Liver Lipidomics Analysis Revealed the Novel Ameliorative Mechanisms of L-Carnitine on High-Fat Diet-Induced NAFLD Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Sample Collection
2.3. Basic Biochemical Measurements
2.4. Histological Analyses
2.5. Western Blot Analysis
2.6. Lipidomics Analysis
2.7. Data Processing and Statistical Analysis
3. Results
3.1. Effects of L-Carnitine on the Growth Parameters of NAFLD Mice
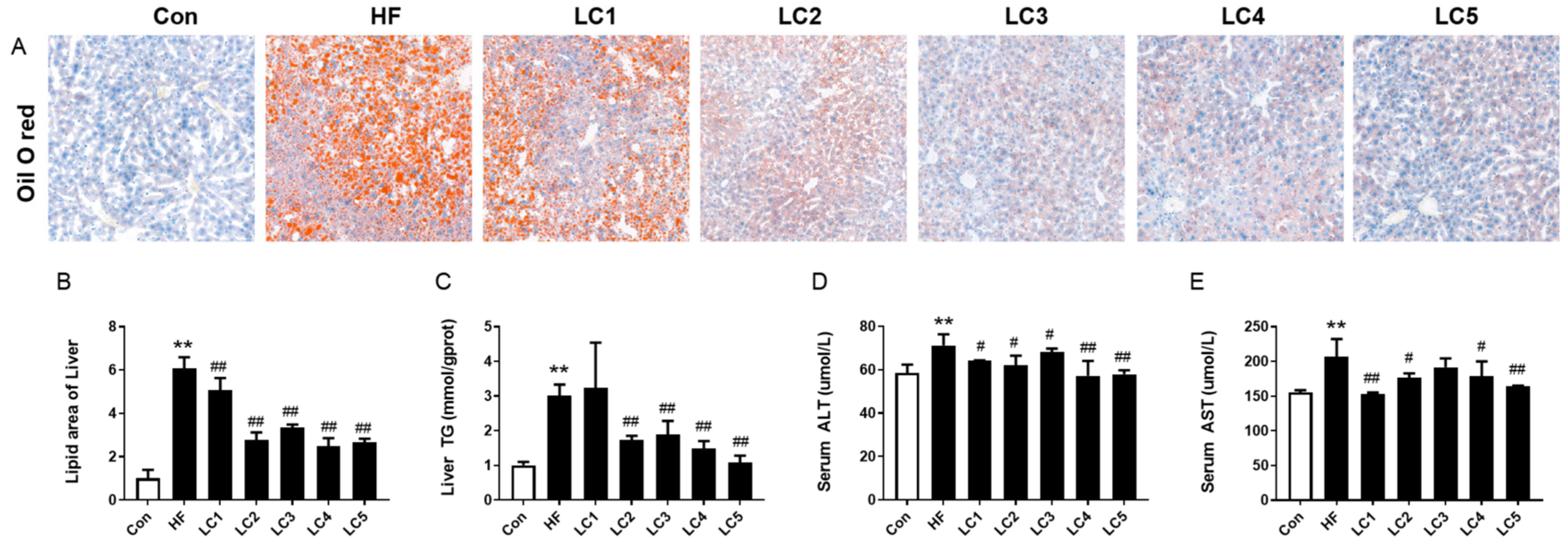
3.2. Effect of L-Carnitine on Hepatic Lipid Accumulation and Liver Function in NAFLD Mice
3.3. L-Carnitine Supplementation Attenuated Hepatic Inflammation
3.4. Lipidomics Analysis of Hepatic Lipid Profiles following L-Carnitine Supplementation
3.5. Correlation Analysis
3.6. Pathway Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdelmalek, M.F. Nonalcoholic fatty liver disease: Another leap forward. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 85–86. [Google Scholar] [CrossRef] [PubMed]
- Cotter, T.G.; Rinella, M. Nonalcoholic Fatty Liver Disease 2020: The State of the Disease. Gastroenterology 2020, 158, 1851–1864. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.-G.; Kim, S.-U.; Wong, V.W.-S. New trends on obesity and NAFLD in Asia. J. Hepatol. 2017, 67, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Portillo-Sanchez, P.; Bril, F.; Maximos, M.; Lomonaco, R.; Biernacki, D.; Orsak, B.; Subbarayan, S.; Webb, A.; Hecht, J.; Cusi, K. High Prevalence of Nonalcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Mellitus and Normal Plasma Aminotransferase Levels. J. Clin. Endocrinol. Metab. 2015, 100, 2231–2238. [Google Scholar] [CrossRef]
- Pal, P.; Palui, R.; Ray, S.J. Heterogeneity of non-alcoholic fatty liver disease: Implications for clinical practice and research activity. World J. Hepatol. 2021, 13, 1584–1610. [Google Scholar] [CrossRef]
- Nobili, V.; Alisi, A.; Valenti, L.; Miele, L.; Feldstein, A.E.; Alkhouri, N. NAFLD in children: New genes, new diagnostic modalities and new drugs. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 517–530. [Google Scholar] [CrossRef]
- Sanyal, A.J. Past, present and future perspectives in nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 377–386. [Google Scholar] [CrossRef]
- Su, C.-C.; Chang, C.-S.; Chou, C.-H.; Wu, Y.-H.S.; Yang, K.-T.; Tseng, J.-K.; Chang, Y.-Y.; Chen, Y.-C. L-carnitine ameliorates dyslipidemic and hepatic disorders induced by a high-fat diet via regulating lipid metabolism, self-antioxidant capacity, and inflammatory response. J. Funct. Foods 2015, 15, 497–508. [Google Scholar] [CrossRef]
- Mollica, G.; Senesi, P.; Codella, R.; Vacante, F.; Montesano, A.; Luzi, L.; Terruzzi, I. L-carnitine supplementation attenuates NAFLD progression and cardiac dysfunction in a mouse model fed with methionine and choline-deficient diet. Dig. Liver Dis. 2020, 52, 314–323. [Google Scholar] [CrossRef]
- Savic, D.; Hodson, L.; Neubauer, S.; Pavlides, M. The Importance of the Fatty Acid Transporter L-carnitine in Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients 2020, 12, 2178. [Google Scholar] [CrossRef] [PubMed]
- Abolfathi, M.; Mohd-Yusof, B.-N.; Hanipah, Z.N.; Mohd Redzwan, S.; Yusof, L.M.; Khosroshahi, M.Z. The effects of carnitine supplementation on clinical characteristics of patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2020, 48, 102273–102286. [Google Scholar] [CrossRef] [PubMed]
- Knottnerus, S.J.G.; Bleeker, J.C.; Wüst, R.C.I.; Ferdinandusse, S.; Ijlst, L.; Wijburg, F.A.; Wanders, R.J.A.; Visser, G.; Houtkooper, R.H. Disorders of mitochondrial long-chain fatty acid oxidation and the carnitine shuttle. Rev. Endocr. Metab. Disord. 2018, 19, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.; Alonso, E.; Rao, S.; Whitington, P.; Fryer, J.; Abecassis, M.; Superina, R.; Flamm, S.L.; Blei, A.T. Body mass index as a predictor of hepatic steatosis in living liver donors. Liver Transplant. 2001, 7, 409–414. [Google Scholar] [CrossRef]
- Malaguarnera, M.; Gargante, M.P.; Russo, C.; Antic, T.; Vacante, M.; Malaguarnera, M.; Avitabile, T.; Li Volti, G.; Galvano, F. L-Carnitine supplementation to diet: A new tool in treatment of nonalcoholic steatohepatitis—A randomized and controlled clinical trial. Am. J. Gastroenterol. 2010, 105, 1338–1345. [Google Scholar] [CrossRef]
- Ten Hove, M.; Pater, L.; Storm, G.; Weiskirchen, S.; Weiskirchen, R.; Lammers, T.; Bansal, R. The hepatic lipidome: From basic science to clinical translation. Adv. Drug Deliv. Rev. 2020, 159, 180–197. [Google Scholar] [CrossRef]
- Yang, K.; Han, X. Lipidomics: Techniques, Applications, and Outcomes Related to Biomedical Sciences. Trends Biochem. Sci. 2016, 41, 954–969. [Google Scholar] [CrossRef]
- Han, X.; Gross, R. Global analyses of cellular lipidomes directly from crude extracts of biological samples by ESI mass spectrometry: A bridge to lipidomics. J. Lipid Res. 2003, 44, 1071–1080. [Google Scholar] [CrossRef]
- Puri, P.; Baillie, R.; Mm, W.; Mirshahi, F.; Sanyal, A. 7088 A lipidomic analysis of non-alcoholic fatty liver disease (NAFLD). J. Hepatol. 2006, 44, S260–S261. [Google Scholar] [CrossRef]
- Kartsoli, S.; Kostara, C.E.; Tsimihodimos, V.; Bairaktari, E.T.; Christodoulou, D.K. Lipidomics in non-alcoholic fatty liver disease. World J. Hepatol. 2020, 12, 436–450. [Google Scholar] [CrossRef]
- Feng, S.; Gan, L.; Yang, C.S.; Liu, A.B.; Lu, W.; Shao, P.; Dai, Z.; Sun, P.; Luo, Z. Effects of Stigmasterol and β-Sitosterol on Nonalcoholic Fatty Liver Disease in a Mouse Model: A Lipidomic Analysis. J. Agric. Food Chem. 2018, 66, 3417–3425. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, T.; Xie, X.; Wang, X.; Luo, Y.; Xu, N.; Sa, Z.; Zhang, M.; Chen, Z.; Hu, X.; et al. Hepatic Lipidomics Analysis Reveals the Ameliorative Effects of Highland Barley β-Glucan on Western Diet-Induced Nonalcoholic Fatty Liver Disease Mice. J. Agric. Food Chem. 2021, 69, 9287–9298. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Sun, C.; Zhang, Y.; Hu, S.; Li, D. Dietary supplementation of L-carnitine ameliorates metabolic syndrome independent of trimethylamine N-oxide produced by gut microbes in high-fat diet-induced obese mice. Food Funct. 2022, 13, 12039–12050. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Gao, X.; Liu, Z.; Zhang, L.; Fang, X.; Sun, J.; Zhang, Z.; Sun, Y. Sodium Alginate Prevents Non-Alcoholic Fatty Liver Disease by Modulating the Gut-Liver Axis in High-Fat Diet-Fed Rats. Nutrients 2022, 14, 4846. [Google Scholar] [CrossRef]
- Song, Y.; Cai, Q.; Wang, S.; Li, L.; Wang, Y.; Zou, S.; Gao, X.; Wei, Y. The Ameliorative Effect and Mechanisms of Ruditapes philippinarum Bioactive Peptides on Obesity and Hyperlipidemia Induced by a High-Fat Diet in Mice. Nutrients 2022, 14, 5066. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cong, P.; Zhang, T.; Wang, R.; Wang, X.; Liu, J.; Wang, X.; Xu, J.; Wang, Y.; Wang, J.; et al. Plasmalogen attenuates the development of hepatic steatosis and cognitive deficit through mechanism involving p75NTR inhibition. Redox Biol. 2021, 43, 102002–102014. [Google Scholar] [CrossRef]
- Xia, Y.; Li, Q.; Zhong, W.; Dong, J.; Wang, Z.; Wang, C. L-carnitine ameliorated fatty liver in high-calorie diet/STZ-induced type 2 diabetic mice by improving mitochondrial function. Redox Biol. 2011, 3, 31–41. [Google Scholar] [CrossRef]
- Montesano, A.; Senesi, P.; Vacante, F.; Mollica, G.; Benedini, S.; Mariotti, M.; Luzi, L.; Terruzzi, I. L-carnitine counteracts in vitro fructose-induced hepatic steatosis through targeting oxidative stress markers. J. Endocrinol. Investig. 2020, 43, 493–503. [Google Scholar] [CrossRef]
- Manne, V.; Handa, P.; Kowdley, K.V. Pathophysiology of Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis. Clin. Liver Dis. 2018, 22, 23–37. [Google Scholar] [CrossRef]
- Szabo, G.; Petrasek, J. Inflammasome activation and function in liver disease. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 387–400. [Google Scholar] [CrossRef]
- Khan, R.S.; Bril, F.; Cusi, K.; Newsome, P.N. Modulation of Insulin Resistance in Nonalcoholic Fatty Liver Disease. Hepatology 2019, 70, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Cheng, X.F.; Liu, Y.; Lv, Q.Z.; Liu, G.L.; Zhang, J.G.; Li, X.Y. Potential Nexus of Non-alcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus: Insulin Resistance between Hepatic and Peripheral Tissues. Front. Pharmacol. 2018, 9, 1566. [Google Scholar] [CrossRef] [PubMed]
- Uemura, H.; Katsuura-Kamano, S.; Yamaguchi, M.; Bahari, T.; Ishizu, M.; Fujioka, M.; Arisawa, K. Relationships of serum high-sensitivity C-reactive protein and body size with insulin resistance in a Japanese cohort. PLoS ONE 2017, 12, e0178672. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hu, D.; Fan, H.; Zhang, Y.; LeSage, G.D.; Caudle, Y.; Stuart, C.; Liu, Z.; Yin, D. β-Arrestin 2 Negatively Regulates Toll-like Receptor 4 (TLR4)-triggered Inflammatory Signaling via Targeting p38 MAPK and Interleukin 10*. J. Biol. Chem. 2014, 289, 23075–23085. [Google Scholar] [CrossRef]
- Wan, X.; Xu, C.; Yu, C.; Li, Y. Role of NLRP3 Inflammasome in the Progression of NAFLD to NASH. Can. J. Gastroenterol. Hepatol. 2016, 2016, 6489012. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Pan, X.; Luo, J.; Xiao, X.; Li, J.; Bestman, P.L.; Luo, M. Association of Inflammatory Cytokines with Non-Alcoholic Fatty Liver Disease. Front. Immunol. 2022, 13, 880298. [Google Scholar] [CrossRef]
- Knorr, J.; Wree, A.; Tacke, F.; Feldstein, A.E. The NLRP3 Inflammasome in Alcoholic and Nonalcoholic Steatohepatitis. Semin. Liver Dis. 2020, 40, 298–306. [Google Scholar] [CrossRef]
- Bykov, I.; Järveläinen, H.; Lindros, K. L-carnitine alleviates alcohol-induced liver damage in rats: Role of tumour necrosis factor-alpha. Alcohol Alcohol. 2003, 38, 400–406. [Google Scholar] [CrossRef]
- Abdel-Emam, R.A.; Ali, M.F. Effect of L-carnitine supplementation on lead acetate-induced liver cell apoptosis and inflammation: Role of caspase-3 and glycogen synthase kinase-3β enzymes. Life Sci. 2022, 291, 120277–120286. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, K.; Yang, L.; Liu, R.; Chu, Y.; Qin, X.; Yang, P.; Yu, H. Lipid metabolism in inflammation-related diseases. Analyst 2018, 143, 4526–4536. [Google Scholar] [CrossRef]
- Sun, T.; Wang, X.; Cong, P.; Xu, J.; Xue, C. Mass spectrometry-based lipidomics in food science and nutritional health: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2530–2558. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Kim, H.; Jung, M.H.; Hong, S.; Song, J. Consumption of barley β-glucan ameliorates fatty liver and insulin resistance in mice fed a high-fat diet. Mol. Nutr. Food Res. 2010, 54, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Puri, P.; Baillie, R.A.; Wiest, M.M.; Mirshahi, F.; Choudhury, J.; Cheung, O.; Sargeant, C.; Contos, M.J.; Sanyal, A.J. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology 2007, 46, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Yang, L.; McCall, S.; Huang, J.; Yu, X.X.; Pandey, S.K.; Bhanot, S.; Monia, B.P.; Li, Y.X.; Diehl, A.M. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology 2007, 45, 1366–1374. [Google Scholar] [CrossRef]
- Alkhouri, N.; Dixon, L.J.; Feldstein, A.E. Lipotoxicity in nonalcoholic fatty liver disease: Not all lipids are created equal. Expert Rev. Gastroenterol. Hepatol. 2009, 3, 445–451. [Google Scholar] [CrossRef]
- Preuss, C.; Jelenik, T.; Bódis, K.; Müssig, K.; Burkart, V.; Szendroedi, J.; Roden, M.; Markgraf, D.F. A New Targeted Lipidomics Approach Reveals Lipid Droplets in Liver, Muscle and Heart as a Repository for Diacylglycerol and Ceramide Species in Non-Alcoholic Fatty Liver. Cells 2019, 8, 277. [Google Scholar] [CrossRef]
- Gorden, D.L.; Ivanova, P.T.; Myers, D.S.; McIntyre, J.O.; VanSaun, M.N.; Wright, J.K.; Matrisian, L.M.; Brown, H.A. Increased diacylglycerols characterize hepatic lipid changes in progression of human nonalcoholic fatty liver disease; comparison to a murine model. PLoS ONE 2011, 6, e22775. [Google Scholar] [CrossRef]
- Ruby, M.A.; Massart, J.; Hunerdosse, D.M.; Schönke, M.; Correia, J.C.; Louie, S.M.; Ruas, J.L.; Näslund, E.; Nomura, D.K.; Zierath, J.R. Human Carboxylesterase 2 Reverses Obesity-Induced Diacylglycerol Accumulation and Glucose Intolerance. Cell Rep. 2017, 18, 636–646. [Google Scholar] [CrossRef]
- Jelenik, T.; Kaul, K.; Séquaris, G.; Flögel, U.; Phielix, E.; Kotzka, J.; Knebel, B.; Fahlbusch, P.; Hörbelt, T.; Lehr, S.; et al. Mechanisms of Insulin Resistance in Primary and Secondary Nonalcoholic Fatty Liver. Diabetes 2017, 66, 2241–2253. [Google Scholar] [CrossRef]
- Hermansson, M.; Hokynar, K.; Somerharju, P. Mechanisms of glycerophospholipid homeostasis in mammalian cells. Prog. Lipid Res. 2011, 50, 240–257. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Cuenca, S.; Pellegrinelli, V.; Campbell, M.; Oresic, M.; Vidal-Puig, A. Sphingolipids and glycerophospholipids—The “ying and yang” of lipotoxicity in metabolic diseases. Prog. Lipid Res. 2017, 66, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y. Effect of carbon tetrachloride administration on the synthesis of triglycerides and phospholipids in rat liver. J. Lipid Res. 1969, 10, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Lyu, Q.; Liu, Y.; Hu, H.; Wang, S.; Pan, C.; Duan, X.; Gao, Y.; Qi, L.W.; Liu, W.; et al. Chitosan Oligosaccharide Ameliorates Nonalcoholic Fatty Liver Disease (NAFLD) in Diet-Induced Obese Mice. Mar. Drugs 2019, 17, 391. [Google Scholar] [CrossRef]
- Apostolopoulou, M.; Gordillo, R.; Koliaki, C.; Gancheva, S.; Jelenik, T.; De Filippo, E.; Herder, C.; Markgraf, D.; Jankowiak, F.; Esposito, I.; et al. Specific Hepatic Sphingolipids Relate to Insulin Resistance, Oxidative Stress, and Inflammation in Nonalcoholic Steatohepatitis. Diabetes Care 2018, 41, 1235–1243. [Google Scholar] [CrossRef]
- Nikolova-Karakashian, M. Alcoholic and non-alcoholic fatty liver disease: Focus on ceramide. Adv. Biol. Regul. 2018, 70, 40–50. [Google Scholar] [CrossRef]
- Simon, J.; Ouro, A.; Ala-Ibanibo, L.; Presa, N.; Delgado, T.C.; Martínez-Chantar, M.L. Sphingolipids in Non-Alcoholic Fatty Liver Disease and Hepatocellular Carcinoma: Ceramide Turnover. Int. J. Mol. Sci. 2019, 21, 40. [Google Scholar] [CrossRef]
- Taniguchi, M.; Okazaki, T. Ceramide/Sphingomyelin Rheostat Regulated by Sphingomyelin Synthases and Chronic Diseases in Murine Models. J. Lipid Atheroscler. 2020, 9, 380–405. [Google Scholar] [CrossRef]
- Sun, Z.; Tang, Z.; Yang, X.; Liu, Q.S.; Zhang, J.; Zhou, Q.; Jiang, G. 3-tert-Butyl-4-hydroxyanisole Impairs Hepatic Lipid Metabolism in Male Mice Fed with a High-Fat Diet. Environ. Sci. Technol. 2022, 56, 3204–3213. [Google Scholar] [CrossRef]
- Lakshman, M.R. Some novel insights into the pathogenesis of alcoholic steatosis. Alcohol 2004, 34, 45–53. [Google Scholar] [CrossRef]
- Shapiro, H.; Tehilla, M.; Attal-Singer, J.; Bruck, R.; Luzzatti, R.; Singer, P. The therapeutic potential of long-chain omega-3 fatty acids in nonalcoholic fatty liver disease. Clin. Nutr. 2011, 30, 6–19. [Google Scholar] [CrossRef]
- Jordão Candido, C.; Silva Figueiredo, P.; Del Ciampo Silva, R.; Candeloro Portugal, L.; Augusto Dos Santos Jaques, J.; Alves de Almeida, J.; de Barros Penteado, B.; Albuquerque Dias, D.; Marcelino, G.; Pott, A.; et al. Protective Effect of α-Linolenic Acid on Non-Alcoholic Hepatic Steatosis and Interleukin-6 and -10 in Wistar Rats. Nutrients 2019, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, X.-J.; Feng, K.; He, C.; Li, P.; Hu, Y.-J.; Su, H.; Wan, J.-B. Dietary α-linolenic acid-rich flaxseed oil prevents against alcoholic hepatic steatosis via ameliorating lipid homeostasis at adipose tissue-liver axis in mice. Sci. Rep. 2016, 6, 26826. [Google Scholar] [CrossRef] [PubMed]
- Masuda, S.; Fujishima, Y.; Maeda, N.; Tsugawa-Shimizu, Y.; Nakamura, Y.; Tanaka, Y.; Obata, Y.; Fukuda, S.; Nagao, H.; Kita, S.; et al. Impact of glycosylphosphatidylinositol-specific phospholipase D on hepatic diacylglycerol accumulation, steatosis, and insulin resistance in diet-induced obesity. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E239–E250. [Google Scholar] [CrossRef] [PubMed]
- Metz, C.N.; Brunner, G.; Choi-Muira, N.H.; Nguyen, H.; Gabrilove, J.; Caras, I.W.; Altszuler, N.; Rifkin, D.B.; Wilson, E.L.; Davitz, M.A. Release of GPI-anchored membrane proteins by a cell-associated GPI-specific phospholipase D. EMBO J. 1994, 13, 1741–1751. [Google Scholar] [CrossRef] [PubMed]
- Davitz, M.A.; Hereld, D.; Shak, S.; Krakow, J.; Englund, P.T.; Nussenzweig, V. A glycan-phosphatidylinositol-specific phospholipase D in human serum. Science 1987, 238, 81–84. [Google Scholar] [CrossRef]
- Low, M.G.; Prasad, A.R. A phospholipase D specific for the phosphatidylinositol anchor of cell-surface proteins is abundant in plasma. Proc. Natl. Acad. Sci. USA 1988, 85, 980–984. [Google Scholar] [CrossRef]
- Chalasani, N.; Vuppalanchi, R.; Raikwar, N.S.; Deeg, M.A. Glycosylphosphatidylinositol-Specific Phospholipase D in Nonalcoholic Fatty Liver Disease: A Preliminary Study. J. Clin. Endocrinol. Metab. 2006, 91, 2279–2285. [Google Scholar] [CrossRef]






| Lipid Species | HF vs. Con | LC5 vs. HF | ||||
|---|---|---|---|---|---|---|
| a VIP | b FC | c Trend | VIP | FC | Trend | |
| Cer(d18:1_16:0) | 1.03 | 0.52 |  | 1.03 | 2.17 |  |
| DG(16:0_18:1) | 1.06 | 2.06 |  | 1.24 | 0.52 |  |
| DG(18:1_18:1) | 1.14 | 2.44 |  | 1.29 | 0.48 |  |
| PC(17:1_20:5) | 1.18 | 0.48 |  | 1.39 | 1.61 |  |
| PC(34:2) | 1.25 | 0.30 |  | 1.11 | 1.99 |  |
| PC(35:2) | 1.10 | 0.36 |  | 1.05 | 1.56 |  |
| PC(35:4) | 1.01 | 0.63 |  | 1.37 | 1.62 |  |
| PC(38:6) | 1.20 | 0.54 |  | 1.45 | 1.78 |  |
| PC(40:5) | 1.02 | 2.01 |  | 1.21 | 2.04 |  |
| PC(40:7) | 1.18 | 0.48 |  | 1.14 | 1.46 |  |
| PE(16:0_18:2) | 1.27 | 0.27 |  | 1.17 | 1.76 |  |
| PE(16:0_20:4) | 1.10 | 0.53 |  | 1.34 | 1.80 |  |
| PE(16:0_22:6) | 1.22 | 0.53 |  | 1.44 | 1.83 |  |
| PE(18:1_20:4) | 1.05 | 0.57 |  | 1.30 | 1.68 |  |
| PE(18:1_22:6) | 1.22 | 0.47 |  | 1.28 | 1.51 |  |
| PI(18:0_20:4) | 1.11 | 0.60 |  | 1.32 | 1.67 |  |
| SM(d18:1_24:1) | 1.26 | 0.35 |  | 1.11 | 1.52 |  |
| SM(d34:1) | 1.14 | 0.62 |  | 1.26 | 2.05 |  |
| TG(15:0_14:0_16:0) | 1.16 | 0.16 |  | 1.25 | 4.17 |  |
| TG(15:0_16:0_16:0) | 1.23 | 0.17 |  | 1.39 | 4.59 |  |
| TG(15:0_16:0_16:1) | 1.17 | 0.24 |  | 1.29 | 3.20 |  |
| TG(15:0_16:0_18:1) | 1.24 | 0.38 |  | 1.23 | 2.02 |  |
| TG(15:0_17:1_17:1) | 1.26 | 0.35 |  | 1.15 | 1.99 |  |
| TG(15:0_18:1_18:1) | 1.04 | 1.44 |  | 1.12 | 0.76 |  |
| TG(16:0_14:0_16:0) | 1.27 | 0.14 |  | 1.22 | 5.36 |  |
| TG(16:0_14:0_16:1) | 1.14 | 0.34 |  | 1.10 | 2.22 |  |
| TG(16:0_16:0_16:0) | 1.20 | 0.18 |  | 1.30 | 4.33 |  |
| TG(16:0_16:0_17:0) | 1.20 | 0.26 |  | 1.32 | 3.24 |  |
| TG(16:0_18:1_20:4) | 1.18 | 2.72 |  | 1.24 | 0.57 |  |
| TG(16:0_18:2_20:4) | 1.05 | 3.91 |  | 1.20 | 0.42 |  |
| TG(16:1_16:1_18:1) | 1.10 | 2.60 |  | 1.42 | 0.39 |  |
| TG(16:1_16:1_18:2) | 1.04 | 3.22 |  | 1.40 | 0.19 |  |
| TG(16:1_16:1_18:3) | 1.10 | 5.35 |  | 1.35 | 0.19 |  |
| TG(16:1_17:1_18:1) | 1.14 | 1.52 |  | 1.46 | 0.57 |  |
| TG(16:1_18:1_18:2) | 1.08 | 2.39 |  | 1.34 | 0.47 |  |
| TG(16:1_18:2_18:2) | 1.04 | 3.25 |  | 1.37 | 0.24 |  |
| TG(17:0_18:1_18:1) | 1.20 | 2.38 |  | 1.31 | 0.60 |  |
| TG(18:0_18:0_18:1) | 1.20 | 5.22 |  | 1.35 | 0.45 |  |
| TG(18:0_18:0_22:4) | 1.27 | 2.36 |  | 1.13 | 0.60 |  |
| TG(18:0_18:1_18:1) | 1.23 | 4.77 |  | 1.28 | 0.57 |  |
| TG(18:1_17:1_18:2) | 1.17 | 2.08 |  | 1.43 | 0.50 |  |
| TG(18:1_18:1_22:0) | 1.18 | 2.28 |  | 1.31 | 0.66 |  |
| TG(18:1_18:1_22:1) | 1.21 | 3.31 |  | 1.38 | 0.50 |  |
| TG(19:0_18:1_18:1) | 1.13 | 1.93 |  | 1.29 | 0.64 |  |
| TG(19:1_18:1_18:1) | 1.08 | 1.69 |  | 1.32 | 0.63 |  |
| TG(20:0_18:1_18:1) | 1.21 | 5.58 |  | 1.42 | 0.39 |  |
| TG(20:1_18:1_18:1) | 1.24 | 4.47 |  | 1.40 | 0.49 |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, C.; Guo, Y.; Cong, P.; Tian, Y.; Gao, X. Liver Lipidomics Analysis Revealed the Novel Ameliorative Mechanisms of L-Carnitine on High-Fat Diet-Induced NAFLD Mice. Nutrients 2023, 15, 1359. https://doi.org/10.3390/nu15061359
Sun C, Guo Y, Cong P, Tian Y, Gao X. Liver Lipidomics Analysis Revealed the Novel Ameliorative Mechanisms of L-Carnitine on High-Fat Diet-Induced NAFLD Mice. Nutrients. 2023; 15(6):1359. https://doi.org/10.3390/nu15061359
Chicago/Turabian StyleSun, Chengyuan, Yan Guo, Peixu Cong, Yuan Tian, and Xiang Gao. 2023. "Liver Lipidomics Analysis Revealed the Novel Ameliorative Mechanisms of L-Carnitine on High-Fat Diet-Induced NAFLD Mice" Nutrients 15, no. 6: 1359. https://doi.org/10.3390/nu15061359
APA StyleSun, C., Guo, Y., Cong, P., Tian, Y., & Gao, X. (2023). Liver Lipidomics Analysis Revealed the Novel Ameliorative Mechanisms of L-Carnitine on High-Fat Diet-Induced NAFLD Mice. Nutrients, 15(6), 1359. https://doi.org/10.3390/nu15061359






