A Microbial-Based Approach to Mental Health: The Potential of Probiotics in the Treatment of Depression
Abstract
1. Introduction
2. Rudiments of Probiotics: Human Gut Microbiota and Gut Dysbiosis
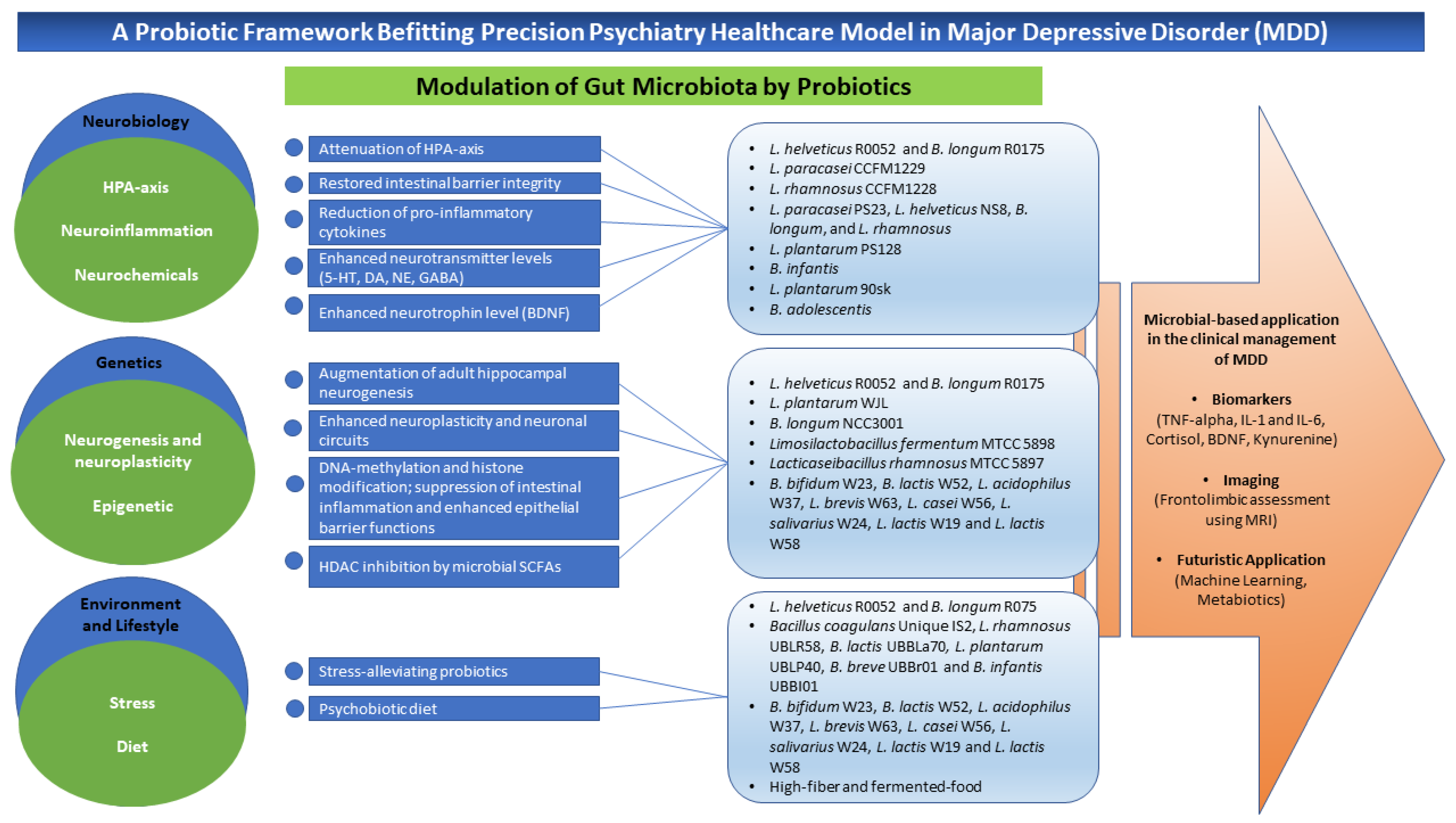
3. A Conceptual Framework of Probiotics Aligned to Precision Psychiatry in Clinical Depression
3.1. Neurobiological Bases
3.2. Genetic Bases
Epigenetics
3.3. Environment and Lifestyle Bases
3.3.1. Stress
3.3.2. Diet
4. Potential Microbial-Based Approach in the Clinical Management of Depression
5. Limitations and Translational Gaps
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bested, A.C.; Logan, A.C.; Selhub, E.M. Intestinal microbiota, probiotics and mental health: From Metchnikoff to modern advances: Part I—Autointoxication revisited. Gut Pathog. 2013, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.; Thurairajasingam, S.; Letchumanan, V.; Chan, K.-G.; Lee, L.-H. Exploring the role and potential of probiotics in the field of mental health: Major depressive disorder. Nutrients 2021, 13, 1728. [Google Scholar] [CrossRef]
- Letchumanan, V.; Thye, A.Y.-K.; Tan, L.T.-H.; Law, J.W.-F.; Johnson, D.; Ser, H.-L.; Bhuvanendran, S.; Thurairajasingam, S.; Lee, L.-H. Gut feelings in depression: Microbiota dysbiosis in response to antidepressants. Gut 2021, 70, A49–A50. [Google Scholar]
- Johnson, D.; Letchumanan, V.; Thurairajasingam, S.; Lee, L.-H. A revolutionizing approach to autism spectrum disorder using the microbiome. Nutrients 2020, 12, 1983. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.; Fenasse, R. Probiotics and depression: The link between the microbiome-gut-brain axis and digestive and mental health. J. Aust. Tradit.-Med. Soc. 2019, 25, 127–132. [Google Scholar]
- McFarland, L.V.; Evans, C.T.; Goldstein, E.J. Strain-specificity and disease-specificity of probiotic efficacy: A systematic review and meta-analysis. Front. Med. 2018, 5, 124. [Google Scholar] [CrossRef]
- Morovic, W.; Budinoff, C.R. Epigenetics: A new frontier in probiotic research. Trends Microbiol. 2021, 29, 117–126. [Google Scholar] [CrossRef]
- Logan, A.C.; Katzman, M. Major depressive disorder: Probiotics may be an adjuvant therapy. Med. Hypotheses 2005, 64, 533–538. [Google Scholar] [CrossRef]
- Mathias, M. Autointoxication and historical precursors of the microbiome–gut–brain axis. Microb. Ecol. Health Dis. 2018, 29, 1548249. [Google Scholar] [CrossRef]
- King, H. Hippocrates Now: The ‘Father of Medicine’in the Internet Age; Bloomsbury Academic: London, UK, 2019; Volume 95. [Google Scholar]
- Régis, E. Précis De Psychiatrie, 5th ed.; Octave Doin: Paris, France, 1914. [Google Scholar]
- Chevalier-Lavaure, F.-A. Des Auto-Intoxications Dans Les Maladies Mentales: Contribution À L’étude De La Pathogénie De La Folie. Ph.D. Thesis, Université de Bordeaux, Bordeaux, France, 1890. [Google Scholar]
- Paykel, E.S. Basic concepts of depression. Dialogues Clin. Neurosci. 2022, 10, 279–289. [Google Scholar] [CrossRef]
- Samtiya, M.; Dhewa, T.; Puniya, A.K. Probiotic Mechanism to Modulate the Gut-Brain Axis (GBA). In Microbiome-Gut-Brain Axis; Springer: Berlin/Heidelberg, Germany, 2022; pp. 237–259. [Google Scholar]
- Cabana, M.D.; Salminen, S.; Sanders, M.E. Probiotic Safety—Reasonable Certainty of No Harm. JAMA Intern. Med. 2019, 179, 276. [Google Scholar] [CrossRef]
- Wallace, C.J.; Milev, R.V. The efficacy, safety, and tolerability of probiotics on depression: Clinical results from an open-label pilot study. Front. Psychiatry 2021, 12, 132. [Google Scholar] [CrossRef] [PubMed]
- El Dib, R.; Periyasamy, A.G.; de Barros, J.L.; França, C.G.; Senefonte, F.L.; Vesentini, G.; Alves, M.G.O.; da Silva Rodrigues, J.V.; Gomaa, H.; Júnior, J.R.G. Probiotics for the treatment of depression and anxiety: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. ESPEN 2021, 45, 75–90. [Google Scholar] [CrossRef]
- Chudzik, A.; Orzyłowska, A.; Rola, R.; Stanisz, G.J. Probiotics, prebiotics and postbiotics on mitigation of depression symptoms: Modulation of the brain–gut–microbiome axis. Biomolecules 2021, 11, 1000. [Google Scholar] [CrossRef] [PubMed]
- Purton, T.; Staskova, L.; Lane, M.M.; Dawson, S.L.; West, M.; Firth, J.; Clarke, G.; Cryan, J.F.; Berk, M.; O’Neil, A. Prebiotic and probiotic supplementation and the tryptophan-kynurenine pathway: A systematic review and meta analysis. Neurosci. Biobehav. Rev. 2021, 123, 1–13. [Google Scholar] [CrossRef]
- Nikolova, V.L.; Cleare, A.J.; Young, A.H.; Stone, J.M. Updated review and meta-analysis of probiotics for the treatment of clinical depression: Adjunctive vs. stand-alone treatment. J. Clin. Med. 2021, 10, 647. [Google Scholar] [CrossRef] [PubMed]
- Alli, S.R.; Gorbovskaya, I.; Liu, J.C.; Kolla, N.J.; Brown, L.; Müller, D.J. The Gut Microbiome in Depression and Potential Benefit of Prebiotics, Probiotics and Synbiotics: A Systematic Review of Clinical Trials and Observational Studies. Int. J. Mol. Sci. 2022, 23, 4494. [Google Scholar] [CrossRef]
- Zhu, H.; Tian, P.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. A psychobiotic approach to the treatment of depression: A systematic review and meta-analysis. J. Funct. Foods 2022, 91, 104999. [Google Scholar] [CrossRef]
- Musazadeh, V.; Zarezadeh, M.; Faghfouri, A.H.; Keramati, M.; Jamilian, P.; Jamilian, P.; Mohagheghi, A.; Farnam, A. Probiotics as an effective therapeutic approach in alleviating depression symptoms: An umbrella meta-analysis. Crit. Rev. Food Sci. Nutr. 2022, 1–9. [Google Scholar] [CrossRef]
- Nadeem, I.; Rahman, M.Z.; Ad-Dab’bagh, Y.; Akhtar, M. Effect of probiotic interventions on depressive symptoms: A narrative review evaluating systematic reviews. Psychiatry Clin. Neurosci. 2019, 73, 154–162. [Google Scholar] [CrossRef]
- Poluektova, E.; Yunes, R.; Danilenko, V. The putative antidepressant mechanisms of probiotic bacteria: Relevant genes and proteins. Nutrients 2021, 13, 1591. [Google Scholar] [CrossRef] [PubMed]
- Yong, S.J.; Tong, T.; Chew, J.; Lim, W.L. Antidepressive mechanisms of probiotics and their therapeutic potential. Front. Neurosci. 2020, 13, 1361. [Google Scholar] [CrossRef]
- Poluektova, E.; Danilenko, V. Probiotic Bacteria in the Correction of Depression Symptoms, Their Active Genes and Proteins. Russ. J. Genet. 2021, 57, 1017–1025. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhai, Q.; Zhang, H.; Chen, W.; Hill, C. Gut colonization mechanisms of Lactobacillus and Bifidobacterium: An argument for personalized designs. Annu. Rev. Food Sci. Technol. 2021, 12, 213–233. [Google Scholar] [CrossRef] [PubMed]
- Collins, F.S.; Varmus, H. A new initiative on precision medicine. N. Engl. J. Med. 2015, 372, 793–795. [Google Scholar] [CrossRef]
- Hodson, R. Precision medicine. Nature 2016, 537, S49. [Google Scholar] [CrossRef] [PubMed]
- Khoury, M.J.; Bowen, S.; Dotson, W.D.; Drzymalla, E.; Green, R.F.; Goldstein, R.; Kolor, K.; Liburd, L.C.; Sperling, L.S.; Bunnell, R. Health equity in the implementation of genomics and precision medicine: A public health imperative. Genet. Med. 2022, 24, 1630–1639. [Google Scholar] [CrossRef]
- Salazar de Pablo, G.; Studerus, E.; Vaquerizo-Serrano, J.; Irving, J.; Catalan, A.; Oliver, D.; Baldwin, H.; Danese, A.; Fazel, S.; Steyerberg, E.W. Implementing precision psychiatry: A systematic review of individualized prediction models for clinical practice. Schizophr. Bull. 2021, 47, 284–297. [Google Scholar] [CrossRef]
- MacEachern, S.J.; Forkert, N.D. Machine learning for precision medicine. Genome 2021, 64, 416–425. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G.; Ahern, A.; Carbone, C.; Temko, A.; Claesson, M.J.; Gasbarrini, A.; Tortora, G. Gut microbiome, big data and machine learning to promote precision medicine for cancer. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 635–648. [Google Scholar] [CrossRef]
- Fernandes, B.S.; Williams, L.M.; Steiner, J.; Leboyer, M.; Carvalho, A.F.; Berk, M. The new field of ‘precision psychiatry’. BMC Med. 2017, 15, 80. [Google Scholar] [CrossRef]
- Maes, M. Precision nomothetic medicine in depression research: A new depression model, and new endophenotype classes and pathway phenotypes, and a digital self. J. Pers. Med. 2022, 12, 403. [Google Scholar] [CrossRef]
- Sullivan, P.F.; Geschwind, D.H. Defining the genetic, genomic, cellular, and diagnostic architectures of psychiatric disorders. Cell 2019, 177, 162–183. [Google Scholar] [CrossRef] [PubMed]
- Menke, A. Precision pharmacotherapy: Psychiatry’s future direction in preventing, diagnosing, and treating mental disorders. Pharm. Pers. Med. 2018, 11, 211–222. [Google Scholar] [CrossRef]
- Menke, A. Is the HPA axis as target for depression outdated, or is there a new hope? Front. Psychiatry 2019, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Abildgaard, A.; Kern, T.; Pedersen, O.; Hansen, T.; Wegener, G.; Lund, S. The antidepressant-like effect of probiotics and their faecal abundance may be modulated by the cohabiting gut microbiota in rats. Eur. Neuropsychopharmacol. 2019, 29, 98–110. [Google Scholar] [CrossRef]
- Feczko, E.; Miranda-Dominguez, O.; Marr, M.; Graham, A.M.; Nigg, J.T.; Fair, D.A. The heterogeneity problem: Approaches to identify psychiatric subtypes. Trends Cogn. Sci. 2019, 23, 584–601. [Google Scholar] [CrossRef]
- Passos, I.C.; Ballester, P.; Rabelo-da-Ponte, F.D.; Kapczinski, F. Precision psychiatry: The future is now. Can. J. Psychiatry 2022, 67, 21–25. [Google Scholar] [CrossRef]
- Zanardi, R.; Prestifilippo, D.; Fabbri, C.; Colombo, C.; Maron, E.; Serretti, A. Precision psychiatry in clinical practice. Int. J. Psychiatry Clin. Pract. 2021, 25, 19–27. [Google Scholar] [CrossRef]
- Arns, M.; van Dijk, H.; Luykx, J.J.; van Wingen, G.; Olbrich, S. Stratified psychiatry: Tomorrow’s precision psychiatry? Eur. Neuropsychopharmacol. 2022, 55, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, Q. A longitudinal study on college students’ depressive symptoms during the COVID-19 pandemic: The trajectories, antecedents, and outcomes. Psychiatry Res. 2023, 321, 115058. [Google Scholar] [CrossRef]
- Kang, P.Y.; Do, K.-H.; Koo, B.-S.; Lee, W.-K. Comparative antimicrobial activity of human and monkey origin lactic acid bacteria on simian enteric bacteria. J. Biomed. Transl. Res. 2022, 23, 55–65. [Google Scholar] [CrossRef]
- Wong, C.; Sugahara, H.; Odamaki, T.; Xiao, J. Different physiological properties of human-residential and non-human-residential bifidobacteria in human health. Benef. Microbes 2018, 9, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Law, J.W.-F.; Letchumanan, V.; Tan, L.T.-H.; Ser, H.-L.; Goh, B.-H.; Lee, L.-H. The rising of “modern actinobacteria” era. Prog. Microbes Mol. Biol. 2020, 3, a0000064. [Google Scholar] [CrossRef]
- Metchnikoff, E.; Williams, H.S. Why not live forever. Cosmopolitan 1912, 53, 436–446. [Google Scholar]
- Anukam, K.C.; Reid, G. Probiotics: 100 years (1907–2007) after Elie Metchnikoff’s observation. Commun. Curr. Res. Educ. Top. Trends Appl. Microbiol. 2007, 1, 466–474. [Google Scholar]
- Lau, A.W.Y.; Tan, L.T.-H.; Ab Mutalib, N.-S.; Wong, S.H.; Letchumanan, V.; Lee, L.-H. The chemistry of gut microbiome in health and diseases. Prog. Microbes Mol. Biol. 2021, 4, a0000175. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.; Sandhu, K.V.; Bastiaanssen, T.F.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V. The microbiota-gut-brain axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Tshikantwa, T.S.; Ullah, M.W.; He, F.; Yang, G. Current trends and potential applications of microbial interactions for human welfare. Front. Microbiol. 2018, 9, 1156. [Google Scholar] [CrossRef] [PubMed]
- Pathak, K.; Saikia, R.; Gogoi, U.; Das, A. Potential application of microbes for human health and welfare. World J. Pharm. Pharm. Sci. 2020, 10, 514–524. [Google Scholar]
- Busnelli, M.; Manzini, S.; Chiesa, G. The gut microbiota affects host pathophysiology as an endocrine organ: A focus on cardiovascular disease. Nutrients 2019, 12, 79. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, L.; Cao, Z.; Li, W.; Li, H.; Lu, C.; Yang, X.; Liu, Y. Gut microbiota as an “invisible organ” that modulates the function of drugs. Biomed. Pharmacother. 2020, 121, 109653. [Google Scholar] [CrossRef]
- Stephens, R.W.; Arhire, L.; Covasa, M. Gut microbiota: From microorganisms to metabolic organ influencing obesity. Obesity 2018, 26, 801–809. [Google Scholar] [CrossRef]
- Seo, D.-O.; Holtzman, D.M. Gut microbiota: From the forgotten organ to a potential key player in the pathology of Alzheimer’s disease. J. Gerontol. Ser. A 2020, 75, 1232–1241. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.Q.; Cheam, J.Y.; Law, J.W.-F.; Letchumanan, V.; Lee, L.-H.; Tan, L.T.-H. Role of Garlic in Chronic Diseases: Focusing on Gut Microbiota Modulation. Prog. Microbes Mol. Biol. 2022, 5, a0000271. [Google Scholar] [CrossRef]
- Selvaraj, S.M.; Wong, S.H.; Ser, H.-L.; Lee, L.-H. Role of low FODMAP diet and probiotics on gut microbiome in irritable bowel syndrome (IBS). Prog. Microbes Mol. Biol. 2020, 3, a0000069. [Google Scholar] [CrossRef]
- Chong, H.-Y.; Tan, L.T.-H.; Law, J.W.-F.; Hong, K.-W.; Ratnasingam, V.; Ab Mutalib, N.-S.; Lee, L.-H.; Letchumanan, V. Exploring the Potential of Human Milk and Formula Milk on Infants’ Gut and Health. Nutrients 2022, 14, 3554. [Google Scholar] [CrossRef]
- Bliss, E.S.; Whiteside, E. The gut-brain axis, the human gut microbiota and their integration in the development of obesity. Front. Physiol. 2018, 9, 900. [Google Scholar] [CrossRef]
- O’Neill, I.J.; Sanchez Gallardo, R.; Saldova, R.; Murphy, E.F.; Cotter, P.D.; McAuliffe, F.M.; van Sinderen, D. Maternal and infant factors that shape neonatal gut colonization by bacteria. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 651–664. [Google Scholar] [CrossRef]
- Rackaityte, E.; Halkias, J.; Fukui, E.; Mendoza, V.; Hayzelden, C.; Crawford, E.; Fujimura, K.; Burt, T.; Lynch, S. Viable bacterial colonization is highly limited in the human intestine in utero. Nat. Med. 2020, 26, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.J.; Ajami, N.J.; O’Brien, J.L.; Hutchinson, D.S.; Smith, D.P.; Wong, M.C.; Ross, M.C.; Lloyd, R.E.; Doddapaneni, H.; Metcalf, G.A. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature 2018, 562, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Forster, S.C.; Tsaliki, E.; Vervier, K.; Strang, A.; Simpson, N.; Kumar, N.; Stares, M.D.; Rodger, A.; Brocklehurst, P. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature 2019, 574, 117–121. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, A.A.; Corr, S.C. Establishing boundaries: The relationship that exists between intestinal epithelial cells and gut-dwelling bacteria. Microorganisms 2019, 7, 663. [Google Scholar] [CrossRef] [PubMed]
- Bittinger, K.; Zhao, C.; Li, Y.; Ford, E.; Friedman, E.S.; Ni, J.; Kulkarni, C.V.; Cai, J.; Tian, Y.; Liu, Q. Bacterial colonization reprograms the neonatal gut metabolome. Nat. Microbiol. 2020, 5, 838–847. [Google Scholar] [CrossRef]
- Sonali, S.; Ray, B.; Ahmed Tousif, H.; Rathipriya, A.G.; Sunanda, T.; Mahalakshmi, A.M.; Rungratanawanich, W.; Essa, M.M.; Qoronfleh, M.W.; Chidambaram, S.B. Mechanistic insights into the link between gut dysbiosis and major depression: An extensive review. Cells 2022, 11, 1362. [Google Scholar] [CrossRef] [PubMed]
- Ong, I.J.; Loo, K.-Y.; Law, L.N.-S.; Law, J.W.-F.; Tan, L.T.-H.; Letchumanan, V. Exploring the impact of Helicobacter pylori and potential gut microbiome modulation. Prog. Microbes Mol. Biol. 2023, 6, 1–19. [Google Scholar] [CrossRef]
- Thye, A.Y.-K.; Bah, Y.-R.; Law, J.W.-F.; Tan, L.T.-H.; He, Y.-W.; Wong, S.-H.; Thurairajasingam, S.; Chan, K.-G.; Lee, L.-H.; Letchumanan, V. Gut–skin axis: Unravelling the connection between the gut microbiome and psoriasis. Biomedicines 2022, 10, 1037. [Google Scholar] [CrossRef]
- Thye, A.Y.-K.; Law, J.W.-F.; Tan, L.T.-H.; Thurairajasingam, S.; Chan, K.-G.; Letchumanan, V.; Lee, L.-H. Exploring the gut microbiome in Myasthenia Gravis. Nutrients 2022, 14, 1647. [Google Scholar] [CrossRef]
- Wang, H.J.; Battousse, O.; Ramadas, A. Modulation of gut microbiota by dietary macronutrients in type 2 diabetes: A review. Prog. Microbes Mol. Biol. 2021, 4, a0000182. [Google Scholar]
- Durganaudu, H.; Kunasegaran, T.; Ramadas, A. Dietary glycaemic index and type 2 diabetes mellitus: Potential modulation of gut microbiota. Prog. Microbes Mol. Biol. 2020, 3, a0000082. [Google Scholar] [CrossRef]
- Zhao, L.; Xiong, Q.; Stary, C.M.; Mahgoub, O.K.; Ye, Y.; Gu, L.; Xiong, X.; Zhu, S. Bidirectional gut-brain-microbiota axis as a potential link between inflammatory bowel disease and ischemic stroke. J. NeuroInflamm. 2018, 15, 339. [Google Scholar] [CrossRef]
- Zhu, F.; Tu, H.; Chen, T. The Microbiota–Gut–Brain Axis in Depression: The Potential Pathophysiological Mechanisms and Microbiota Combined Antidepression Effect. Nutrients 2022, 14, 2081. [Google Scholar] [CrossRef] [PubMed]
- Barrio, C.; Arias-Sánchez, S.; Martín-Monzón, I. The gut microbiota-brain axis, psychobiotics and its influence on brain and behaviour: A systematic review. Psychoneuroendocrinology 2022, 137, 105640. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.; Kalam, A.; Sarker, M.; Li, T.; Yin, J. Probiotic species in the modulation of gut microbiota: An overview. BioMed Res. Int. 2018, 2018, 9478630. [Google Scholar] [CrossRef]
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The gut microbiota–brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-W.; Gao, C.-S.; Zhang, H.; Yang, J.; Wang, Y.-P.; Pan, L.-B.; Yu, H.; He, C.-Y.; Luo, H.-B.; Zhao, Z.-X. Morinda officinalis oligosaccharides increase serotonin in the brain and ameliorate depression via promoting 5-hydroxytryptophan production in the gut microbiota. Acta Pharm. Sin. B 2022, 12, 3298–3312. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, G.; Siopi, E.; Guenin-Macé, L.; Pascal, M.; Laval, T.; Rifflet, A.; Boneca, I.G.; Demangel, C.; Colsch, B.; Pruvost, A. Effect of gut microbiota on depressive-like behaviors in mice is mediated by the endocannabinoid system. Nat. Commun. 2020, 11, 6363. [Google Scholar] [CrossRef]
- Radjabzadeh, D.; Bosch, J.A.; Uitterlinden, A.G.; Zwinderman, A.H.; Ikram, M.A.; van Meurs, J.B.; Luik, A.I.; Nieuwdorp, M.; Lok, A.; van Duijn, C.M. Gut microbiome-wide association study of depressive symptoms. Nat. Commun. 2022, 13, 7128. [Google Scholar] [CrossRef]
- Barandouzi, Z.A.; Starkweather, A.R.; Henderson, W.A.; Gyamfi, A.; Cong, X.S. Altered composition of gut microbiota in depression: A systematic review. Front. Psychiatry 2020, 11, 541. [Google Scholar] [CrossRef]
- Borkent, J.; Ioannou, M.; Laman, J.D.; Haarman, B.C.; Sommer, I.E. Role of the gut microbiome in three major psychiatric disorders. Psychol. Med. 2022, 52, 1222–1242. [Google Scholar] [CrossRef]
- McGuinness, A.; Davis, J.; Dawson, S.; Loughman, A.; Collier, F.; O’Hely, M.; Simpson, C.; Green, J.; Marx, W.; Hair, C. A systematic review of gut microbiota composition in observational studies of major depressive disorder, bipolar disorder and schizophrenia. Mol. Psychiatry 2022, 27, 1920–1935. [Google Scholar] [CrossRef]
- Inserra, A.; Rogers, G.B.; Licinio, J.; Wong, M.L. The microbiota-inflammasome hypothesis of major depression. Bioessays 2018, 40, 1800027. [Google Scholar] [CrossRef]
- Huang, Y.; Shi, X.; Li, Z.; Shen, Y.; Shi, X.; Wang, L.; Li, G.; Yuan, Y.; Wang, J.; Zhang, Y. Possible association of Firmicutes in the gut microbiota of patients with major depressive disorder. Neuropsychiatr. Dis. Treat. 2018, 14, 3329–3337. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, E.; Tsuji, H.; Asahara, T.; Takahashi, T.; Teraishi, T.; Yoshida, S.; Ota, M.; Koga, N.; Hattori, K.; Kunugi, H. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J. Affect. Disord. 2016, 202, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Kanoujia, J.; Lakshmi, S.M.; Patil, C.; Gupta, G.; Chellappan, D.K.; Dua, K. Role of Brain-Gut-Microbiota Axis in Depression: Emerging Therapeutic Avenues. CNS Neurol. Disord.-Drug Targets (Former. Curr. Drug Targets-CNS Neurol. Disord.) 2023, 22, 276–288. [Google Scholar]
- Kong, G.Y.-E.; Letchumanan, V.; Tan, L.T.-H.; Law, J.W.-F. Gut Microbiome in Obsessive Compulsive Disorder: Potential of Probiotics as an Adjuvant Therapy. Prog. Microbes Mol. Biol. 2022, 5, a0000272. [Google Scholar] [CrossRef]
- Zendeboodi, F.; Khorshidian, N.; Mortazavian, A.M.; da Cruz, A.G. Probiotic: Conceptualization from a new approach. Curr. Opin. Food Sci. 2020, 32, 103–123. [Google Scholar] [CrossRef]
- Millette, M.; Nguyen, A.; Amine, K.M.; Lacroix, M. Gastrointestinal survival of bacteria in commercial probiotic products. Int. J. Probiotics Prebiotics 2013, 8, 149. [Google Scholar]
- Domig, K.; Kiss, H.; Petricevic, L.; Viernstein, H.; Unger, F.; Kneifel, W. Strategies for the evaluation and selection of potential vaginal probiotics from human sources: An exemplary study. Benef. Microbes 2014, 5, 263–272. [Google Scholar] [CrossRef]
- Hofmeister, M.; Clement, F.; Patten, S.; Li, J.; Dowsett, L.E.; Farkas, B.; Mastikhina, L.; Egunsola, O.; Diaz, R.; Cooke, N.C. The effect of interventions targeting gut microbiota on depressive symptoms: A systematic review and meta-analysis. Can. Med. Assoc. Open Access J. 2021, 9, E1195–E1204. [Google Scholar] [CrossRef] [PubMed]
- Saarela, M.; Mogensen, G.; Fonden, R.; Mättö, J.; Mattila-Sandholm, T. Probiotic bacteria: Safety, functional and technological properties. J. Biotechnol. 2000, 84, 197–215. [Google Scholar] [CrossRef] [PubMed]
- Barros-Santos, T.; Silva, K.S.O.; Libarino-Santos, M.; Cata-Preta, E.G.; Reis, H.S.; Tamura, E.K.; de Oliveira-Lima, A.J.; Berro, L.F.; Uetanabaro, A.P.T.; Marinho, E.A.V. Effects of chronic treatment with new strains of Lactobacillus plantarum on cognitive, anxiety-and depressive-like behaviors in male mice. PLoS ONE 2020, 15, e0234037. [Google Scholar] [CrossRef] [PubMed]
- van Reenen, C.A.; Dicks, L.M. Horizontal gene transfer amongst probiotic lactic acid bacteria and other intestinal microbiota: What are the possibilities? A review. Arch. Microbiol. 2011, 193, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Saez-Lara, M.J.; Gomez-Llorente, C.; Plaza-Diaz, J.; Gil, A. The role of probiotic lactic acid bacteria and bifidobacteria in the prevention and treatment of inflammatory bowel disease and other related diseases: A systematic review of randomized human clinical trials. BioMed Res. Int. 2015, 2015, 505878. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lv, J.; Pan, L.; Zhang, Y. Roles and applications of probiotic Lactobacillus strains. Appl. Microbiol. Biotechnol. 2018, 102, 8135–8143. [Google Scholar] [CrossRef]
- Sharma, M.; Wasan, A.; Sharma, R.K. Recent developments in probiotics: An emphasis on Bifidobacterium. Food Biosci. 2021, 41, 100993. [Google Scholar] [CrossRef]
- Tanaka, K.; Satoh, T.; Kitahara, J.; Uno, S.; Nomura, I.; Kano, Y.; Suzuki, T.; Niimura, Y.; Kawasaki, S. O2-inducible H2O2-forming NADPH oxidase is responsible for the hyper O2 sensitivity of Bifidobacterium longum subsp. infantis. Sci. Rep. 2018, 8, 10750. [Google Scholar] [CrossRef] [PubMed]
- Guarner, F.; Sanders, M.; Eliakim, R.; Fedorak, R.; Gangl, A.; Garisch, J. Probiotics and prebiotics. In World Gastroenterology Organisation Global Guidelines; World Gastroenterology Organisation: Milwaukee, WI, USA, 2017; Volume 46, pp. 468–481. [Google Scholar]
- Bzdok, D.; Meyer-Lindenberg, A. Machine learning for precision psychiatry: Opportunities and challenges. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2018, 3, 223–230. [Google Scholar] [CrossRef]
- Gandal, M.J.; Leppa, V.; Won, H.; Parikshak, N.N.; Geschwind, D.H. The road to precision psychiatry: Translating genetics into disease mechanisms. Nat. Neurosci. 2016, 19, 1397–1407. [Google Scholar] [CrossRef]
- Namkung, J. Machine learning methods for microbiome studies. J. Microbiol. 2020, 58, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.M. Precision psychiatry: A neural circuit taxonomy for depression and anxiety. Lancet Psychiatry 2016, 3, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Khanra, S.; Khess, C.R.; Munda, S.K. “Precision psychiatry”: A promising direction so far. Indian J. Psychiatry 2018, 60, 373–374. [Google Scholar] [CrossRef]
- Edition, F. Diagnostic and statistical manual of mental disorders. Am. Psychiatr. Assoc. 2013, 21, 591–643. [Google Scholar]
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; El Hage, W.; Surget, A.; Belzung, C. Neuroinflammation and depression: A review. Eur. J. Neurosci. 2021, 53, 151–171. [Google Scholar] [CrossRef]
- Phillips, C. Brain-derived neurotrophic factor, depression, and physical activity: Making the neuroplastic connection. Neural Plast. 2017, 2017, 7260130. [Google Scholar] [CrossRef]
- Alemi, F.; Min, H.; Yousefi, M.; Becker, L.K.; Hane, C.A.; Nori, V.S.; Wojtusiak, J. Effectiveness of common antidepressants: A post market release study. eClinicalMedicine 2021, 41, 101171. [Google Scholar] [CrossRef]
- Rush, A.J.; Trivedi, M.H.; Wisniewski, S.R.; Stewart, J.W.; Nierenberg, A.A.; Thase, M.E.; Ritz, L.; Biggs, M.M.; Warden, D.; Luther, J.F. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N. Engl. J. Med. 2006, 354, 1231–1242. [Google Scholar] [CrossRef]
- Czéh, B.; Fuchs, E.; Wiborg, O.; Simon, M. Animal models of major depression and their clinical implications. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 64, 293–310. [Google Scholar] [CrossRef]
- Pereira, V.S.; Hiroaki-Sato, V.A. A brief history of antidepressant drug development: From tricyclics to beyond ketamine. Acta Neuropsychiatr. 2018, 30, 307–322. [Google Scholar] [CrossRef]
- Suda, K.; Matsuda, K. How Microbes Affect Depression: Underlying Mechanisms via the Gut–Brain Axis and the Modulating Role of Probiotics. Int. J. Mol. Sci. 2022, 23, 1172. [Google Scholar] [CrossRef]
- Freimer, D.; Yang, T.T.; Ho, T.C.; Tymofiyeva, O.; Leung, C. The gut microbiota, HPA axis, and brain in adolescent-onset depression: Probiotics as a novel treatment. Brain Behav. Immun.-Health 2022, 26, 100541. [Google Scholar] [CrossRef] [PubMed]
- Delgado, P.L. Depression: The case for a monoamine deficiency. J. Clin. Psychiatry 2000, 61, 7–11. [Google Scholar] [PubMed]
- Zhang, F.F.; Peng, W.; Sweeney, J.A.; Jia, Z.Y.; Gong, Q.Y. Brain structure alterations in depression: Psychoradiological evidence. CNS Neurosci. Ther. 2018, 24, 994–1003. [Google Scholar] [CrossRef]
- Yang, T.; Nie, Z.; Shu, H.; Kuang, Y.; Chen, X.; Cheng, J.; Yu, S.; Liu, H. The role of BDNF on neural plasticity in depression. Front. Cell. Neurosci. 2020, 14, 82. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.B.; Park, S.-C. Neural circuitry–neurogenesis coupling model of depression. Int. J. Mol. Sci. 2021, 22, 2468. [Google Scholar] [CrossRef]
- Młynarska, E.; Gadzinowska, J.; Tokarek, J.; Forycka, J.; Szuman, A.; Franczyk, B.; Rysz, J. The Role of the Microbiome-Brain-Gut Axis in the Pathogenesis of Depressive Disorder. Nutrients 2022, 14, 1921. [Google Scholar] [CrossRef] [PubMed]
- Mikulska, J.; Juszczyk, G.; Gawrońska-Grzywacz, M.; Herbet, M. HPA Axis in the pathomechanism of depression and schizophrenia: New therapeutic strategies based on its participation. Brain Sci. 2021, 11, 1298. [Google Scholar] [CrossRef]
- Köhler, C.A.; Freitas, T.H.; Maes, M.; De Andrade, N.; Liu, C.S.; Fernandes, B.S.; Stubbs, B.; Solmi, M.; Veronese, N.; Herrmann, N. Peripheral cytokine and chemokine alterations in depression: A meta-analysis of 82 studies. Acta Psychiatr. Scand. 2017, 135, 373–387. [Google Scholar] [CrossRef]
- Mac Giollabhui, N.; Ng, T.H.; Ellman, L.M.; Alloy, L.B. The longitudinal associations of inflammatory biomarkers and depression revisited: Systematic review, meta-analysis, and meta-regression. Mol. Psychiatry 2021, 26, 3302–3314. [Google Scholar] [CrossRef]
- Rudzki, L.; Ostrowska, L.; Pawlak, D.; Małus, A.; Pawlak, K.; Waszkiewicz, N.; Szulc, A. Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology 2019, 100, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Samochowies, J. Probiotics Therapy of Mood Disorders. Available online: https://clinicaltrials.gov/show/NCT04753944 (accessed on 1 December 2022).
- Giri, R.; Sharma, R.K. Psychobiotics in diet: Significance and applications of neuroactive and psychoactive microbial metabolites. Nutr. Rev. 2022, 80, 2002–2016. [Google Scholar] [CrossRef]
- Dicks, L.M. Gut Bacteria and Neurotransmitters. Microorganisms 2022, 10, 1838. [Google Scholar] [CrossRef] [PubMed]
- Tracey, K.J.; Chavan, S.S. Nerve Stimulation for Treatment of Diseases and Disorders. U.S. Patent No. 10,507,327, 17 December 2019. [Google Scholar]
- Angelucci, F.; Brene, S.; Mathe, A. BDNF in schizophrenia, depression and corresponding animal models. Mol. Psychiatry 2005, 10, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Dowlatshahi, D.; MacQueen, G.M.; Wang, J.F.; Young, L.T. Increased temporal cortex CREB concentrations and antidepressant treatment in major depression. Lancet 1998, 352, 1754. [Google Scholar]
- Aygun, H.; Akin, A.T.; Kızılaslan, N.; Sumbul, O.; Karabulut, D. Probiotic supplementation alleviates absence seizures and anxiety-and depression-like behavior in WAG/Rij rat by increasing neurotrophic factors and decreasing proinflammatory cytokines. Epilepsy Behav. 2022, 128, 108588. [Google Scholar] [CrossRef]
- Schmidt, H.D.; Duman, R.S. Peripheral BDNF produces antidepressant-like effects in cellular and behavioral models. Neuropsychopharmacology 2010, 35, 2378–2391. [Google Scholar] [CrossRef]
- Dulawa, S.C.; Hen, R. Recent advances in animal models of chronic antidepressant effects: The novelty-induced hypophagia test. Neurosci. Biobehav. Rev. 2005, 29, 771–783. [Google Scholar] [CrossRef]
- Hasan, N.; Yang, H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ 2019, 7, e7502. [Google Scholar] [CrossRef]
- Lai, C.-H. Fronto-limbic neuroimaging biomarkers for diagnosis and prediction of treatment responses in major depressive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 107, 110234. [Google Scholar] [CrossRef]
- Iob, E.; Kirschbaum, C.; Steptoe, A. Persistent depressive symptoms, HPA-axis hyperactivity, and inflammation: The role of cognitive-affective and somatic symptoms. Mol. Psychiatry 2020, 25, 1130–1140. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Tian, P.; Zhu, H.; Zou, R.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Lactobacillus paracasei CCFM1229 and Lactobacillus rhamnosus CCFM1228 Alleviated Depression-and Anxiety-Related Symptoms of Chronic Stress-Induced Depression in Mice by Regulating Xanthine Oxidase Activity in the Brain. Nutrients 2022, 14, 1294. [Google Scholar] [CrossRef]
- Messaoudi, M.; Lalonde, R.; Violle, N.; Javelot, H.; Desor, D.; Nejdi, A.; Bisson, J.-F.; Rougeot, C.; Pichelin, M.; Cazaubiel, M. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 2011, 105, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Ait-Belgnaoui, A.; Colom, A.; Braniste, V.; Ramalho, L.; Marrot, A.; Cartier, C.; Houdeau, E.; Theodorou, V.; Tompkins, T. Probiotic gut effect prevents the chronic psychological stress-induced brain activity abnormality in mice. Neurogastroenterol. Motil. 2014, 26, 510–520. [Google Scholar] [CrossRef]
- Arseneault-Bréard, J.; Rondeau, I.; Gilbert, K.; Girard, S.-A.; Tompkins, T.A.; Godbout, R.; Rousseau, G. Combination of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 reduces post-myocardial infarction depression symptoms and restores intestinal permeability in a rat model. Br. J. Nutr. 2012, 107, 1793–1799. [Google Scholar] [CrossRef]
- Ait-Belgnaoui, A.; Payard, I.; Rolland, C.; Harkat, C.; Braniste, V.; Théodorou, V.; Tompkins, T.A. Bifidobacterium longum and Lactobacillus helveticus synergistically suppress stress-related visceral hypersensitivity through hypothalamic-pituitary-adrenal axis modulation. J. Neurogastroenterol. Motil. 2018, 24, 138. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, G.; Dargahi, L.; Naserpour, T.; Mirzanejad, Y.; Alizadeh, S.A.; Peymani, A.; Nassiri-Asl, M. Probiotic mixture of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 attenuates hippocampal apoptosis induced by lipopolysaccharide in rats. Int. Microbiol. 2019, 22, 317–323. [Google Scholar] [CrossRef]
- Partrick, K.A.; Rosenhauer, A.M.; Auger, J.; Arnold, A.R.; Ronczkowski, N.M.; Jackson, L.M.; Lord, M.N.; Abdulla, S.M.; Chassaing, B.; Huhman, K.L. Ingestion of probiotic (Lactobacillus helveticus and Bifidobacterium longum) alters intestinal microbial structure and behavioral expression following social defeat stress. Sci. Rep. 2021, 11, 3763. [Google Scholar] [CrossRef]
- Kazemi, A.; Noorbala, A.A.; Azam, K.; Eskandari, M.H.; Djafarian, K. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: A randomized clinical trial. Clin. Nutr. 2019, 38, 522–528. [Google Scholar] [CrossRef]
- Wallace, C.J.; Milev, R. The effects of probiotics on depressive symptoms in humans: A systematic review. Ann. Gen. Psychiatry 2017, 16, 14. [Google Scholar] [CrossRef]
- Wei, C.-L.; Wang, S.; Yen, J.-T.; Cheng, Y.-F.; Liao, C.-L.; Hsu, C.-C.; Wu, C.-C.; Tsai, Y.-C. Antidepressant-like activities of live and heat-killed Lactobacillus paracasei PS23 in chronic corticosterone-treated mice and possible mechanisms. Brain Res. 2019, 1711, 202–213. [Google Scholar] [CrossRef]
- Cheng, L.-H.; Chou, P.-Y.; Hou, A.-T.; Huang, C.-L.; Shiu, W.-L.; Wang, S. Lactobacillus paracasei PS23 improves cognitive deficits via modulating the hippocampal gene expression and the gut microbiota in D-galactose-induced aging mice. Food Funct. 2022, 13, 5240–5251. [Google Scholar] [CrossRef]
- Huang, F.; Wu, X. Brain neurotransmitter modulation by gut microbiota in anxiety and depression. Front. Cell Dev. Biol. 2021, 9, 649103. [Google Scholar] [CrossRef]
- Liu, W.-H.; Chuang, H.-L.; Huang, Y.-T.; Wu, C.-C.; Chou, G.-T.; Wang, S.; Tsai, Y.-C. Alteration of behavior and monoamine levels attributable to Lactobacillus plantarum PS128 in germ-free mice. Behav. Brain Res. 2016, 298, 202–209. [Google Scholar] [CrossRef]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Kiely, B.; Cryan, J.F.; Dinan, T. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 2010, 170, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Yunes, R.; Poluektova, E.; Vasileva, E.; Odorskaya, M.; Marsova, M.; Kovalev, G.; Danilenko, V. A multi-strain potential probiotic formulation of GABA-producing Lactobacillus plantarum 90sk and Bifidobacterium adolescentis 150 with antidepressant effects. Probiotics Antimicrob. Proteins 2020, 12, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Altaib, H.; Nakamura, K.; Abe, M.; Badr, Y.; Yanase, E.; Nomura, I.; Suzuki, T. Differences in the concentration of the fecal neurotransmitters GABA and glutamate are associated with microbial composition among healthy human subjects. Microorganisms 2021, 9, 378. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, F.; Abdollahi, S.; Shidfar, F.; Clark, C.C.; Soltani, S. Probiotics supplementation and brain-derived neurotrophic factor (BDNF): A systematic review and meta-analysis of randomized controlled trials. Nutr. Neurosci. 2022, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Heidarzadeh-Rad, N.; Gökmen-Özel, H.; Kazemi, A.; Almasi, N.; Djafarian, K. Effects of a psychobiotic supplement on serum brain-derived neurotrophic factor levels in depressive patients: A post hoc analysis of a randomized clinical trial. J. Neurogastroenterol. Motil. 2020, 26, 486. [Google Scholar] [CrossRef]
- Romijn, A.R.; Rucklidge, J.J.; Kuijer, R.G.; Frampton, C. A double-blind, randomized, placebo-controlled trial of Lactobacillus helveticus and Bifidobacterium longum for the symptoms of depression. Aust. N. Z. J. Psychiatry 2017, 51, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Sanchez, M.I.; Hall, G.B.; Ghajar, K.; Nardelli, A.; Bolino, C.; Lau, J.T.; Martin, F.-P.; Cominetti, O.; Welsh, C.; Rieder, A. Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: A pilot study in patients with irritable bowel syndrome. Gastroenterology 2017, 153, 448–459.e8. [Google Scholar] [CrossRef]
- Akkasheh, G.; Kashani-Poor, Z.; Tajabadi-Ebrahimi, M.; Jafari, P.; Akbari, H.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z.; Esmaillzadeh, A. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: A randomized, double-blind, placebo-controlled trial. Nutrition 2016, 32, 315–320. [Google Scholar] [CrossRef]
- Khatri, I.; Tomar, R.; Ganesan, K.; Prasad, G.; Subramanian, S. Complete genome sequence and comparative genomics of the probiotic yeast Saccharomyces boulardii. Sci. Rep. 2017, 7, 371. [Google Scholar] [CrossRef]
- Bai, S.; Wang, W.; Wang, T.; Li, J.; Zhang, S.; Chen, Z.; Qi, X.; Chen, J.; Cheng, K.; Xie, P. CD36 deficiency affects depressive-like behaviors possibly by modifying gut microbiota and the inflammasome pathway in mice. Transl. Psychiatry 2021, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Nasca, C.; Gray, J.D. Stress effects on neuronal structure: Hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology 2016, 41, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, M.H.; La Fata, G.; Steinert, R.E.; Weber, P. Relationship between the gut microbiome and brain function. Nutr. Rev. 2018, 76, 481–496. [Google Scholar] [CrossRef]
- Kumari, A.; Bhawal, S.; Kapila, S.; Kapila, R. Strain-specific effects of probiotic Lactobacilli on mRNA expression of epigenetic modifiers in intestinal epithelial cells. Arch. Microbiol. 2022, 204, 411. [Google Scholar] [CrossRef]
- Eliwa, H.; Brizard, B.; Le Guisquet, A.-M.; Hen, R.; Belzung, C.; Surget, A. Adult neurogenesis augmentation attenuates anhedonia and HPA axis dysregulation in a mouse model of chronic stress and depression. Psychoneuroendocrinology 2021, 124, 105097. [Google Scholar] [CrossRef] [PubMed]
- Kupfer, D.J.; Frank, E.; Phillips, M.L. Major depressive disorder: New clinical, neurobiological, and treatment perspectives. Lancet 2012, 379, 1045–1055. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, Y.; Xiao, L.; Van Cleemput, J.; Ji, S.-P.; Bai, G.; Zhang, X. Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects. J. Clin. Investig. 2005, 115, 3104–3116. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.C.; Ortega, Z.; Palazuelos, J.; Fogaça, M.V.; Aguiar, D.C.; Díaz-Alonso, J.; Ortega-Gutiérrez, S.; Vázquez-Villa, H.; Moreira, F.A.; Guzmán, M. The anxiolytic effect of cannabidiol on chronically stressed mice depends on hippocampal neurogenesis: Involvement of the endocannabinoid system. Int. J. Neuropsychopharmacol. 2013, 16, 1407–1419. [Google Scholar] [CrossRef]
- Monteleone, P.; Bifulco, M.; Maina, G.; Tortorella, A.; Gazzerro, P.; Proto, M.C.; Di Filippo, C.; Monteleone, F.; Canestrelli, B.; Buonerba, G. Investigation of CNR1 and FAAH endocannabinoid gene polymorphisms in bipolar disorder and major depression. Pharmacol. Res. 2010, 61, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Aguado, T.; Romero, E.; Monory, K.; Palazuelos, J.; Sendtner, M.; Marsicano, G.; Lutz, B.; Guzmán, M.; Galve-Roperh, I. The CB1 cannabinoid receptor mediates excitotoxicity-induced neural progenitor proliferation and neurogenesis. J. Biol. Chem. 2007, 282, 23892–23898. [Google Scholar] [CrossRef]
- Leuchter, A.F.; Hunter, A.M.; Krantz, D.E.; Cook, I.A. Intermediate phenotypes and biomarkers of treatment outcome in major depressive disorder. Dialogues Clin. Neurosci. 2022, 16, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, P.F.; Neale, M.C.; Kendler, K.S. Genetic epidemiology of major depression: Review and meta-analysis. Am. J. Psychiatry 2000, 157, 1552–1562. [Google Scholar] [CrossRef]
- Bagot, R.C.; Labonté, B.; Peña, C.J.; Nestler, E.J. Epigenetic signaling in psychiatric disorders: Stress and depression. Dialogues Clin. Neurosci. 2022, 16, 281–295. [Google Scholar] [CrossRef]
- Berding, K.; Bastiaanssen, T.F.; Moloney, G.M.; Boscaini, S.; Strain, C.R.; Anesi, A.; Long-Smith, C.; Mattivi, F.; Stanton, C.; Clarke, G. Feed your microbes to deal with stress: A psychobiotic diet impacts microbial stability and perceived stress in a healthy adult population. Mol. Psychiatry 2022, 28, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Stilling, R.M.; Dinan, T.G.; Cryan, J.F. Microbial genes, brain & behaviour–epigenetic regulation of the gut–brain axis. Genes Brain Behav. 2014, 13, 69–86. [Google Scholar]
- Penner-Goeke, S.; Binder, E.B. Epigenetics and depression. Dialogues Clin. Neurosci. 2022, 21, 397–405. [Google Scholar] [CrossRef]
- Park, C.; Rosenblat, J.D.; Brietzke, E.; Pan, Z.; Lee, Y.; Cao, B.; Zuckerman, H.; Kalantarova, A.; McIntyre, R.S. Stress, epigenetics and depression: A systematic review. Neurosci. Biobehav. Rev. 2019, 102, 139–152. [Google Scholar] [CrossRef]
- Torres-Berrío, A.; Issler, O.; Parise, E.M.; Nestler, E.J. Unraveling the epigenetic landscape of depression: Focus on early life stress. Dialogues Clin. Neurosci. 2022, 21, 341–357. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Alvarez-Mon, M.A.; García-Montero, C.; Fraile-Martinez, O.; Guijarro, L.G.; Lahera, G.; Monserrat, J.; Valls, P.; Mora, F.; Rodríguez-Jiménez, R. Gut microbiota metabolites in major depressive disorder—Deep insights into their pathophysiological role and potential translational applications. Metabolites 2022, 12, 50. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.I.; Kapila, R. Dietary metabolites derived from gut microbiota: Critical modulators of epigenetic changes in mammals. Nutr. Rev. 2017, 75, 374–389. [Google Scholar] [CrossRef] [PubMed]
- Begum, N.; Mandhare, A.; Tryphena, K.P.; Srivastava, S.; Shaikh, M.F.; Singh, S.B.; Khatri, D.K. Epigenetics in depression and gut-brain axis: A molecular crosstalk. Front. Aging Neurosci. 2022, 14, 1048333. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.; Dam Ha, S.; Lee, S.B.; Buratowski, S.; Kim, T. Modulation of gene expression dynamics by co-transcriptional histone methylations. Exp. Mol. Med. 2017, 49, e326. [Google Scholar] [CrossRef]
- Halaburkova, A.; Cahais, V.; Cuenin, C.; Khoueiry, R.; Ouzounova, M.; Ghantous, A.; Herceg, Z. Identifying and characterizing epigenetic «driver» genes («epidrivers») in regulatory pathways involved in tumorigenesis and tumour cell plasticity. Cancer Res. 2019, 79, 4313. [Google Scholar] [CrossRef]
- Bhat, M.I.; Kumari, A.; Kapila, S.; Kapila, R. Probiotic lactobacilli mediated changes in global epigenetic signatures of human intestinal epithelial cells during Escherichia coli challenge. Ann. Microbiol. 2019, 69, 603–612. [Google Scholar] [CrossRef]
- Ledwaba, S.E.; Costa, D.V.; Bolick, D.T.; Giallourou, N.; Medeiros, P.H.; Swann, J.R.; Traore, A.N.; Potgieter, N.; Nataro, J.P.; Guerrant, R.L. Enteropathogenic Escherichia coli infection induces diarrhea, intestinal damage, metabolic alterations, and increased intestinal permeability in a murine model. Front. Cell. Infect. Microbiol. 2020, 10, 595266. [Google Scholar] [CrossRef]
- Covington, H.E., III; Vialou, V.F.; LaPlant, Q.; Ohnishi, Y.N.; Nestler, E.J. Hippocampal-dependent antidepressant-like activity of histone deacetylase inhibition. Neurosci. Lett. 2011, 493, 122–126. [Google Scholar] [CrossRef]
- Jaworska, J.; Zalewska, T.; Sypecka, J.; Ziemka-Nalecz, M. Effect of the HDAC inhibitor, sodium butyrate, on neurogenesis in a rat model of neonatal hypoxia–ischemia: Potential mechanism of action. Mol. Neurobiol. 2019, 56, 6341–6370. [Google Scholar] [CrossRef]
- Yamawaki, Y.; Fuchikami, M.; Morinobu, S.; Segawa, M.; Matsumoto, T.; Yamawaki, S. Antidepressant-like effect of sodium butyrate (HDAC inhibitor) and its molecular mechanism of action in the rat hippocampus. World J. Biol. Psychiatry 2012, 13, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, F.A.; Lewis, M.C.; Fass, D.M.; Wagner, F.F.; Zhang, Y.-L.; Hennig, K.M.; Gale, J.; Zhao, W.-N.; Reis, S.; Barker, D.D. A selective HDAC 1/2 inhibitor modulates chromatin and gene expression in brain and alters mouse behavior in two mood-related tests. PLoS ONE 2013, 8, e71323. [Google Scholar] [CrossRef]
- Li, J.-M.; Yu, R.; Zhang, L.-P.; Wen, S.-Y.; Wang, S.-J.; Zhang, X.-Y.; Xu, Q.; Kong, L.-D. Dietary fructose-induced gut dysbiosis promotes mouse hippocampal neuroinflammation: A benefit of short-chain fatty acids. Microbiome 2019, 7, 98. [Google Scholar] [CrossRef] [PubMed]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Sanada, K.; Nakajima, S.; Kurokawa, S.; Barceló-Soler, A.; Ikuse, D.; Hirata, A.; Yoshizawa, A.; Tomizawa, Y.; Salas-Valero, M.; Noda, Y. Gut microbiota and major depressive disorder: A systematic review and meta-analysis. J. Affect. Disord. 2020, 266, 1–13. [Google Scholar] [CrossRef]
- Reininghaus, E.Z.; Platzer, M.; Kohlhammer-Dohr, A.; Hamm, C.; Mörkl, S.; Bengesser, S.A.; Fellendorf, F.T.; Lahousen-Luxenberger, T.; Leitner-Afschar, B.; Schöggl, H. PROVIT: Supplementary probiotic treatment and vitamin B7 in depression—A randomized controlled trial. Nutrients 2020, 12, 3422. [Google Scholar] [CrossRef]
- Birmann, P.T.; Casaril, A.M.; Pesarico, A.P.; Caballero, P.S.; Smaniotto, T.Â.; Rodrigues, R.R.; Moreira, Â.N.; Conceição, F.R.; Sousa, F.S.; Collares, T. Komagataella pastoris KM71H modulates neuroimmune and oxidative stress parameters in animal models of depression: A proposal for a new probiotic with antidepressant-like effect. Pharmacol. Res. 2021, 171, 105740. [Google Scholar] [CrossRef]
- Cole, J.; Costafreda, S.G.; McGuffin, P.; Fu, C.H. Hippocampal atrophy in first episode depression: A meta-analysis of magnetic resonance imaging studies. J. Affect. Disord. 2011, 134, 483–487. [Google Scholar] [CrossRef]
- Lussier, A.A.; Hawrilenko, M.; Wang, M.J.; Choi, K.W.; Cerutti, J.; Zhu, Y.; Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium; Dunn, E.C. Genetic susceptibility for major depressive disorder associates with trajectories of depressive symptoms across childhood and adolescence. J. Child Psychol. Psychiatry 2021, 62, 895–904. [Google Scholar] [CrossRef]
- Mindus, C.; Ellis, J.; Van Staaveren, N.; Harlander-Matauschek, A. Lactobacillus-based probiotics reduce the adverse effects of stress in rodents: A meta-analysis. Front. Behav. Neurosci. 2021, 15, 642757. [Google Scholar] [CrossRef]
- Venkataraman, R.; Madempudi, R.S.; Neelamraju, J.; Ahire, J.J.; Vinay, H.; Lal, A.; Thomas, G.; Stephen, S. Effect of multi-strain probiotic formulation on students facing examination stress: A double-blind, placebo-controlled study. Probiotics Antimicrob. Proteins 2021, 13, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Steenbergen, L.; Sellaro, R.; van Hemert, S.; Bosch, J.A.; Colzato, L.S. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav. Immun. 2015, 48, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Papalini, S.; Michels, F.; Kohn, N.; Wegman, J.; van Hemert, S.; Roelofs, K.; Arias-Vasquez, A.; Aarts, E. Stress matters: Randomized controlled trial on the effect of probiotics on neurocognition. Neurobiol. Stress 2019, 10, 100141. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Jin, H.; Kwok, L.-Y.; Sun, Z.; Liong, M.-T.; Zhang, H. Probiotic consumption relieved human stress and anxiety symptoms possibly via modulating the neuroactive potential of the gut microbiota. Neurobiol. Stress 2021, 14, 100294. [Google Scholar] [CrossRef]
- Okumura, R.; Takeda, K. Maintenance of intestinal homeostasis by mucosal barriers. Inflamm. Regen. 2018, 38, 5. [Google Scholar] [CrossRef] [PubMed]
- Hosomi, K.; Kiyono, H.; Kunisawa, J. Fatty acid metabolism in the host and commensal bacteria for the control of intestinal immune responses and diseases. Gut Microbes 2020, 11, 276–284. [Google Scholar] [CrossRef]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A novel class of psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef]
- Oliver, A.; Chase, A.B.; Weihe, C.; Orchanian, S.B.; Riedel, S.F.; Hendrickson, C.L.; Lay, M.; Sewall, J.M.; Martiny, J.B.; Whiteson, K. High-fiber, whole-food dietary intervention alters the human gut microbiome but not fecal short-chain fatty acids. Msystems 2021, 6, e00115-21. [Google Scholar] [CrossRef]
- Cannavale, C.N.; Mysonhimer, A.R.; Bailey, M.A.; Cohen, N.J.; Holscher, H.D.; Khan, N.A. Consumption of a fermented dairy beverage improves hippocampal-dependent relational memory in a randomized, controlled cross-over trial. Nutr. Neurosci. 2023, 26, 265–274. [Google Scholar] [CrossRef]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Feiqiao, B.Y.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S. Gut-microbiota-targeted diets modulate human immune status. Cell 2021, 184, 4137–4153.e14. [Google Scholar] [CrossRef]
- Amirani, E.; Milajerdi, A.; Mirzaei, H.; Jamilian, H.; Mansournia, M.A.; Hallajzadeh, J.; Ghaderi, A. The effects of probiotic supplementation on mental health, biomarkers of inflammation and oxidative stress in patients with psychiatric disorders: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2020, 49, 102361. [Google Scholar] [CrossRef]
- Kelly, J.R.; Borre, Y.; O’Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef]
- Labus, J.S.; Hollister, E.B.; Jacobs, J.; Kirbach, K.; Oezguen, N.; Gupta, A.; Acosta, J.; Luna, R.A.; Aagaard, K.; Versalovic, J. Differences in gut microbial composition correlate with regional brain volumes in irritable bowel syndrome. Microbiome 2017, 5, 49. [Google Scholar] [CrossRef] [PubMed]
- LeWinn, K.Z.; Connolly, C.G.; Wu, J.; Drahos, M.; Hoeft, F.; Ho, T.C.; Simmons, A.N.; Yang, T.T. White matter correlates of adolescent depression: Structural evidence for frontolimbic disconnectivity. J. Am. Acad. Child Adolesc. Psychiatry 2014, 53, 899–909.e7. [Google Scholar] [CrossRef] [PubMed]
- Touron, E.; Moulinet, I.; Kuhn, E.; Sherif, S.; Ourry, V.; Landeau, B.; Mézenge, F.; Vivien, D.; Klimecki, O.M.; Poisnel, G. Depressive symptoms in cognitively unimpaired older adults are associated with lower structural and functional integrity in a frontolimbic network. Mol. Psychiatry 2022, 27, 5086–5095. [Google Scholar] [CrossRef] [PubMed]
- Sheline, Y.I.; Wang, P.W.; Gado, M.H.; Csernansky, J.G.; Vannier, M.W. Hippocampal atrophy in recurrent major depression. Proc. Natl. Acad. Sci. USA 1996, 93, 3908–3913. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Tian, S.; Wang, H.; Jiang, H.; Wang, X.; Shao, J.; Wang, Q.; Yan, R.; Tao, S.; Liu, H. Discriminating suicide attempters and predicting suicide risk using altered frontolimbic resting-state functional connectivity in patients with bipolar II disorder. Front. Psychiatry 2020, 11, 1352. [Google Scholar] [CrossRef]
- Connolly, C.G.; Ho, T.C.; Blom, E.H.; LeWinn, K.Z.; Sacchet, M.D.; Tymofiyeva, O.; Simmons, A.N.; Yang, T.T. Resting-state functional connectivity of the amygdala and longitudinal changes in depression severity in adolescent depression. J. Affect. Disord. 2017, 207, 86–94. [Google Scholar] [CrossRef]
- Kaur, H.; Singh, Y.; Singh, S.; Singh, R.B. Gut microbiome-mediated epigenetic regulation of brain disorder and application of machine learning for multi-omics data analysis. Genome 2021, 64, 355–371. [Google Scholar] [CrossRef]
- Davenport, T.; Kalakota, R. The potential for artificial intelligence in healthcare. Future Healthc. J. 2019, 6, 94. [Google Scholar] [CrossRef]
- Sun, Y.; Li, H.; Zheng, L.; Li, J.; Hong, Y.; Liang, P.; Kwok, L.-Y.; Zuo, Y.; Zhang, W.; Zhang, H. iProbiotics: A machine learning platform for rapid identification of probiotic properties from whole-genome primary sequences. Brief. Bioinform. 2022, 23, bbab477. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.; Panahi, B.; Mazlumi, A.; Hejazi, M.A.; Komi, D.E.A.; Nami, Y. Screening of potential probiotic lactic acid bacteria with antimicrobial properties and selection of superior bacteria for application as biocontrol using machine learning models. LWT 2022, 162, 113471. [Google Scholar] [CrossRef]
- Westfall, S.; Carracci, F.; Estill, M.; Zhao, D.; Wu, Q.-l.; Shen, L.; Simon, J.; Pasinetti, G.M. Optimization of probiotic therapeutics using machine learning in an artificial human gastrointestinal tract. Sci. Rep. 2021, 11, 1067. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.K.-M.; Lin, J.-J.; Tseng, H.-H.; Wang, X.-Y.; Jang, F.-L.; Chen, P.-S.; Huang, C.-C.; Hsieh, S.; Lin, S.-H. DNA methylation signature aberration as potential biomarkers in treatment-resistant schizophrenia: Constructing a methylation risk score using a machine learning method. J. Psychiatr. Res. 2023, 157, 57–65. [Google Scholar] [CrossRef]
- Shenderov, B.A. Metabiotics: Novel idea or natural development of probiotic conception. Microb. Ecol. Health Dis. 2013, 24, 20399. [Google Scholar] [CrossRef]
- Ansari, F.; Pourjafar, H.; Tabrizi, A.; Homayouni, A. The Effects of Probiotics and Prebiotics on Mental Disorders: A Review on Depression, Anxiety, Alzheimer, and Autism Spectrum Disorders. Curr. Pharm. Biotechnol. 2020, 21, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Bruce-Keller, A.J.; Salbaum, J.M.; Berthoud, H.R. Harnessing Gut Microbes for Mental Health: Getting from Here to There. Biol. Psychiatry 2018, 83, 214–223. [Google Scholar] [CrossRef]
- Cang, W.; Wu, J.; Ding, R.; Wang, W.; Li, N.; Shi, H.; Shi, L.; Lee, Y.; Wu, R. Potential of Probiotics as an Adjunct for Patients with Major Depressive Disorder. Mol. Nutr. Food Res. 2022, 66, e2101057. [Google Scholar] [CrossRef]
- Cepeda, M.S.; Katz, E.G.; Blacketer, C. Microbiome-Gut-Brain Axis: Probiotics and Their Association with Depression. J. Neuropsychiatry Clin. Neurosci. 2017, 29, 39–44. [Google Scholar] [CrossRef]
- Su, G.L.; Ko, C.W.; Bercik, P.; Falck-Ytter, Y.; Sultan, S.; Weizman, A.V.; Morgan, R.L. AGA clinical practice guidelines on the role of probiotics in the management of gastrointestinal disorders. Gastroenterology 2020, 159, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Morelli, L. In Vitro selection of probiotic lactobacilli: A critical appraisal. Curr. Issues Intest. Microbiol. 2000, 1, 59–67. [Google Scholar] [PubMed]
- Sotoudegan, F.; Daniali, M.; Hassani, S.; Nikfar, S.; Abdollahi, M. Reappraisal of probiotics’ safety in human. Food Chem. Toxicol. 2019, 129, 22–29. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, D.; Letchumanan, V.; Thum, C.C.; Thurairajasingam, S.; Lee, L.-H. A Microbial-Based Approach to Mental Health: The Potential of Probiotics in the Treatment of Depression. Nutrients 2023, 15, 1382. https://doi.org/10.3390/nu15061382
Johnson D, Letchumanan V, Thum CC, Thurairajasingam S, Lee L-H. A Microbial-Based Approach to Mental Health: The Potential of Probiotics in the Treatment of Depression. Nutrients. 2023; 15(6):1382. https://doi.org/10.3390/nu15061382
Chicago/Turabian StyleJohnson, Dinyadarshini, Vengadesh Letchumanan, Chern Choong Thum, Sivakumar Thurairajasingam, and Learn-Han Lee. 2023. "A Microbial-Based Approach to Mental Health: The Potential of Probiotics in the Treatment of Depression" Nutrients 15, no. 6: 1382. https://doi.org/10.3390/nu15061382
APA StyleJohnson, D., Letchumanan, V., Thum, C. C., Thurairajasingam, S., & Lee, L.-H. (2023). A Microbial-Based Approach to Mental Health: The Potential of Probiotics in the Treatment of Depression. Nutrients, 15(6), 1382. https://doi.org/10.3390/nu15061382






