Type 2 Diabetes: Also a “Clock Matter”?
Abstract
1. Introduction
2. Materials and Methods
2.1. On Line Questionnaire
2.2. Clinical Parameters
2.3. Statistics
3. Results
3.1. Descriptive Statistics
3.2. Comparison between Chronotype Categories
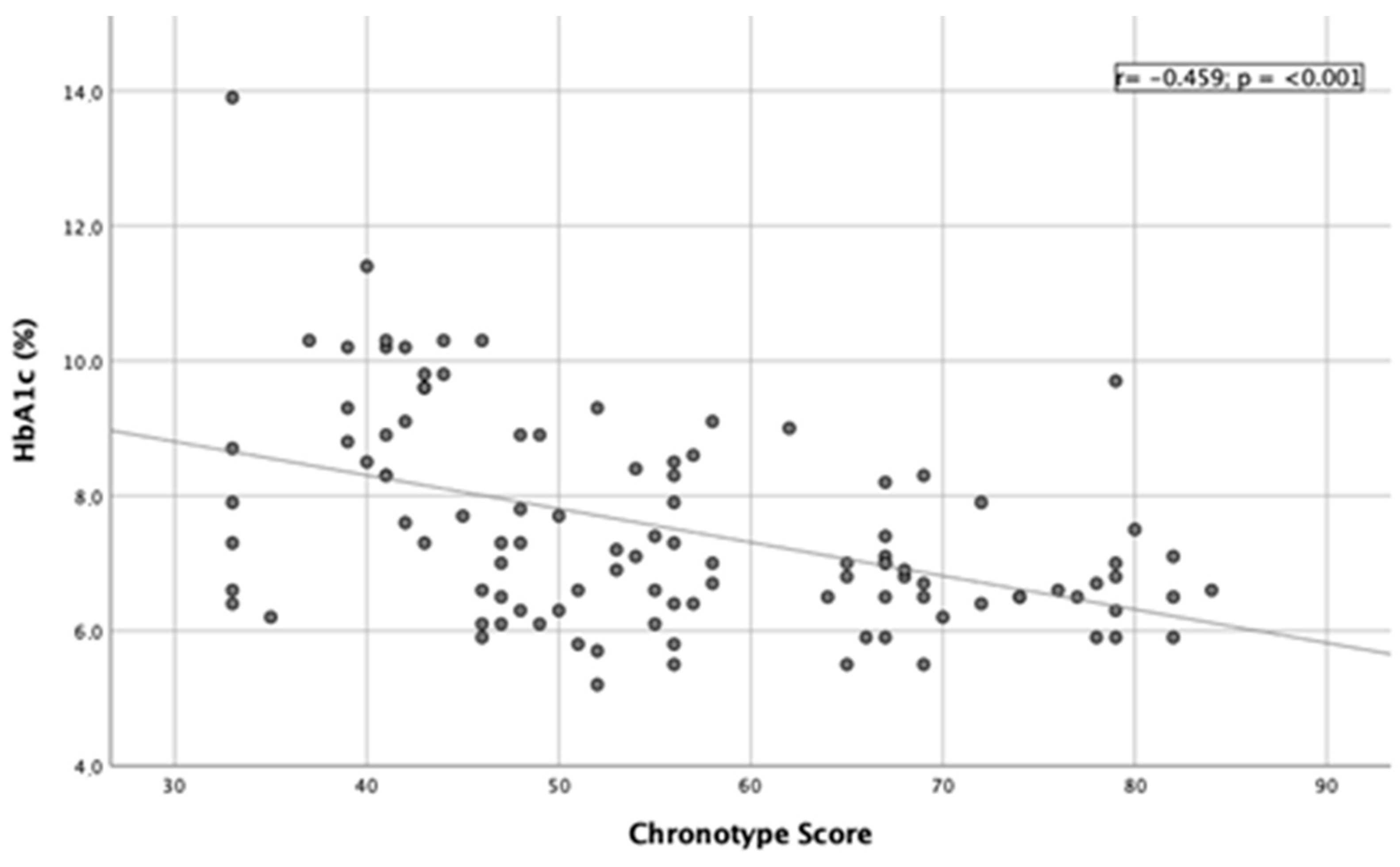
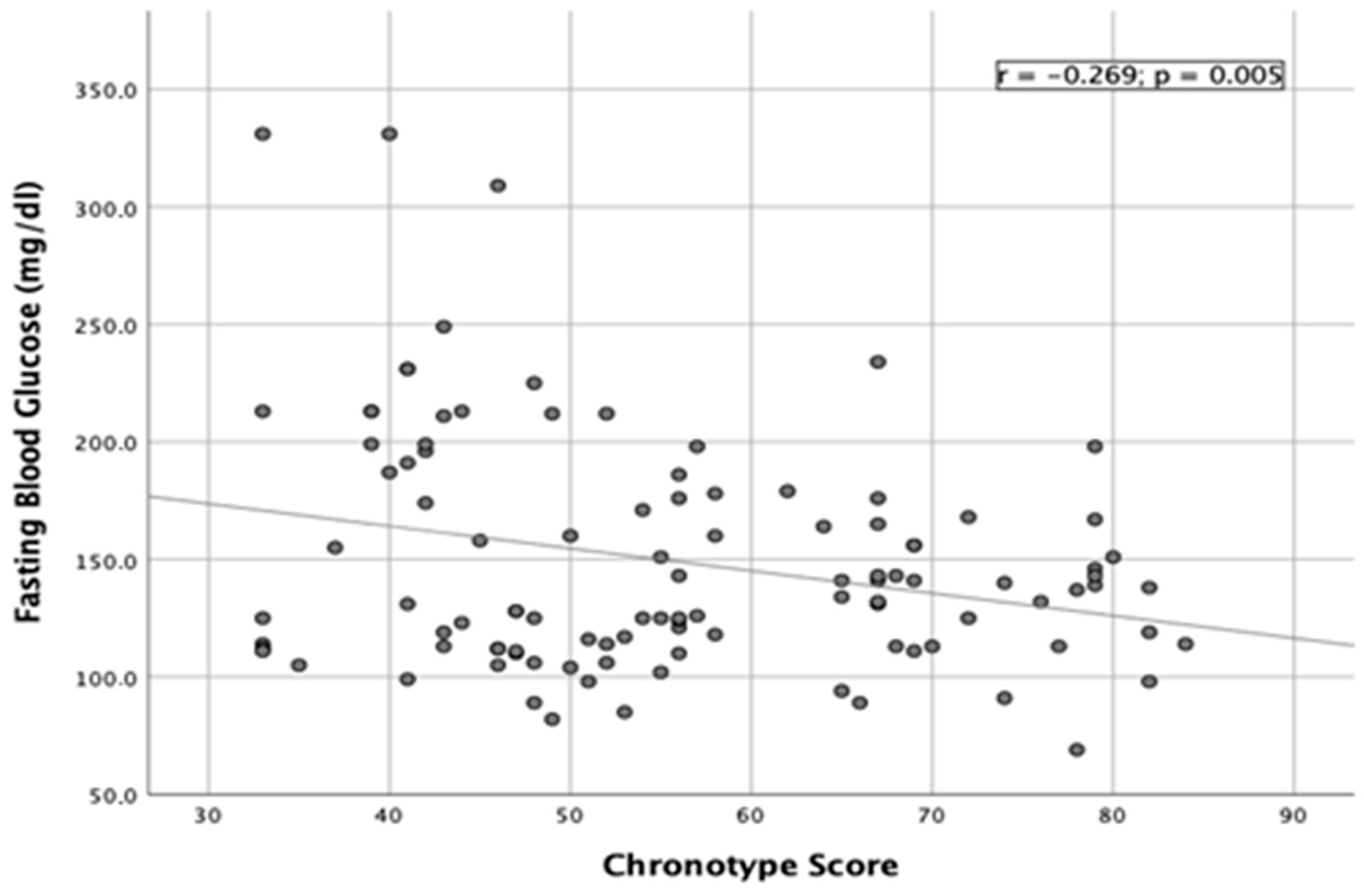
3.3. Correlation Studies
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barrea, L.; Muscogiuri, G.; Pugliese, G.; Modica, R.; Laudisio, D.; Aprano, S.; Faggiano, A.; Colao, A.; Savastano, S. Chronotype: What role in the context of gastroenteropancreatic neuroendocrine tumors? J. Transl. Med. 2021, 19, 324. [Google Scholar] [CrossRef]
- Barrea, L.; Vetrani, C.; Altieri, B.; Verde, L.; Savastano, S.; Colao, A.; Muscogiuri, G. The Importance of Being a ‘Lark’ in Post-Menopausal Women with Obesity: A Ploy to Prevent Type 2 Diabetes Mellitus? Nutrients 2021, 13, 3762. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Barrea, L.; Aprano, S.; Framondi, L.; Di Matteo, R.; Altieri, B.; Laudisio, D.; Pugliese, G.; Savastano, S.; Colao, A. Chronotype and cardio metabolic health in obesity: Does nutrition matter? Int. J. Food Sci. Nutr. 2021, 72, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Barrea, L.; Aprano, S.; Framondi, L.; Di Matteo, R.; Laudisio, D.; Pugliese, G.; Savastano, S.; Colao, A.; On Behalf Of The Opera Prevention, P. Chronotype and Adherence to the Mediterranean Diet in Obesity: Results from the Opera Prevention Project. Nutrients 2020, 12, 1354. [Google Scholar] [CrossRef] [PubMed]
- Kivela, L.; Papadopoulos, M.R.; Antypa, N. Chronotype and Psychiatric Disorders. Curr. Sleep Med. Rep. 2018, 4, 94–103. [Google Scholar] [CrossRef]
- Maukonen, M.; Kanerva, N.; Partonen, T.; Kronholm, E.; Konttinen, H.; Wennman, H.; Mannisto, S. The associations between chronotype, a healthy diet and obesity. Chronobiol. Int. 2016, 33, 972–981. [Google Scholar] [CrossRef]
- Rodriguez-Cortes, F.J.; Morales-Cane, I.; Rodriguez-Munoz, P.M.; Cappadona, R.; De Giorgi, A.; Manfredini, R.; Rodriguez-Borrego, M.A.; Fabbian, F.; Lopez-Soto, P.J. Individual Circadian Preference, Eating Disorders and Obesity in Children and Adolescents: A Dangerous Liaison? A Systematic Review and a Meta-Analysis. Children 2022, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- Fabbian, F.; Zucchi, B.; De Giorgi, A.; Tiseo, R.; Boari, B.; Salmi, R.; Cappadona, R.; Gianesini, G.; Bassi, E.; Signani, F.; et al. Chronotype, gender and general health. Chronobiol. Int. 2016, 33, 863–882. [Google Scholar] [CrossRef]
- Lim, S.T.; Kim, D.Y.; Kwon, H.T.; Lee, E. Sleep quality and athletic performance according to chronotype. BMC Sports Sci. Med. Rehabil. 2021, 13, 2. [Google Scholar] [CrossRef]
- Chaput, J.P. Sleep patterns, diet quality and energy balance. Physiol. Behav. 2014, 134, 86–91. [Google Scholar] [CrossRef]
- Rawat, A.; Gangwar, A.K.; Tiwari, S.; Kant, S.; Garg, R.K.; Singh, P.K. Sleep quality and insulin resistance in adolescent subjects with different circadian preference: A cross-sectional study. J. Family Med. Prim. Care 2019, 8, 2502–2505. [Google Scholar] [CrossRef]
- Chaput, J.P.; Despres, J.P.; Bouchard, C.; Tremblay, A. Association of sleep duration with type 2 diabetes and impaired glucose tolerance. Diabetologia 2007, 50, 2298–2304. [Google Scholar] [CrossRef]
- Anothaisintawee, T.; Lertrattananon, D.; Thamakaison, S.; Knutson, K.L.; Thakkinstian, A.; Reutrakul, S. Later chronotype is associated with higher hemoglobin A1c in prediabetes patients. Chronobiol. Int. 2017, 34, 393–402. [Google Scholar] [CrossRef]
- Afroz-Hossain, A.; Dawkins, M.; Myers, A.K. Sleep and Environmental Factors Affecting Glycemic Control in People with Type 2 Diabetes Mellitus. Curr. Diab. Rep. 2019, 19, 40. [Google Scholar] [CrossRef] [PubMed]
- Linea Guida della Società Italiana di Diabetologia (SID) e dell’Associazione dei Medici Diabetologi (AMD). In La Terapia del Diabete Mellito di Tipo 2; Sistema Nazionale Linee Gu: Roma, Italy, 2021.
- Reutrakul, S.; Hood, M.M.; Crowley, S.J.; Morgan, M.K.; Teodori, M.; Knutson, K.L.; Van Cauter, E. Chronotype is independently associated with glycemic control in type 2 diabetes. Diabetes Care 2013, 36, 2523–2529. [Google Scholar] [CrossRef]
- Hashemipour, S.; Yazdi, Z.; Mahabad, N. Association of Evening Chronotype with Poor Control of Type 2 Diabetes: Roles of Sleep Duration and Insomnia Level. Int. J. Endocrinol. Metab. 2020, 18, e99701. [Google Scholar] [CrossRef] [PubMed]
- von Schnurbein, J.; Boettcher, C.; Brandt, S.; Karges, B.; Dunstheimer, D.; Galler, A.; Denzer, C.; Denzer, F.; Vollbach, H.; Wabitsch, M.; et al. Sleep and glycemic control in adolescents with type 1 diabetes. Pediatr. Diabetes 2018, 19, 143–149. [Google Scholar] [CrossRef]
- Jakubowicz, D.; Wainstein, J.; Ahren, B.; Bar-Dayan, Y.; Landau, Z.; Rabinovitz, H.R.; Froy, O. High-energy breakfast with low-energy dinner decreases overall daily hyperglycaemia in type 2 diabetic patients: A randomised clinical trial. Diabetologia 2015, 58, 912–919. [Google Scholar] [CrossRef]
- O’Byrne, N.A.; Yuen, F.; Butt, W.Z.; Liu, P.Y. Sleep and Circadian Regulation of Cortisol: A Short Review. Curr. Opin. Endocr. Metab. Res. 2021, 18, 178–186. [Google Scholar] [CrossRef]
- Scheer, F.A.; Hilton, M.F.; Mantzoros, C.S.; Shea, S.A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. USA 2009, 106, 4453–4458. [Google Scholar] [CrossRef] [PubMed]
- Buxton, O.M.; Cain, S.W.; O’Connor, S.P.; Porter, J.H.; Duffy, J.F.; Wang, W.; Czeisler, C.A.; Shea, S.A. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci. Transl. Med. 2012, 4, 129ra143. [Google Scholar] [CrossRef] [PubMed]
- Oster, H.; Challet, E.; Ott, V.; Arvat, E.; de Kloet, E.R.; Dijk, D.J.; Lightman, S.; Vgontzas, A.; Van Cauter, E. The Functional and Clinical Significance of the 24-Hour Rhythm of Circulating Glucocorticoids. Endocr. Rev. 2017, 38, 3–45. [Google Scholar] [CrossRef]
- Tan, X.; Ciuculete, D.M.; Schioth, H.B.; Benedict, C. Associations between chronotype, MTNR1B genotype and risk of type 2 diabetes in UK Biobank. J. Intern. Med. 2020, 287, 189–196. [Google Scholar] [CrossRef]
- Aguilar-Galarza, A.; Garcia-Gasca, T.; Mejia, C.; Diaz-Munoz, M.; Perez-Mendoza, M.; Anaya-Loyola, M.A.; Garaulet, M. Evening chronotype associates with increased triglyceride levels in young adults in two independent populations. Clin. Nutr. 2021, 40, 2373–2380. [Google Scholar] [CrossRef]
- Barrea, L.; Verde, L.; Vetrani, C.; Savastano, S.; Colao, A.; Muscogiuri, G. Chronotype: A Tool to Screen Eating Habits in Polycystic Ovary Syndrome? Nutrients 2022, 14, 955. [Google Scholar] [CrossRef] [PubMed]
- Schuppelius, B.; Peters, B.; Ottawa, A.; Pivovarova-Ramich, O. Time Restricted Eating: A Dietary Strategy to Prevent and Treat Metabolic Disturbances. Front. Endocrinol. 2021, 12, 683140. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Morning Chronotype n = 38 (35.8%) | Intermediate Chronotype n = 50 (47.2%) | Evening Chronotype n = 18 (17%) | p-Value |
|---|---|---|---|---|
| Gender | 0.272 | |||
| Female (n, %) | 17 (44.7%) | 31 (62%) | 10 (55.6%) | |
| Male (n, %) | 21 (55.3%) | 19 (38%) | 8 (44.4%) | |
| Age | 60.8 ± 10.3 | 65.4 ± 11 | 62.5 ± 7.8 | 0.111 |
| Anthropometric measurement BMI (kg/m2) | 27.6 ± 3.8 | 29.3 ± 5.3 | 29.9 ± 5.3 | |
| Normal weight (n, %) | 9 (23.7%) | 9 (18%) | 4 (22.2%) | |
| Overweight (n, %) | 19 (50%) | 23 (46%) | 6 (33.4%) | |
| Grade I obesity (n, %) | 7 (18.4%) | 12 (24%) | 4 (22.2%) | 0.146 |
| Grade II obesity (n, %) | 3 (7.9%) | 3 (6%) | 4 (22.2%) | 0.374 |
| Grade III obesity (n, %) | 0 (0%) | 3 (6%) | 0 (0%) | |
| Type 2 diabetes mellitus duration (years) | 10.7 ± 8.3 | 10.1 ± 8 | 10.3 ± 9.1 | 0.953 |
| Glycemic profile | ||||
| Glycemia levels (mg/dL) | 138 ± 31 b | 145 ± 48 a | 183 ± 71 a | 0.005 |
| HbA1c (%) | 6.8 ± 0.9 b | 7.5 ± 1.4 a | 8.9 ± 1.9 a | <0.001 |
| Diseases | ||||
| Arterial hypertension | 26 (68.4%) | 39 (78%) | 17 (94.4%) a | 0.930 |
| Dyslipidemia | 23 (60.5%) | 31 (62%) | 15 (83.3%) | 0.202 |
| Retinopathy | 3 (7.9%) | 5 (10%) | 4 (22.2%) | 0.264 |
| Neuropathy | 3 (7.9%) | 5 (10%) | 4 (22.2%) | 0.264 |
| Nephropathy | 5 (13.2%) | 13 (26%) | 5 (27.8%) | 0.277 |
| Coronary heart disease | 5 (13.2%) | 13 (26%) | 7 (38.9%) a | 0.91 |
| Treatment | ||||
| Metformin | 31 (81.6%) | 39 (78%) | 18 (100%) | 0.99 |
| GLP1-RA | 11 (28.9%) | 18 (36%) | 7 (38.9%) | 0.700 |
| SGLT-2i | 13 (34.2%) | 18 (36%) | 9 (50%) | 0.492 |
| Basal insulin | 1 (2.6%) | 16 (32%) a | 8 (44.4%) a | <0.001 |
| Rapid insulin | 0 (0%) | 8 (16%) a | 3 (16.7%) a | 0.032 |
| DPP4I | 5 (13.2%) | 2 (4%) | 1 (5.6%) | 0.257 |
| Pioglitazone | 2 (5.3%) | 2 (4%) | 0 (0%) | 0.623 |
| Acarbose | 1 (2.6%) | 0 (0%) | 0 (0%) | 0.405 |
| 1° line treatment | 6 (15.8%) | 8 (16%) | 2 (11.1%) | 0.319 |
| 2° line treatment | 23 (60.5%) | 22 (44%) | 7 (38.9%) | |
| 3° line treatment | 9 (23.7%) | 20 (40%) | 9 (50%) |
| Parameters | Linear Regression Model | ||||
|---|---|---|---|---|---|
| Non-Standard Coefficients | Standardized Coefficients | ||||
| T | SE | β | t | p-Value | |
| Age | −0.032 | 0.013 | −0.220 | −2.483 | 0.015 |
| Body mass index | 0.066 | 0.027 | 0.209 | 2.467 | 0.015 |
| Type 2 diabetes mellitus duration | 0.022 | 0.016 | 0.120 | 1.370 | 0.174 |
| Chronotype score | −0.050 | 0.009 | −0.460 | −5.333 | 0.000 |
| Parameters | Linear Regression Model | ||||
|---|---|---|---|---|---|
| Non-Standard Coefficients | Standardized Coefficients | ||||
| T | SE | β | t | p-Value | |
| Age | −1.485 | 0.463 | −0.310 | −3.203 | 0.002 |
| Body mass index | 0.905 | 0.949 | 0.088 | 0.954 | 0.343 |
| Type 2 diabetes mellitus duration | 0.763 | 0.578 | 0.126 | 1.319 | 0.190 |
| Chronotype score | −1.087 | 0.333 | −0.307 | −3.265 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Docimo, A.; Verde, L.; Barrea, L.; Vetrani, C.; Memoli, P.; Accardo, G.; Colella, C.; Nosso, G.; Orio, M.; Renzullo, A.; et al. Type 2 Diabetes: Also a “Clock Matter”? Nutrients 2023, 15, 1427. https://doi.org/10.3390/nu15061427
Docimo A, Verde L, Barrea L, Vetrani C, Memoli P, Accardo G, Colella C, Nosso G, Orio M, Renzullo A, et al. Type 2 Diabetes: Also a “Clock Matter”? Nutrients. 2023; 15(6):1427. https://doi.org/10.3390/nu15061427
Chicago/Turabian StyleDocimo, Annamaria, Ludovica Verde, Luigi Barrea, Claudia Vetrani, Pasqualina Memoli, Giacomo Accardo, Caterina Colella, Gabriella Nosso, Marcello Orio, Andrea Renzullo, and et al. 2023. "Type 2 Diabetes: Also a “Clock Matter”?" Nutrients 15, no. 6: 1427. https://doi.org/10.3390/nu15061427
APA StyleDocimo, A., Verde, L., Barrea, L., Vetrani, C., Memoli, P., Accardo, G., Colella, C., Nosso, G., Orio, M., Renzullo, A., Savastano, S., Colao, A., & Muscogiuri, G. (2023). Type 2 Diabetes: Also a “Clock Matter”? Nutrients, 15(6), 1427. https://doi.org/10.3390/nu15061427







