Association between Serum Magnesium and Fractures: A Systematic Review and Meta-Analysis of Observational Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Searches
2.2. Study Selection
2.3. Data Extraction
2.4. Outcomes
2.5. Quality Assessment
2.6. Data Synthesis and Analysis
3. Results
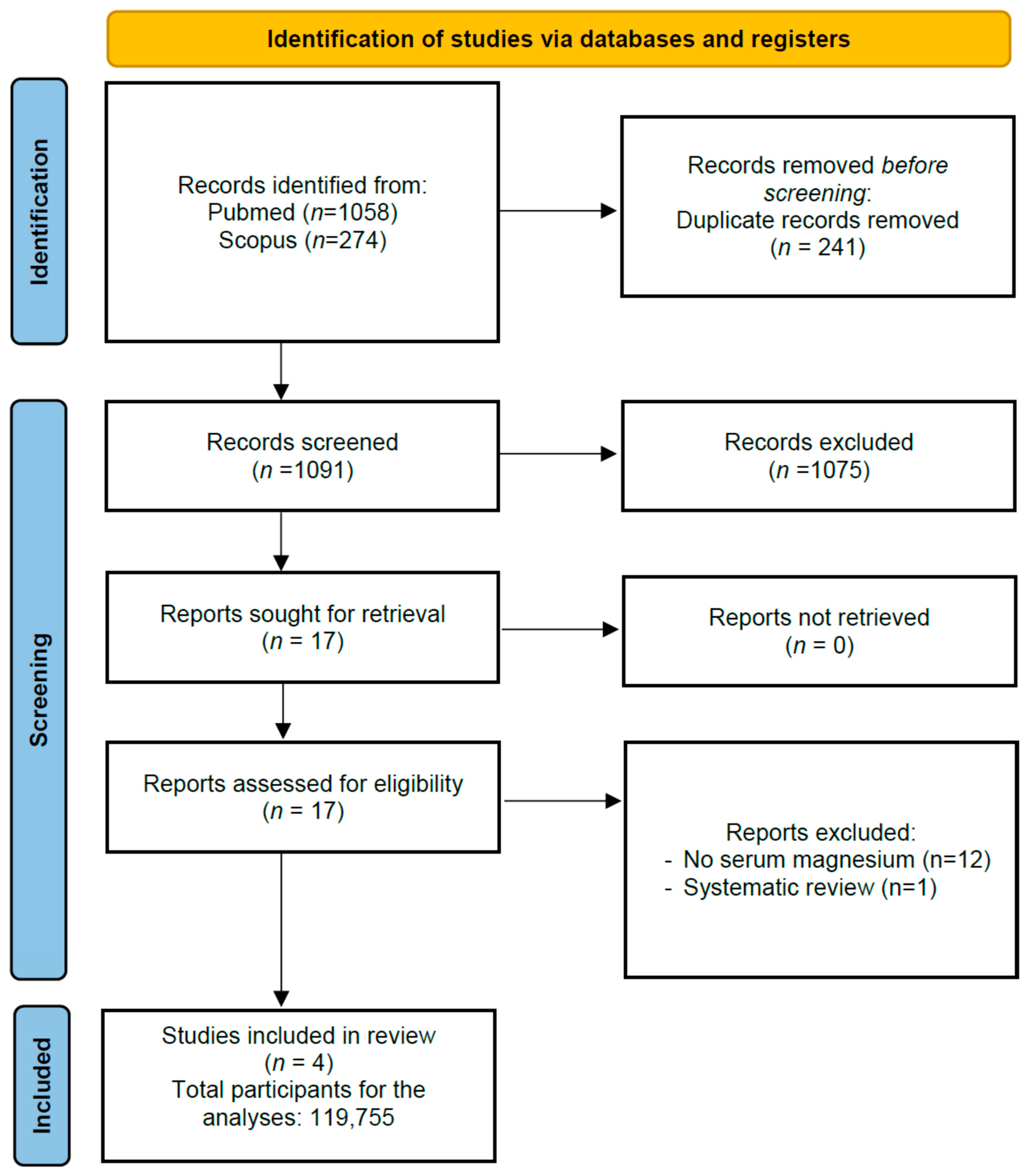
3.1. Search Results
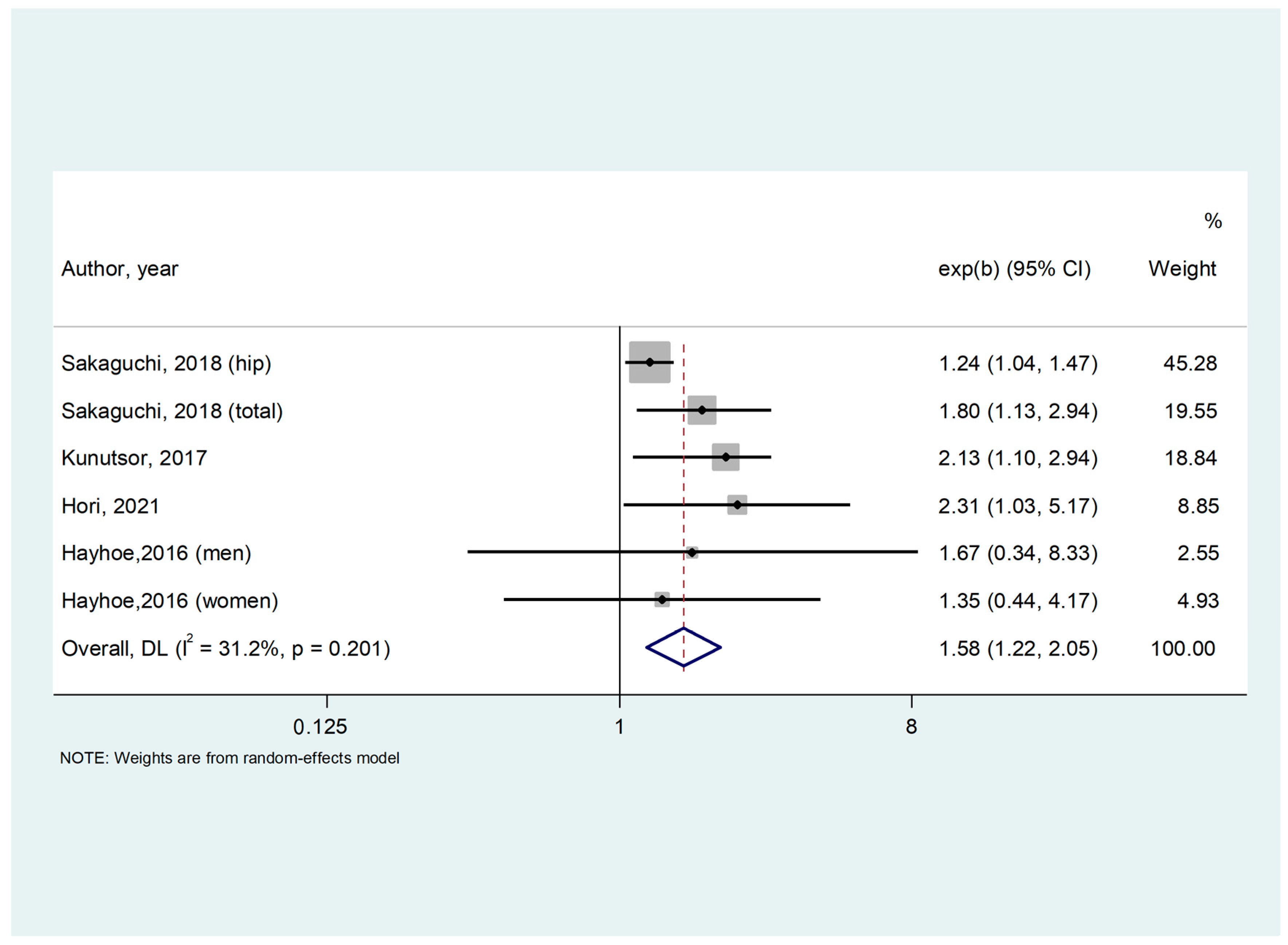
3.2. Study and Patient Characteristics; Meta-Analysis
3.3. Risk of Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lorentzon, M.; Abrahamsen, B. Osteoporosis epidemiology using international cohorts. Curr. Opin. Rheumatol. 2022, 34, 280–288. [Google Scholar] [CrossRef]
- Borgstrom, F.; Karlsson, L.; Ortsater, G.; Norton, N.; Halbout, P.; Cooper, C.; Lorentzon, M.; McCloskey, E.V.; Harvey, N.C.; Javaid, M.K.; et al. Fragility fractures in Europe: Burden, management and opportunities. Arch. Osteoporos. 2020, 15, 59. [Google Scholar] [CrossRef]
- Kanis, J.A.; Norton, N.; Harvey, N.C.; Jacobson, T.; Johansson, H.; Lorentzon, M.; McCloskey, E.V.; Willers, C.; Borgstrom, F. SCOPE 2021: A new scorecard for osteoporosis in Europe. Arch. Osteoporos. 2021, 16, 82. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.; Cole, Z.A.; Holroyd, C.R.; Earl, S.C.; Harvey, N.C.; Dennison, E.M.; Melton, L.J.; Cummings, S.R.; Kanis, J.A. Secular trends in the incidence of hip and other osteoporotic fractures. Osteoporos. Int. 2011, 22, 1277–1288. [Google Scholar] [CrossRef] [PubMed]
- Oden, A.; McCloskey, E.V.; Kanis, J.A.; Harvey, N.C.; Johansson, H. Burden of high fracture probability worldwide: Secular increases 2010–2040. Osteoporos. Int. 2015, 26, 2243–2248. [Google Scholar] [CrossRef]
- Beard, J.R.; Officer, A.; de Carvalho, I.A.; Sadana, R.; Pot, A.M.; Michel, J.P.; Lloyd-Sherlock, P.; Epping-Jordan, J.E.; Peeters, G.; Mahanani, W.R.; et al. The World report on ageing and health: A policy framework for healthy ageing. Lancet 2016, 387, 2145–2154. [Google Scholar] [CrossRef]
- Johnell, O.; Kanis, J.A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 2006, 17, 1726–1733. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Faliva, M.A.; Barrile, G.C.; Cavioni, A.; Mansueto, F.; Mazzola, G.; Oberto, L.; Patelli, Z.; Pirola, M.; Tartara, A.; et al. Nutrition, Physical Activity, and Dietary Supplementation to Prevent Bone Mineral Density Loss: A Food Pyramid. Nutrients 2021, 14, 74. [Google Scholar] [CrossRef]
- Rude, R.K.; Gruber, H.E.; Norton, H.J.; Wei, L.Y.; Frausto, A.; Kilburn, J. Reduction of dietary magnesium by only 50% in the rat disrupts bone and mineral metabolism. Osteoporos. Int. 2006, 17, 1022–1032. [Google Scholar] [CrossRef]
- Rude, R.K.; Gruber, H.E.; Norton, H.J.; Wei, L.Y.; Frausto, A.; Mills, B.G. Bone loss induced by dietary magnesium reduction to 10% of the nutrient requirement in rats is associated with increased release of substance P and tumor necrosis factor-alpha. J. Nutr. 2004, 134, 79–85. [Google Scholar] [CrossRef]
- Rude, R.K.; Singer, F.R.; Gruber, H.E. Skeletal and hormonal effects of magnesium deficiency. J. Am. Coll. Nutr. 2009, 28, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Luthringer, B.J.; Feyerabend, F.; Schilling, A.F.; Willumeit, R. Effects of extracellular magnesium on the differentiation and function of human osteoclasts. Acta Biomater. 2014, 10, 2843–2854. [Google Scholar] [CrossRef] [PubMed]
- Belluci, M.M.; Schoenmaker, T.; Rossa-Junior, C.; Orrico, S.R.; de Vries, T.J.; Everts, V. Magnesium deficiency results in an increased formation of osteoclasts. J. Nutr. Biochem. 2013, 24, 1488–1498. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Kim, K.J.; Cheon, S.; Kim, E.M.; Kim, Y.A.; Park, C.; Kim, K.K. Biochemical activity of magnesium ions on human osteoblast migration. Biochem. Biophys. Res. Commun. 2020, 531, 588–594. [Google Scholar] [CrossRef]
- Wu, L.; Feyerabend, F.; Schilling, A.F.; Willumeit-Romer, R.; Luthringer, B.J.C. Effects of extracellular magnesium extract on the proliferation and differentiation of human osteoblasts and osteoclasts in coculture. Acta Biomater. 2015, 27, 294–304. [Google Scholar] [CrossRef]
- Barbagallo, M.; Veronese, N.; Dominguez, L.J. Magnesium in Type 2 Diabetes Mellitus, Obesity, and Metabolic Syndrome. Nutrients 2022, 14, 714. [Google Scholar] [CrossRef]
- Barbagallo, M.; Dominguez, L.J.; Galioto, A.; Ferlisi, A.; Cani, C.; Malfa, L.; Pineo, A.; Busardo, A.; Paolisso, G. Role of magnesium in insulin action, diabetes and cardio-metabolic syndrome X. Mol. Aspects Med. 2003, 24, 39–52. [Google Scholar] [CrossRef]
- Orchard, T.S.; Larson, J.C.; Alghothani, N.; Bout-Tabaku, S.; Cauley, J.A.; Chen, Z.; LaCroix, A.Z.; Wactawski-Wende, J.; Jackson, R.D. Magnesium intake, bone mineral density, and fractures: Results from the Women’s Health Initiative Observational Study. Am. J. Clin. Nutr. 2014, 99, 926–933. [Google Scholar] [CrossRef]
- Tucker, K.L.; Hannan, M.T.; Chen, H.; Cupples, L.A.; Wilson, P.W.; Kiel, D.P. Potassium, magnesium, and fruit and vegetable intakes are associated with greater bone mineral density in elderly men and women. Am. J. Clin. Nutr. 1999, 69, 727–736. [Google Scholar] [CrossRef]
- Groenendijk, I.; van Delft, M.; Versloot, P.; van Loon, L.J.C.; de Groot, L. Impact of magnesium on bone health in older adults: A systematic review and meta-analysis. Bone 2022, 154, 116233. [Google Scholar] [CrossRef]
- Ryder, K.M.; Shorr, R.I.; Bush, A.J.; Kritchevsky, S.B.; Harris, T.; Stone, K.; Cauley, J.; Tylavsky, F.A. Magnesium intake from food and supplements is associated with bone mineral density in healthy older white subjects. J. Am. Geriatr. Soc. 2005, 53, 1875–1880. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Stubbs, B.; Solmi, M.; Noale, M.; Vaona, A.; Demurtas, J.; Maggi, S. Dietary magnesium intake and fracture risk: Data from a large prospective study. Br. J. Nutr. 2017, 117, 1570–1576. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef] [PubMed]
- Luchini, C.; Stubbs, B.; Solmi, M.; Veronese, N. Assessing the quality of studies in meta-analyses: Advantages and limitations of the Newcastle Ottawa Scale. World J. Meta-Anal. 2017, 5, 80–84. [Google Scholar] [CrossRef]
- Luchini, C.; Veronese, N.; Nottegar, A.; Shin, J.I.; Gentile, G.; Granziol, U.; Soysal, P.; Alexinschi, O.; Smith, L. Assessing the quality of studies in meta-research: Review/guidelines on the most important quality assessment tools. Pharm. Stat. 2021, 20, 185–195. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. Bmj 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef]
- Hayhoe, R.P.; Lentjes, M.A.; Luben, R.N.; Khaw, K.T.; Welch, A.A. Dietary magnesium and potassium intakes and circulating magnesium are associated with heel bone ultrasound attenuation and osteoporotic fracture risk in the EPIC-Norfolk cohort study. Am. J. Clin. Nutr. 2015, 102, 376–384. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Whitehouse, M.R.; Blom, A.W.; Laukkanen, J.A. Low serum magnesium levels are associated with increased risk of fractures: A long-term prospective cohort study. Eur. J. Epidemiol. 2017, 32, 593–603. [Google Scholar] [CrossRef]
- Sakaguchi, Y.; Hamano, T.; Wada, A.; Hoshino, J.; Masakane, I. Magnesium and Risk of Hip Fracture among Patients Undergoing Hemodialysis. J. Am. Soc. Nephrol. 2018, 29, 991–999. [Google Scholar] [CrossRef]
- Hori, M.; Yasuda, K.; Takahashi, H.; Yamazaki, C.; Morozumi, K.; Maruyama, S. Impact of serum magnesium and bone mineral density on systemic fractures in chronic hemodialysis patients. PLoS ONE 2021, 16, e0251912. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; van der Velde, M.; Astor, B.C.; Woodward, M.; Levey, A.S.; de Jong, P.E.; Coresh, J.; Gansevoort, R.T. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 2010, 375, 2073–2081. [Google Scholar] [CrossRef] [PubMed]
- Leenders, N.H.J.; Vervloet, M.G. Magnesium: A Magic Bullet for Cardiovascular Disease in Chronic Kidney Disease? Nutrients 2019, 11, 455. [Google Scholar] [CrossRef] [PubMed]
- Zelt, J.G.; McCabe, K.M.; Svajger, B.; Barron, H.; Laverty, K.; Holden, R.M.; Adams, M.A. Magnesium Modifies the Impact of Calcitriol Treatment on Vascular Calcification in Experimental Chronic Kidney Disease. J. Pharmacol. Exp. Ther. 2015, 355, 451–462. [Google Scholar] [CrossRef]
- Blacher, J.; Guerin, A.P.; Pannier, B.; Marchais, S.J.; London, G.M. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension 2001, 38, 938–942. [Google Scholar] [CrossRef]
- London, G.M.; Guerin, A.P.; Marchais, S.J.; Metivier, F.; Pannier, B.; Adda, H. Arterial media calcification in end-stage renal disease: Impact on all-cause and cardiovascular mortality. Nephrol. Dial Transplant. 2003, 18, 1731–1740. [Google Scholar] [CrossRef]
- Peeters, M.J.; van den Brand, J.A.; van Zuilen, A.D.; Koster, Y.; Bots, M.L.; Vervloet, M.G.; Blankestijn, P.J.; Wetzels, J.F. Abdominal aortic calcification in patients with CKD. J. Nephrol. 2017, 30, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Toussaint, N.D.; Lau, K.K.; Strauss, B.J.; Polkinghorne, K.R.; Kerr, P.G. Determination and validation of aortic calcification measurement from lateral bone densitometry in dialysis patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 119–127. [Google Scholar] [CrossRef]
- Chen, J.; Budoff, M.J.; Reilly, M.P.; Yang, W.; Rosas, S.E.; Rahman, M.; Zhang, X.; Roy, J.A.; Lustigova, E.; Nessel, L.; et al. Coronary Artery Calcification and Risk of Cardiovascular Disease and Death among Patients with Chronic Kidney Disease. JAMA Cardiol. 2017, 2, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Barreto, D.V.; Barreto Fde, C.; Carvalho, A.B.; Cuppari, L.; Draibe, S.A.; Dalboni, M.A.; Moyses, R.M.; Neves, K.R.; Jorgetti, V.; Miname, M.; et al. Association of changes in bone remodeling and coronary calcification in hemodialysis patients: A prospective study. Am. J. Kidney Dis. 2008, 52, 1139–1150. [Google Scholar] [CrossRef]
- London, G.M.; Marchais, S.J.; Guerin, A.P.; Boutouyrie, P.; Metivier, F.; de Vernejoul, M.C. Association of bone activity, calcium load, aortic stiffness, and calcifications in ESRD. J. Am. Soc. Nephrol. 2008, 19, 1827–1835. [Google Scholar] [CrossRef]
- London, G.M.; Marty, C.; Marchais, S.J.; Guerin, A.P.; Metivier, F.; de Vernejoul, M.C. Arterial calcifications and bone histomorphometry in end-stage renal disease. J. Am. Soc. Nephrol. 2004, 15, 1943–1951. [Google Scholar] [CrossRef] [PubMed]
- Louvet, L.; Buchel, J.; Steppan, S.; Passlick-Deetjen, J.; Massy, Z.A. Magnesium prevents phosphate-induced calcification in human aortic vascular smooth muscle cells. Nephrol. Dial Transplant. 2013, 28, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Villa-Bellosta, R. Impact of magnesium:calcium ratio on calcification of the aortic wall. PLoS ONE 2017, 12, e0178872. [Google Scholar] [CrossRef]
- Mortazavi, M.; Moeinzadeh, F.; Saadatnia, M.; Shahidi, S.; McGee, J.C.; Minagar, A. Effect of magnesium supplementation on carotid intima-media thickness and flow-mediated dilatation among hemodialysis patients: A double-blind, randomized, placebo-controlled trial. Eur. Neurol. 2013, 69, 309–316. [Google Scholar] [CrossRef]
- Bressendorff, I.; Hansen, D.; Schou, M.; Pasch, A.; Brandi, L. The Effect of Increasing Dialysate Magnesium on Serum Calcification Propensity in Subjects with End Stage Kidney Disease: A Randomized, Controlled Clinical Trial. Clin. J. Am. Soc. Nephrol. 2018, 13, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Bressendorff, I.; Hansen, D.; Schou, M.; Silver, B.; Pasch, A.; Bouchelouche, P.; Pedersen, L.; Rasmussen, L.M.; Brandi, L. Oral Magnesium Supplementation in Chronic Kidney Disease Stages 3 and 4: Efficacy, Safety, and Effect on Serum Calcification Propensity-A Prospective Randomized Double-Blinded Placebo-Controlled Clinical Trial. Kidney Int. Rep. 2017, 2, 380–389. [Google Scholar] [CrossRef]
- Farsinejad-Marj, M.; Saneei, P.; Esmaillzadeh, A. Dietary magnesium intake, bone mineral density and risk of fracture: A systematic review and meta-analysis. Osteoporos. Int. 2016, 27, 1389–1399. [Google Scholar] [CrossRef]
- Castiglioni, S.; Cazzaniga, A.; Albisetti, W.; Maier, J.A. Magnesium and osteoporosis: Current state of knowledge and future research directions. Nutrients 2013, 5, 3022–3033. [Google Scholar] [CrossRef]
- Aydin, H.; Deyneli, O.; Yavuz, D.; Gozu, H.; Mutlu, N.; Kaygusuz, I.; Akalin, S. Short-term oral magnesium supplementation suppresses bone turnover in postmenopausal osteoporotic women. Biol. Trace Elem. Res. 2010, 133, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Yu, D.; Ji, J.; Wang, N.; Yu, S.; Yu, B. The Association between the Concentration of Serum Magnesium and Postmenopausal Osteoporosis. Front. Med. 2020, 7, 381. [Google Scholar] [CrossRef]
- Rude, R.K.; Adams, J.S.; Ryzen, E.; Endres, D.B.; Niimi, H.; Horst, R.L.; Haddad, J.G., Jr.; Singer, F.R. Low serum concentrations of 1,25-dihydroxyvitamin D in human magnesium deficiency. J. Clin. Endocrinol. Metab. 1985, 61, 933–940. [Google Scholar] [CrossRef]
- Reddy, V.; Sivakumar, B. Magnesium-dependent vitamin-D-resistant rickets. Lancet 1974, 1, 963–965. [Google Scholar] [CrossRef] [PubMed]
- Uwitonze, A.M.; Razzaque, M.S. Role of Magnesium in Vitamin D Activation and Function. J. Am. Osteopath. Assoc. 2018, 118, 181–189. [Google Scholar] [CrossRef]
- Zittermann, A. Magnesium deficit? Overlooked cause of low vitamin D status? BMC Med. 2013, 11, 229. [Google Scholar] [CrossRef] [PubMed]
- Risco, F.; Traba, M.L. Influence of magnesium on the in vitro synthesis of 24,25-dihydroxyvitamin D3 and 1 alpha, 25-dihydroxyvitamin D3. Magnes Res. 1992, 5, 5–14. [Google Scholar]
- Anast, C.S.; Mohs, J.M.; Kaplan, S.L.; Burns, T.W. Evidence for parathyroid failure in magnesium deficiency. Science 1972, 177, 606–608. [Google Scholar] [CrossRef]
- Medalle, R.; Waterhouse, C.; Hahn, T.J. Vitamin D resistance in magnesium deficiency. Am. J. Clin. Nutr. 1976, 29, 854–858. [Google Scholar] [CrossRef]
- Rude, R.K.; Oldham, S.B.; Sharp, C.F., Jr.; Singer, F.R. Parathyroid hormone secretion in magnesium deficiency. J. Clin. Endocrinol. Metab. 1978, 47, 800–806. [Google Scholar] [CrossRef]
- Mutnuri, S.; Fernandez, I.; Kochar, T. Suppression of Parathyroid Hormone in a Patient with Severe Magnesium Depletion. Case Rep. Nephrol. 2016, 2016, 2608538. [Google Scholar] [CrossRef]
- Rude, R.K.; Oldham, S.B.; Singer, F.R. Functional hypoparathyroidism and parathyroid hormone end-organ resistance in human magnesium deficiency. Clin. Endocrinol. 1976, 5, 209–224. [Google Scholar] [CrossRef]
- Rosler, A.; Rabinowitz, D. Magnesium-induced reversal of vitamin-D resistance in hypoparathyroidism. Lancet 1973, 1, 803–804. [Google Scholar] [CrossRef]
- Fuss, M.; Bergmann, P.; Bergans, A.; Bagon, J.; Cogan, E.; Pepersack, T.; Van Gossum, M.; Corvilain, J. Correction of low circulating levels of 1,25-dihydroxyvitamin D by 25-hydroxyvitamin D during reversal of hypomagnesaemia. Clin. Endocrinol. 1989, 31, 31–38. [Google Scholar] [CrossRef]
- Bussiere, F.I.; Tridon, A.; Zimowska, W.; Mazur, A.; Rayssiguier, Y. Increase in complement component C3 is an early response to experimental magnesium deficiency in rats. Life Sci. 2003, 73, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Mazur, A.; Maier, J.A.; Rock, E.; Gueux, E.; Nowacki, W.; Rayssiguier, Y. Magnesium and the inflammatory response: Potential physiopathological implications. Arch. Biochem. Biophys. 2007, 458, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Malpuech-Brugere, C.; Nowacki, W.; Daveau, M.; Gueux, E.; Linard, C.; Rock, E.; Lebreton, J.; Mazur, A.; Rayssiguier, Y. Inflammatory response following acute magnesium deficiency in the rat. Biochim. Biophys. Acta 2000, 1501, 91–98. [Google Scholar] [CrossRef]
- Galland, L. Magnesium and immune function: An overview. Magnesium 1988, 7, 290–299. [Google Scholar] [PubMed]
- Zhou, F.; Zhang, G.; Wu, Y.; Xiong, Y. Inflammasome Complexes: Crucial mediators in osteoimmunology and bone diseases. Int. Immunopharmacol. 2022, 110, 109072. [Google Scholar] [CrossRef] [PubMed]
- Antoniac, I.; Miculescu, M.; Manescu Paltanea, V.; Stere, A.; Quan, P.H.; Paltanea, G.; Robu, A.; Earar, K. Magnesium-Based Alloys Used in Orthopedic Surgery. Materials 2022, 15, 1148. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Ward, R.E.; Orkaby, A.R.; Chen, J.; Hshieh, T.T.; Driver, J.A.; Gaziano, J.M.; Djousse, L. Association between Diet Quality and Frailty Prevalence in the Physicians’ Health Study. J. Am. Geriatr. Soc. 2020, 68, 770–776. [Google Scholar] [CrossRef]
- Erem, S.; Atfi, A.; Razzaque, M.S. Anabolic effects of vitamin D and magnesium in aging bone. J. Steroid. Biochem. Mol. Biol. 2019, 193, 105400. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Pizzol, D.; Smith, L.; Dominguez, L.J.; Barbagallo, M. Effect of Magnesium Supplementation on Inflammatory Parameters: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 679. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, T.Y.; van Dam, R.M.; Manson, J.E.; Hu, F.B. Magnesium intake and plasma concentrations of markers of systemic inflammation and endothelial dysfunction in women. Am. J. Clin. Nutr. 2007, 85, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- King, D.E.; Mainous, A.G., 3rd; Geesey, M.E.; Woolson, R.F. Dietary magnesium and C-reactive protein levels. J. Am. Coll. Nutr. 2005, 24, 166–171. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Bermudez-Pena, C.; Rodriguez-Moran, M. Severe hypomagnesemia and low-grade inflammation in metabolic syndrome. Magnes Res. 2011, 24, 45–53. [Google Scholar] [CrossRef]
- Song, Y.; Ridker, P.M.; Manson, J.E.; Cook, N.R.; Buring, J.E.; Liu, S. Magnesium intake, C-reactive protein, and the prevalence of metabolic syndrome in middle-aged and older U.S. women. Diabetes Care 2005, 28, 1438–1444. [Google Scholar] [CrossRef]
- Konstari, S.; Sares-Jaske, L.; Heliovaara, M.; Rissanen, H.; Knekt, P.; Arokoski, J.; Sundvall, J.; Karppinen, J. Dietary magnesium intake, serum high sensitivity C-reactive protein and the risk of incident knee osteoarthritis leading to hospitalization-A cohort study of 4953 Finns. PLoS ONE 2019, 14, e0214064. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services; U.S. Department of Agriculture; U.S. Dietary Guidelines Advisory Committee. Dietary Guidelines for Americans, 2015–2020, 8th ed.; For Sale by the Superintendent of Documents; U.S. Government Printing Office: Washington, DC, USA, 2015; p. xvii. 122p.
- Workinger, J.L.; Doyle, R.P.; Bortz, J. Challenges in the Diagnosis of Magnesium Status. Nutrients 2018, 10, 1202. [Google Scholar] [CrossRef]
- Dominguez, L.; Veronese, N.; Barbagallo, M. Magnesium and Hypertension in Old Age. Nutrients 2020, 13, 139. [Google Scholar] [CrossRef] [PubMed]
| Author, Year | Country | Study Design | Sample Size | Condition | Mean Age (SD) | Females (%) | Mean Follow-Up (Months) | Type of Fractures | Confounders | Newcastle–Ottawa Scale |
|---|---|---|---|---|---|---|---|---|---|---|
| Hayhoe, 2016 [29] | Multicentric Europe | Population-based Prospective Cohort | 3469 | General population | 62.5 (9.3) | 57.9 | 161 | Total | Age, BMI, smoking status, physical activity, family history of osteoporosis, menopausal and HRT status in women, corticosteroid use. | 9 |
| Kunutsor, 2017 [30] | Finland | Population-based Prospective Cohort | 2245 | General population | 53.1 (5) | 0 | 69 | Hip | Age, BMI, height, systolic BP, smoking, history of diabetes, alcohol consumption, physical activity, estimated GFR, SS, total energy intake, serum zinc, serum potassium, and serum ionized calcium | 9 |
| Sakaguchi, 2018 [31] | Japan | Observational, Cohort Study | 113,683 | Hemodyalisis | 64.9 (12.3) | 37.5 | 24 | Hip and total | Age, sex, BMI, dialysis duration, urea reduction rate, dialysis vintage, physical activity, DM, Ca, P, ALP, intact PTH, albumin, CRP, hemoglobin, past history of CVD (MI, cerebral infarction, cerebral hemorrhage, and amputation), medication (Ca carbonate, sevelamer hydrochloride, lanthanum carbonate, active vitamin D analog [i.v. and p.o.], and cinacalcet hydrochloride), history of parathyroidectomy | 9 |
| Hori, 2021 [32] | Japan | Retrospective | 358 | Hemodyalisis | 65.6 (14.3) | 36 | 36 | Total | Age, BMI, HD duration, past incident fracture, use of phosphate binders, total hip BMD | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dominguez, L.J.; Veronese, N.; Ciriminna, S.; Pérez-Albela, J.L.; Vásquez-López, V.F.; Rodas-Regalado, S.; Di Bella, G.; Parisi, A.; Tagliaferri, F.; Barbagallo, M. Association between Serum Magnesium and Fractures: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2023, 15, 1304. https://doi.org/10.3390/nu15061304
Dominguez LJ, Veronese N, Ciriminna S, Pérez-Albela JL, Vásquez-López VF, Rodas-Regalado S, Di Bella G, Parisi A, Tagliaferri F, Barbagallo M. Association between Serum Magnesium and Fractures: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients. 2023; 15(6):1304. https://doi.org/10.3390/nu15061304
Chicago/Turabian StyleDominguez, Ligia J., Nicola Veronese, Stefano Ciriminna, José Luis Pérez-Albela, Vania Flora Vásquez-López, Santiago Rodas-Regalado, Giovanna Di Bella, Angela Parisi, Federica Tagliaferri, and Mario Barbagallo. 2023. "Association between Serum Magnesium and Fractures: A Systematic Review and Meta-Analysis of Observational Studies" Nutrients 15, no. 6: 1304. https://doi.org/10.3390/nu15061304
APA StyleDominguez, L. J., Veronese, N., Ciriminna, S., Pérez-Albela, J. L., Vásquez-López, V. F., Rodas-Regalado, S., Di Bella, G., Parisi, A., Tagliaferri, F., & Barbagallo, M. (2023). Association between Serum Magnesium and Fractures: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients, 15(6), 1304. https://doi.org/10.3390/nu15061304








