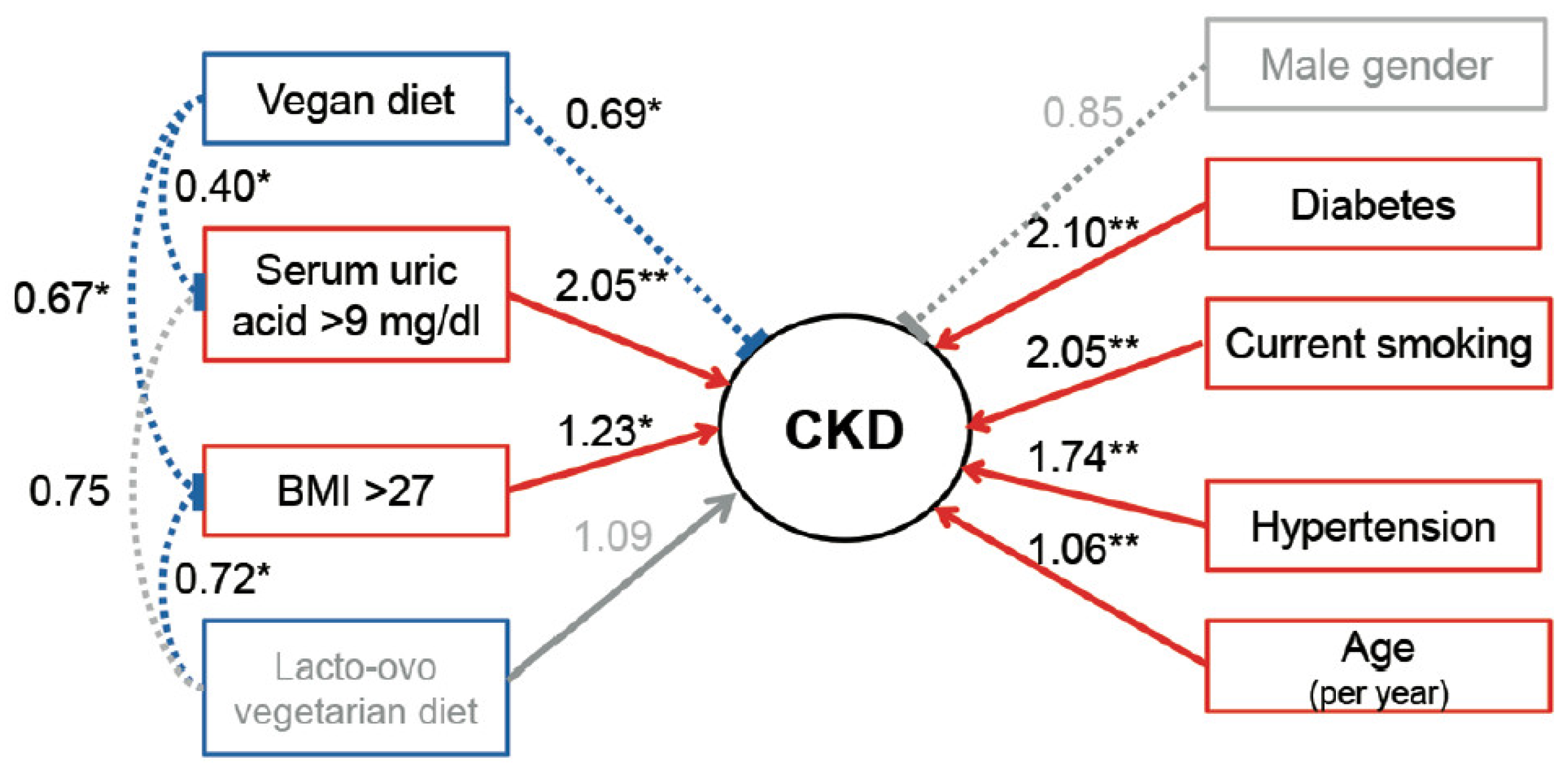
3.2. Associations between Dietary Habits and CKD in Patients with Hyperuricemia
3.3. Interactive Effects of Potential Risk Factors for CKD in Patients with Hyperuricemia
3.4. Sensitivity Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cockwell, P.; Fisher, L.A. The global burden of chronic kidney disease. Lancet 2020, 395, 662–664. [Google Scholar] [CrossRef]
- Wen, C.P.; Cheng, T.Y.; Tsai, M.K.; Chang, Y.C.; Chan, H.T.; Tsai, S.P.; Chiang, P.H.; Hsu, C.C.; Sung, P.K.; Hsu, Y.H.; et al. All-cause mortality attributable to chronic kidney disease: A prospective cohort study based on 462 293 adults in Taiwan. Lancet 2008, 371, 2173–2182. [Google Scholar] [CrossRef] [PubMed]
- Kazancioğlu, R. Risk factors for chronic kidney disease: An update. Kidney Int. 2013, 3, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Seegmiller, J.E. The acute attack of gouty arthritis. Arthritis Rheum. 1965, 8, 714–725. [Google Scholar] [CrossRef]
- So, A.; Thorens, B. Uric acid transport and disease. J. Clin. Investig. 2010, 120, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, K.; Malhotra, K.; Sowers, J.; Aroor, A. Uric Acid—Key ingredient in the recipe for cardiorenal metabolic syndrome. Cardiorenal Med. 2013, 3, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Obermayr, R.P.; Temml, C.; Gutjahr, G.; Knechtelsdorfer, M.; Oberbauer, R.; Klauser-Braun, R. Elevated uric acid increases the risk for kidney disease. J. Am. Soc. Nephrol. 2008, 19, 2407–2413. [Google Scholar] [CrossRef]
- Joo, H.J.; Kim, G.R.; Choi, D.W.; Joo, J.H.; Park, E.C. Uric acid level and kidney function: A cross-sectional study of the Korean national health and nutrition examination survey (2016–2017). Sci. Rep. 2020, 10, 21672. [Google Scholar] [CrossRef]
- Sellmayr, M.; Hernandez Petzsche, M.R.; Ma, Q.; Kruger, N.; Liapis, H.; Brink, A.; Lenz, B.; Angelotti, M.L.; Gnemmi, V.; Kuppe, C.; et al. Only Hyperuricemia with Crystalluria, but not Asymptomatic Hyperuricemia, Drives Progression of Chronic Kidney Disease. J. Am. Soc. Nephrol. 2020, 31, 2773–2792. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.J. Nutrition concerns and health effects of vegetarian diets. Nutr. Clin. Pract. 2010, 25, 613–620. [Google Scholar] [CrossRef]
- Chiu, T.H.T.; Liu, C.H.; Chang, C.C.; Lin, M.N.; Lin, C.L. Vegetarian diet and risk of gout in two separate prospective cohort studies. Clin. Nutr. 2020, 39, 837–844. [Google Scholar] [CrossRef]
- Szeto, Y.T.; Kwok, T.C.; Benzie, I.F. Effects of a long-term vegetarian diet on biomarkers of antioxidant status and cardiovascular disease risk. Nutrition 2004, 20, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Ausman, L.M.; Oliver, L.M.; Goldin, B.R.; Woods, M.N.; Gorbach, S.L.; Dwyer, J.T. Estimated net acid excretion inversely correlates with urine pH in vegans, lacto-ovo vegetarians, and omnivores. J. Ren. Nutr. 2008, 18, 456–465. [Google Scholar] [CrossRef]
- Chen, T.K.; Knicely, D.H.; Grams, M.E. Chronic Kidney Disease Diagnosis and Management: A Review. JAMA 2019, 322, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.C.; Huang, H.F.; Tsai, W.H.; Huang, S.Y.; Liu, H.W.; Liu, J.S.; Kuo, K.L. Vegetarian Diet Was Associated With a Lower Risk of Chronic Kidney Disease in Diabetic Patients. Front. Nutr. 2022, 9, 843357. [Google Scholar] [CrossRef]
- Liu, H.W.; Tsai, W.H.; Liu, J.S.; Kuo, K.L. Association of Vegetarian Diet with Chronic Kidney Disease. Nutrients 2019, 11, 279. [Google Scholar] [CrossRef] [PubMed]
- Chiu, T.H.T.; Chang, H.R.; Wang, L.Y.; Chang, C.C.; Lin, M.N.; Lin, C.L. Vegetarian diet and incidence of total, ischemic, and hemorrhagic stroke in 2 cohorts in Taiwan. Neurology 2020, 94, e1112–e1121. [Google Scholar] [CrossRef] [PubMed]
- Hwang, L.C.; Bai, C.H.; Chen, C.J. Prevalence of obesity and metabolic syndrome in Taiwan. J. Formos. Med. Assoc. 2006, 105, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Stevens, L.A.; Claybon, M.A.; Schmid, C.H.; Chen, J.; Horio, M.; Imai, E.; Nelson, R.G.; Van Deventer, M.; Wang, H.Y.; Zuo, L.; et al. Evaluation of the Chronic Kidney Disease Epidemiology Collaboration equation for estimating the glomerular filtration rate in multiple ethnicities. Kidney Int. 2011, 79, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.J.; Mangels, A.R.; Fresan, U.; Marsh, K.; Miles, F.L.; Saunders, A.V.; Haddad, E.H.; Heskey, C.E.; Johnston, P.; Larson-Meyer, E.; et al. The Safe and Effective Use of Plant-Based Diets with Guidelines for Health Professionals. Nutrients 2021, 13, 4144. [Google Scholar] [CrossRef]
- Adeva-Andany, M.M.; Fernandez-Fernandez, C.; Carneiro-Freire, N.; Vila-Altesor, M.; Ameneiros-Rodriguez, E. The differential effect of animal versus vegetable dietary protein on the clinical manifestations of diabetic kidney disease in humans. Clin. Nutr. ESPEN 2022, 48, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wei, G.; Jalili, T.; Metos, J.; Giri, A.; Cho, M.E.; Boucher, R.; Greene, T.; Beddhu, S. The Associations of Plant Protein Intake with All-Cause Mortality in CKD. Am. J. Kidney Dis. 2016, 67, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Alvirdizadeh, S.; Yuzbashian, E.; Mirmiran, P.; Eghtesadi, S.; Azizi, F. A prospective study on total protein, plant protein and animal protein in relation to the risk of incident chronic kidney disease. BMC Nephrol. 2020, 21, 489. [Google Scholar] [CrossRef]
- Ferraro, P.M.; Bargagli, M.; Trinchieri, A.; Gambaro, G. Risk of Kidney Stones: Influence of Dietary Factors, Dietary Patterns, and Vegetarian-Vegan Diets. Nutrients 2020, 12, 779. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.; Heilbronn, L.; Chen, D.; Coster, A.; Greenfield, J.; Samocha-Bonet, D. Dietary acid load, metabolic acidosis and insulin resistance—Lessons from cross-sectional and overfeeding studies in humans. Clin. Nutr. 2016, 35, 1084–1090. [Google Scholar] [CrossRef]
- Muller, A.; Zimmermann-Klemd, A.M.; Lederer, A.K.; Hannibal, L.; Kowarschik, S.; Huber, R.; Storz, M.A. A Vegan Diet Is Associated with a Significant Reduction in Dietary Acid Load: Post Hoc Analysis of a Randomized Controlled Trial in Healthy Individuals. Int. J. Environ. Res. Public Health 2021, 18, 9998. [Google Scholar] [CrossRef] [PubMed]
- Storz, M.A.; Ronco, A.L. Reduced dietary acid load in U.S. vegetarian adults: Results from the National Health and Nutrition Examination Survey. Food Sci. Nutr. 2022, 10, 2091–2100. [Google Scholar] [CrossRef]
- Cosgrove, K.; Johnston, C.S. Examining the Impact of Adherence to a Vegan Diet on Acid-Base Balance in Healthy Adults. Plant Foods Hum. Nutr. 2017, 72, 308–313. [Google Scholar] [CrossRef]
- Goraya, N.; Simoni, J.; Jo, C.H.; Wesson, D.E. Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int. 2014, 86, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Gajski, G.; Gerić, M.; Vučić Lovrenčić, M.; Božičević, S.; Rubelj, I.; Nanić, L.; Škrobot Vidaček, N.; Bendix, L.; Peraica, M.; Rašić, D.; et al. Analysis of Health-Related Biomarkers between Vegetarians and Non-Vegetarians: A Multi-Biomarker Approach. J. Funct. Foods 2018, 48, 643–653. [Google Scholar] [CrossRef]
- Schmidt, L.; Crowe, F.; Appleby, P.; Key, T.; Travis, R. Serum uric acid concentrations in meat eaters, fish eaters, vegetarians and vegans: A cross-sectional analysis in the EPIC-Oxford cohort. PLoS ONE 2013, 8, e56339. [Google Scholar] [CrossRef]
- Choi, H.K.; Atkinson, K.; Karlson, E.W.; Willett, W.; Curhan, G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N. Engl. J. Med. 2004, 350, 1093–1103. [Google Scholar] [CrossRef]
- Carrero, J.J.; Hecking, M.; Chesnaye, N.C.; Jager, K.J. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat. Rev. Nephrol. 2018, 14, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Suleymanlar, G.; Utas, C.; Arinsoy, T.; Ates, K.; Altun, B.; Altiparmak, M.R.; Ecder, T.; Yilmaz, M.E.; Camsari, T.; Basci, A.; et al. A population-based survey of Chronic REnal Disease in Turkey—The CREDIT study. Nephrol. Dial. Transplant. 2011, 26, 1862–1871. [Google Scholar] [CrossRef]
- Inker, L.A.; Levey, A.S.; Tighiouart, H.; Shafi, T.; Eckfeldt, J.H.; Johnson, C.; Okparavero, A.; Post, W.S.; Coresh, J.; Shlipak, M.G. Performance of glomerular filtration rate estimating equations in a community-based sample of Blacks and Whites: The multiethnic study of atherosclerosis. Nephrol. Dial. Transplant. 2018, 33, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Eneanya, N.D.; Coresh, J.; Tighiouart, H.; Wang, D.; Sang, Y.; Crews, D.C.; Doria, A.; Estrella, M.M.; Froissart, M.; et al. New Creatinine- and Cystatin C-Based Equations to Estimate GFR without Race. N. Engl. J. Med. 2021, 385, 1737–1749. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; An, K.; Mou, X.; Zhang, M.; Su, Q.; Li, S. Effect of Urate-Lowering Therapy on the Progression of Kidney Function in Patients With Asymptomatic Hyperuricemia: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2021, 12, 795082. [Google Scholar] [CrossRef] [PubMed]
- Shetty, A.A.; Magadum, S.; Managanvi, K. Vegetables as Sources of Antioxidants. J. Food Nutr. Disord. 2013, 2, 1000104. [Google Scholar] [CrossRef]
- Chiavaroli, L.; Mirrahimi, A.; Sievenpiper, J.L.; Jenkins, D.J.; Darling, P.B. Dietary fiber effects in chronic kidney disease: A systematic review and meta-analysis of controlled feeding trials. Eur. J. Clin. Nutr. 2015, 69, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Cases, A.; Cigarran-Guldris, S.; Mas, S.; Gonzalez-Parra, E. Vegetable-Based Diets for Chronic Kidney Disease? It Is Time to Reconsider. Nutrients 2019, 11, 1263. [Google Scholar] [CrossRef]
| Characteristics | Vegans (n = 225) | Lacto-Ovo Vegetarians (n = 509) | Omnivores (n = 2884) | p-Value |
|---|---|---|---|---|
| Demographics | ||||
| Age group, years old, n (%) | <0.001 a | |||
| 40–49, n (%) | 12 (5.3) | 52 (10.2) | 474 (16.4) | |
| 50–69, n (%) | 40 (17.8) | 159 (31.2) | 838 (29.1) | |
| 60–69, n (%) | 99 (44.0) | 193 (37.9) | 920 (31.9) | |
| >70, n (%) | 74 (32.9) | 105 (20.6) | 652 (22.6) | |
| Age, years old, mean (SD) | 66.3 (10.6) | 62 (9.4) | 61.3 (11.4) | <0.001 b |
| Gender | <0.001 a | |||
| Male, n (%) | 116 (51.6) | 282 (55.4) | 2100 (72.8) | |
| Female, n (%) | 109 (48.4) | 227 (44.6) | 784 (27.2) | |
| Comorbid conditions | ||||
| Current smoking, n (%) | 0 (<0.1) | 4 (0.8) | 173 (6.0) | <0.001 a |
| Alcohol drinking, n (%) | 17 (7.6) | 44 (8.6) | 1066 (37.0) | <0.001 a |
| BMI, Kg/m2, mean (SD) | 25.2 (3.8) | 25.3 (3.8) | 25.9 (3.8) | <0.001 b |
| >27, n (%) | 56 (24.9) | 133 (26.1) | 951 (33.0) | 0.001 a |
| Hypertension, n (%) | 86 (38.2) | 179 (35.2) | 1075 (37.3) | 0.73 a |
| SBP, mmHg, mean (SD) | 129 (18) | 125 (15) | 127 (16) | 0.59 b |
| DBP, mmHg, mean (SD) | 77 (12) | 76 (11) | 79 (12) | 0.001 b |
| Diabetes mellitus, n (%) | 23 (10.2) | 51 (10.0) | 322 (11.2) | 0.77 a |
| Gout, n (%) | 101 (44.9) | 195 (38.3) | 1383 (48.0) | <0.001 a |
| CKD, n (%) | 51 (23.1) | 133 (26.3) | 744 (25.9) | 0.55 a |
| Stage 3, n (%) | 39 (17.6) | 112 (22.2) | 602 (20.9) | 0.35 a |
| Stages 4–5, n (%) | 3 (1.4) | 9 (1.8) | 49 (1.7) | 0.91 a |
| Biochemistry | ||||
| Uric acid, mg/dL, mean (SD) | 7.3 (1.3) | 7.5 (1.2) | 7.8 (1.4) | <0.001 b |
| >9 mg/dL, n (%) | 12 (5.3) | 48 (9.4) | 353 (12.2) | 0.002 a |
| HbA1c, %, mean (SD) | 5.8 (0.7) | 5.8 (1.0) | 5.8 (1.0) | 0.64 b |
| HDL-C, mg/dL, mean (SD) | 42.1 (10.3) | 44.3 (12.0) | 44.1 (12.7) | 0.50 b |
| LDL-C, mg/dL, mean (SD) | 118.6 (32.4) | 122.2 (30.3) | 129.9 (34.9) | <0.001 b |
| Serum albumin, mg/dL, mean (SD) | 4.3 (0.4) | 4.2 (0.4) | 4.3 (0.4) | 0.006 b |
| Serum creatinine, mg/dL, mean (SD) | 0.9 (0.3) | 1.0 (0.4) | 1.0 (0.6) | 0.001 b |
| CKD-EPI eGFR, mL/min/1.73 m2, mean (SD) | 76 (17) | 77 (17) | 77 (17) | 0.51 b |
| Proteinuria, n (%) | 30 (13.6) | 77 (15.2) | 423 (14.7) | 0.84 a |
| Model 1 a | Model 2 b | Model 3 c | ||||
|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) | p-Value | Odds Ratio (95% CI) | p-Value | Odds Ratio (95% CI) | p-Value | |
| Dietary habits | ||||||
| Vegan vs. omnivores | 0.86 (0.62–1.19) | 0.36 | 0.62 (0.45–0.97) | 0.006 | 0.69 (0.48–0.99) | 0.04 |
| Lacto-ovo vegetarian vs. omnivores | 1.02 (0.83–1.27) | 0.83 | 0.99 (0.78–1.25) | 0.99 | 1.15 (0.91–1.45) | 0.23 |
| Age, per year | 1.06 (1.05–1.07) | <0.001 | 1.06 (1.05–1.07) | <0.001 | 1.06 (1.05–1.06) | <0.001 |
| Male gender | 0.71 (0.61–0.83) | <0.001 | 0.89 (0.56–0.86) | 0.19 | 0.83 (0.70–0.99) | 0.04 |
| Diabetes mellitus | 3.04 (2.46–3.77) | <0.001 | 2.12 (1.68–2.67) | <0.001 | ||
| Hypertension | 2.69 (2.31–3.14) | <0.001 | 1.73 (1.45–2.05) | <0.001 | ||
| BMI > 27 kg/m2 | 1.20 (1.03–1.41) | 0.02 | 1.24 (1.04–1.47) | 0.02 | ||
| Current smoking | 1.28 (0.92–1.78) | 0.14 | 2.05 (1.44–2.94) | <0.001 | ||
| Serum uric acid > 9 mg/dL | 1.92 (1.55–2.38) | <0.001 | 2.08 (1.64–2.63) | <0.001 | ||
| SBP, per 10 mmHg | 1.22 (1.16–1.28) | <0.001 | ||||
| HbA1c, % | 1.17 (1.09–1.26) | <0.001 | ||||
| Hyperlipidemia | 1.00 (0.99–1.02) | 0.63 | ||||
| LDL-C, per 10 mg/dL | 0.98 (0.95–1.01) | 0.20 | ||||
| Alcohol drinking | 1.28 (0.92–1.78) | <0.001 | ||||
| Model 1 a | Model 2 b | Model 3 c | ||||
|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) | p-Value | Odds Ratio (95% CI) | p-Value | Odds Ratio (95% CI) | p-Value | |
| Dietary habits | ||||||
| Vegan vs. omnivores | 0.85 (0.61–1.17) | 0.31 | 0.59 (0.42–0.82) | 0.002 | 0.65 (0.46–0.93) | 0.02 |
| Lacto-ovo vegetarian vs. omnivores | 1.08 (0.88–1.33) | 0.45 | 1.03 (0.83–1.27) | 0.8 | 1.21 (0.97–1.51) | 0.09 |
| Age, per year | 0.88 (0.83–0.93) | <0.001 | 1.06 (1.05–1.06) | <0.001 | 0.86 (0.81–0.91) | <0.001 |
| Male gender | 0.58 (0.48–0.69) | <0.001 | 0.75 (0.63–0.91) | 0.003 | 0.76 (0.63–0.93) | 0.006 |
| Diabetes mellitus | 3.03 (2.46–3.73) | <0.001 | 2.12 (1.69–2.66) | <0.001 | ||
| Hypertension | 2.52 (2.18–2.91) | <0.001 | 1.63 (1.38–1.91) | <0.001 | ||
| BMI > 27 kg/m2 | 1.13 (0.97–1.31) | 0.11 | 1.13 (0.95–1.33) | 0.16 | ||
| Current smoking | 1.35 (1.02–1.79) | 0.033 | 2.10 (1.56–2.84) | <0.001 | ||
| Serum uric acid >9 mg/dL | 1.99 (1.61–2.46) | <0.001 | 2.00 (1.59–2.52) | <0.001 | ||
| SBP, per 10 mmHg | 1.21 (1.16–1.26) | <0.001 | ||||
| HbA1c, % | 1.26 (1.13–1.41) | <0.001 | ||||
| Hyperlipidemia | 1.00 (0.99–1.02) | 0.63 | ||||
| LDL-C, per 10 mg/dL | 0.97 (0.94–1.00) | 0.07 | ||||
| Alcohol drinking | 0.73 (0.63–0.85) | <0.001 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-L.; Tsai, W.-H.; Liu, J.-S.; Liu, H.-W.; Huang, S.-Y.; Kuo, K.-L. Vegan Diet Is Associated with a Lower Risk of Chronic Kidney Disease in Patients with Hyperuricemia. Nutrients 2023, 15, 1444. https://doi.org/10.3390/nu15061444
Wu C-L, Tsai W-H, Liu J-S, Liu H-W, Huang S-Y, Kuo K-L. Vegan Diet Is Associated with a Lower Risk of Chronic Kidney Disease in Patients with Hyperuricemia. Nutrients. 2023; 15(6):1444. https://doi.org/10.3390/nu15061444
Chicago/Turabian StyleWu, Chia-Lin, Wen-Hsin Tsai, Jia-Sin Liu, Hao-Wen Liu, Sin-Yi Huang, and Ko-Lin Kuo. 2023. "Vegan Diet Is Associated with a Lower Risk of Chronic Kidney Disease in Patients with Hyperuricemia" Nutrients 15, no. 6: 1444. https://doi.org/10.3390/nu15061444
APA StyleWu, C.-L., Tsai, W.-H., Liu, J.-S., Liu, H.-W., Huang, S.-Y., & Kuo, K.-L. (2023). Vegan Diet Is Associated with a Lower Risk of Chronic Kidney Disease in Patients with Hyperuricemia. Nutrients, 15(6), 1444. https://doi.org/10.3390/nu15061444








