Iodine Availability through Iodized Salt in Portugal: 2010–2021 Sales Evolution and Distribution
Abstract
1. Introduction
1.1. Iodine Intake Recommendations, Dietary Sources, and Deficiency Prevention Strategies
1.2. Global Iodine Status
1.3. Iodine Status and Iodine Deficiency Prevention Strategies in Portugal
2. Materials and Methods
3. Results
3.1. Iodized Salt Sales from 2010 to 2021
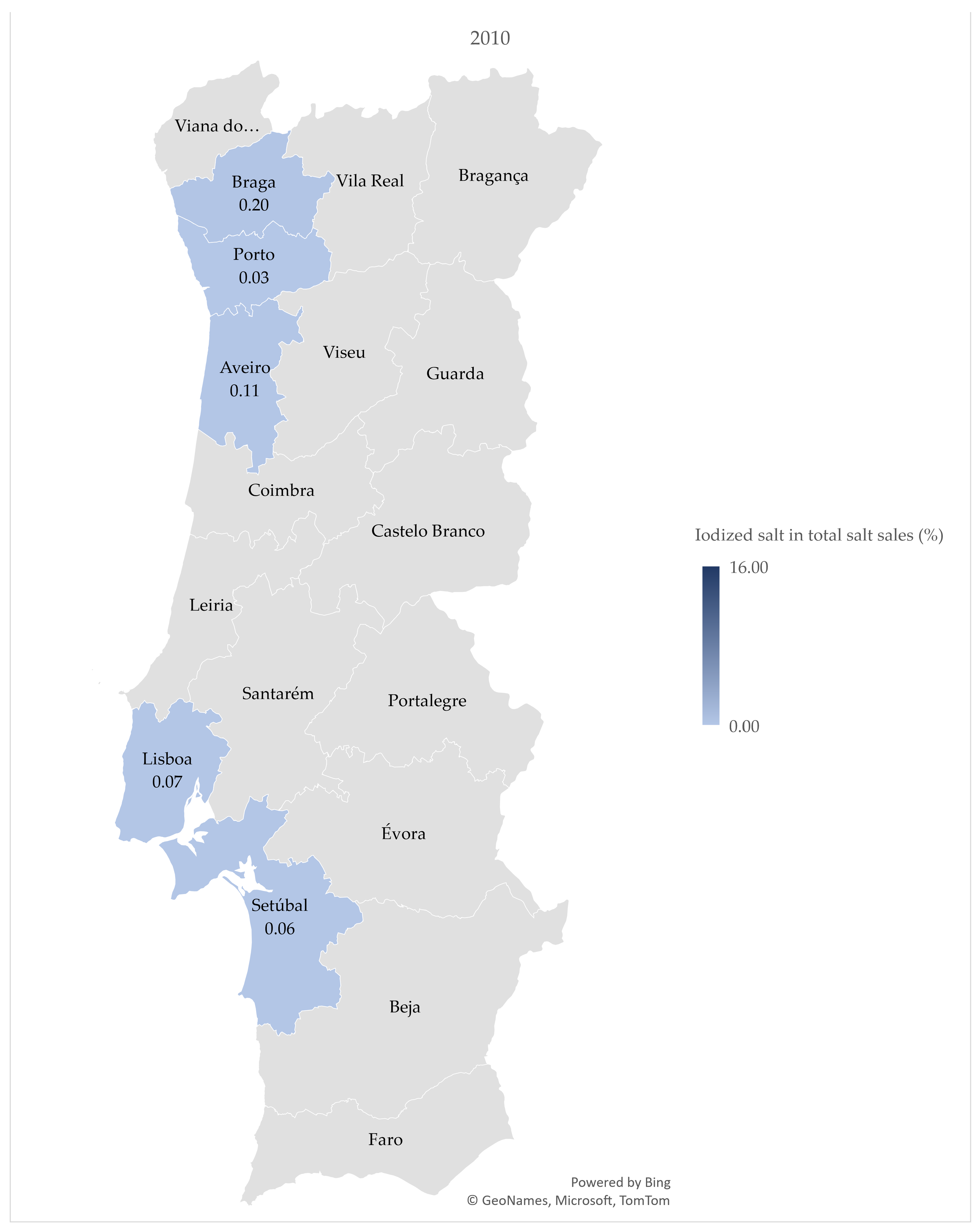
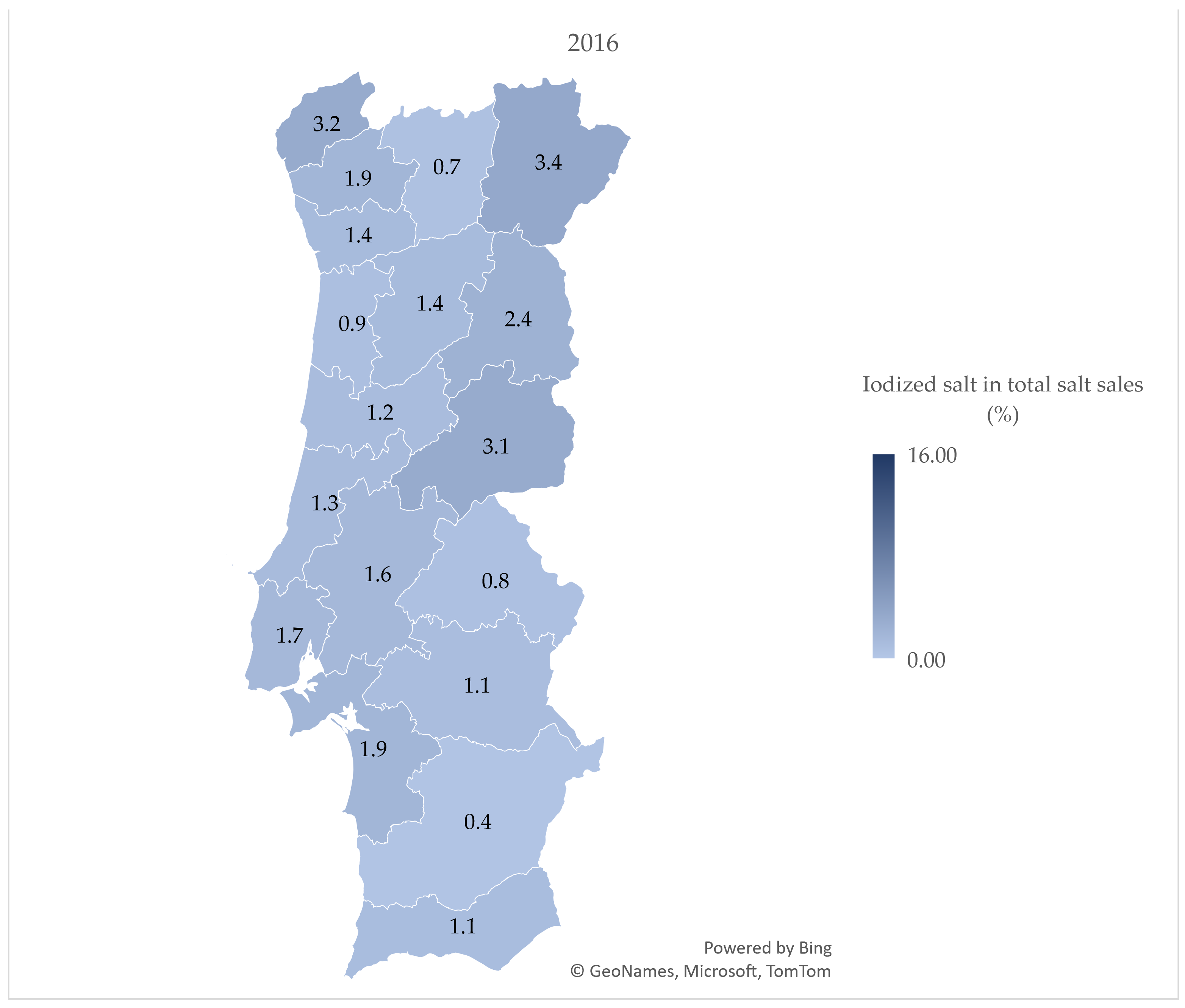
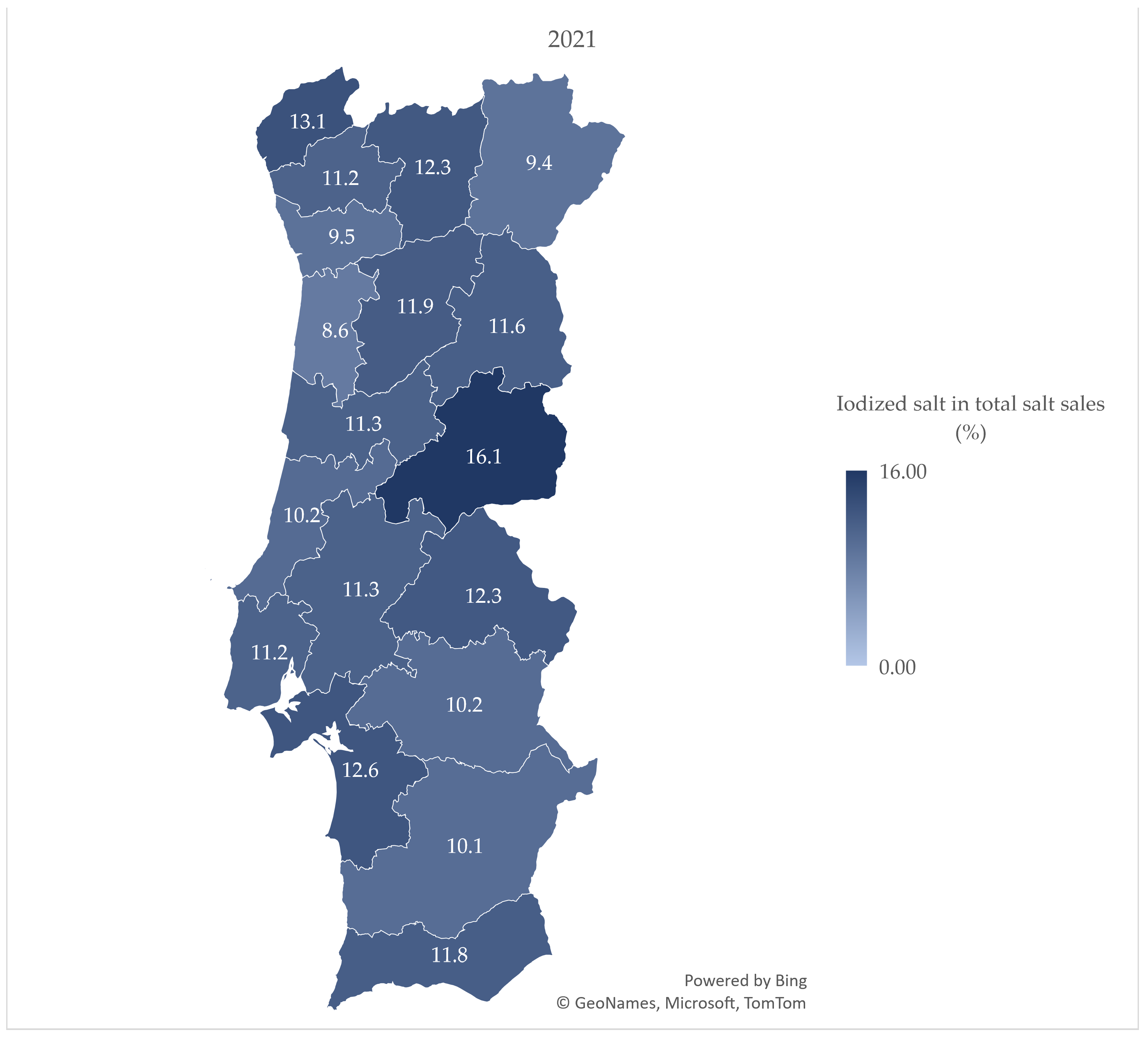
3.2. Iodized Salt Sales by District
3.3. Estimates of Household Availability of Salt and Iodine per Capita, from 2010 to 2021
4. Discussion
4.1. Iodized Salt Sales from 2010 to 2021
4.2. Iodized Salt Sales by District
4.3. Estimates of Household Availability of Salt and Iodine per Capita, from 2010 to 2021
4.4. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glinoer, D. Pregnancy and iodine. Thyroid 2001, 11, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B. The role of iodine in human growth and development. Semin. Cell Dev. Biol. 2011, 22, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Costeira, M.J.; Oliveira, P.; Ares, S.; de Escobar, G.M.; Palha, J.A. Iodine Status of Pregnant Women and Their Progeny in the Minho Region of Portugal. Thyroid 2009, 19, 157–163. [Google Scholar] [CrossRef]
- Delange, F.; de Benoist, B.; Pretell, E.; Dunn, J.T. Iodine deficiency in the world: Where do we stand at the turn of the century? Thyroid 2001, 11, 437–447. [Google Scholar] [CrossRef]
- Eastman, C.J.; Zimmermann, M.B. The Iodine Deficiency Disorders. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Zimmermann, M.B. Iodine deficiency. Endocr. Rev. 2009, 30, 376–408. [Google Scholar] [CrossRef]
- United Nations Children’s Fund (UNICEF). Guidance on the Monitoring of Salt Iodization Programmes and Determination of Population Iodine Status; UNICEF: New York, NY, USA, 2018. [Google Scholar]
- World Health Organization (WHO); United Nations Children’s Fund (UNICEF); International Council for the Control of Iodine Deficiency Disorders (ICCIDD). Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination: A Guide for Programme Managers, 3rd ed.; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- World Health Organization (WHO), Nutrition Unit. Recommended Iodine Levels in Salt and Guidelines for Monitoring their Adequacy and Effectiveness; World Health Organization: Geneva, Switzerland, 1996. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on Dietary Reference Values for iodine. EFSA J. 2014, 12, 3660. [Google Scholar] [CrossRef]
- Rohner, F.; Zimmermann, M.; Jooste, P.; Pandav, C.; Caldwell, K.; Raghavan, R.; Raiten, D.J. Biomarkers of nutrition for development--iodine review. J. Nutr. 2014, 144, 1322S–1342S. [Google Scholar] [CrossRef] [PubMed]
- Delgado, I.; Coelho, I.; Calhau, M.; Albuquerque, J.; Breda, J. Scientific Update on the Iodine Content of Portuguese Foods; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Dold, S.; Zimmermann, M.B.; Jukic, T.; Kusic, Z.; Jia, Q.; Sang, Z.; Quirino, A.; San Luis, T.O.L.; Fingerhut, R.; Kupka, R.; et al. Universal Salt Iodization Provides Sufficient Dietary Iodine to Achieve Adequate Iodine Nutrition during the First 1000 Days: A Cross-Sectional Multicenter Study. J. Nutr. 2018, 148, 587–598. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Guideline: Fortification of Food-Grade Salt with Iodine for the Prevention and Control of Iodine Deficiency Disorders; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- World Health Organization (WHO). Universal Salt Iodization and Sodium Intake Reduction: Compatible, Cost-Effective Strategies of Great Public Health Benefit; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Andersson, M.; Braegger, C.P. The Role of Iodine for Thyroid Function in Lactating Women and Infants. Endocr. Rev. 2022, 43, 469–506. [Google Scholar] [CrossRef]
- Bath, S.C.; Verkaik-Kloosterman, J.; Sabatier, M.; Ter Borg, S.; Eilander, A.; Hora, K.; Aksoy, B.; Hristozova, N.; van Lieshout, L.; Tanju Besler, H.; et al. A systematic review of iodine intake in children, adults, and pregnant women in Europe-comparison against dietary recommendations and evaluation of dietary iodine sources. Nutr. Rev. 2022, 80, 2154–2177. [Google Scholar] [CrossRef]
- Global Fortification Data Exchange (GFDx). Available online: https://fortificationdata.org (accessed on 25 October 2022).
- Pearce, E.N.; Zimmermann, M.B. The Prevention of Iodine Deficiency: A History. Thyroid 2023, 33, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B.; Andersson, M. Global Endocrinology: Global perspectives in endocrinology: Coverage of iodized salt programs and iodine status in 2020. Eur. J. Endocrinol. 2021, 185, R13–R21. [Google Scholar] [CrossRef]
- Costeira, M.J.; Oliveira, P.; Ares, S.; Roque, S.; de Escobar, G.M.; Palha, J.A. Parameters of thyroid function throughout and after pregnancy in an iodine-deficient population. Thyroid 2010, 20, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Costeira, M.J.; Oliveira, P.; Santos, N.C.; Ares, S.; Saenz-Rico, B.; de Escobar, G.M.; Palha, J.A. Psychomotor development of children from an iodine-deficient region. J. Pediatr. 2011, 159, 447–453. [Google Scholar] [CrossRef]
- Direção-Geral da Saúde (DGS). Aporte de Iodo em Mulheres na Preconceção, Gravidez e Amamentação; DGS: Lisboa, Portugal, 2013.
- Limbert, E.; Prazeres, S.; São Pedro, M.; Madureira, D.; Miranda, A.; Ribeiro, M.; Jacome de Castro, J.; Carrilho, F.; Oliveira, M.J.; Reguengo, H.; et al. Iodine intake in Portuguese pregnant women: Results of a countrywide study. Eur. J. Endocrinol. 2010, 163, 631–635. [Google Scholar] [CrossRef]
- Direção-Geral da Educação (DGE). Circular n.º3/DSEEAS/DGE/2013—Orientações sobre Ementas e Refeitórios Escolares—2013/2014; DGS: Lisboa, Portugal, 2013.
- Sousa, I.; Carvalho, R.; Mendes, I.; Prazeres, S.; César, R.; Limbert, E. Carência de iodo nas grávidas da Ilha de São Miguel, Açores: Avaliação do impacto das medidas corretivas. Rev. Port. Endocrinol. Diabetes Metab. 2020, 15, 142–145. [Google Scholar]
- Lopes-Pereira, M.; Quialheiro, A.; Costa, P.; Roque, S.; Correia Santos, N.; Correia-Neves, M.; Goios, A.; Carvalho, I.; Korevaar, T.I.M.; Vilarinho, L.; et al. Iodine supplementation: Compliance and association with adverse obstetric and neonatal outcomes. Eur. Thyroid. J. 2022, 11, e210035. [Google Scholar] [CrossRef]
- Costa Leite, J.; Keating, E.; Pestana, D.; Cruz Fernandes, V.; Maia, M.L.; Norberto, S.; Pinto, E.; Moreira-Rosario, A.; Sintra, D.; Moreira, B.; et al. Iodine Status and Iodised Salt Consumption in Portuguese School-Aged Children: The Iogeneration Study. Nutrients 2017, 9, 458. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.; Moniz, C.S.; Mendes, I.; Mendes, A.; Sousa, I. Iodine status, dietary iodine intake and iodized salt in school-aged children in São Miguel Island, Azores, Portugal. Nutrition 2022, 99–100, 111681. [Google Scholar] [CrossRef]
- Moniz, C.S.; Carvalho, R.; Prazeres, S.; Limbert, E.; Mendes, I.; César, R. Efficacy of a Salt Iodization Program on Iodine Status and Intakes in Schoolchildren of São Miguel Island, Azores, Portugal. Eur. Thyroid J. 2021, 10, 109–113. [Google Scholar] [CrossRef]
- da Silva, C.P. Awareness for Increased Iodine Consumption—Current Status and Future Perspectives. Acta Port. Nutr. 2022, 30, 24–29. [Google Scholar] [CrossRef]
- Lopes-Pereira, M.; Roque, S.; Costa, P.; Quialheiro, A.; Santos, N.C.; Goios, A.; Vilarinho, L.; Correia-Neves, M.; Palha, J.A. Impact of iodine supplementation during preconception, pregnancy and lactation on maternal thyroid homeostasis and offspring psychomotor development: Protocol of the IodineMinho prospective study. BMC Pregnancy Childbirth 2020, 20, 693. [Google Scholar] [CrossRef] [PubMed]
- Ministério da Saúde e Assistência; Gabinete do Ministro (Eds.) Diário do Governo n.º 226/1969, Série I de 1969-09-26. Decreto-Lei n.º 49271, de 26 de Setembro. 1969, p. 1318. Available online: https://files.dre.pt/1s/1969/09/22600/13181318.pdf (accessed on 18 October 2022).
- Lobato, C.B.; Machado, A.; Mesquita, R.B.R.; Lima, L.; Bordalo, A.A. Can non-fortified marine salt cover human needs for iodine? Int. J. Food Sci. Nutr. 2019, 70, 349–354. [Google Scholar] [CrossRef]
- Polonia, J.; Martins, L.; Pinto, F.; Nazare, J. Prevalence, awareness, treatment and control of hypertension and salt intake in Portugal: Changes over a decade. The PHYSA study. J. Hypertens. 2014, 32, 1211–1221. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Guideline: Sodium Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Abreu, D.; Sousa, P.; Matias-Dias, C.; Pinto, F.J. Cardiovascular disease and high blood pressure trend analyses from 2002 to 2016: After the implementation of a salt reduction strategy. BMC Public Health 2018, 18, 722. [Google Scholar] [CrossRef] [PubMed]
- Quilez, J.; Salas-Salvado, J. Salt in bread in Europe: Potential benefits of reduction. Nutr. Rev. 2012, 70, 666–678. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Regional Office for Europe & McColl, K. Meeting of the WHO Action Network on Salt Reduction in the Population in the European Region (ESAN) 20–21 April 2016 Lisbon, Portugal: Meeting Report; World Health Organization: Geneva, Switzerland; Regional Office for Europe: Copenhagen, Denmark, 2016. [Google Scholar]
- Nista, F.; Bagnasco, M.; Gatto, F.; Albertelli, M.; Vera, L.; Boschetti, M.; Musso, N.; Ferone, D. The effect of sodium restriction on iodine prophylaxis: A review. J. Endocrinol. Investig. 2022, 45, 1121–1138. [Google Scholar] [CrossRef]
- Direção-Geral da Saúde (DGS). Programa Nacional para a Promoção da Alimentação Saudável. In Programa Nacional para a Promoção da Alimentação Saudável—Orientações Programáticas; Direção-Geral da Saúde: Lisboa, Portugal, 2012. [Google Scholar]
- Wang, D.; Thielecke, F.; Fleith, M.; Afeiche, M.C.; De Castro, C.A.; Martínez-Costa, C.; Haaland, K.; Marchini, G.; Agosti, M.; Domellöf, M.; et al. Analysis of dietary patterns and nutritional adequacy in lactating women: A multicentre European cohort (ATLAS study). J. Nutr. Sci. 2021, 10, e17. [Google Scholar] [CrossRef]
- Ferreira, P.; Pinheiro, C.; Matta Coelho, C.; Guimarães, J.; Pereira, G.; Xavier Moreira, N.; Cortez, A.; Bracchi, I.; Pestana, D.; Barreiros Mota, I.; et al. The association of milk and dairy consumption with iodine status in pregnant women in Oporto region. Br. J. Nutr. 2021, 126, 1314–1322. [Google Scholar] [CrossRef]
- Delgado, I.; Ventura, M.; Gueifão, S.; Assunção, R.; Coelho, I.; Bento, A.; Silva, J.A.L.; Castanheira, I. Studying iodine intake of Portuguese children school meals. J. Food Compos. Anal. 2023, 116, 105061. [Google Scholar] [CrossRef]
- Eveleigh, E.R.; Coneyworth, L.J.; Avery, A.; Welham, S.J.M. Vegans, Vegetarians, and Omnivores: How Does Dietary Choice Influence Iodine Intake? A Systematic Review. Nutrients 2020, 12, 1606. [Google Scholar] [CrossRef] [PubMed]
- Iacone, R.; Iaccarino Idelson, P.; Russo, O.; Donfrancesco, C.; Krogh, V.; Sieri, S.; Macchia, P.E.; Formisano, P.; Lo Noce, C.; Palmieri, L.; et al. Iodine Intake from Food and Iodized Salt as Related to Dietary Salt Consumption in the Italian Adult General Population. Nutrients 2021, 13, 3486. [Google Scholar] [CrossRef] [PubMed]
- Esche, J.; Thamm, M.; Remer, T. Contribution of iodized salt to total iodine and total salt intake in Germany. Eur. J. Nutr. 2020, 59, 3163–3169. [Google Scholar] [CrossRef] [PubMed]
- Maalouf, J.; Barron, J.; Gunn, J.P.; Yuan, K.; Perrine, C.G.; Cogswell, M.E. Iodized salt sales in the United States. Nutrients 2015, 7, 1691–1695. [Google Scholar] [CrossRef] [PubMed]
- Bath, S.C.; Button, S.; Rayman, M.P. Availability of iodised table salt in the UK—Is it likely to influence population iodine intake? Public Health Nutr. 2014, 17, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Verkaik-Kloosterman, J.; Buurma-Rethans, E.J.M.; Dekkers, A.L.M.; van Rossum, C.T.M. Decreased, but still sufficient, iodine intake of children and adults in the Netherlands. Br. J. Nutr. 2017, 117, 1020–1031. [Google Scholar] [CrossRef]
- Ministério da Saúde (Ed.) Diário da República Série I-A, n.º 152. Decreto-Lei n.º 87/96, de 3 de Julho. 1986. Available online: https://files.dre.pt/1s/1996/07/152a00/17081709.pdf (accessed on 12 October 2022).
- Dasgupta, P.K.; Liu, Y.; Dyke, J.V. Iodine nutrition: Iodine content of iodized salt in the United States. Environ. Sci. Technol. 2008, 42, 1315–1323. [Google Scholar] [CrossRef]
- Vila, L.; Lucas, A.; Donnay, S.; de la Vieja, A.; Wengrovicz, S.; Santiago, P.; Bandrés, O.; Velasco, I.; Garcia-Fuentes, E.; Ares, S.; et al. La nutrición de yodo en España. Necesidades para el futuro. Endocrinol. Diabetes Nutr. 2020, 67, 61–69. [Google Scholar] [CrossRef]
| Salt Type | No. of Salt Products | Sales (kg) | Sales (%) |
|---|---|---|---|
| Iodized salt | 3 | 2,815,956 | 4.1 |
| Coarse | 2 | 2,771,297 | 4.0 |
| Fine | 1 | 44,659 | 0.1 |
| Non-iodized salt | 30 | 66,299,501 | 95.9 |
| Coarse | 20 | 62,767,079 | 90.8 |
| Fine | 10 | 3,532,421 | 5.1 |
| Total | 33 | 69,133,401 | 100 |
| Percentage of Salt Sales in Total Salt Sales (%) | ||||||
|---|---|---|---|---|---|---|
| Year | Total Non-Iodized Salt | Coarse Non-Iodized Salt | Fine Non-Iodized Salt | Total Iodized Salt | Coarse Iodized Salt | Fine Iodized Salt |
| 2010 | 99.95 | 94.62 | 5.33 | 0.05 | 0.05 | 0.01 |
| 2011 | 99.94 | 94.65 | 5.29 | 0.06 | 0.05 | 0.01 |
| 2012 | 99.94 | 94.88 | 5.06 | 0.06 | 0.05 | 0.01 |
| 2013 | 99.74 | 94.89 | 4.85 | 0.26 | 0.24 | 0.02 |
| 2014 | 99.39 | 94.51 | 4.89 | 0.61 | 0.56 | 0.04 |
| 2015 | 99.07 | 94.23 | 4.85 | 0.93 | 0.87 | 0.05 |
| 2016 | 98.45 | 93.65 | 4.80 | 1.55 | 1.48 | 0.07 |
| 2017 | 95.41 | 90.72 | 4.68 | 4.59 | 4.52 | 0.08 |
| 2018 | 91.03 | 86.57 | 4.46 | 8.97 | 8.86 | 0.11 |
| 2019 | 90.70 | 85.65 | 5.05 | 9.30 | 9.18 | 0.12 |
| 2020 | 89.65 | 83.73 | 5.92 | 10.35 | 10.22 | 0.13 |
| 2021 | 89.08 | 82.72 | 6.36 | 10.92 | 10.80 | 0.12 |
| Year | Population Older than Four Years of Age | Estimates of Salt per Capita (g/day) | Estimates of Iodine per Capita (μg/day) |
|---|---|---|---|
| 2010 | 9,579,729 | 6.0 | 0.1 |
| 2011 | 9,576,046 | 6.2 | 0.1 |
| 2012 | 9,543,700 | 7.2 | 0.1 |
| 2013 | 9,499,685 | 7.6 | 0.5 |
| 2014 | 9,460,800 | 7.3 | 1.0 |
| 2015 | 9,433,777 | 7.7 | 1.6 |
| 2016 | 9,413,499 | 7.2 | 2.6 |
| 2017 | 9,395,108 | 7.0 | 7.4 |
| 2018 | 9,379,021 | 6.8 | 14.5 |
| 2019 | 9,376,734 | 6.9 | 15.2 |
| 2020 | 9,437,325 | 6.9 | 16.9 |
| 2021 | 9,484,013 | 6.8 | 17.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, S.I.; Pereira, M.L.; Roque, S.; Costeira, M.J.; Bordalo, A.A.; Miranda, A.; Costa, P.; Borges, N.; Palha, J.A. Iodine Availability through Iodized Salt in Portugal: 2010–2021 Sales Evolution and Distribution. Nutrients 2023, 15, 1324. https://doi.org/10.3390/nu15061324
Machado SI, Pereira ML, Roque S, Costeira MJ, Bordalo AA, Miranda A, Costa P, Borges N, Palha JA. Iodine Availability through Iodized Salt in Portugal: 2010–2021 Sales Evolution and Distribution. Nutrients. 2023; 15(6):1324. https://doi.org/10.3390/nu15061324
Chicago/Turabian StyleMachado, Sarai Isabel, Maria Lopes Pereira, Susana Roque, Maria José Costeira, Adriano A. Bordalo, André Miranda, Patrício Costa, Nuno Borges, and Joana Almeida Palha. 2023. "Iodine Availability through Iodized Salt in Portugal: 2010–2021 Sales Evolution and Distribution" Nutrients 15, no. 6: 1324. https://doi.org/10.3390/nu15061324
APA StyleMachado, S. I., Pereira, M. L., Roque, S., Costeira, M. J., Bordalo, A. A., Miranda, A., Costa, P., Borges, N., & Palha, J. A. (2023). Iodine Availability through Iodized Salt in Portugal: 2010–2021 Sales Evolution and Distribution. Nutrients, 15(6), 1324. https://doi.org/10.3390/nu15061324







