Integrated Analysis of Gut Microbiome and Liver Metabolome to Evaluate the Effects of Fecal Microbiota Transplantation on Lipopolysaccharide/D-galactosamine-Induced Acute Liver Injury in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Protocol
2.3. Determination of Liver Enzymes and Biochemical Indexes
2.4. Terminal Deoxynucleotidyl Transferase-Mediated Nucleotide Nick-End Labeling (TUNEL) Assay
2.5. Histology and Immunohistochemistry
2.6. Microbial 16S rRNA Gene Sequencing Analysis
2.7. Metabonomics
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
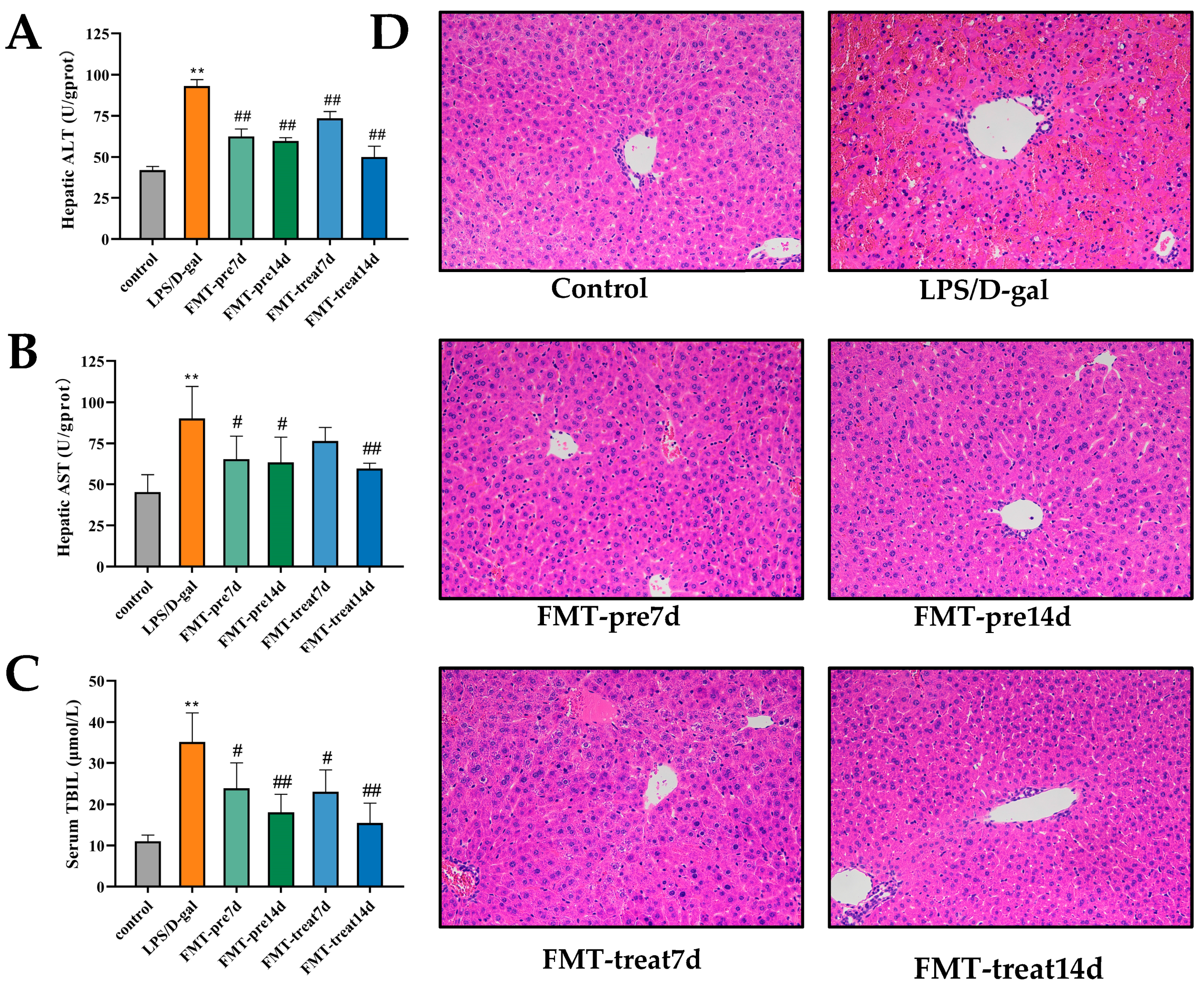
3.1. FMT Relieved LPS/D-Gal-Induced Liver Injury in Mice
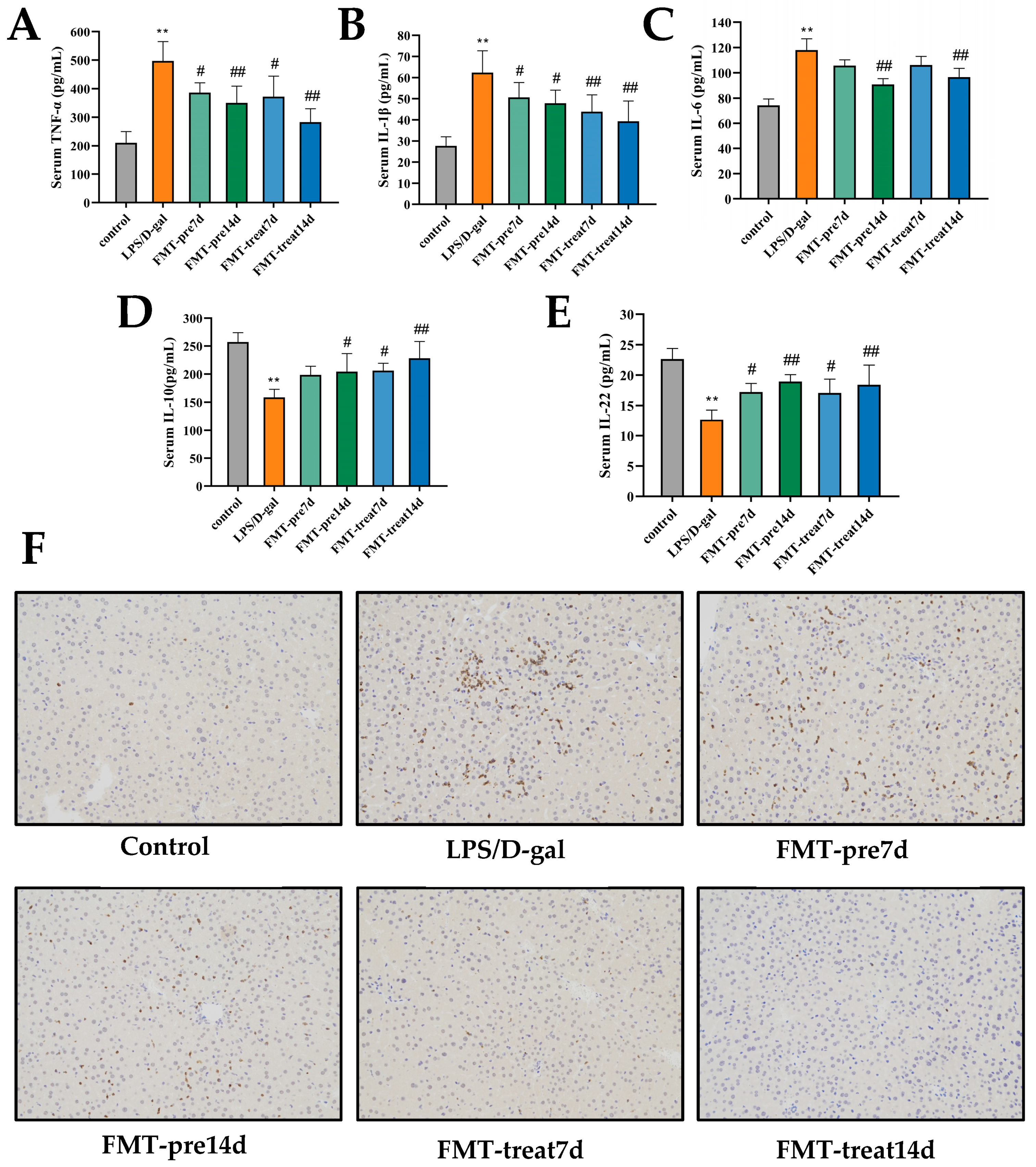
3.2. FMT Ameliorated Inflammatory Response in LPS/D-Gal-Challenged Mice
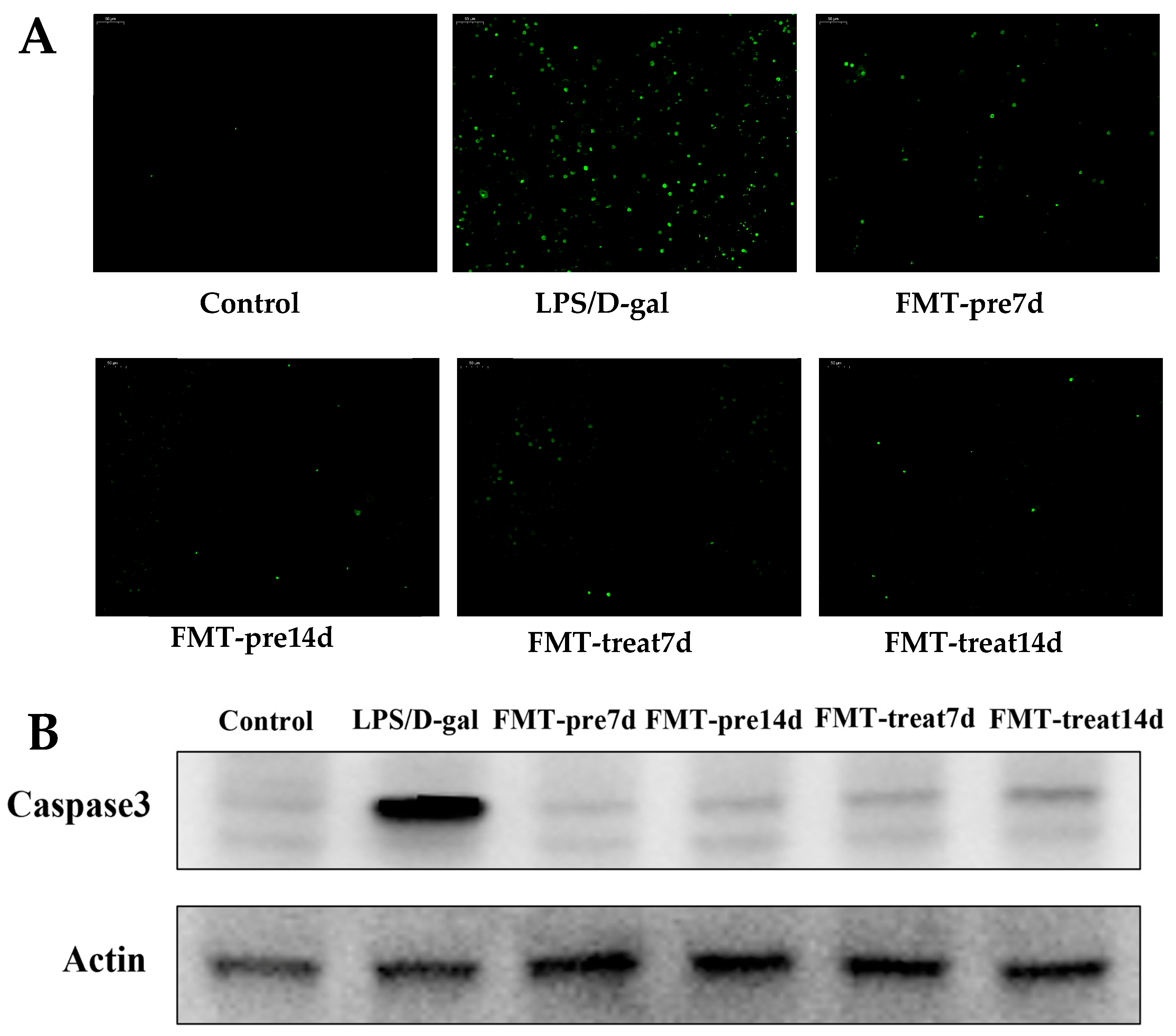
3.3. FMT Inhibited Apoptosis of Hepatocytes in LPS/D-Gal-Induced Mice
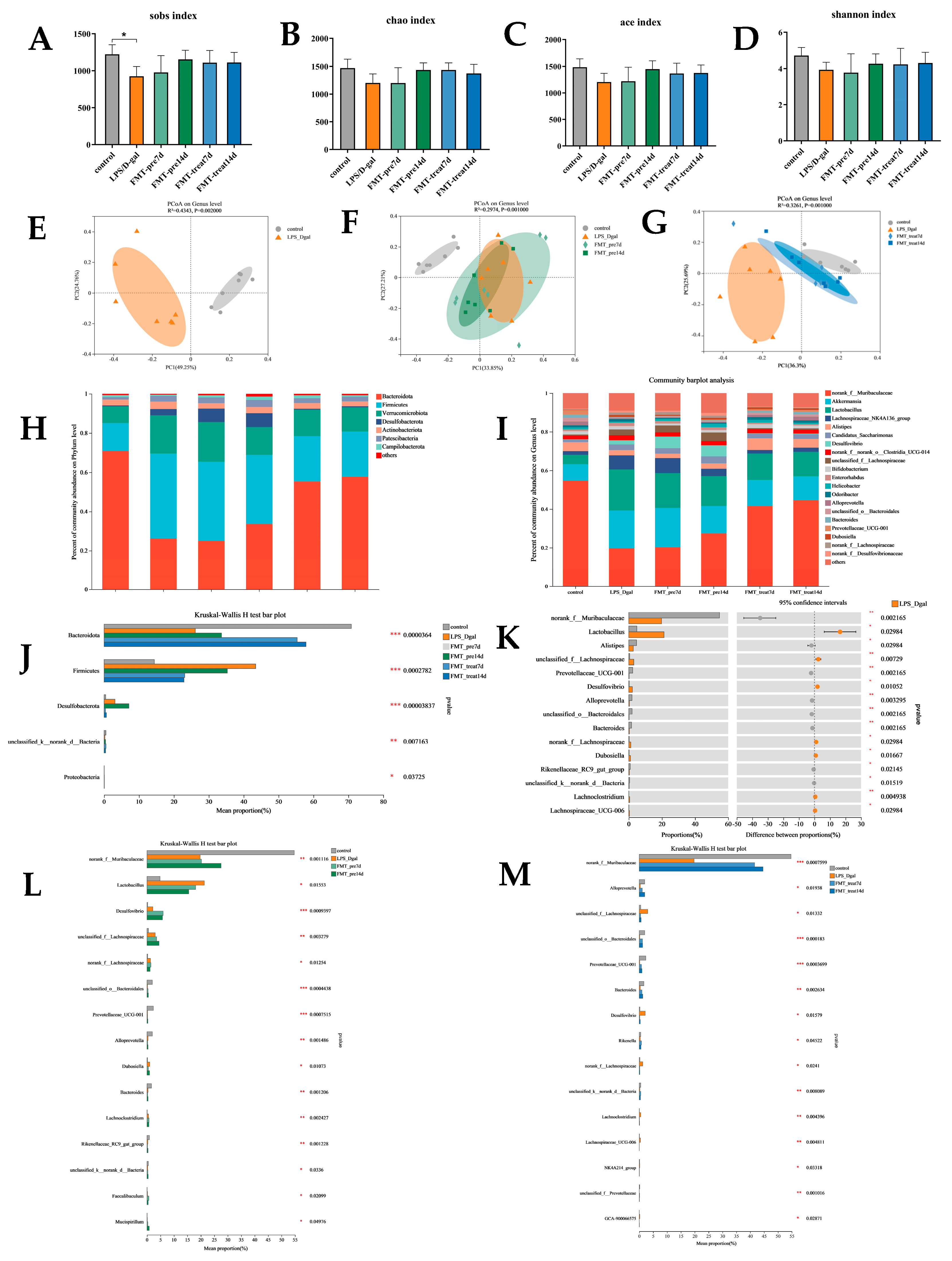
3.4. FMT Modulated Gut Microbiota Composition in LPS/D-Gal-Induced Mice
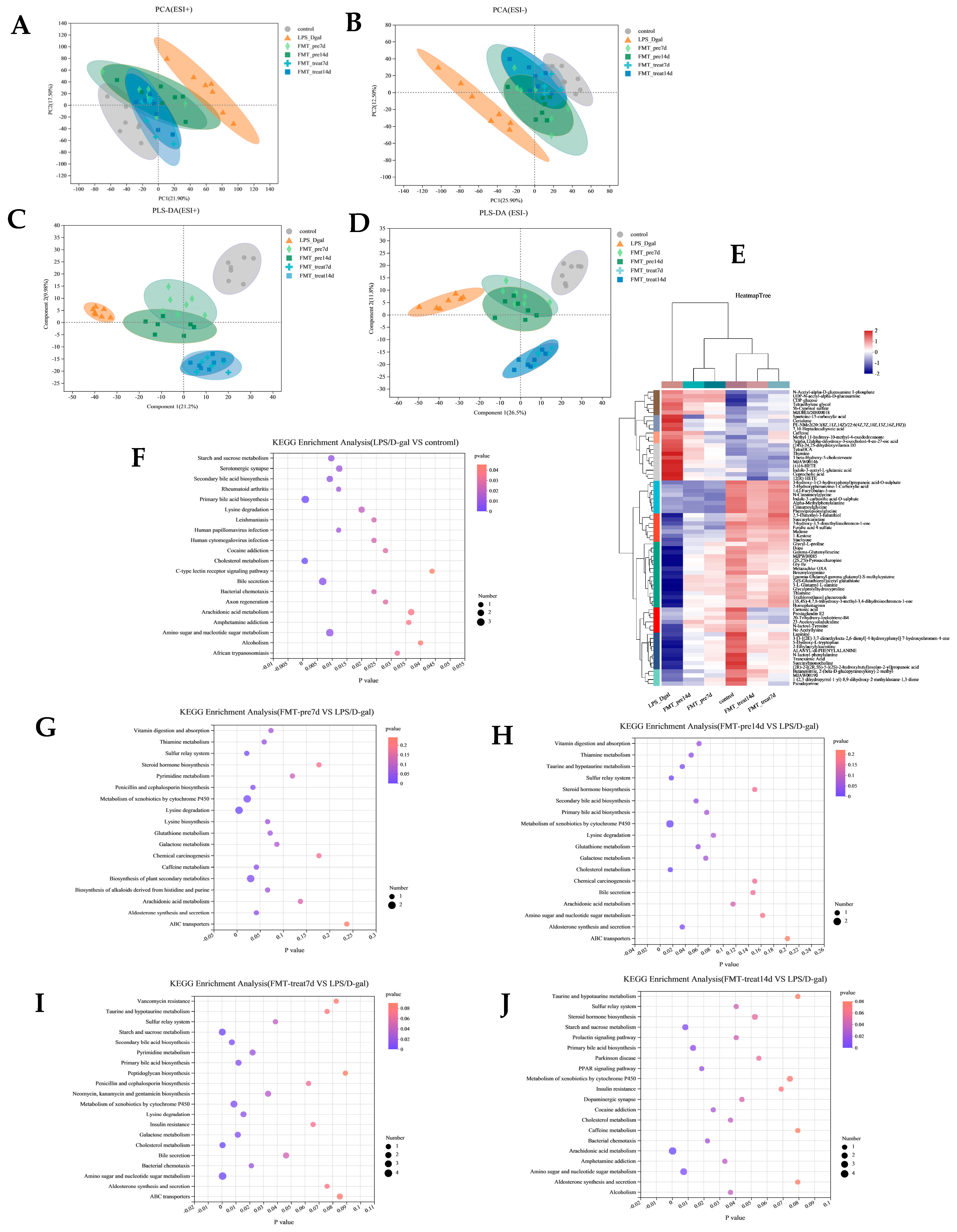
3.5. FMT Altered Liver Metabolome in LPS/D-Gal-Induced Mice
3.6. Gut Microbiota Is Associated with Liver Metabolites and Inflammatory Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arroyo, V.; Moreau, R.; Jalan, R. Acute-on-Chronic Liver Failure. N. Engl. J. Med. 2020, 382, 2137–2145. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Ying, H.; Zheng, Y.; Ma, H.; Li, L.; Zhao, Y. Alanyl-Glutamine Protects against Lipopolysaccharide-Induced Liver Injury in Mice via Alleviating Oxidative Stress, Inhibiting Inflammation, and Regulating Autophagy. Antioxidants 2022, 11, 1070. [Google Scholar] [CrossRef]
- Zhou, H.H.; Tang, L.; Yang, Y.Q.; Lin, L.; Dai, J.; Ge, P.; Ai, Q.; Jiang, R.; Zhang, L. Dopamine alleviated acute liver injury induced by lipopolysaccharide/d-galactosamine in mice. Int. Immunopharmacol. 2018, 61, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Davis, A.M.; Parker, W.F. Managing Adult Acute and Acute-on-Chronic Liver Failure in the ICU. JAMA 2021, 326, 1964–1965. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.M.; Fan, L.D.; Cheng, Z.; Yu, L.; Cong, S.; Hu, Y.; Zhu, L.L.; Zhang, B.F.; Cheng, Y.J.; Zhao, P.L.; et al. Fecal transplantation alleviates acute liver injury in mice through regulating Treg/Th17 cytokines balance. Sci. Rep. 2021, 11, 1611. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.H.; Onufer, E.J.; Huang, L.H.; Sprung, R.W.; Davidson, W.S.; Czepielewski, R.S.; Wohltmann, M.; Sorci-Thomas, M.G.; Warner, B.W.; Randolph, G.J. Enterically derived high-density lipoprotein restrains liver injury through the portal vein. Science 2021, 373, 6553. [Google Scholar] [CrossRef]
- Stuart, W.D.; Kulkarni, R.M.; Gray, J.K.; Vasiliauskas, J.; Leonis, M.A.; Waltz, S.E. Ron receptor regulates Kupffer cell-dependent cytokine production and hepatocyte survival following endotoxin exposure in mice. Hepatology 2011, 53, 1618–1628. [Google Scholar] [CrossRef] [PubMed]
- Tuñón, M.J.; Alvarez, M.; Culebras, J.M.; González-Gallego, J. An overview of animal models for investigating the pathogenesis and therapeutic strategies in acute hepatic failure. World J. Gastroenterol. 2009, 15, 3086–3098. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, Z.; Ying, H.; Yao, J.; Ma, H.; Li, L.; Zhao, Y. Oleoylethanolamide Protects Against Acute Liver Injury by Regulating Nrf-2/HO-1 and NLRP3 Pathways in Mice. Front. Pharmacol. 2020, 11, 605065. [Google Scholar] [CrossRef]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med 2016, 375, 2369–2379. [Google Scholar] [CrossRef]
- Tilg, H.; Cani, P.D.; Mayer, E.A. Gut microbiome and liver diseases. Gut 2016, 65, 2035–2044. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.Y.; Yan, R.; Wang, K.C.; Wang, Q.Q.; Chen, X.X.; Chen, L.F.; Li, L.J.; Lv, L.X. Lactobacillus reuteri DSM 17938 alleviates d-galactosamine-induced liver failure in rats. Biomed. Pharmacother. 2021, 133, 111000. [Google Scholar] [CrossRef]
- Tripathi, A.; Debelius, J.; Brenner, D.A.; Karin, M.; Loomba, R.; Schnabl, B.; Knight, R. The gut-liver axis and the intersection with the microbiome. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Bahl, M.I.; Baunwall, S.M.D.; Dahlerup, J.F.; Hvas, C.L.; Licht, T.R. Gut microbiota differs between treatment outcomes early after fecal microbiota transplantation against recurrent Clostridioides difficile infection. Gut Microbes 2022, 14, 2084306. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, P.; Wang, P.; Hu, X.; Chen, F. The Gut Microbiota, A Treasure for Human Health. Biotechnol. Adv. 2016, 34, 1210–1224. [Google Scholar] [CrossRef]
- Zhang, F.; Cui, B.; He, X.; Nie, Y.; Wu, K.; Fan, D.; FMT-standardization Study Group. Microbiota transplantation: Concept, methodology and strategy for its modernization. Protein Cell 2018, 9, 462–473. [Google Scholar] [CrossRef]
- Juul, F.E.; Garborg, K.; Bretthauer, M.; Skudal, H.; Oines, M.N.; Wiig, H.; Rose, Ø.; Seip, B.; Lamont, J.T.; Midtvedt, T.; et al. Fecal microbiota transplantation for primary clostridium difficile infection. N. Engl. J. Med. 2018, 378, 2535–2536. [Google Scholar] [CrossRef]
- Wang, W.W.; Zhang, Y.; Huang, X.B.; You, N.; Zheng, L.; Li, J. Fecal microbiota transplantation prevents hepatic encephalopathy in rats with carbon tetrachloride-induced acute hepatic dysfunction. World J. Gastroenterol. 2017, 23, 6983–6994. [Google Scholar] [CrossRef]
- Smits, L.P.; Bouter, K.E.; de Vos, W.M.; Borody, T.J.; Nieuwdorp, M. Therapeutic potential of fecal microbiota transplantation. Gastroenterology 2013, 145, 946–953. [Google Scholar] [CrossRef]
- Schneider, K.M.; Elfers, C.; Ghallab, A.; Ghallab, A.; Schneider, C.V.; Galvez, E.J.C.; Mohs, A.; Gui, W.; Candels, L.S.; Wirtz, T.H.; et al. Intestinal Dysbiosis Amplifies Acetaminophen-Induced Acute Liver Injury. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 909–933. [Google Scholar] [CrossRef]
- Ferrere, G.; Wrzosek, L.; Cailleux, F.; Turpin, W.; Puchois, V.; Spatz, M.; Ciocan, D.; Rainteau, D.; Humbert, L.; Hugot, C.; et al. Fecal microbiota manipulation prevents dysbiosis and alcohol-induced liver injury in mice. J. Hepatol. 2017, 66, 806–815. [Google Scholar] [CrossRef]
- Schnabl, B.; Brenner, D.A. Interactions between the intestinal microbiome and liver diseases. Gastroenterology 2014, 146, 1513–1524. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.P.; Li, L.L.; Han, X.; Li, Q.W.; Zhang, X.H.; Liu, J.J.; Wang, Y. Fecal microbiota transplantation improves metabolism and gut microbiome composition in db/db mice. Acta Pharmacol. Sin. 2020, 41, 678–685. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Stackebrandt, E.; Goebel, B.M. Taxonomic Note: A Place for DNA-DNA Reassociation and 16S rRNA Sequence Analysis in the Present Species Definition in Bacteriology. Int. J. Syst. Bacteriol. 1994, 44, 846–849. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, X.J.; Liu, X.L.; Wang, C.; Gao, P.; Wang, J.S.; Li, L.; Gu, J.R.; Yang, S.L.; Xu, G.W. High performance liquid chromatography-mass spectrometry for metabonomics: Potential biomarkers for acute deterioration of liver function in chronic hepatitis B. J. Proteome Res. 2006, 5, 554–561. [Google Scholar] [CrossRef]
- Zhou, L.L.; Zhang, W.P.; Xie, W.P.; Chen, H.M.; Yu, W.L.; Li, H.S.; Shen, G.L. Tributyl phosphate impairs the urea cycle and alters liver pathology and metabolism in mice after short-term exposure based on a metabonomics study. Sci. Total Environ. 2017, 603–604, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yang, W.; Yin, Y.; Zhang, P.; Wang, Y.; Tao, K. Protective Role of 4-Octyl Itaconate in Murine LPS/D-GalN-Induced Acute Liver Failure via Inhibiting Inflammation, Oxidative Stress, and Apoptosis. Oxid. Med. Cell Longev. 2021, 2021, 9932099. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, L.; Chen, J.; Li, L.; Zheng, Z.; Ren, J.; Qiu, Y. Oleoylethanolamide; an endogenous PPAR-alpha ligand, attenuates liver fibrosis targeting hepatic stellate cells. Oncotarget. 2015, 6, 42530–42540. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Karin, M. NF-κB and STAT3—Key players in liver inflammation and cancer. Cell Res. 2011, 21, 159–168. [Google Scholar] [CrossRef]
- Liu, L.M.; Zhang, J.X.; Luo, J.; Guo, H.X.; Deng, H.; Chen, J.Y.; Sun, S.L. A Role of Cell Apoptosis in Lipopolysaccharide (LPS)-induced Nonlethal Liver Injury in D-galactosamine (D-GalN)-sensitized Rats. Dig. Dis. Sci. 2008, 53, 1316–1324. [Google Scholar] [CrossRef]
- Bernal, W.; Auzinger, G.; Dhawan, A.; Wendon, J. Acute liver failure. Lancet 2010, 376, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Grek, A.; Arasi, L. Acute Liver Failure. AACN Adv. Crit. Care 2016, 27, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Keppler, D.; Lesch, R.; Reutter, W.; Decker, K. Experimental hepatitis induced by D-galactosamine. Exp. Mol. Pathol. 1968, 9, 279–290. [Google Scholar] [CrossRef]
- Rahman, N.; Pervin, M.; Kuramochi, M.; Karim, M.R.; Izawa, T.; Kuwamura, M.; Yamate, J. M1/M2-macrophage Polarization-based Hepatotoxicity in d-galactosamine-induced Acute Liver Injury in Rats. Toxicol. Pathol. 2018, 46, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Li, R.; Chen, P. Gut Microbiota and Chemical-Induced Acute Liver Injury. Front. Physiol. 2021, 12, 688780. [Google Scholar] [CrossRef]
- Bouri, S.; Hart, A. Fecal microbial transplantation: An update. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.X.; Gao, X.J.; Li, T.; Wei, B.; Wang, P.P.; Yang, Y.; Yan, R. Fecal Microbiota Transplantation in Experimental Ulcerative Colitis Reveals Associated Gut Microbial and Host Metabolic Reprogramming. Appl. Environ. Microbiol. 2018, 84, e00434-18. [Google Scholar] [CrossRef]
- Farhana, A.; Khan, Y.S. Biochemistry; Lipopolysaccharide; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Yang, H.; Young, D.W.; Gusovsky, F.; Chow, J.C. Cellular events mediated by lipopolysaccharide-stimulated toll-like receptor 4. MD-2 is required for activation of mitogen-activated protein kinases and Elk-1. J. Biol. Chem. 2000, 275, 20861–20866. [Google Scholar] [CrossRef]
- Siore, A.M.; Parker, R.E.; Stecenko, A.A.; Cuppels, C.; McKean, M.; Christman, B.W.; Cruz-Gervis, R.; Brigham, K.L. Endotoxin-induced acute lung injury requires interaction with the liver. American Journal of physiology. Lung Cell. Mol. Physiol. 2005, 289, L769–L776. [Google Scholar] [CrossRef]
- Li, C.; Si, J.; Tan, F.; Park, K.Y.; Zhao, X. Lactobacillus plantarum KSFY06 Prevents Inflammatory Response and Oxidative Stress in Acute Liver Injury Induced by D-Gal/LPS in Mice. Drug Des Devel Ther. 2021, 15, 37–50. [Google Scholar] [CrossRef]
- Jiang, Z.; Meng, Y.; Bo, L.; Wang, C.; Bian, J.; Deng, X. Sophocarpine attenuates LPS-induced liver injury and improves survival of mice through suppressing oxidative stress, inflammation, and apoptosis. Mediat. Inflamm. 2018, 2018, 5871431. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Ren, Y.; Cui, Y.; Li, R.; Wang, C.; Zhang, Y. Obeticholic acid protects mice against lipopolysaccharide-induced liver injury and inflammation. Biomed. Pharmacother. 2017, 96, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Jiang, Y. Protective effects of Lactobacillus plantarum NDC 75017 against lipopolysaccharide-induced liver injury in mice. Inflammation 2014, 37, 1599–1607. [Google Scholar] [CrossRef] [PubMed]
- Zhuge, A.; Li, B.; Yuan, Y.; Lv, L.; Li, Y.; Wu, J.; Yang, L.; Bian, X.; Wang, K.; Wang, Q.; et al. Lactobacillus salivarius LI01 encapsulated in alginate-pectin microgels ameliorates D-galactosamine-induced acute liver injury in rats. Appl. Microbiol. Biotechnol. 2020, 104, 7437–7455. [Google Scholar] [CrossRef] [PubMed]
- Streetz, K.; Leifeld, L.; Grundmann, D.; Ramakers, J.; Eckert, K.; Spengler, U.; Brenner, D.; Manns, M.; Trautwein, C. Tumor necrosis factor alpha in the pathogenesis of human and murine fulminant hepatic failure. Gastroenterology 2000, 119, 446–460. [Google Scholar] [CrossRef]
- Nowak, M.; Gaines, G.C.; Rosenberg, J.; Minter, R.; Bahjat, F.R.; Rectenwald, J.; MacKay, S.L.D.; Edwards, C.K., III; Moldawer, L.L. LPS-induced liver injury in d-galactosamine-sensitized mice requires secreted TNF-alpha and the TNF-p55 receptor. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 278, R1202–R1209. [Google Scholar] [CrossRef]
- Josephs, M.D.; Bahjat, F.R.; Fukuzuka, K.; Ksontini, R.; Solorzano, C.C.; Edwards, C.K., III; Tannahill, C.L.; Copeland, E.M., III; Moldawer, L.L. Lipopolysaccharide and D-galactosamine-induced hepatic injury is mediated by TNF-α and not by Fas ligand. Am. J. Physiology. Regul. Integr. Comp. Physiol. 2000, 278, R1196–R1201. [Google Scholar] [CrossRef] [PubMed]
- Leist, M.; Gantner, F.; Bohlinger, I.; Tiegs, G.; Germann, P.G.; Wendel, A. Tumor necrosis factor-induced hepatocyte apoptosis precedes liver failure in experimental murine shock models. Am. J. Pathol. 1995, 146, 1220–1234.5. [Google Scholar]
- Masaki, T.; Chiba, S.; Tatsukawa, H.; Yasuda, T.; Noguchi, H.; Seike, M.; Yoshimatsu, H. Adiponectin protects LPS-induced liver injury through modulation of TNF-alpha in KK-ay obese mice. Hepatology 2004, 40, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Daubioul, C.A.; Horsmans, Y.; Lambert, P.; Danse, E.; Delzenne, N.M. Effects of oligofructose on glucose and lipid metabolism in patients with nonalcoholic steatohepatitis: Results of a pilot study. Eur. J. Clin. Nutr. 2005, 59, 723–726. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, M.; Vacante, M.; Antic, T.; Giordano, M.; Chisari, G.; Acquaviva, R.; Mastrojeni, S.; Malaguarnera, G.; Mistretta, A.; Li Volti, G.; et al. Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig. Dis. Sci. 2012, 57, 545–553. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Zhu, W.; Ren, H.Z.; Zhao, X.; Wang, S.; Ma, H.C.; Shi, X.-L. Mesenchymal stem cells increase expression of heme oxygenase-1 leading to anti-inflammatory activity in treatment of acute liver failure. Stem Cell Res. Ther. 2017, 8, 70. [Google Scholar] [CrossRef]
- Yang, B.S.; Ma, Y.J.; Wang, Y.; Chen, L.Y.; Bi, M.R.; Yan, B.Z.; Bai, L.; Zhou, H.; Wang, F.-X. Protective effect and mechanism of stronger neo-minophagen C against fulminant hepatic failure. World J. Gastroenterol. 2007, 13, 462–466. [Google Scholar] [CrossRef]
- Li, P.; Nijhawan, D.; Budihardjo, I.; Srinivasula, S.M.; Ahmad, M.; Alnemri, E.S.; Wang, X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 1997, 91, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human nutrition, the gut microbiome and the immune system. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef]
- Suk, K.T.; Koh, H. New perspective on fecal microbiota transplantation in liver diseases. J. Gastroenterol. Hepatol. 2022, 37, 24–33. [Google Scholar] [CrossRef]
- Yang, X.; Lu, D.; Zhuo, J.; Lin, Z.; Yang, M.; Xu, X. The Gut-liver Axis in Immune Remodeling: New insight into Liver Diseases. Int. J. Biol. Sci. 2020, 16, 2357–2366. [Google Scholar] [CrossRef]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef]
- Kassam, Z.; Lee, C.H.; Yuan, Y.; Hunt, R.H. Fecal microbiota transplantation for Clostridium difficile infection: Systematic review and meta-analysis. Am. J. Gastroenterol. 2013, 108, 500–508. [Google Scholar] [CrossRef]
- Colman, R.J.; Rubin, D.T. Fecal microbiota transplantation as therapy for inflammatory bowel disease: A systematic review and meta-analysis. J. Crohn’s Colitis 2014, 8, 1569–1581. [Google Scholar] [CrossRef]
- Vrieze, A.; Nood, V.E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012, 143, 913–916.e7. [Google Scholar] [CrossRef] [PubMed]
- Heath, R.D.; Cockerell, C.; Mankoo, R.; Ibdah, J.A.; Tahan, V. Fecal microbiota transplantation and its potential therapeutic uses in gastrointestinal disorders. North. Clin. Istanb. 2018, 5, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Grigor’eva, I.N. Gallstone Disease, Obesity and the Firmicutes/Bacteroidetes Ratio as a Possible Biomarker of Gut Dysbiosis. J. Personalized Med. 2020, 11, 13. [Google Scholar] [CrossRef]
- Yu, L.; Zhao, X.K.; Cheng, M.L.; Yang, G.Z.; Wang, B.; Liu, H.J.; Hu, Y.-X.; Zhu, L.-L.; Zhang, S.; Xiao, Z.-W.; et al. Saccharomyces boulardii Administration Changes Gut Microbiota and Attenuates D-Galactosamine-Induced Liver Injury. Sci. Rep. 2017, 7, 1359. [Google Scholar] [CrossRef] [PubMed]
- Zoetendal, E.G.; Rajilic-Stojanovic, M.; de Vos, W.M. High-throughput diversity and functionality analysis of the gastrointestinal tract microbiota. Gut 2008, 57, 1605–1615. [Google Scholar] [CrossRef]
- Tan, H.; Zhai, Q.; Chen, W. Investigations of Bacteroides spp. towards next-generation probiotics. Food Res. Int. 2019, 116, 637–644. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Xu, J.; Xue, Z.; Zhang, M.; Pang, X.; Zhang, X.; Zhao, L. Modulation of gut microbiota by berberine and metformin during the treatment of high-fat diet-induced obesity in rats. Sci. Rep. 2015, 5, 14405. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, X. Effects of cyclophosphamide on immune system and gut microbiota in mice. Microbiol. Res. 2014, 171, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The Genus Alistipes: Gut Bacteria with Emerging Implications to Inflammation, Cancer, and Mental Health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Vargas, H.E.; Reddy, K.R.; Lai, J.C.; O’Leary, J.G.; Tandon, P.; Wong, F.; Mitrani, R.; White, M.B.; Kelly, M.; et al. Association Between Intestinal Microbiota Collected at Hospital Admission and Outcomes of Patients With Cirrhosis. Clin. Gastroenterol. Hepatol. 2019, 17, 756–765.e3. [Google Scholar] [CrossRef]
- Wong, J.; Piceno, Y.M.; DeSantis, T.Z.; Pahl, M.; Andersen, G.L.; Vaziri, N.D. Expansion of urease-and uricase-containing, indole-and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am. J. Nephrol. 2014, 39, 230–237. [Google Scholar] [CrossRef]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef]
- Compare, D.; Coccoli, P.; Rocco, A.; Nardone, O.M.; Maria, S.D.; Cartenì, M.; Nardone, G. Gut–liver axis: The impact of gut microbiota on non alcoholic fatty liver disease. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 471–476. [Google Scholar] [CrossRef]
- Chen, C.C.; Lai, C.C.; Huang, H.L.; Huang, W.Y.; Toh, H.S.; Weng, T.C.; Chuang, Y.-C.; Lu, Y.-C.; Tang, H.-J. Antimicrobial Activity of Lactobacillus Species Against Carbapenem-Resistant Enterobacteriaceae. Front. Microbiol. 2019, 10, 789. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Wang, K.; Wang, Q.; Jiang, H.; Lu, Y.; Chen, X.; Zhang, H.; Su, X.; Du, Y.; Chen, L.; et al. Probiotic Lactobacillus casei Shirota prevents acute liver injury by reshaping the gut microbiota to alleviate excessive inflammation and metabolic disorders. Microb. Biotechnol. 2022, 15, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Muramatsu, N.; Ito, Y.; Yamamoto, Y.; Nagasawa, T. L-Lysine Attenuates Hepatic Steatosis in Senescence-Accelerated Mouse Prone 8 Mice. J. Nutr. Sci. Vitaminol. 2018, 64, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Gao, Q.; Yang, Y.; Xia, J.; Zhang, W.; Chen, Y.; Zhou, Z.; Chang, L.; Hu, Y.; Zhou, H.; et al. Hexosamine biosynthetic pathway promotes the antiviral activity of SAMHD1 by enhancing O-GlcNAc transferase-mediated protein O-GlcNAcylation. Theranostics 2021, 11, 805–823. [Google Scholar] [CrossRef]
- Herzog, K.; Bandiera, S.; Pernot, S.; Fauvelle, C.; Jühling, F.; Weiss, A.; Bull, A.; Durand, S.C.; Chane-Woon-Ming, B.; Pfeffer, S.; et al. Functional microRNA screen uncovers O-linked N-acetylglucosamine transferase as a host factor modulating hepatitis C virus morphogenesis and infectivity. Gut 2020, 69, 380–392. [Google Scholar] [CrossRef] [PubMed]
- McClain, D.A.; Crook, E.D. Hexosamines and insulin resistance. Diabetes 1996, 45, 1003–1009. [Google Scholar] [CrossRef]
- Weinhage, T.; Wirth, T.; Schütz, P.; Becker, P.; Lueken, A.; Skryabin, B.V.; Wittkowski, H.; Foell, D. The Receptor for Advanced Glycation Endproducts (RAGE) Contributes to Severe Inflammatory Liver Injury in Mice. Front. Immunol. 2020, 11, 1157. [Google Scholar] [CrossRef]
- Manikandan, P.; Nagini, S. Cytochrome P450 Structure; Function and Clinical Significance: A Review. Curr. Drug Targets 2018, 19, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, J.; Wang, J.; Jiang, R.; Zhao, T. Gut microbiota combined with metabolomics reveal the mechanism of curcumol on liver fibrosis in mice. Biomed. Pharmacother. 2022, 152, 113204. [Google Scholar] [CrossRef]
- Chauhan, S.; Devi, U.; Kumar, V.R.; Kumar, V.; Anwar, F.; Kaithwas, G. Dual inhibition of arachidonic acid pathway by mulberry leaf extract. Inflammopharmacology 2015, 23, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Khadge, S.; Sharp, J.G.; Thiele, G.M.; McGuire, T.R.; Klassen, L.W.; Duryee, M.J.; Britton, H.C.; Dafferner, A.J.; Beck, J.; Black, P.N.; et al. Dietary omega-3 and omega-6 polyunsaturated fatty acids modulate hepatic pathology. J. Nutr. Biochem. 2018, 52, 92–102. [Google Scholar] [CrossRef]
- Monteiro, J.; Leslie, M.; Moghadasian, M.H.; Arendt, B.M.; Allard, J.P.; Ma, D.W. The role of n-6 and n-3 polyunsaturated fatty acids in the manifestation of the metabolic syndrome in cardiovascular disease and non-alcoholic fatty liver disease. Food Funct. 2014, 5, 426–435. [Google Scholar] [CrossRef]

| LPS/D-Gal vs. Control | FMT-Pre7d vs. LPS/D-Gal | FMT-Pre14d vs. LPS/D-Gal | FMT-Treat7d vs. LPS/D-Gal | FMT-Treat14d vs. LPS/D-Gal | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Metabolite | Formula | Subclass | Mass (M/Z) | RT (min) 1 | FC 2 | p Value | FC | p Value | FC | p Value | FC | p Value | FC | p Value |
| 1 | 2-(S-Glutathionyl)acetyl glutathione | C22H34N6O13S2 | Amino acids, peptides, and analogues | 675.1366 | 2.7876 | 0.7921 | 0.0002 | 1.2361 | 0.0006 | 1.2411 | 0.0005 | 1.2571 | 0.0006 | 1.2290 | 0.0007 |
| 2 | Stachyose | C24H42O21 | Carbohydrates and carbohydrate conjugates | 701.1902 | 0.9816 | 0.6992 | 0.0000 | 1.2223 | 0.0233 | 1.2190 | 0.0290 | 1.3679 | 0.0008 | 1.3206 | 0.0010 |
| 3 | 12(R)-HETE | C20H32O3 | Others | 319.2273 | 6.7749 | 1.2619 | 0.0000 | 0.8245 | 0.0001 | 0.8154 | 0.0000 | 0.7490 | 0.0000 | 0.6675 | 0.0000 |
| 4 | Carnosic acid | C20H28O4 | Diterpenoids | 331.1909 | 6.4796 | 0.8202 | 0.0032 | / 3 | / | / | / | / | / | / | / |
| 5 | Trichloroethanol glucuronide | C8H11Cl3O7 | Carbohydrates and carbohydrate conjugates | 646.9061 | 4.3392 | 0.7386 | 0.0010 | 1.2427 | 0.0156 | 1.2537 | 0.0090 | 1.3571 | 0.0019 | 1.2849 | 0.0041 |
| 6 | Maltose | C12H22O11 | Carbohydrates and carbohydrate conjugates | 387.1138 | 0.9994 | 0.7691 | 0.0110 | / | / | / | / | 1.2519 | 0.0394 | 1.2345 | 0.0373 |
| 7 | UDP-N-acetyl-alpha- D-glucosamine | C17H27N3O17P2 | Others | 628.0553 | 1.0787 | 2.5670 | 0.0000 | / | / | / | / | 0.7503 | 0.0292 | 0.5881 | 0.0001 |
| 8 | L-2-Aminoadipic acid | C6H11NO4 | Amino acids, peptides, and analogues | 160.0605 | 0.9994 | 1.2102 | 0.0029 | 0.7811 | 0.0006 | / | / | 0.8275 | 0.0090 | 1.3381 | 0.0106 |
| 9 | Ne-Acetyllysine | C8H16N2O3 | Indolyl carboxylic acids and derivatives | 187.1079 | 2.6841 | 0.6637 | 0.0002 | 1.4079 | 0.0015 | 1.3163 | 0.0146 | 1.2880 | 0.0363 | / | / |
| 10 | Lupinine | C10H19NO | Others | 214.1442 | 6.5576 | 0.7915 | 0.0000 | / | / | / | / | / | / | / | / |
| 11 | Prostaglandin E2 | C20H32O5 | Eicosanoids | 373.2008 | 6.6143 | 0.7992 | 0.0297 | / | / | / | / | / | / | / | / |
| 12 | Cortolone | C21H34O5 | Hydroxysteroids | 387.2148 | 6.7749 | 1.4877 | 0.0000 | / | / | / | / | 0.7391 | 0.0000 | 0.7845 | 0.0000 |
| 13 | Glycocholate | C26H43NO6 | Amino acids, peptides, and analogues | 464.3007 | 6.4862 | 1.2422 | 0.0000 | / | / | / | / | 0.8218 | 0.0000 | / | / |
| 14 | Geranic acid | C10H16O2 | Monoterpenoids | 213.1125 | 5.3076 | 0.8306 | 0.0000 | / | / | / | / | / | / | / | / |
| 15 | 5-Hydroxy-L-tryptophan | C11H12N2O3 | Tryptamines and derivatives | 219.0769 | 2.9159 | 0.7723 | 0.0000 | / | / | / | / | / | / | / | / |
| 16 | 5-L-Glutamyl-L-alanine | C8H14N2O5 | Amino acids, peptides, and analogues | 217.0823 | 1.4357 | 0.7984 | 0.0000 | 1.2053 | 0.0000 | 1.2088 | 0.0000 | 1.2413 | 0.0000 | / | / |
| 17 | CDP-glucose | C15H25N3O16P2 | Pyrimidine nucleotide sugars | 564.0628 | 0.9816 | 1.9616 | 0.0000 | / | / | / | / | 0.7848 | 0.0000 | 0.7113 | 0.0000 |
| 18 | Carglumic Acid | C6H10N2O5 | Amino acids, peptides, and analogues | 189.0508 | 0.9504 | 0.8204 | 0.0000 | / | / | / | / | / | / | / | / |
| 19 | Taurocholate | C26H45NO7S | Bile acids, alcohols and derivatives | 480.2791 | 8.8787 | 1.2538 | 0.0011 | / | / | 0.8226 | 0.0000 | 0.7505 | 0.0002 | 0.7524 | 0.0016 |
| 20 | Dopa | C9H11NO4 | Amino acids, peptides, and analogues | 239.1030 | 2.8047 | 0.8176 | 0.0000 | / | / | / | / | / | / | 1.2127 | 0.0000 |
| 21 | 5b-Cyprinol sulfate | C27H48O8S | Bile acids, alcohols and derivatives | 550.3423 | 6.5822 | 1.3112 | 0.0001 | / | / | / | / | / | / | 0.8069 | 0.0062 |
| 22 | Thiamine | C12H16N4OS | Pyrimidines and pyrimidine derivatives | 265.1122 | 0.9118 | 0.7550 | 0.0001 | 1.2805 | 0.0004 | 1.2224 | 0.0066 | 1.3269 | 0.0003 | 1.2948 | 0.0003 |
| 23 | Caffeine | C8H10N4O2 | Purines and purine derivatives | 195.0882 | 3.6826 | 1.6653 | 0.0002 | 0.5319 | 0.0011 | / | / | / | / | 0.5070 | 0.0021 |
| 24 | Thymine | C5H6N2O2 | Pyrimidines and pyrimidine derivatives | 127.0507 | 2.8586 | 1.4026 | 0.0000 | 0.8138 | 0.0000 | / | / | 0.7455 | 0.0000 | 0.7439 | 0.0000 |
| 25 | N-Acetyl-alpha-D-glucosamine 1-phosphate | C8H16NO9P | Carbohydrates and carbohydrate conjugates | 324.0466 | 0.9748 | 4.1378 | 0.0000 | / | / | 0.8112 | 0.0153 | 0.4614 | 0.0096 | 0.4818 | 0.0036 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, C.; Fan, J.; Jiang, L.; Ye, W.; Chen, Z.; Wu, W.; Huang, Q.; Qian, L. Integrated Analysis of Gut Microbiome and Liver Metabolome to Evaluate the Effects of Fecal Microbiota Transplantation on Lipopolysaccharide/D-galactosamine-Induced Acute Liver Injury in Mice. Nutrients 2023, 15, 1149. https://doi.org/10.3390/nu15051149
Yuan C, Fan J, Jiang L, Ye W, Chen Z, Wu W, Huang Q, Qian L. Integrated Analysis of Gut Microbiome and Liver Metabolome to Evaluate the Effects of Fecal Microbiota Transplantation on Lipopolysaccharide/D-galactosamine-Induced Acute Liver Injury in Mice. Nutrients. 2023; 15(5):1149. https://doi.org/10.3390/nu15051149
Chicago/Turabian StyleYuan, Chunchun, Jinghui Fan, Lai Jiang, Wenxin Ye, Zhuo Chen, Wenzi Wu, Qixin Huang, and Lichun Qian. 2023. "Integrated Analysis of Gut Microbiome and Liver Metabolome to Evaluate the Effects of Fecal Microbiota Transplantation on Lipopolysaccharide/D-galactosamine-Induced Acute Liver Injury in Mice" Nutrients 15, no. 5: 1149. https://doi.org/10.3390/nu15051149
APA StyleYuan, C., Fan, J., Jiang, L., Ye, W., Chen, Z., Wu, W., Huang, Q., & Qian, L. (2023). Integrated Analysis of Gut Microbiome and Liver Metabolome to Evaluate the Effects of Fecal Microbiota Transplantation on Lipopolysaccharide/D-galactosamine-Induced Acute Liver Injury in Mice. Nutrients, 15(5), 1149. https://doi.org/10.3390/nu15051149





