Early Probiotic Supplementation of Healthy Term Infants with Bifidobacterium longum subsp. infantis M-63 Is Safe and Leads to the Development of Bifidobacterium-Predominant Gut Microbiota: A Double-Blind, Placebo-Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Design
2.3. Infant Gastrointestinal (GI) Tolerability and Health Examination
2.4. Fecal Sample Collection
2.5. Fecal DNA Extraction and 16S rRNA Gene Sequencing
2.6. Quantification of Bifidobacterium Species by Real-Time PCR
2.7. Fecal pH, Short-Chain Fatty Acids (SCFAs) and Biomarker Analysis
2.8. Sample Size
2.9. Primary and Secondary Outcomes
2.10. Statistics
3. Results
3.1. Infant and Maternal Characteristics
3.2. Infant Feeding
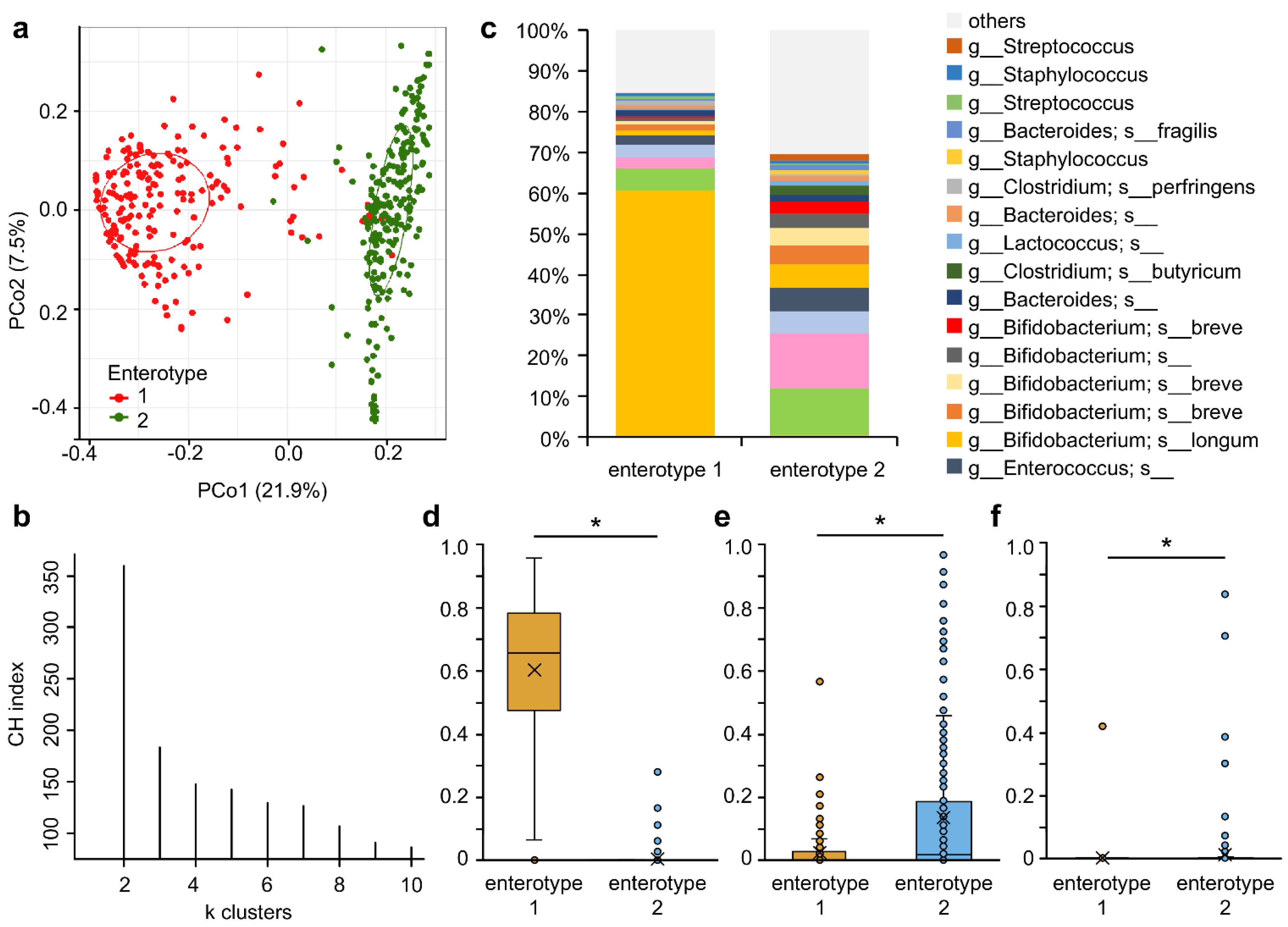
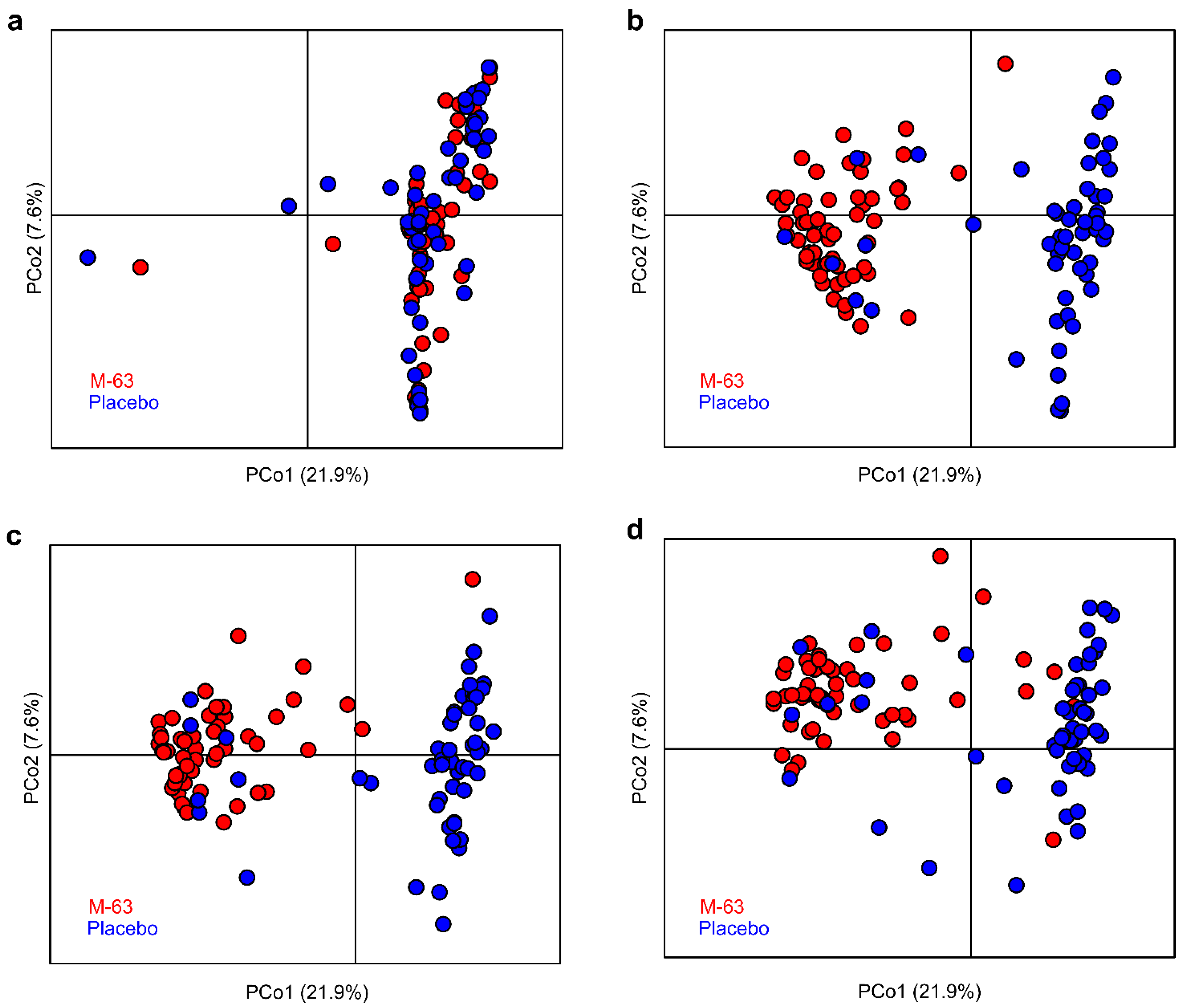
3.3. Microbiota Analysis
3.4. Bifidobacterial Colonization
3.5. Bacterial Species of Colonized Bifidobacteria
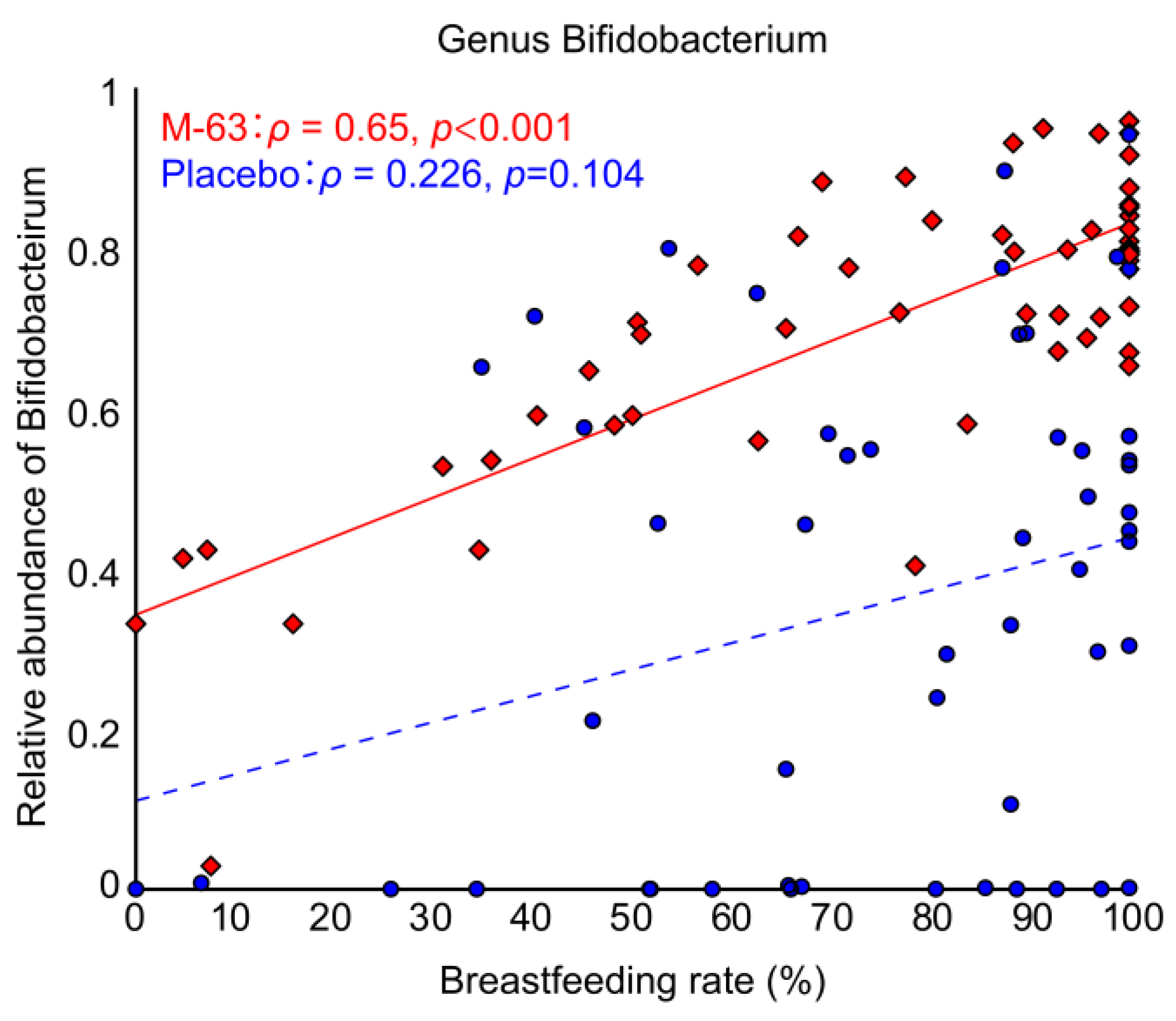
3.6. Correlation between Breastfeeding and Bifidobacterial Occupancy
3.7. Gut Fermentation Patterns and Immunologic Parameters in Stools
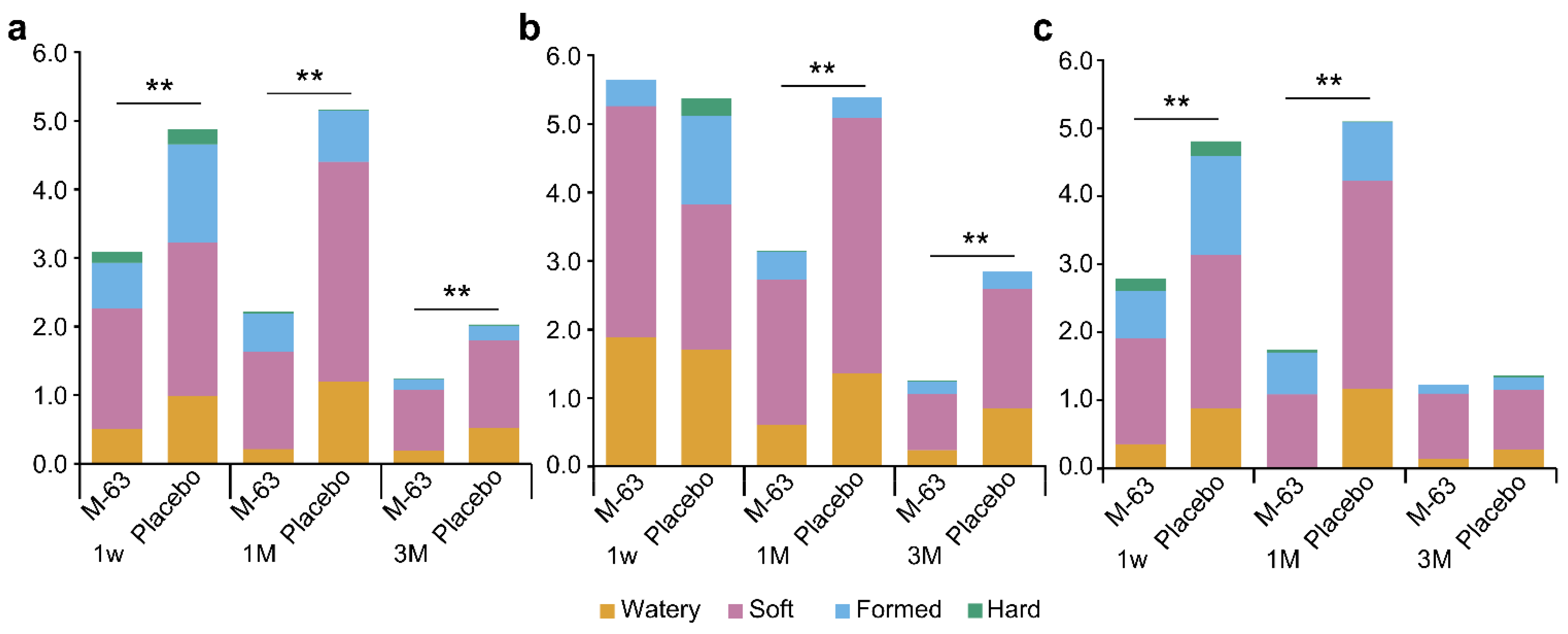
3.8. GI Tolerability and Health Condition of Infants
3.9. Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Korpela, K.; de Vos, W.M. Infant gut microbiota restoration: State of the art. Gut Microbes 2022, 14, 2118811. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.B.; Sugahara, H.; Odamaki, T.; Xiao, J.Z. Different physiological properties of human-residential and non-human-residential bifidobacteria in human health. Benef. Microbes 2018, 9, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Giugliano, F.P.; Quitadamo, P.; Mancusi, V.; Miele, E.; Staiano, A. Efficacy of a mixture of probiotic agents as complementary therapy for chronic functional constipation in childhood. Ital. J. Pediatr. 2017, 43, 24. [Google Scholar] [CrossRef]
- Huda, M.N.; Lewis, Z.; Kalanetra, K.M.; Rashid, M.; Ahmad, S.M.; Raqib, R.; Qadri, F.; Underwood, M.A.; Mills, D.A.; Stephensen, C.B. Stool microbiota and vaccine responses of infants. Pediatrics 2014, 134, e362–e372. [Google Scholar] [CrossRef]
- Huda, M.N.; Ahmad, S.M.; Alam, M.J.; Khanam, A.; Kalanetra, K.M.; Taft, D.H.; Raqib, R.; Underwood, M.A.; Mills, D.A.; Stephensen, C.B. Bifidobacterium abundance in early infancy and vaccine response at 2 years of age. Pediatrics 2019, 143, e20181489. [Google Scholar] [CrossRef]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Henrick, B.M.; Chew, S.; Casaburi, G.; Brown, H.K.; Frese, S.A.; Zhou, Y.; Underwood, M.A.; Ra, J.T. Colonization by B. infantis EVC001 modulates enteric inflammation in exclusively breastfed infants. Pediatr. Res. 2019, 86, 749–757. [Google Scholar] [CrossRef]
- Fiocchi, A.; Pawankar, R.; Cuello-Garcia, C.; Ahn, K.; Al-Hammadi, S.; Agarwal, A.; Beyer, K.; Burks, W.; Canonica, G.W.; Ebisawa, M.; et al. World allergy organization-mcmaster university guidelines for allergic disease prevention (GLAD-P): Probiotics. World Allergy Organ. J. 2015, 8, 4. [Google Scholar] [CrossRef]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Palacio, S.D.; Montes, S.A.; Mancabelli, L.; et al. The first microbial colonizers of the human gut: Composition, activities, and health implications of the infant gut microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef]
- Sutharsan, R.; Mannan, M.; Doi, S.A.; Mamun, A.A. Caesarean delivery and the risk of offspring overweight and obesity over the life course: A systematic review and bias-adjusted meta-analysis. Clin. Obes. 2015, 5, 293–301. [Google Scholar] [CrossRef]
- Korpela, K.; Zijlmans, M.A.C.; Kuitunen, M.; Kukkonen, K.; Savilahti, E.; Salonen, A.; de Weerth, C.; de Vos, W.M. Childhood BMI in relation to microbiota in infancy and lifetime antibiotic use. Microbiome 2017, 5, 26. [Google Scholar] [CrossRef]
- Mitselou, N.; Hallberg, J.; Stephansson, O.; Almqvist, C.; Melén, E.; Ludvigsson, J.F. Cesarean delivery, preterm birth, and risk of food allergy: Nationwide Swedish cohort study of more than 1 million children. J. Allergy Clin. Immunol. 2018, 142, 1510–1514.e2. [Google Scholar] [CrossRef] [PubMed]
- Imoto, N.; Morita, H.; Amanuma, F.; Maruyama, H.; Watanabe, S.; Hashiguchi, N. Maternal antimicrobial use at delivery has a stronger impact than mode of delivery on bifidobacterial colonization in infants: A pilot study. J. Perinatol. 2018, 38, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Henderickx, J.G.E.; Zwittink, R.D.; van Lingen, R.A.; Knol, J.; Belzer, C. The preterm gut microbiota: An inconspicuous challenge in nutritional neonatal care. Front. Cell. Infect. Microbiol. 2019, 9, 85. [Google Scholar] [CrossRef]
- Matsuki, T.; Yahagi, K.; Mori, H.; Matsumoto, H.; Hara, T.; Tajima, S.; Ogawa, E.; Kodama, H.; Yamamoto, K.; Yamada, T.; et al. A key genetic factor for fucosyllactose utilization affects infant gut microbiota development. Nat. Commun. 2016, 7, 11939. [Google Scholar] [CrossRef] [PubMed]
- Underwood, M.A.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Bifidobacterium longum subspecies infantis: Champion colonizer of the infant gut. Pediatr. Res. 2014, 77, 229–235. [Google Scholar] [CrossRef]
- Hickey, L.; Jacobs, S.E.; Garland, S.M.; ProPrems Study Group. Probiotics in neonatology. J. Paediatr. Child Health 2012, 48, 777–783. [Google Scholar] [CrossRef]
- Bajorek, S.; Duar, R.M.; Corrigan, M.; Matrone, C.; Winn, K.A.; Norman, S.; Mitchell, R.D.; Cagney, O.; Aksenov, A.A.; Melnik, A.V.; et al. B. infantis EVC001 is well-tolerated and improves human milk oligosaccharide utilization in preterm infants in the neonatal intensive care unit. Front. Pediatr. 2021, 9, 795970. [Google Scholar] [CrossRef]
- Ishizeki, S.; Sugita, M.; Takata, M.; Yaeshima, T. Effect of administration of bifidobacteria on intestinal microbiota in low-birth-weight infants and transition of administered bifidobacteria: A comparison between one-species and three-species administration. Anaerobe 2013, 23, 38–44. [Google Scholar] [CrossRef]
- Patole, S.K.; Rao, S.C.; Keil, A.D.; Nathan, E.A.; Doherty, D.A.; Simmer, K.N. Benefits of Bifidobacterium breve M-16V supplementation in preterm neonates—A retrospective cohort study. PLoS ONE 2016, 11, e0150775. [Google Scholar] [CrossRef]
- Tobias, J.; Olyaei, A.; Laraway, B.; Jordan, B.K.; Dickinson, S.L.; Golzarri-Arroyo, L.; Fialkowski, E.; Owora, A.; Scottoline, B. Bifidobacterium longum subsp. infantis EVC001 administration is associated with a significant reduction in the incidence of necrotizing enterocolitis in very low birth weight infants. J. Pediatr. 2022, 244, 64–71.e2. [Google Scholar] [CrossRef] [PubMed]
- Abe, F.; Yaeshima, T.; Iwatsuki, K. Safety evaluation of two probiotic bifidobacterial strains, Bifidobacterium breve M-16V and Bifidobacterium infantis M-63, by oral toxicity tests using rats. Biosci. Microflora 2009, 28, 7–15. [Google Scholar] [CrossRef]
- Walsh, C.; Lane, J.A.; van Sinderen, D.; Hickey, R.M. Human milk oligosaccharide-sharing by a consortium of infant derived Bifidobacterium species. Sci. Rep. 2022, 12, 4143. [Google Scholar] [CrossRef] [PubMed]
- Thongaram, T.; Hoeflinger, J.L.; Chow, J.; Miller, M.J. Human milk oligosaccharide consumption by probiotic and human-associated bifidobacteria and lactobacilli. J. Dairy Sci. 2017, 100, 7825–7833. [Google Scholar] [CrossRef] [PubMed]
- Minami, J.; Odamaki, T.; Hashikura, N.; Abe, F.; Xiao, J.Z. Lysozyme in breast milk is a selection factor for bifidobacterial colonisation in the infant intestine. Benef. Microbes 2016, 7, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Esvaran, M.; Chen, L.; Keil, A.D.; Gollow, I.; Simmer, K.; Wemheuer, B.; Conway, P.; Patole, S. Probiotic supplementation in neonates with congenital gastrointestinal surgical conditions: A pilot randomised controlled trial. Pediatr. Res. 2022, 92, 1122–1131. [Google Scholar] [CrossRef]
- Dupont, C.; Rivero, M.; Grillon, C.; Belaroussi, N.; Kalindjian, A.; Marin, V. α-Lactalbumin-enriched and probiotic-supplemented infant formula in infants with colic: Growth and gastrointestinal tolerance. Eur. J. Clin. Nutr. 2010, 64, 765–767. [Google Scholar] [CrossRef]
- Rozé, J.C.; Barbarot, S.; Butel, M.J.; Kapel, N.; Waligora-Dupriet, A.J.; De Montgolfier, I.; Leblanc, M.; Godon, N.; Soulaines, P.; Darmaun, D.; et al. An α-lactalbumin-enriched and symbiotic-supplemented v. a standard infant formula: A multicentre, double-blind, randomised trial. Br. J. Nutr. 2012, 107, 1616–1622. [Google Scholar] [CrossRef]
- Del Giudice, M.M.; Indolfi, C.; Capasso, M.; Maiello, N.; Decimo, F.; Ciprandi, G. Bifidobacterium mixture (B longum BB536, B infantis M-63, B breve M-16V) treatment in children with seasonal allergic rhinitis and intermittent asthma. Ital. J. Pediatr. 2017, 43, 25. [Google Scholar] [CrossRef]
- Giannetti, E.; Maglione, M.; Alessandrella, A.; Strisciuglio, C.; De Giovanni, D.; Campanozzi, A.; Miele, E.; Staiano, A. A Mixture of 3 bifidobacteria decreases abdominal pain and improves the quality of life in children with irritable bowel syndrome. J. Clin. Gastroenterol. 2017, 51, e5–e10. [Google Scholar] [CrossRef]
- Martin, R.; Makino, H.; Yavuz, A.C.; Ben-Amor, K.; Roelofs, M.; Ishikawa, E.; Kubota, H.; Swinkels, S.; Sakai, T.; Oishi, K.; et al. Early-life events, including mode of delivery and type of feeding, siblings and gender, shape the developing gut microbiota. PLoS ONE 2016, 11, e0158498. [Google Scholar] [CrossRef]
- Bekkali, N.; Hamers, S.L.; Reitsma, J.B.; Van Toledo, L.; Benninga, M.A. Infant stool form scale: Development and results. J. Pediatr. 2009, 154, 521–526.e1. [Google Scholar] [CrossRef]
- Wolke, D.; Bilgin, A.; Samara, M. Systematic review and meta-analysis: Fussing and crying durations and prevalence of colic in infants. J. Pediatr. 2017, 185, 55–61.e4. [Google Scholar] [CrossRef]
- Komatsu, Y.; Kumakura, D.; Seto, N.; Izumi, H.; Takeda, Y.; Ohnishi, Y.; Nakaoka, S.; Aizawa, T. Dynamic associations of milk components with the infant gut microbiome and fecal metabolites in a mother–infant model by microbiome, NMR metabolomic, and time-series clustering analyses. Front. Nutr. 2022, 8, 813690. [Google Scholar] [CrossRef] [PubMed]
- Odamaki, T.; Bottacini, F.; Kato, K.; Mitsuyama, E.; Yoshida, K.; Horigome, A.; Xiao, J.Z.; van Sinderen, D. Genomic diversity and distribution of Bifidobacterium longum subsp. longum across the human lifespan. Sci. Rep. 2018, 8, 85. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Tomita, J.; Nishioka, K.; Hisada, T.; Nishijima, M. Development of a prokaryotic universal primer for simultaneous analysis of bacteria and archaea using next-generation sequencing. PLoS ONE 2014, 9, e105592. [Google Scholar] [CrossRef]
- Tso, L.; Bonham, K.S.; Fishbein, A.; Rowland, S.; Klepac-Ceraj, V. Targeted high-resolution taxonomic identification of Bifidobacterium longum subsp. infantis using human milk oligosaccharide metabolizing genes. Nutrients 2021, 13, 2833. [Google Scholar] [CrossRef]
- Murakami, R.; Hashikura, N.; Yoshida, K.; Xiao, J.Z.; Odamaki, T. Growth-promoting effect of alginate on Faecalibacterium prausnitzii through cross-feeding with Bacteroides. Food Res. Int. 2021, 144, 110326. [Google Scholar] [CrossRef]
- Kato, K.; Odamaki, T.; Mitsuyama, E.; Sugahara, H.; Xiao, J.Z.; Osawa, R. Age-related changes in the composition of gut Bifidobacterium species. Curr. Microbiol. 2017, 74, 987–995. [Google Scholar] [CrossRef] [PubMed]
- García-Villalba, R.; Giménez-Bastida, J.A.; García-Conesa, M.T.; Tomás-Barberán, F.A.; Espín, J.C.; Larrosa, M. Alternative method for gas chromatography-mass spectrometry analysis of short-chain fatty acids in faecal samples. J. Sep. Sci. 2012, 35, 1906–1913. [Google Scholar] [CrossRef]
- Fernandes, A.D.; Reid, J.N.S.; Macklaim, J.M.; McMurrough, T.A.; Edgell, D.R.; Gloor, G.B. Unifying the analysis of high-throughput sequencing datasets: Characterizing RNA-seq, 16S rRNA gene sequencing and selective growth experiments by compositional data analysis. Microbiome 2014, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Weaver, L.T.; Steiner, H. The bowel habit of young children. Arch. Dis. Child. 1984, 59, 649–652. [Google Scholar] [CrossRef]
- Weaver, L.T.; Ewing, G.; Taylor, L.C. The bowel habit of milk-fed infants. J. Pediatr. Gastroenterol. Nutr. 1988, 7, 568–571. [Google Scholar] [CrossRef]
- Tham, E.B.A.; Nathan, R.; Davidson, G.P.; Moore, D.J. Bowel habits of healthy Australian children aged 0–2 years. J. Paediatr. Child Health 1996, 32, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Nyhan, W.L. Stool frequency of normal infants in the first week of life. Pediatrics 1952, 10, 414–425. [Google Scholar] [CrossRef]
- Smilowitz, J.T.; Moya, J.; Breck, M.A.; Cook, C.; Fineberg, A.; Angkustsiri, K.; Underwood, M.A. Safety and tolerability of Bifidobacterium longum subspecies infantis EVC001 supplementation in healthy term breastfed infants: A phase I clinical trial. BMC Pediatr. 2017, 17, 180. [Google Scholar] [CrossRef]
- Chichlowski, M.; De Lartigue, G.; German, J.B.; Raybould, H.E.; Mills, D.A. Bifidobacteria isolated from infants and cultured on human milk oligosaccharides affect intestinal epithelial function. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 321–327. [Google Scholar] [CrossRef]
- Subramanian, S.; Blanton, L.V.; Frese, S.A.; Charbonneau, M.; Mills, D.A.; Gordon, J.I. Cultivating healthy growth and nutrition through the gut microbiota. Cell 2015, 161, 36–48. [Google Scholar] [CrossRef]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 2015, 17, 852. [Google Scholar] [CrossRef]
- Taft, D.H.; Lewis, Z.T.; Nguyen, N.; Ho, S.; Masarweh, C.; Dunne-Castagna, V.; Tancredi, D.J.; Huda, M.N.; Stephensen, C.B.; Hinde, K.; et al. Bifidobacterium species colonization in infancy: A global cross-sectional comparison by population history of breastfeeding. Nutrients 2022, 14, 1423. [Google Scholar] [CrossRef] [PubMed]
- Imoto, N.; Kano, C.; Aoyagi, Y.; Morita, H.; Amanuma, F.; Maruyama, H.; Nojiri, S.; Hashiguchi, N.; Watanabe, S. Administration of β-lactam antibiotics and delivery method correlate with intestinal abundances of Bifidobacteria and Bacteroides in early infancy, in Japan. Sci. Rep. 2021, 11, 6231. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shoji, H.; Sato, H.; Nagata, S.; Ohtsuka, Y.; Shimizu, T.; Yamashiro, Y. Effects of oral administration of Bifidobacterium breve on fecal lactic acid and short-chain fatty acids in low birth weight infants. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Tsukuda, N.; Yahagi, K.; Hara, T.; Watanabe, Y.; Matsumoto, H.; Mori, H.; Higashi, K.; Tsuji, H.; Matsumoto, S.; Kurokawa, K.; et al. Key bacterial taxa and metabolic pathways affecting gut short-chain fatty acid profiles in early life. ISME J. 2021, 15, 2574–2590. [Google Scholar] [CrossRef]
- Duar, R.M.; Kyle, D.; Casaburi, G. Colonization resistance in the infant gut: The role of B. infantis in reducing pH and preventing pathogen growth. High Throughput 2020, 9, 7. [Google Scholar] [CrossRef]
- Wu, W.; Sun, M.; Chen, F.; Cao, A.T.; Liu, H.; Zhao, Y.; Huang, X.; Xiao, Y.; Yao, S.; Zhao, Q.; et al. Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal Immunol. 2017, 10, 946–956. [Google Scholar] [CrossRef]
- Frese, S.A.; Hutton, A.A.; Contreras, L.N.; Shaw, C.A.; Palumbo, M.C.; Casaburi, G.; Xu, G.; Davis, J.C.C.; Lebrilla, C.B.; Henrick, B.M.; et al. Persistence of supplemented Bifidobacterium longum subsp. infantis EVC001 in breastfed infants. mSphere 2017, 2, e00501-17. [Google Scholar] [CrossRef]
- Ioannou, A.; Knol, J.; Belzer, C. Microbial glycoside hydrolases in the first year of life: An analysis review on their presence and importance in infant gut. Front. Microbiol. 2021, 12, 631282. [Google Scholar] [CrossRef]
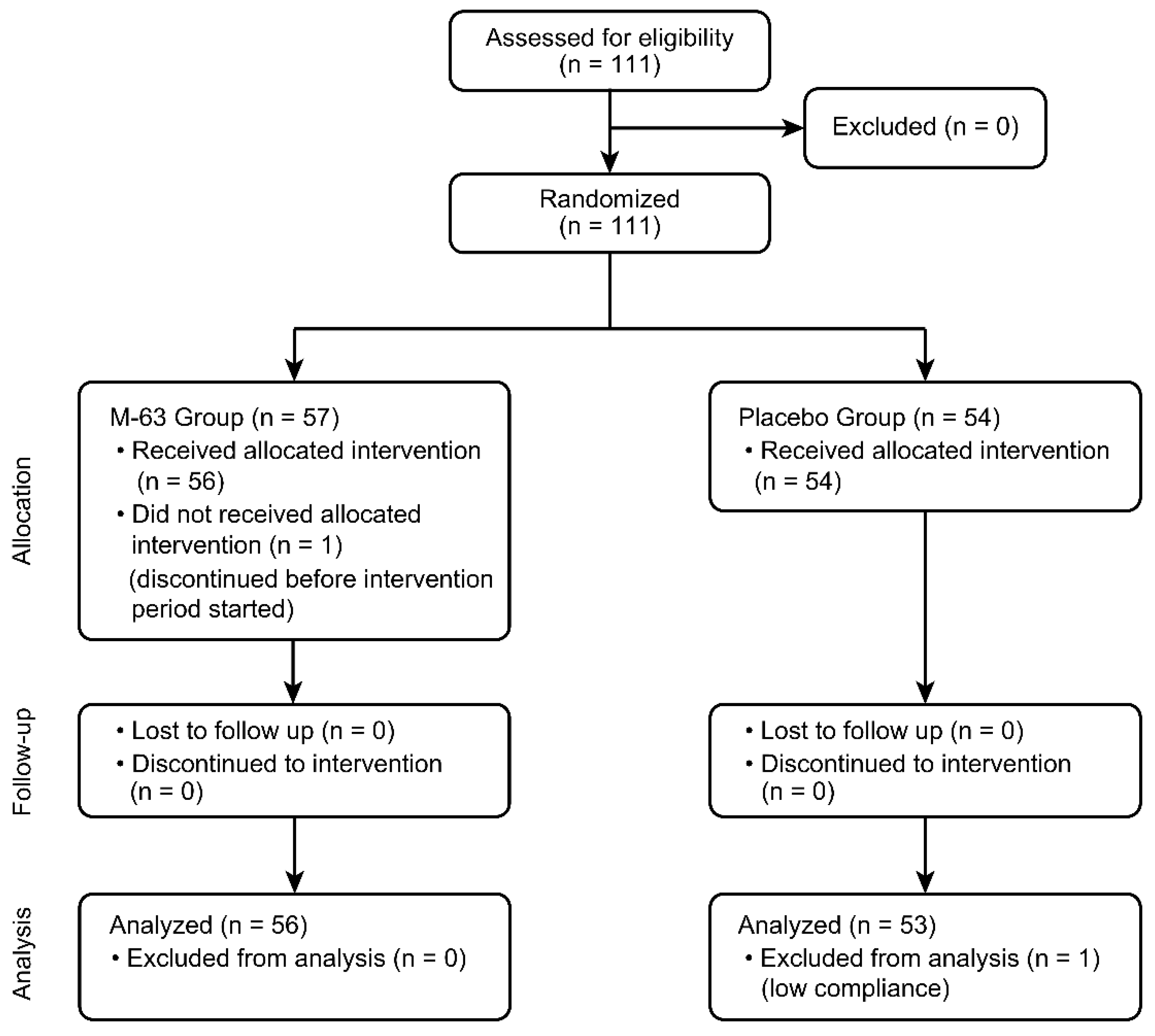
| Placebo (n = 53) | M-63 (n = 56) | p Value | ||
|---|---|---|---|---|
| Infant | ||||
| Gestational age, weeks | 39.0 ± 0.2 | 39.1 ± 0.2 | 0.704 a | |
| Singleton, n (%) | 53 (100) | 56 (100) | - | |
| Sex, n male/female (%) | 24/29 (45.3/54.7) | 28/28 (50.0/50.0) | 0.702 b | |
| Birth weight, g | 3037.5 ± 43.6 | 3058.7 ± 35.4 | 0.706 a | |
| Birth height, cm | 49.2 ± 0.2 | 49.5 ± 0.2 | 0.319 a | |
| Birth head circumference, cm | 33.4 ± 0.2 | 33.5 ± 0.2 | 0.496 a | |
| Cesarean births, n (%) | 8 (15.1) | 11 (19.6) | 0.618 b | |
| APGAR score (5 min after birth) | 8.96 ± 0.05 | 9.07 ± 0.04 | 0.087 a | |
| Maternal | ||||
| Maternal age, years | 31.8 ± 0.7 | 31.3 ± 0.6 | 0.534 a | |
| Multiparous woman, n (%) | 36 (68.0) | 32 (57.1) | 0.323 b | |
| Prepregnancy body mass index (BMI) | 21.0 ± 0.4 | 21.8 ± 0.4 | 0.119 a | |
| Pregnancy weight gain, kg | 9.9 ± 0.4 | 9.7 ± 0.5 | 0.759 a | |
| Antibiotics during labor, n (%) | 30 (56.6) | 30 (53.6) | 0.848 b | |
| Pregnancy smoking habits, n (%) | 3 (5.7) | 3 (5.4) | 1.00 b | |
| Relative Abundance of Bifidobacterium (%) | Infants Where Bifidobacterium Is The Most Dominant Genus, n (%) | ||||||
|---|---|---|---|---|---|---|---|
| Placebo | M-63 | p Value a | Placebo | M-63 | p Value b | ||
| PPS population | |||||||
| Before ingestion | 18.7 ± 4.0 | 17.1 ± 3.4 | 0.531 | 16 (30.2) | 15 (26.8) | 0.832 | |
| 1 week after ingestion | 28.3 ± 3.8 | 63.8 ± 2.2 | <0.001 | 22 (41.5) | 53 (94.6) | <0.001 | |
| 1 month of age | 35.8 ± 4.1 | 71.0 ± 2.5 | <0.001 | 27 (51.0) | 53 (94.6) | <0.001 | |
| 3 months of age | 44.3 ± 3.3 | 64.5 ± 3.0 | <0.001 | 36 (68.0) | 47 (83.9) | 0.072 | |
| Vaginal delivery | |||||||
| Before ingestion | 20.2 ± 4.2 | 18.4 ± 3.8 | 0.669 | 15 (33.3) | 13 (29.0) | 0.82 | |
| 1 week after ingestion | 29.8 ± 4.2 | 62.1 ± 2.5 | <0.001 | 20 (44.4) | 42 (93.3) | <0.001 | |
| 1 month of age | 37.6 ± 4.4 | 69.4 ± 2.9 | <0.001 | 24 (53.3) | 42 (93.3) | <0.001 | |
| 3 months of age | 43.5 ± 3.7 | 63.4 ± 3.4 | <0.001 | 29 (64.4) | 38 (84.4) | 0.052 | |
| Cesarean section | |||||||
| Before ingestion | 10.3 ± 10.2 | 11.8 ± 8.0 | 0.560 | 1 (12.5) | 2 (18.2) | 1.00 | |
| 1 week after ingestion | 20.4 ± 8.3 | 70.7 ± 4.2 | <0.001 | 2 (25.0) | 11 (100.0) | 0.001 | |
| 1 month of age | 26.0 ± 12.1 | 77.7 ± 3.5 | 0.004 | 3 (37.5) | 11 (100.0) | 0.005 | |
| 3 months of age | 48.5 ± 9.8 | 69.0 ± 6.2 | 0.099 | 7 (87.5) | 9 (81.8) | 1.00 | |
| Not using antibiotics during labor | |||||||
| Before ingestion | 27.5 ± 5.5 | 27.3 ± 5.4 | 0.992 | 11 (47.8) | 11 (42.3) | 0.778 | |
| 1 week after ingestion | 37.8 ± 5.9 | 64.4 ± 3.8 | 0.001 | 14 (60.9) | 25 (96.2) | 0.003 | |
| 1 month of age | 40.5 ± 5.7 | 67.6 ± 4.3 | <0.001 | 12 (52.2) | 23 (88.5) | 0.010 | |
| 3 months of age | 42.7 ± 5.1 | 62.4 ± 4.8 | 0.006 | 14 (60.9) | 21 (80.8) | 0.205 | |
| Using antibiotics during labor | |||||||
| Before ingestion | 12.0 ± 5.2 | 8.3 ± 3.6 | 0.324 | 5 (16.7) | 4 (13.3) | 1.00 | |
| 1 week after ingestion | 21.1 ± 4.6 | 63.3 ± 2.5 | <0.001 | 8 (26.7) | 28 (93.3) | <0.001 | |
| 1 month of age | 32.2 ± 5.2 | 74.0 ± 2.6 | <0.001 | 15 (50.0) | 30 (100.0) | <0.001 | |
| 3 months of age | 44.1 ± 4.5 | 66.4 ± 3.8 | 0.001 | 22 (73.3) | 26 (86.7) | 0.333 | |
| Breast-fed infants | |||||||
| 1 week after ingestion | 36.1 ± 10.3 | 68.0 ± 4.4 | 0.022 | 4 (57.1) | 6 (100.0) | 0.192 | |
| 1 month of age | 45.5 ± 8.5 | 81.3 ± 1.8 | <0.001 | 7 (63.6) | 19 (100.0) | 0.012 | |
| 3 months of age | 49.5 ± 5.6 | 72.9 ± 2.7 | <0.001 | 18 (75.0) | 32 (97.0) | 0.034 | |
| Mixed-fed infants and formula-fed infants | |||||||
| 1 week after ingestion | 27.2 ± 4.1 | 63.3 ± 2.4 | <0.001 | 18 (39.1) | 47 (94.0) | <0.001 | |
| 1 month of age | 33.3 ± 4.7 | 65.7 ± 3.3 | <0.001 | 20 (47.6) | 34 (91.9) | <0.001 | |
| 3 months of age | 38.5 ± 3.9 | 52.5 ± 5.3 | 0.052 | 18 (62.1) | 15 (65.2) | 1.00 | |
| Abundance (Log10 CFU/g Feces) | Number of Infants Where Each Bifidobacterium Was Detected (Detection Rate, %) | ||||||
|---|---|---|---|---|---|---|---|
| Placebo | M-63 | p Value a | Placebo | M-63 | p Value b | ||
| Genus Bifidobacterium | |||||||
| Before ingestion | 7.57 ± 0.27 | 7.85 ± 0.27 | 0.487 | 24 (45.3) | 29 (51.2) | 0.567 | |
| 1 month of age | 9.10 ± 0.29 | 10.73 ± 0.11 | <0.001 | 38 (71.7) | 55 (98.2) | <0.001 | |
| Bifidobacterium bifidum | |||||||
| Before ingestion | 6.20 ± 0.12 | 6.25 ± 0.11 | 0.374 | 3 (5.7) | 6 (10.7) | 0.490 | |
| 1 month of age | 6.42 ± 0.17 | 6.52 ± 0.17 | 0.425 | 6 (11.3) | 10 (17.9) | 0.421 | |
| Bifidobacterium breve | |||||||
| Before ingestion | 6.50 ± 0.16 | 6.68 ± 0.18 | 0.543 | 10 (18.9) | 13 (23.2) | 0.643 | |
| 1 month of age | 7.36 ± 0.25 | 7.07 ± 0.19 | 0.523 | 21 (39.6) | 24 (42.9) | 0.846 | |
| Bifidobacterium longum | |||||||
| Before ingestion | 6.85 ± 0.19 | 7.19 ± 0.24 | 0.192 | 15 (28.3) | 21 (37.5) | 0.318 | |
| 1 month of age | 7.39 ± 0.25 | 7.22 ± 0.20 | 0.735 | 24 (45.3) | 27 (48.2) | 0.848 | |
| Bifidobacterium infantis | |||||||
| Before ingestion | 6.15 ± 0.11 | 6.00 ± 0.00 | 0.144 | 2 (3.8) | 0 (0) | 0.234 | |
| 1 month of age | 6.35 ± 0.17 | 10.36 ± 0.13 | <0.001 | 4 (7.6) | 54 (96.4) | <0.001 | |
| Placebo | M-63 | p Value 1 | |||
|---|---|---|---|---|---|
| pH | |||||
| Before ingestion | 5.92 ± 0.08 | 6.05 ± 0.08 | 0.209 | ||
| 1 month of age | 6.05 ± 0.11 | 5.53 ± 0.07 | <0.001 | ||
| Short-chain fatty acids (µmol/g feces) | |||||
| acetic acid | |||||
| Before ingestion | 16.09 ± 1.49 | 15.45 ± 1.16 | 0.884 | ||
| 1 month of age | 22.01 ± 1.62 | 30.0 ± 1.44 | <0.001 | ||
| propionic acid | |||||
| Before ingestion | 0.54 ± 0.26 | 0.64 ± 0.31 | 0.644 | ||
| 1 month of age | 2.32 ± 0.66 | 2.54 ± 0.69 | 0.404 | ||
| n-butanoic acid | |||||
| Before ingestion | 0.43 ± 0.36 | 0.75 ± 0.34 | 0.105 | ||
| 1 month of age | 0.49 ± 0.17 | 0.15 ± 0.11 | 0.027 | ||
| iso-butanoic acid | |||||
| Before ingestion | 0 | 0.01 ± 0.01 | 0.326 | ||
| 1 month of age | 0.06 ± 0.02 | 0.08 ± 0.04 | 0.734 | ||
| n-valeric acid | |||||
| Before ingestion | 0 | 0 | 1.00 | ||
| 1 month of age | 0.02 ± 0.02 | 0.01 ± 0.01 | 0.931 | ||
| iso-valeric acid | |||||
| Before ingestion | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.989 | ||
| 1 month of age | 0.15 ± 0.09 | 0.06 ± 0.03 | 0.795 | ||
| n-caproic acid | |||||
| Before ingestion | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.989 | ||
| 1 month of age | 0.01 ± 0.01 | 0 | 0.317 | ||
| Calprotectin (µg/g feces) | |||||
| Before ingestion | 277.06 ± 35.16 | 346.07 ± 54.22 | 0.535 | ||
| 1 month of age | 212.54 ± 30.0 | 257.52 ± 37.46 | 0.537 | ||
| IgA (µg/g feces) | |||||
| Before ingestion | 1528.85 ± 311.53 | 1700.63 ± 373.62 | 0.661 | ||
| 1 month of age | 1393.19 ± 152.91 | 1971.25 ± 200.90 | 0.033 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hiraku, A.; Nakata, S.; Murata, M.; Xu, C.; Mutoh, N.; Arai, S.; Odamaki, T.; Iwabuchi, N.; Tanaka, M.; Tsuno, T.; et al. Early Probiotic Supplementation of Healthy Term Infants with Bifidobacterium longum subsp. infantis M-63 Is Safe and Leads to the Development of Bifidobacterium-Predominant Gut Microbiota: A Double-Blind, Placebo-Controlled Trial. Nutrients 2023, 15, 1402. https://doi.org/10.3390/nu15061402
Hiraku A, Nakata S, Murata M, Xu C, Mutoh N, Arai S, Odamaki T, Iwabuchi N, Tanaka M, Tsuno T, et al. Early Probiotic Supplementation of Healthy Term Infants with Bifidobacterium longum subsp. infantis M-63 Is Safe and Leads to the Development of Bifidobacterium-Predominant Gut Microbiota: A Double-Blind, Placebo-Controlled Trial. Nutrients. 2023; 15(6):1402. https://doi.org/10.3390/nu15061402
Chicago/Turabian StyleHiraku, Akari, Setsuko Nakata, Mai Murata, Chendong Xu, Natsumi Mutoh, Satoshi Arai, Toshitaka Odamaki, Noriyuki Iwabuchi, Miyuki Tanaka, Takahisa Tsuno, and et al. 2023. "Early Probiotic Supplementation of Healthy Term Infants with Bifidobacterium longum subsp. infantis M-63 Is Safe and Leads to the Development of Bifidobacterium-Predominant Gut Microbiota: A Double-Blind, Placebo-Controlled Trial" Nutrients 15, no. 6: 1402. https://doi.org/10.3390/nu15061402
APA StyleHiraku, A., Nakata, S., Murata, M., Xu, C., Mutoh, N., Arai, S., Odamaki, T., Iwabuchi, N., Tanaka, M., Tsuno, T., & Nakamura, M. (2023). Early Probiotic Supplementation of Healthy Term Infants with Bifidobacterium longum subsp. infantis M-63 Is Safe and Leads to the Development of Bifidobacterium-Predominant Gut Microbiota: A Double-Blind, Placebo-Controlled Trial. Nutrients, 15(6), 1402. https://doi.org/10.3390/nu15061402






