The Impact of a Low-Carbohydrate Diet on Micronutrient Intake and Status in Adolescents with Type 1 Diabetes
Abstract
1. Introduction
2. Materials and Methods
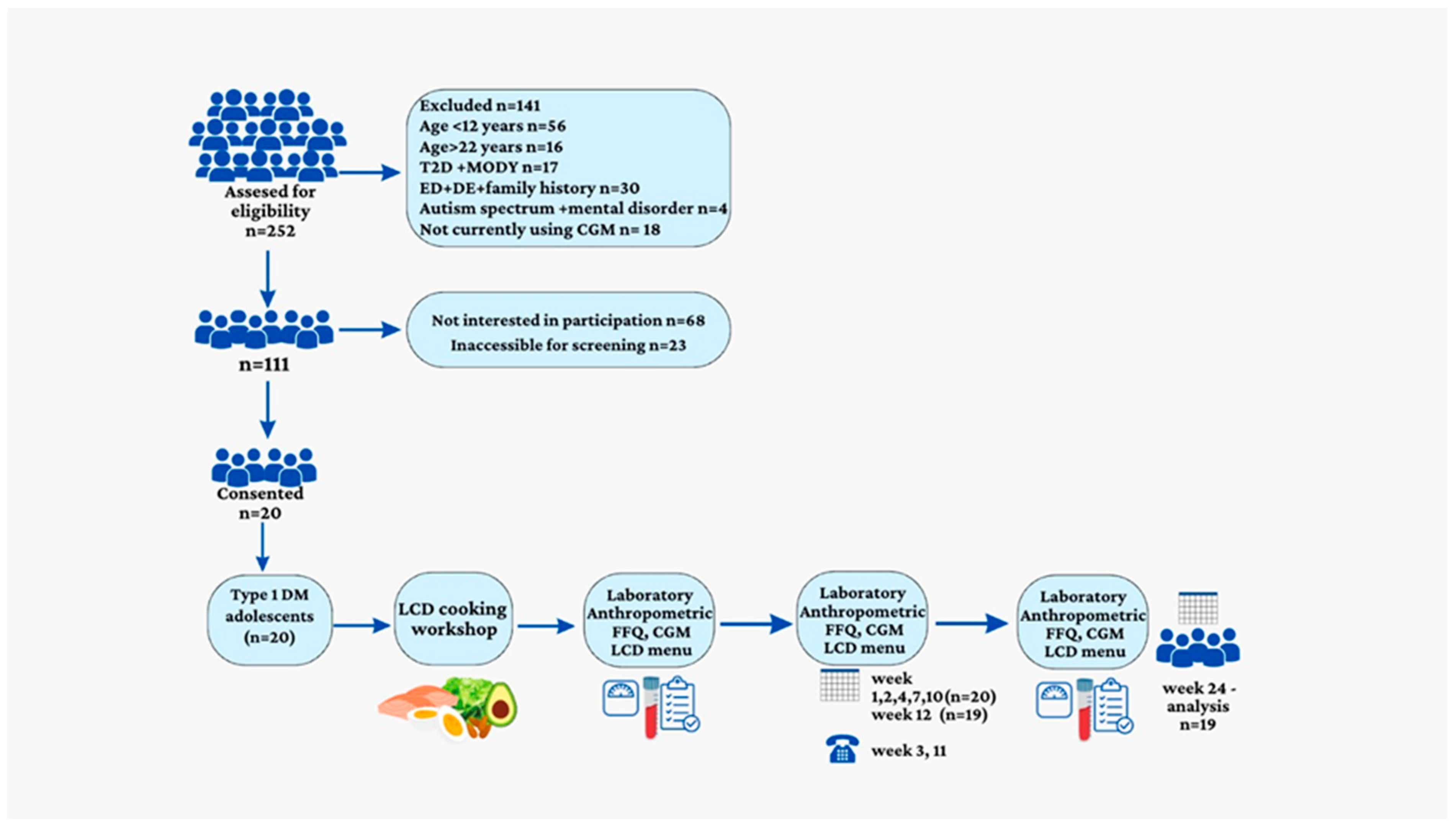
2.1. Participants and Study Design
2.2. Diet Intervention
2.3. Low-Carbohydrate Diet
2.4. Assessment of Nutritional Composition
2.5. Medical History and Anthropometric Measurements
2.6. Biochemical Parameters
2.7. Trial Outcomes
2.8. Statistical Analysis
3. Results
3.1. Study Group Characteristics
3.2. FQQ
3.3. Weight Loss and Waist Circumference
3.4. Blood Laboratory Measurements
3.5. Correlations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiang, J.L.; Maahs, D.M.; Garvey, K.C.; Hood, K.K.; Laffel, L.M.; Weinzimer, S.A.; Wolfsdorf, J.I.; Schatz, D. Type 1 Diabetes in Children and Adolescents: A Position Statement by the American Diabetes Association. Diabetes Care 2018, 41, 2026–2044. [Google Scholar] [CrossRef]
- Gregory, J.W.; Cameron, F.J.; Joshi, K.; Eiswirth, M.; Garrett, C.; Garvey, K.; Agarwal, S.; Codner, E. ISPAD Clinical Practice Consensus Guidelines 2022: Diabetes in Adolescence. Pediatr. Diabetes 2022, 23, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Foster, N.C.; Beck, R.W.; Miller, K.M.; Clements, M.A.; Rickels, M.R.; DiMeglio, L.A.; Maahs, D.M.; Tamborlane, W.V.; Bergenstal, R.; Smith, E.; et al. State of Type 1 Diabetes Management and Outcomes from the T1D Exchange in 2016–2018. Diabetes Technol. Ther. 2019, 21, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Neuman, V.; Plachy, L.; Pruhova, S.; Kolouskova, S.; Petruzelkova, L.; Obermannova, B.; Vyzralkova, J.; Konecna, P.; Vosahlo, J.; Romanova, M.; et al. Low-Carbohydrate Diet among Children with Type 1 Diabetes: A Multi-Center Study. Nutrients 2021, 13, 3903. [Google Scholar] [CrossRef] [PubMed]
- Lennerz, B.S.; Barton, A.; Bernstein, R.K.; Dikeman, R.D.; Diulus, C.; Hallberg, S.; Rhodes, E.T.; Ebbeling, C.B.; Westman, E.C.; Yancy, W.S.; et al. Management of Type 1 Diabetes with a Very Low–Carbohydrate Diet. Pediatrics 2018, 141, e20173349. [Google Scholar] [CrossRef] [PubMed]
- De Bock, M.; Lobley, K.; Anderson, D.; Davis, E.; Donaghue, K.; Pappas, M.; Siafarikas, A.; Cho, Y.H.; Jones, T.; Smart, C. Endocrine and Metabolic Consequences Due to Restrictive Carbohydrate Diets in Children with Type 1 Diabetes: An Illustrative Case Series. Pediatr. Diabetes 2018, 19, 129–137. [Google Scholar] [CrossRef]
- Seckold, R.; Fisher, E.; de Bock, M.; King, B.R.; Smart, C.E. The Ups and Downs of Low-Carbohydrate Diets in the Management of Type 1 Diabetes: A Review of Clinical Outcomes. Diabet. Med. 2019, 36, 326–334. [Google Scholar] [CrossRef]
- Shai, I.; Rosner, B.A.; Shahar, D.R.; Vardi, H.; Azrad, A.B.; Kanfi, A.; Schwarzfuchs, D.; Fraser, D. DEARR study Dietary Evaluation and Attenuation of Relative Risk: Multiple Comparisons between Blood and Urinary Biomarkers, Food Frequency, and 24-h Recall Questionnaires: The DEARR Study. J. Nutr. 2005, 135, 573–579. [Google Scholar] [CrossRef]
- Ministry of Health Public Health Services Nutrition Division. Tzameret-Israeli National Nutrient Database 2015; Ministry of Health Public Health Services Nutrition Division: Jerusalem, Israel, 2015.
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M.; Food and Nutrition Board of the Institute of Medicine, The National Academies. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids. J. Am. Diet. Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef]
- Lumeng, J.C.; Kaciroti, N.; Frisvold, D.E. Changes in Body Mass Index z Score over the Course of the Academic Year among Children Attending Head Start. Acad. Pediatr. 2010, 10, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D.; Ayuketah, A.; Brychta, R.; Cai, H.; Cassimatis, T.; Chen, K.Y.; Chung, S.T.; Costa, E.; Courville, A.; Darcey, V.; et al. Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell. Metab. 2019, 30, 67–77.e3. [Google Scholar] [CrossRef]
- Gardner, C.D.; Landry, M.J.; Perelman, D.; Petlura, C.; Durand, L.R.; Aronica, L.; Crimarco, A.; Cunanan, K.M.; Chang, A.; Dant, C.C.; et al. Effect of a Ketogenic Diet versus Mediterranean Diet on Glycated Hemoglobin in Individuals with Prediabetes and Type 2 Diabetes Mellitus: The Interventional Keto-Med Randomized Crossover Trial. Am. J. Clin. Nutr. 2022, 116, 640–652. [Google Scholar] [CrossRef] [PubMed]
- Storz, M.A.; Ronco, A.L. Nutrient Intake in Low-Carbohydrate Diets in Comparison to the 2020–2025 Dietary Guidelines for Americans: A Cross-Sectional Study. Br. J. Nutr. 2022, 129, 1023–1036. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, S.J. The Association between Dietary Fibre Deficiency and High-Income Lifestyle-Associated Diseases: Burkitt’s Hypothesis Revisited. Lancet Gastroenterol. Hepatol. 2019, 4, 984–996. [Google Scholar] [CrossRef]
- Schmidt, S.; Christensen, M.B.; Serifovski, N.; Damm-Frydenberg, C.; Jensen, J.-E.B.; Fløyel, T.; Størling, J.; Ranjan, A.; Nørgaard, K. Low versus High Carbohydrate Diet in Type 1 Diabetes: A 12-Week Randomized Open-Label Crossover Study. Diabetes Obes. Metab. 2019, 21, 1680–1688. [Google Scholar] [CrossRef]
- Churuangsuk, C.; Griffiths, D.; Lean, M.E.J.; Combet, E. Impacts of Carbohydrate-restricted Diets on Micronutrient Intakes and Status: A Systematic Review. Obes. Rev. 2019, 20, 1132–1147. [Google Scholar] [CrossRef] [PubMed]
- Christodoulides, S.S.; Neal, E.G.; Fitzsimmons, G.; Chaffe, H.M.; Jeanes, Y.M.; Aitkenhead, H.; Cross, J.H. The Effect of the Classical and Medium Chain Triglyceride Ketogenic Diet on Vitamin and Mineral Levels. J. Hum. Nutr. Diet. 2012, 25, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Hanna, M.; Jaqua, E.; Nguyen, V.; Clay, J. B Vitamins: Functions and Uses in Medicine. Perm. J. 2022, 26, 89–97. [Google Scholar] [CrossRef]
- King, J.C.; Brown, K.H.; Gibson, R.S.; Krebs, N.F.; Lowe, N.M.; Siekmann, J.H.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND)—Zinc Review. J. Nutr. 2015, 146, 858S–885S. [Google Scholar] [CrossRef]
- Lin, C.-C.; Huang, Y.-L. Chromium, Zinc and Magnesium Status in Type 1 Diabetes. Curr. Opin. Clin. Nutr. Metab Care 2015, 18, 588–592. [Google Scholar] [CrossRef]
- Salmonowicz, B.; Krzystek-Korpacka, M.; Noczyńska, A. Trace Elements, Magnesium, and the Efficacy of Antioxidant Systems in Children with Type 1 Diabetes Mellitus and in Their Siblings. Adv. Clin. Exp. Med. 2014, 23, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Moran, V.H.; Stammers, A.-L.; Medina, M.W.; Patel, S.; Dykes, F.; Souverein, O.W.; Dullemeijer, C.; Pérez-Rodrigo, C.; Serra-Majem, L.; Nissensohn, M.; et al. The Relationship between Zinc Intake and Serum/Plasma Zinc Concentration in Children: A Systematic Review and Dose-Response Meta-Analysis. Nutrients 2012, 4, 841–858. [Google Scholar] [CrossRef] [PubMed]
- Hennigar, S.R.; Lieberman, H.R.; Fulgoni, V.L.; McClung, J.P. Serum Zinc Concentrations in the US Population Are Related to Sex, Age, and Time of Blood Draw but Not Dietary or Supplemental Zinc. J. Nutr. 2018, 148, 1341–1351. [Google Scholar] [CrossRef]
- Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press: Washington, DC, USA, 2001; ISBN 978-0-309-07279-3.
- De Romaña, D.L.; Olivares, M.; Uauy, R.; Araya, M. Risks and Benefits of Copper in Light of New Insights of Copper Homeostasis. J. Trace Elem. Med. Biol. 2011, 25, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Chin, A. Copper Deficiency Anemia and Neutropenia Due to Ketogenic Diet. Pediatrics 2018, 141, e20173286. [Google Scholar] [CrossRef]
- Galli-Tsinopoulou, A.; Maggana, I.; Kyrgios, I.; Mouzaki, K.; Grammatikopoulou, M.G.; Stylianou, C.; Karavanaki, K. Association between Magnesium Concentration and HbA1c in Children and Adolescents with Type 1 Diabetes Mellitus. J. Diabetes 2014, 6, 369–377. [Google Scholar] [CrossRef]
- Toh, P.; Nicholson, J.L.; Vetter, A.M.; Berry, M.J.; Torres, D.J. Selenium in Bodily Homeostasis: Hypothalamus, Hormones, and Highways of Communication. Int. J. Mol. Sci. 2022, 23, 15445. [Google Scholar] [CrossRef]
- Bergqvist, A.G.C.; Chee, C.M.; Lutchka, L.; Rychik, J.; Stallings, V.A. Selenium Deficiency Associated with Cardiomyopathy: A Complication of the Ketogenic Diet. Epilepsia 2003, 44, 618–620. [Google Scholar] [CrossRef] [PubMed]
- Grabia, M.; Markiewicz-Żukowska, R.; Socha, K. Prevalence of Metabolic Syndrome in Children and Adolescents with Type 1 Diabetes Mellitus and Possibilities of Prevention and Treatment: A Systematic Review. Nutrients 2021, 13, 1782. [Google Scholar] [CrossRef]
- Liu, Y.-M.C.; Williams, S.; Basualdo-Hammond, C.; Stephens, D.; Curtis, R. A Prospective Study: Growth and Nutritional Status of Children Treated with the Ketogenic Diet. J. Am. Diet. Assoc. 2003, 103, 707–712. [Google Scholar] [CrossRef]
- Grandjean, A.C. Dietary Intake Data Collection: Challenges and Limitations. Nutr. Rev. 2012, 70 (Suppl. S2), S101–S104. [Google Scholar] [CrossRef] [PubMed]
| At Baseline | After Six Months | Percent Change | p-Value | Adjusted p-Value a | ||
|---|---|---|---|---|---|---|
| Macronutrients | Carbohydrate, g | 265 (204; 315) | 86 (68; 95) | 67.4 | 0.0001 | <0.001 |
| Sugar alcohols, g | 0.0 (0.0; 8.6) | 0.0 (0.0; 2.5) | 0 | 0.133 | 0.197 | |
| Fiber, g | 33 (28; 43) | 21 (17; 28) | 30.1 | 0.0001 | <0.001 | |
| Total sugars, g | 120 (87; 156) | 51 (42; 69) | 56.9 | 0.0001 | <0.001 | |
| Fructose, g | 25.8 (22.1; 36.0) | 11.9 (6.7; 23.1) | 53.9 | 0.0001 | <0.001 | |
| Protein, g | 116 (92; 138) | 91 (69; 108) | 21.6 | 0.020 | 0.040 | |
| Fat, g | 85 (78; 108) | 85 (65; 96) | 0 | 0.445 | 0.531 | |
| Cholesterol, mg | 429 (369; 580) | 420 (365; 617) | 2 | 0.960 | 0.997 | |
| Saturated fat, mg | 25.0 (22.3; 34.5) | 25.5 (19.4; 29.0) | 2 | 0.460 | 0.536 | |
| Minerals | Iron, mg | 15.6 (11.7; 17.1) | 11.2 (8.8; 12.6) | 28.2 | 0.001 | 0.004 |
| Magnesium, mg | 508 (429; 604. | 398 (361; 433) | 21,6 | 0.002 | 0.007 | |
| Phosphorus, mg | 1793 (1615; 2323) | 1462 (1181; 1637) | 18.5 | 0.005 | 0.01 | |
| Calcium, mg | 1315 (902; 1468) | 966 (635; 1232) | 26.6 | 0.036 | 0.067 | |
| Zinc, mg | 12.6 (10.5; 15.8) | 10.5 (8.3; 12.4) | 16.7 | 0.016 | 0.034 | |
| Copper, mg | 2.1 (1.7; 2.3) | 1.5 (1.3; 1.8) | 23.8 | 0.001 | 0.004 | |
| Selenium, mcg | 160 (125; 206) | 111 (89; 136) | 30.1 | 0.010 | 0.022 | |
| Potassium, mg | 4803 (3763; 5768) | 3605 (2990; 4316) | 20 | 0.005 | 0.014 | |
| Sodium, mg | 4209 (3645; 5250) | 3529 (2966; 4410) | 16.1 | 0.070 | 0.122 | |
| Vitamins | Thiamine B1, mg | 1.6 (1.5; 2.1) | 0.9 (0.8; 1.2) | 43.8 | 0.0001 | <0.001 |
| Riboflavin B2, mg | 2.6 (2.1; 3.0) | 1.8 (1.4; 2.4) | 31 | 0.001 | 0.004 | |
| Niacin B3, mg | 29.1 (22.6; 35.0) | 24.7 (17.2; 28.4) | 15 | 0.064 | 0.116 | |
| Vitamin B6, mg | 3.2 (2.6; 3.6) | 2.1 (1.7; 2.6) | 34.4 | 0.001 | 0.004 | |
| Folate B9, mcg | 492 (422; 602) | 361 (286; 484) | 26.5 | 0.004 | 0.01 | |
| Vitamin B12, mcg | 5.5 (4.7; 7.1) | 5.8 (4.8; 7.7) | +5.4 | 0.904 | 0.984 | |
| Vitamin C, mg | 293 (156; 350) | 212 (142; 309) | 27.8 | 0.099 | 0.156 | |
| Vitamin D, mcg | 8.1 (6.3; 11.7) | 8.7 (6.3; 10.4) | +7.4 | 0.930 | 0.990 | |
| Carotene, mcg | 7332 (5610; 10193) | 6439 (3997; 8773) | 12.2 | 0.126 | 0.984 | |
| Vitamin A, mcg | 1951 (1592; 3484) | 1375 (1152; 2091) | 29.6 | 0.084 | 0.141 | |
| Vitamin E, mg | 14.0 (11.2; 17.0) | 12.7 (10.4; 15.8) | 9.3 | 0.159 | 0.222 |
| Before | After | Change | p-Value | Adjusted p-Value * | ||
|---|---|---|---|---|---|---|
| Anthropometric measurements | BMI z-score | 1.30 (0.65; −1.58) | 1.20 (0.54; −1.49) | −0.13 (−0.29; −0.02) | 0.008 | 0.042 |
| Waist circumference percentile | 76.5 (55.5; 83.5) | 65.5 (37.6; −77.5) | −6.5 (−19.0; −4.0) | 0.002 | 0.021 | |
| Treatment | Insulin unit/kg | 0.8 (0.63; −1.95) | 0.7 (0.50; −0.92) | −0.12 (−0.18; −0.04) | 0.006 | 0.01 |
| Blood tests | HbA1c % | 8.1 (7.5; 9.4) | 7.7 (6.9; 8.2) | −0.8 (−1.3; −0.3) | 0.001 | 0.021 |
| Total cholesterol mg/dL | 176 (160; 194) | 160 (155; 190) | 0.00 (−22; 18) | 0.289 | 0.379 | |
| LDL cholesterol mg/dL | 105 (97; 119) | 110 (98; 120) | −3.5 (−14; 18) | 0.737 | 0.814 | |
| HDL cholesterol mg/dL | 64 (53; 71) | 58 (45; 65) | −11 (−4; 0) | 0.02 | 0.06 | |
| Triglycerides mg/dL | 84 (64; 89) | 69 (56; 88) | −8 (−20; 0) | 0.019 | 0.06 | |
| CRP <0.20–5.00 mg/L | 3.5 (1.1; 7.1) | 2.5 (1.0; 4.9) | −0.77 (−1.1; 0) | 0.010 | 0.042 | |
| Urea 17–45 mg/dL | 28.0 (23.5; 33.25) | 27.0 (21.0; 31.0) | −1.0 (−7.5; 0.5) | 0.195 | 0.364 | |
| Creatinine 0.62–1.10 mg/dL | 0.63 (0.56; 0.7) | 0.64 (0.56; 0.7) | 0.0 (0.0; 0.0) | 0.530 | 0.618 | |
| Zinc 50.0–150.0 mcg/dL | 130.5 (104.7; 150.0) | 98.0 (82.5; 119.0) | −28 (−63; 0) | 0.006 | 0.042 | |
| Magnesium 1.90–2.70 mg/dL | 1.9 (1.8; 2.0) | 1.9 (1.8; 2.0) | 0.0 (−0.2; 0) | 0.260 | 0.364 | |
| Phosphorus 2.00–4.00 mg/dL | 4.1 (3.8; 4.4) | 4.1 (3.7; 4.4) | 0.0 (−0.2; 0.3) | 1.000 | >0.999 | |
| Calcium 8.1–10.4 md/dL | 9.7 (9.6; 10.1) | 9.8 (9.5; 9.9) | 0.1 (−0.4; 0.2) | 0.260 | 0.364 | |
| Vitamin b1 66.5–200.0 nmol/L | 131.8 (119.4; 150.8) | 129.0 (120.0; 36.9) | −4.2 (−15.4;−1.5) | 0.260 | 0.364 | |
| Vitamin C 4.6–14.9 mg/L | 11.0 (8.0; 12.8) | 11.8 (9.8; 14.4) | 0.8 (−0.8; 3.8) | 0.190 | 0.364 | |
| Folic acid 5.9–24.0 ng/mL | 9.9 (7.1; 13.5) | 10.1 (7.4; 16.1) | 0.6 (−1.8; 1.5) | 0.230 | 0.364 |
| Correlation with Decreased Carbohydrate Intake (g) | |||
|---|---|---|---|
| R | p-Value | p-Value adj | |
| Delta calories | 0.749 | <0.001 | <0.001 |
| Delta fiber | 0.75 | <0.001 | <0.001 |
| Delta sugar alcohols | 0.180 | 0.447 | 0.448 |
| Delta protein | 0.341 | 0.141 | 0.172 |
| Delta fat | 0.412 | 0.079 | 0.108 |
| Delta iron | 0.598 | 0.005 | 0.011 |
| Delta calcium | 0.352 | 0.128 | 0.14 |
| Delta copper | 0.720 | <0.001 | <0.001 |
| Delta potassium | 0.653 | 0.002 | 0.005 |
| Delta sodium | 0.292 | 0.212 | 0.24 |
| Delta magnesium | 0.576 | 0.008 | 0.014 |
| Delta zinc | 0.37 | 0.108 | 0.132 |
| Delta thiamine | 0.701 | 0.001 | 0.002 |
| Delta vitamin B6 | 0.538 | 0.014 | 0.002 |
| Delta riboflavin | 0.538 | 0.014 | 0.023 |
| Delta vitamin C | 0.675 | 0.001 | 0.002 |
| Correlations with Decreased Protein Intake (g) | |||
| R | p-Value | p-Value adj | |
| Delta calories | 0.827 | <0.0001 | <0.0001 |
| Delta fiber | 0.214 | 0.366 | 0.402 |
| Delta sugar alcohols | −0.039 | 0.869 | 0.869 |
| Delta fat | 0.826 | <0.0001 | <0.0001 |
| Delta iron | 0.627 | 0.003 | 0.005 |
| Delta calcium | 0.687 | 0.001 | 0.002 |
| Delta copper | 0.460 | 0.041 | 0.061 |
| Delta potassium | 0.595 | 0.006 | 0.011 |
| Delta sodium | 0.502 | 0.024 | 0.04 |
| Delta magnesium | 0.403 | 0.078 | 0.107 |
| Delta zinc | 0.947 | <0.001 | <0.0001 |
| Delta thiamine | 0.448 | 0.048 | 0.075 |
| Delta vitamin B6 | 0.85 | <0.0001 | <0.0001 |
| Delta riboflavin | 0.850 | <0.0001 | <0.0001 |
| Delta vitamin C | 0.37 | 0.108 | 0.132 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levran, N.; Levek, N.; Sher, B.; Gruber, N.; Afek, A.; Monsonego-Ornan, E.; Pinhas-Hamiel, O. The Impact of a Low-Carbohydrate Diet on Micronutrient Intake and Status in Adolescents with Type 1 Diabetes. Nutrients 2023, 15, 1418. https://doi.org/10.3390/nu15061418
Levran N, Levek N, Sher B, Gruber N, Afek A, Monsonego-Ornan E, Pinhas-Hamiel O. The Impact of a Low-Carbohydrate Diet on Micronutrient Intake and Status in Adolescents with Type 1 Diabetes. Nutrients. 2023; 15(6):1418. https://doi.org/10.3390/nu15061418
Chicago/Turabian StyleLevran, Neriya, Noah Levek, Bruria Sher, Noah Gruber, Arnon Afek, Efrat Monsonego-Ornan, and Orit Pinhas-Hamiel. 2023. "The Impact of a Low-Carbohydrate Diet on Micronutrient Intake and Status in Adolescents with Type 1 Diabetes" Nutrients 15, no. 6: 1418. https://doi.org/10.3390/nu15061418
APA StyleLevran, N., Levek, N., Sher, B., Gruber, N., Afek, A., Monsonego-Ornan, E., & Pinhas-Hamiel, O. (2023). The Impact of a Low-Carbohydrate Diet on Micronutrient Intake and Status in Adolescents with Type 1 Diabetes. Nutrients, 15(6), 1418. https://doi.org/10.3390/nu15061418






