Taste Function in Adult Humans from Lean Condition to Stage II Obesity: Interactions with Biochemical Regulators, Dietary Habits, and Clinical Aspects
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. All the Participants Underwent
2.2.1. Anthropometric Parameters and Bioelectrical Impedance Analysis (BIA) Measurements
2.2.2. Taste Function Testing
2.2.3. Biochemical Assays
2.2.4. Data Handling and Statistical Analysis
3. Results
4. Discussion
5. Limitations of the Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Micarelli, A.; Malacrida, S.; Strapazzon, G.; Mrakic-Sposta, S.; Micarelli, B.; Alessandrini, N.; Carbini, V.; Caputo, S.; Falla, M.; Alessandrini, M. Impact of Nutritional Intervention on Taste Perception—A Scoping Review. Foods 2021, 10, 2747. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas Fuentes, G.; Bawaked, R.A.; Martínez González, M.; Corella, D.; Subirana Cachinero, I.; Salas-Salvadó, J.; Estruch, R.; Serra-Majem, L.; Ros, E.; Lapetra Peralta, J.; et al. Association of physical activity with body mass index, waist circumference and incidence of obesity in older adults. Eur. J. Public Health 2018, 28, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, J.A.; Drewnowski, A.; Christakis, D.A. Dietary energy density is associated with obesity and the metabolic syndrome in U.S. adults. Diabetes Care 2007, 30, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, L.F.; Bennett, L.; Baic, S.; Melichar, J.K. Taste and weight: Is there a link? Am. J. Clin. Nutr. 2009, 90, 800s–803s. [Google Scholar] [CrossRef]
- Ochs-Balcom, H.M.; Preus, L.; Nie, J.; Wactawski-Wende, J.; Agyemang, L.; Neuhouser, M.L.; Tinker, L.; Zheng, C.; Kazlauskaite, R.; Qi, L.; et al. Physical activity modifies genetic susceptibility to obesity in postmenopausal women. Menopause 2018, 25, 1131–1137. [Google Scholar] [CrossRef]
- Han, P.; Bagenna, B.; Fu, M. The sweet taste signalling pathways in the oral cavity and the gastrointestinal tract affect human appetite and food intake: A review. Int. J. Food Sci. Nutr. 2019, 70, 125–135. [Google Scholar] [CrossRef]
- Low, Y.Q.; Lacy, K.; Keast, R. The role of sweet taste in satiation and satiety. Nutrients 2014, 6, 3431–3450. [Google Scholar] [CrossRef]
- Berthoud, H.R.; Zheng, H. Modulation of taste responsiveness and food preference by obesity and weight loss. Physiol. Behav. 2012, 107, 527–532. [Google Scholar] [CrossRef]
- Shoar, S.; Naderan, M.; Shoar, N.; Modukuru, V.R.; Mahmoodzadeh, H. Alteration Pattern of Taste Perception After Bariatric Surgery: A Systematic Review of Four Taste Domains. Obes. Surg. 2019, 29, 1542–1550. [Google Scholar] [CrossRef]
- Swinburn, B.A.; Sacks, G.; Hall, K.D.; McPherson, K.; Finegood, D.T.; Moodie, M.L.; Gortmaker, S.L. The global obesity pandemic: Shaped by global drivers and local environments. Lancet 2011, 378, 804–814. [Google Scholar] [CrossRef]
- Webb, J.; Bolhuis, D.P.; Cicerale, S.; Hayes, J.E.; Keast, R. The Relationships Between Common Measurements of Taste Function. Chemosens. Percept. 2015, 8, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, S.N.; Kruger, R.; Walsh, D.C.I.; Cao, G.; Rivers, S.; Richter, M.; Breier, B.H. Is Sweet Taste Perception Associated with Sweet Food Liking and Intake? Nutrients 2017, 9, 750. [Google Scholar] [CrossRef] [PubMed]
- Duffy, V.B.; Hayes, J.E.; Sullivan, B.S.; Faghri, P. Surveying food and beverage liking: A tool for epidemiological studies to connect chemosensation with health outcomes. Ann. N. Y. Acad. Sci. 2009, 1170, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, A.; Choo, E.; Koh, A.; Dando, R. Inflammation arising from obesity reduces taste bud abundance and inhibits renewal. PLoS Biol. 2018, 16, e2001959. [Google Scholar] [CrossRef] [PubMed]
- Kubasova, N.; Burdakov, D.; Domingos, A.I. Sweet and Low on Leptin: Hormonal Regulation of Sweet Taste Buds. Diabetes 2015, 64, 3651–3652. [Google Scholar] [CrossRef]
- Rohde, K.; Schamarek, I.; Blüher, M. Consequences of Obesity on the Sense of Taste: Taste Buds as Treatment Targets? Diabetes Metab. J. 2020, 44, 509–528. [Google Scholar] [CrossRef]
- Trellakis, S.; Tagay, S.; Fischer, C.; Rydleuskaya, A.; Scherag, A.; Bruderek, K.; Schlegl, S.; Greve, J.; Canbay, A.E.; Lang, S.; et al. Ghrelin, leptin and adiponectin as possible predictors of the hedonic value of odors. Regul. Pept. 2011, 167, 112–117. [Google Scholar] [CrossRef]
- Ozturk, S.; Baltaci, D.; Turker, Y.; Kutlucan, A.; Yengil, E.; Deler, M.H.; Gur, M.; Ankarali, H. Effects of the degree of obesity on achieving target blood pressure and metabolic deterioration in obese individuals: A population-based study. Kidney Blood Press Res. 2013, 37, 531–539. [Google Scholar] [CrossRef]
- Tzamaloukas, A.H.; Murata, G.H.; Hoffman, R.M.; Schmidt, D.W.; Hill, J.E.; Leger, A.; Macdonald, L.; Caswell, C.; Janis, L.; White, R.E. Classification of the degree of obesity by body mass index or by deviation from ideal weight. JPEN J. Parenter. Enteral. Nutr. 2003, 27, 340–348. [Google Scholar] [CrossRef]
- Tepper, B.J.; Banni, S.; Melis, M.; Crnjar, R.; Tomassini Barbarossa, I. Genetic sensitivity to the bitter taste of 6-n-propylthiouracil (PROP) and its association with physiological mechanisms controlling body mass index (BMI). Nutrients 2014, 6, 3363–3381. [Google Scholar] [CrossRef]
- Micarelli, A.; Mrakic-Sposta, S.; Micarelli, B.; Malacrida, S.; Misici, I.; Carbini, V.; Iennaco, I.; Caputo, S.; Vezzoli, A.; Alessandrini, M. Smell Impairment in Stage I-II Obesity: Correlation with Biochemical Regulators and Clinical Aspects. Laryngoscope 2022, 132, 2028–2035. [Google Scholar] [CrossRef] [PubMed]
- Poessel, M.; Breuer, N.; Joshi, A.; Pampel, A.; Villringer, A.; Hummel, T.; Horstmann, A. Reduced Olfactory Bulb Volume in Obesity and Its Relation to Metabolic Health Status. Front. Hum. Neurosci. 2020, 14, 586998. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, S.; Rosafio, G.; Vasto, S.; Massenti, F.M.; Grosso, G.; Galvano, F.; Rini, N.; Barile, A.M.; Maniaci, V.; Cosentino, L.; et al. Validation of a food frequency questionnaire for use in Italian adults living in Sicily. Int. J. Food Sci. Nutr. 2015, 66, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Black, A.E. Critical evaluation of energy intake using the Goldberg cut-off for energy intake:basal metabolic rate. A practical guide to its calculation, use and limitations. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Korth, A.L.; Bhutani, S.; Neuhouser, M.L.; Beresford, S.A.; Snetselaar, L.; Tinker, L.F.; Schoeller, D.A. Comparison of Methods Used to Correct Self-Reported Protein Intake for Systematic Variation in Reported Energy Intake Using Quantitative Biomarkers of Dietary Intake. J. Nutr. 2020, 150, 1330–1336. [Google Scholar] [CrossRef]
- Streppel, M.T.; de Vries, J.H.; Meijboom, S.; Beekman, M.; de Craen, A.J.; Slagboom, P.E.; Feskens, E.J. Relative validity of the food frequency questionnaire used to assess dietary intake in the Leiden Longevity Study. Nutr. J. 2013, 12, 75. [Google Scholar] [CrossRef]
- Micarelli, A.; Viziano, A.; Granito, I.; Micarelli, R.X.; Felicioni, A.; Alessandrini, M. Changes in body composition in unilateral vestibular hypofunction: Relationships between bioelectrical impedance analysis and neuro-otological parameters. Eur. Arch. Otorhinolaryngol. 2021, 278, 2603–2611. [Google Scholar] [CrossRef]
- Ahmad, N.; Adam, S.I.; Nawi, A.M.; Hassan, M.R.; Ghazi, H.F. Abdominal Obesity Indicators: Waist Circumference or Waist-to-hip Ratio in Malaysian Adults Population. Int. J. Prev. Med. 2016, 7, 82. [Google Scholar] [CrossRef]
- Alessandrini, M.; Viziano, A.; Pistillo, R.; Granito, I.; Basso, L.; Preziosi, N.; Micarelli, A. Changes in daily energy expenditure and movement behavior in unilateral vestibular hypofunction: Relationships with neuro-otological parameters. J. Clin. Neurosci. 2021, 91, 200–208. [Google Scholar] [CrossRef]
- Kushner, R.F.; Gudivaka, R.; Schoeller, D.A. Clinical characteristics influencing bioelectrical impedance analysis measurements. Am. J. Clin. Nutr. 1996, 64, 423s–427s. [Google Scholar] [CrossRef]
- Fuller, P.M.; Jones, T.A.; Jones, S.M.; Fuller, C.A. Neurovestibular modulation of circadian and homeostatic regulation: Vestibulohypothalamic connection? Proc. Natl. Acad. Sci. USA 2002, 99, 15723–15728. [Google Scholar] [CrossRef] [PubMed]
- Bosy-Westphal, A.; Later, W.; Hitze, B.; Sato, T.; Kossel, E.; Gluer, C.C.; Heller, M.; Muller, M.J. Accuracy of bioelectrical impedance consumer devices for measurement of body composition in comparison to whole body magnetic resonance imaging and dual X-ray absorptiometry. Obes. Facts. 2008, 1, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Demura, S.; Sato, S.; Kitabayashi, T. Percentage of total body fat as estimated by three automatic bioelectrical impedance analyzers. J. Physiol. Anthropol. Appl. Hum. Sci. 2004, 23, 93–99. [Google Scholar] [CrossRef]
- Ginieis, R.; Abeywickrema, S.; Oey, I.; Peng, M. Testing Links of Food-Related Olfactory Perception to Peripheral Ghrelin and Leptin Concentrations. Front. Nutr. 2022, 9, 888608. [Google Scholar] [CrossRef]
- Mueller, C.; Kallert, S.; Renner, B.; Stiassny, K.; Temmel, A.F.; Hummel, T.; Kobal, G. Quantitative assessment of gustatory function in a clinical context using impregnated “taste strips”. Rhinology 2003, 41, 2–6. [Google Scholar] [PubMed]
- Landis, B.N.; Welge-Luessen, A.; Brämerson, A.; Bende, M.; Mueller, C.A.; Nordin, S.; Hummel, T. “Taste Strips”-a rapid, lateralized, gustatory bedside identification test based on impregnated filter papers. J. Neurol. 2009, 256, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Uygun, B.; Kiyici, S.; Ozmen, S.; Gul, Z.; Sigirli, D.; Cavun, S. The Association Between Olfaction and Taste Functions with Serum Ghrelin and Leptin Levels in Obese Women. Metab. Syndr. Relat. Disord. 2019, 17, 452–457. [Google Scholar] [CrossRef]
- Besser, G.; Erlacher, B.; Aydinkoc-Tuzcu, K.; Liu, D.T.; Pablik, E.; Niebauer, V.; Koenighofer, M.; Renner, B.; Mueller, C.A. Body-Mass-Index Associated Differences in Ortho- and Retronasal Olfactory Function and the Individual Significance of Olfaction in Health and Disease. J. Clin. Med. 2020, 9, 366. [Google Scholar] [CrossRef]
- Micarelli, A.; Viziano, A.; Panella, M.; Micarelli, E.; Alessandrini, M. Power spectra prognostic aspects of impulsive eye movement traces in superior vestibular neuritis. Med. Biol. Eng. Comput. 2019, 57, 1617–1627. [Google Scholar] [CrossRef]
- Micarelli, A.; Cormano, A.; Caccamo, D.; Alessandrini, M. Olfactory-Related Quality of Life in Multiple Chemical Sensitivity: A Genetic-Acquired Factors Model. Int. J. Mol. Sci. 2019, 21, 156. [Google Scholar] [CrossRef]
- Winkvist, A.; Hultén, B.; Kim, J.L.; Johansson, I.; Torén, K.; Brisman, J.; Bertéus Forslund, H. Dietary intake, leisure time activities and obesity among adolescents in Western Sweden: A cross-sectional study. Nutr. J. 2016, 15, 41. [Google Scholar] [CrossRef] [PubMed]
- Vignini, A.; Borroni, F.; Sabbatinelli, J.; Pugnaloni, S.; Alia, S.; Taus, M.; Ferrante, L.; Mazzanti, L.; Fabri, M. General Decrease of Taste Sensitivity Is Related to Increase of BMI: A Simple Method to Monitor Eating Behavior. Dis. Markers 2019, 2019, 2978026. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, L.B.; Møller, P.; Flint, A.; Martens, M.; Raben, A. Effect of sensory perception of foods on appetite and food intake: A review of studies on humans. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 1152–1166. [Google Scholar] [CrossRef] [PubMed]
- Hardikar, S.; Höchenberger, R.; Villringer, A.; Ohla, K. Higher sensitivity to sweet and salty taste in obese compared to lean individuals. Appetite 2017, 111, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Pepino, M.Y.; Finkbeiner, S.; Beauchamp, G.K.; Mennella, J.A. Obese women have lower monosodium glutamate taste sensitivity and prefer higher concentrations than do normal-weight women. Obesity 2010, 18, 959–965. [Google Scholar] [CrossRef]
- Sartor, F.; Donaldson, L.F.; Markland, D.A.; Loveday, H.; Jackson, M.J.; Kubis, H.P. Taste perception and implicit attitude toward sweet related to body mass index and soft drink supplementation. Appetite 2011, 57, 237–246. [Google Scholar] [CrossRef]
- Skrandies, W.; Zschieschang, R. Olfactory and gustatory functions and its relation to body weight. Physiol. Behav. 2015, 142, 1–4. [Google Scholar] [CrossRef]
- Bartoshuk, L.M.; Duffy, V.B.; Hayes, J.E.; Moskowitz, H.R.; Snyder, D.J. Psychophysics of sweet and fat perception in obesity: Problems, solutions and new perspectives. Philos. Trans. R Soc. Lond. B Biol. Sci. 2006, 361, 1137–1148. [Google Scholar] [CrossRef]
- Makaronidis, J.M.; Neilson, S.; Cheung, W.H.; Tymoszuk, U.; Pucci, A.; Finer, N.; Doyle, J.; Hashemi, M.; Elkalaawy, M.; Adamo, M.; et al. Reported appetite, taste and smell changes following Roux-en-Y gastric bypass and sleeve gastrectomy: Effect of gender, type 2 diabetes and relationship to post-operative weight loss. Appetite 2016, 107, 93–105. [Google Scholar] [CrossRef]
- Van Vuuren, M.A.J.; Strodl, E.; White, K.M.; Lockie, P.D. Taste, Enjoyment, and Desire of Flavors Change After Sleeve Gastrectomy-Short Term Results. Obes. Surg. 2017, 27, 1466–1473. [Google Scholar] [CrossRef]
- Altun, H.; Hanci, D.; Altun, H.; Batman, B.; Serin, R.K.; Karip, A.B.; Akyuz, U. Improved Gustatory Sensitivity in Morbidly Obese Patients After Laparoscopic Sleeve Gastrectomy. Ann. Otol. Rhinol. Laryngol. 2016, 125, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Holinski, F.; Menenakos, C.; Haber, G.; Olze, H.; Ordemann, J. Olfactory and Gustatory Function After Bariatric Surgery. Obes. Surg. 2015, 25, 2314–2320. [Google Scholar] [CrossRef] [PubMed]
- Cicerale, S.; Riddell, L.J.; Keast, R.S. The association between perceived sweetness intensity and dietary intake in young adults. J. Food Sci. 2012, 77, H31–H35. [Google Scholar] [CrossRef] [PubMed]
- Fábián, T.K.; Beck, A.; Fejérdy, P.; Hermann, P.; Fábián, G. Molecular mechanisms of taste recognition: Considerations about the role of saliva. Int. J. Mol. Sci. 2015, 16, 5945–5974. [Google Scholar] [CrossRef] [PubMed]
- Horio, N.; Jyotaki, M.; Yoshida, R.; Sanematsu, K.; Shigemura, N.; Ninomiya, Y. New frontiers in gut nutrient sensor research: Nutrient sensors in the gastrointestinal tract: Modulation of sweet taste sensitivity by leptin. J. Pharmacol. Sci. 2010, 112, 8–12. [Google Scholar] [CrossRef]
- Perello, M.; Dickson, S.L. Ghrelin signalling on food reward: A salient link between the gut and the mesolimbic system. J. Neuroendocrinol. 2015, 27, 424–434. [Google Scholar] [CrossRef]
- Lv, Y.; Liang, T.; Wang, G.; Li, Z. Ghrelin, a gastrointestinal hormone, regulates energy balance and lipid metabolism. Biosci. Rep. 2018, 38, BSR20181061. [Google Scholar] [CrossRef]
- Akalu, Y.; Molla, M.D.; Dessie, G.; Ayelign, B. Physiological Effect of Ghrelin on Body Systems. Int. J. Endocrinol. 2020, 2020, 1385138. [Google Scholar] [CrossRef]
- Nakazato, M.; Murakami, N.; Date, Y.; Kojima, M.; Matsuo, H.; Kangawa, K.; Matsukura, S. A role for ghrelin in the central regulation of feeding. Nature 2001, 409, 194–198. [Google Scholar] [CrossRef]
- Shin, Y.K.; Martin, B.; Kim, W.; White, C.M.; Ji, S.; Sun, Y.; Smith, R.G.; Sévigny, J.; Tschöp, M.H.; Maudsley, S.; et al. Ghrelin is produced in taste cells and ghrelin receptor null mice show reduced taste responsivity to salty (NaCl) and sour (citric acid) tastants. PLoS ONE 2010, 5, e12729. [Google Scholar] [CrossRef]
- Kouno, T.; Akiyama, N.; Fujieda, K.; Nanchi, I.; Okuda, T.; Iwasaki, T.; Oka, S.; Yukioka, H. Reduced intake of carbohydrate prevents the development of obesity and impaired glucose metabolism in ghrelin O-acyltransferase knockout mice. Peptides 2016, 86, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Takai, S.; Watanabe, Y.; Sanematsu, K.; Yoshida, R.; Margolskee, R.F.; Jiang, P.; Atsuta, I.; Koyano, K.; Ninomiya, Y.; Shigemura, N. Effects of insulin signaling on mouse taste cell proliferation. PLoS ONE 2019, 14, e0225190. [Google Scholar] [CrossRef] [PubMed]
- Shigemura, N.; Shirosaki, S.; Ohkuri, T.; Sanematsu, K.; Islam, A.A.; Ogiwara, Y.; Kawai, M.; Yoshida, R.; Ninomiya, Y. Variation in umami perception and in candidate genes for the umami receptor in mice and humans. Am. J. Clin. Nutr. 2009, 90, 764s–769s. [Google Scholar] [CrossRef] [PubMed]
- Tepper, B.J.; Koelliker, Y.; Zhao, L.; Ullrich, N.V.; Lanzara, C.; d’ Adamo, P.; Ferrara, A.; Ulivi, S.; Esposito, L.; Gasparini, P. Variation in the bitter-taste receptor gene TAS2R38, and adiposity in a genetically isolated population in Southern Italy. Obesity 2008, 16, 2289–2295. [Google Scholar] [CrossRef] [PubMed]
- Shigemura, N.; Miura, H.; Kusakabe, Y.; Hino, A.; Ninomiya, Y. Expression of leptin receptor (Ob-R) isoforms and signal transducers and activators of transcription (STATs) mRNAs in the mouse taste buds. Arch. Histol. Cytol. 2003, 66, 253–260. [Google Scholar] [CrossRef]
- Aliasghari, F.; Yaghin, N.L.; Mahdavi, R. Relationship between hedonic hunger and serum levels of insulin, leptin and BDNF in the Iranian population. Physiol. Behav. 2019, 199, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Sanematsu, K.; Nakamura, Y.; Nomura, M.; Shigemura, N.; Ninomiya, Y. Diurnal Variation of Sweet Taste Recognition Thresholds Is Absent in Overweight and Obese Humans. Nutrients 2018, 10, 297. [Google Scholar] [CrossRef]
- Zolotukhin, S. Metabolic hormones in saliva: Origins and functions. Oral Dis. 2013, 19, 219–229. [Google Scholar] [CrossRef]
- Benedix, F.; Westphal, S.; Patschke, R.; Luley, C.; Lippert, H.; Wolff, S. Comparison of serum and salivary ghrelin in healthy adults, morbidly obese, and patients with metastatic carcinoma. Obes. Surg. 2011, 21, 1265–1271. [Google Scholar] [CrossRef]
- Ogawa, T.; Annear, M.J.; Ikebe, K.; Maeda, Y. Taste-related sensations in old age. J. Oral Rehabil. 2017, 44, 626–635. [Google Scholar] [CrossRef]
- Uota, M.; Ogawa, T.; Ikebe, K.; Arai, Y.; Kamide, K.; Gondo, Y.; Masui, Y.; Ishizaki, T.; Inomata, C.; Takeshita, H.; et al. Factors related to taste sensitivity in elderly: Cross-sectional findings from SONIC study. J. Oral Rehabil. 2016, 43, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Coltell, O.; Sorlí, J.V.; Asensio, E.M.; Fernández-Carrión, R.; Barragán, R.; Ortega-Azorín, C.; Estruch, R.; González, J.I.; Salas-Salvadó, J.; Lamon-Fava, S.; et al. Association between taste perception and adiposity in overweight or obese older subjects with metabolic syndrome and identification of novel taste-related genes. Am. J. Clin. Nutr. 2019, 109, 1709–1723. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010, 11, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Item, F.; Konrad, D. Visceral fat and metabolic inflammation: The portal theory revisited. Obes. Rev. 2012, 13 (Suppl. 2), 30–39. [Google Scholar] [CrossRef]
- Ahne, G.; Erras, A.; Hummel, T.; Kobal, G. Assessment of gustatory function by means of tasting tablets. Laryngoscope 2000, 110, 1396–1401. [Google Scholar] [CrossRef]
- Hummel, T.; Erras, A.; Kobal, G. A test for the screening of taste function. Rhinology 1997, 35, 146–148. [Google Scholar]
- Hyde, R.J.; Feller, R.P. Age and sex effects on taste of sucrose, NaCl, citric acid and caffeine. Neurobiol. Aging 1981, 2, 315–318. [Google Scholar] [CrossRef]
- Yousem, D.M.; Maldjian, J.A.; Siddiqi, F.; Hummel, T.; Alsop, D.C.; Geckle, R.J.; Bilker, W.B.; Doty, R.L. Gender effects on odor-stimulated functional magnetic resonance imaging. Brain Res. 1999, 818, 480–487. [Google Scholar] [CrossRef]
- Doty, R.L.; Cameron, E.L. Sex differences and reproductive hormone influences on human odor perception. Physiol. Behav. 2009, 97, 213–228. [Google Scholar] [CrossRef]
- Larsson, M.; Lövdén, M.; Nilsson, L.G. Sex differences in recollective experience for olfactory and verbal information. Acta Psychol. 2003, 112, 89–103. [Google Scholar] [CrossRef]
- Caruso, S.; Grillo, C.; Agnello, C.; Di Mari, L.; Farina, M.; Serra, A. Olfactometric and rhinomanometric outcomes in post-menopausal women treated with hormone therapy: A prospective study. Hum. Reprod. 2004, 19, 2959–2964. [Google Scholar] [CrossRef] [PubMed]
- Dhong, H.J.; Chung, S.K.; Doty, R.L. Estrogen protects against 3-methylindole-induced olfactory loss. Brain Res. 1999, 824, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Landis, B.N.; Konnerth, C.G.; Hummel, T. A study on the frequency of olfactory dysfunction. Laryngoscope 2004, 114, 1764–1769. [Google Scholar] [CrossRef] [PubMed]
- Kölble, N.; Hummel, T.; von Mering, R.; Huch, A.; Huch, R. Gustatory and olfactory function in the first trimester of pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 99, 179–183. [Google Scholar] [CrossRef]
- May, C.E.; Rosander, J.; Gottfried, J.; Dennis, E.; Dus, M. Dietary sugar inhibits satiation by decreasing the central processing of sweet taste. eLife 2020, 9, 54530. [Google Scholar] [CrossRef]
- Weiss, M.S.; Hajnal, A.; Czaja, K.; Di Lorenzo, P.M. Taste Responses in the Nucleus of the Solitary Tract of Awake Obese Rats Are Blunted Compared With Those in Lean Rats. Front. Integr. Neurosci. 2019, 13, 35. [Google Scholar] [CrossRef]
- Stewart, J.E.; Keast, R.S. Recent fat intake modulates fat taste sensitivity in lean and overweight subjects. Int. J. Obes. 2012, 36, 834–842. [Google Scholar] [CrossRef]
- Newman, L.P.; Bolhuis, D.P.; Torres, S.J.; Keast, R.S. Dietary fat restriction increases fat taste sensitivity in people with obesity. Obesity 2016, 24, 328–334. [Google Scholar] [CrossRef]
- Stewart, J.E.; Feinle-Bisset, C.; Golding, M.; Delahunty, C.; Clifton, P.M.; Keast, R.S. Oral sensitivity to fatty acids, food consumption and BMI in human subjects. Br. J. Nutr. 2010, 104, 145–152. [Google Scholar] [CrossRef]
- Martin, C.; Passilly-Degrace, P.; Gaillard, D.; Merlin, J.F.; Chevrot, M.; Besnard, P. The lipid-sensor candidates CD36 and GPR120 are differentially regulated by dietary lipids in mouse taste buds: Impact on spontaneous fat preference. PLoS ONE 2011, 6, e24014. [Google Scholar] [CrossRef]
- Zhao, G.Q.; Zhang, Y.; Hoon, M.A.; Chandrashekar, J.; Erlenbach, I.; Ryba, N.J.; Zuker, C.S. The receptors for mammalian sweet and umami taste. Cell 2003, 115, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Kusaba, T.; Mori, Y.; Masami, O.; Hiroko, N.; Adachi, T.; Sugishita, C.; Sonomura, K.; Kimura, T.; Kishimoto, N.; Nakagawa, H.; et al. Sodium restriction improves the gustatory threshold for salty taste in patients with chronic kidney disease. Kidney Int. 2009, 76, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Noel, C.A.; Finlayson, G.; Dando, R. Prolonged Exposure to Monosodium Glutamate in Healthy Young Adults Decreases Perceived Umami Taste and Diminishes Appetite for Savory Foods. J. Nutr. 2018, 148, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.; Paik, H.Y.; Kim, J.; Chung, J. Salty taste acuity is affected by the joint action of αENaC A663T gene polymorphism and available zinc intake in young women. Nutrients 2013, 5, 4950–4963. [Google Scholar] [CrossRef] [PubMed]
- Breslin, P.A. An evolutionary perspective on food and human taste. Curr. Biol. 2013, 23, R409–R418. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Aranda, F.; Agüera, Z.; Fernández-García, J.C.; Garrido-Sanchez, L.; Alcaide-Torres, J.; Tinahones, F.J.; Giner-Bartolomé, C.; Baños, R.M.; Botella, C.; Cebolla, A.; et al. Smell-taste dysfunctions in extreme weight/eating conditions: Analysis of hormonal and psychological interactions. Endocrine 2016, 51, 256–267. [Google Scholar] [CrossRef]
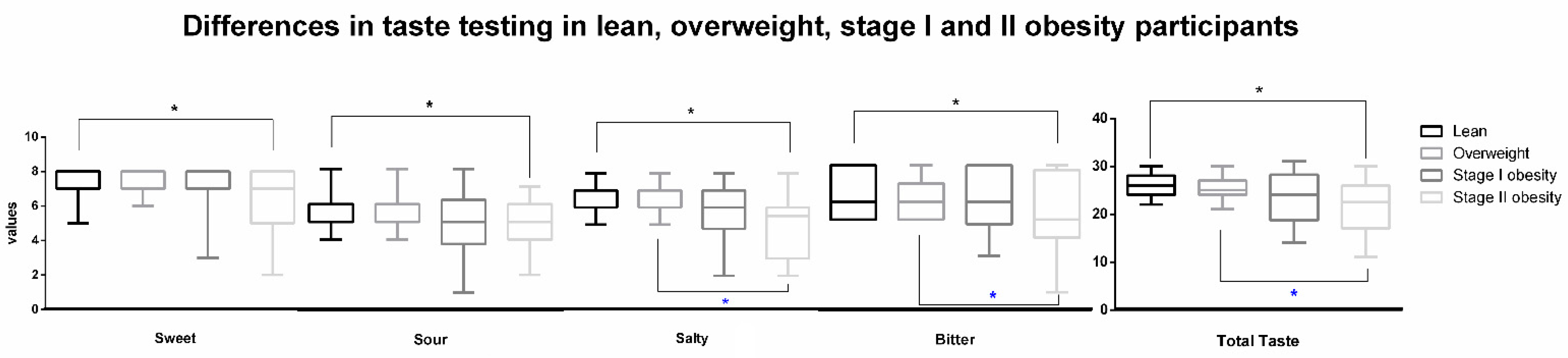
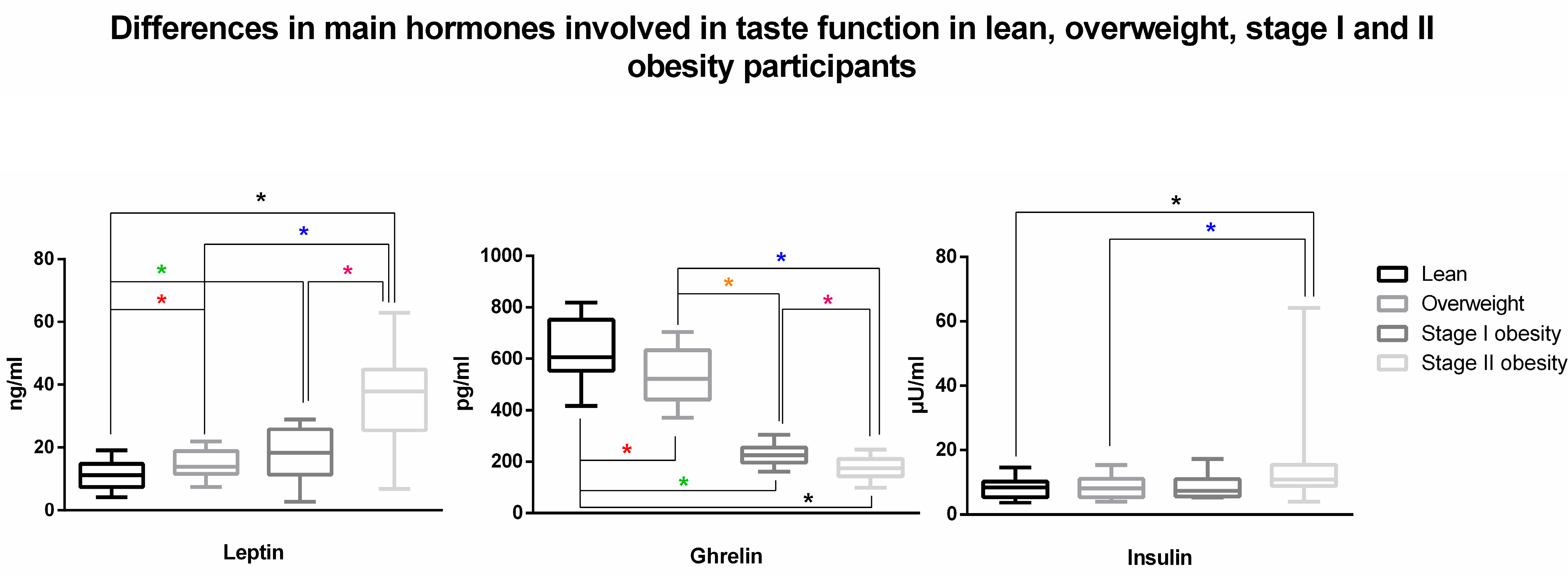


| Lean (n = 60) | Overweight (n = 39) | Stage I Obesity (n = 18) | Stage II Obesity (n = 20) | |
|---|---|---|---|---|
| Socio-demographic aspects | ||||
| Age (years) | 54.04 ± 10.27 | 53.51 ± 11.17 | 54.3 ± 13.1 | 55 ± 11.81 |
| Male | 31 | 20 | 7 | 10 |
| Female | 29 | 19 | 11 | 10 |
| Anthropometric and BIA measurements | ||||
| Waist circumference (cm) | 86.99 ± 5.4 *#ƚ | 97.46 ± 4.37 £$ | 105.72 ± 6.89 & | 120.82 ± 12.55 |
| Height (cm) | 165.55 ± 11.38 | 168.28 ± 13.53 | 165.85 ± 9.62 | 166.91 ± 11.65 |
| Weight (Kg) | 69.16 ± 10.64 *#ƚ | 79.38 ± 16.62 $ | 86.43 ± 10.76 & | 106.77 ± 18.73 |
| BMI (Kg/m2) | 23.64 ± 1.47 *#ƚ | 27.66 ± 1.4 £$ | 31.46 ± 1.38 & | 38.08 ± 2.99 |
| FM% | 18.85 ± 5.17 *#ƚ | 33.42 ± 4.13 £$ | 40.68 ± 6.99 | 45.23 ± 7.23 |
| VFlevel | 7.68 ± 2.69 *#ƚ | 10.23 ± 2.54 £$ | 26.17 ± 4.16 & | 24.17 ± 3.38 |
| MM% | 31.59 ± 4.58 | 28.16 ± 1.88 £$ | 12.61 ± 3.16 | 18.2 ± 5.38 |
| REE (Kcal) | 1509.15 ± 183.34 | 1574.1 ± 141.4 $ | 1650.94 ± 257.6 & | 1915.65 ± 346.2 |
| Lean (n = 31) | Overweight (n = 26) | Stage I Obesity (n = 11) | Stage II Obesity (n = 11) | |
|---|---|---|---|---|
| Energy intake (kcal/day) | 1897.37 ± 202.51 *#ƚ | 3507.8 ± 603.67 £$ | 7108.58 ±1928.03 | 7133.42 ± 1584.5 |
| Carbohydrate (g/day) | 140.69 ± 27.43 *#ƚ | 356.05 ± 139.2 | 485.82 ± 147.5 | 482.72 ± 176.51 |
| Protein (g/day) | 84.63 ± 18.06 *#ƚ | 211.68 ± 59.46 £$ | 424.01 ± 138.63 | 460.55 ± 134.96 |
| Fat (g/day) | 100.41 ± 23.1 *#ƚ | 189.96 ± 58.77 £$ | 297.25 ± 95.36 | 318.58 ± 63.57 |
| Saturated fat (g/day) | 26.27 ± 9.25 *#ƚ | 82.2 ± 10.15 $ | 87.22 ± 33.24 | 108.62 ± 33.99 |
| Monounsaturated fatty acids (g/day) | 35.64 ± 8.19 *#ƚ | 87.5 ± 24.12 £$ | 144.38 ± 55.12 | 158.1 ± 44.27 |
| Polyunsaturated fatty acids (g/day) | 6.19 ± 2.33 *#ƚ | 17.07 ± 3.03 £$ | 28.46 ± 10.37 | 37.93 ± 29.6 |
| n-3 Fatty acids (g/day) | 1.01 ± 0.53 *#ƚ | 4.16 ± 0.9 £ | 5.85 ± 2.71 | 19.92 ± 47.31 |
| n-6 Fatty acids (g/day) | 5.92 ± 1.9 *#ƚ | 11.62 ± 2.35 £$ | 22.13 ± 8 | 37.05 ± 46.57 |
| Cholesterol (mg/day) | 232.55 ± 46.82 *#ƚ | 836.09 ± 320.43 £$ | 1362.52 ± 606.19 | 1396.44 ± 395.68 |
| Alcohol (g/day) | 5.73 ± 3.48 *#ƚ | 20.14 ± 8.49 £$ | 50.17 ± 54.59 | 53.13 ± 62.23 |
| Fiber (g/day) | 14.72 ± 5.29 *#ƚ | 62.31 ± 16.16 £$ | 109.72 ± 35.65 | 108.2 ± 52.46 |
| Sodium (mg/day) | 1207.86 ± 379.23 *#ƚ | 1838.93 ± 735.08 £$ | 6683.02 ± 4380.24 | 8456.19 ± 2803.17 |
| Potassium (mg/day) | 2253.88 ± 715.06 #ƚ | 2706.15 ± 805.1 £$ | 14092.83 ± 3329.49 | 14069.59 ± 4337.26 |
| Lean (n = 60) | Overweight (n = 39) | Stage I Obesity (n = 18) | Stage II Obesity (n = 20) | |
|---|---|---|---|---|
| Total cholesterol (mg/dl) | 167.58 ± 18.76 *#ƚ | 188.41 ± 20.03 £$ | 216.27 ± 49.64 | 206.2 ± 28.95 |
| LDL (mg/dl) | 91.88 ± 22.71 #ƚ | 100.68 ± 19.8 £$ | 135.43 ± 35.8 | 131.86 ± 33.76 |
| HDL (mg/dl) | 50.91 ± 8.8 *# | 61.43 ± 5.68 | 62.22 ± 18.4 | 57.85 ± 21.42 |
| Triglycerides (mg/dl) | 123.9 ± 22.44 ƚ | 131.43 ± 23.34 | 116 ± 70.56 | 153.8 ± 79.28 |
| Serum glucose (mg/dl) | 83.63 ± 8.94 *#ƚ | 92.97 ± 9 $ | 96.44 ± 8.21 & | 114.65 ± 22.35 |
| Creatinine (mg/dl) | 0.85 ± 0.19 | 0.88 ± 0.18 | 0.82 ± 0.15 | 0.88 ± 0.2 |
| AST (U/L) | 12.4 ± 3.42 *#ƚ | 16.1 ± 4.29 £$ | 23.11 ± 6.29 | 23.83 ± 7.64 |
| ALT (U/L) | 13.3 ± 3.57 *#ƚ | 16.84 ± 5.01 £$ | 23.5 ± 10.98 | 27.46 ± 15.02 |
| Partial Regression Coefficient | Std. Err | t | p-Value | Cnf. Lmt−95.00% | Cnf. Lmt+95.00% | Partial Correlation Coefficient (ß) | Std. Err. ß | Cnf. Lmt−95.00% | Cnf. Lmt+95.00% | |
|---|---|---|---|---|---|---|---|---|---|---|
| Socio-demographic and anthropometric factors | ||||||||||
| Intercept | 38.499 | 22.65 | 1.699 | 0.041 | −6.332 | 83.331 | ||||
| Age | −0.146 | 0.023 | −6.153 | <0.001 | −0.193 | −0.099 | −0.453 | 0.073 | −0.599 | −0.307 |
| VFlevel | −0.167 | 0.082 | −2.038 | 0.043 | −0.329 | −0.004 | −0.173 | 0.084 | −0.341 | −0.005 |
| Gender | −1.335 | 0.413 | −3.232 | 0.001 | −2.153 | −0.517 | −0.201 | 0.062 | −0.325 | −0.078 |
| FFQ-based nutrients intakes | ||||||||||
| Intercept | 26.111 | 0.717 | 36.37 | <0.001 | 24.689 | 27.532 | ||||
| Monounsaturated fatty acids | −0.013 | 0.005 | −2.183 | 0.032 | −0.024 | −0.001 | −0.203 | 0.093 | −0.389 | −0.017 |
| Biochemical assays | ||||||||||
| Intercept | 33.139 | 4.113 | 8.056 | <0.001 | 24.997 | 41.281 | ||||
| Leptin | −0.201 | 0.06 | −3.36 | 0.001 | −0.32 | −0.082 | −0.367 | 0.109 | −0.584 | −0.151 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Micarelli, A.; Vezzoli, A.; Malacrida, S.; Micarelli, B.; Misici, I.; Carbini, V.; Iennaco, I.; Caputo, S.; Mrakic-Sposta, S.; Alessandrini, M. Taste Function in Adult Humans from Lean Condition to Stage II Obesity: Interactions with Biochemical Regulators, Dietary Habits, and Clinical Aspects. Nutrients 2023, 15, 1114. https://doi.org/10.3390/nu15051114
Micarelli A, Vezzoli A, Malacrida S, Micarelli B, Misici I, Carbini V, Iennaco I, Caputo S, Mrakic-Sposta S, Alessandrini M. Taste Function in Adult Humans from Lean Condition to Stage II Obesity: Interactions with Biochemical Regulators, Dietary Habits, and Clinical Aspects. Nutrients. 2023; 15(5):1114. https://doi.org/10.3390/nu15051114
Chicago/Turabian StyleMicarelli, Alessandro, Alessandra Vezzoli, Sandro Malacrida, Beatrice Micarelli, Ilaria Misici, Valentina Carbini, Ilaria Iennaco, Sara Caputo, Simona Mrakic-Sposta, and Marco Alessandrini. 2023. "Taste Function in Adult Humans from Lean Condition to Stage II Obesity: Interactions with Biochemical Regulators, Dietary Habits, and Clinical Aspects" Nutrients 15, no. 5: 1114. https://doi.org/10.3390/nu15051114
APA StyleMicarelli, A., Vezzoli, A., Malacrida, S., Micarelli, B., Misici, I., Carbini, V., Iennaco, I., Caputo, S., Mrakic-Sposta, S., & Alessandrini, M. (2023). Taste Function in Adult Humans from Lean Condition to Stage II Obesity: Interactions with Biochemical Regulators, Dietary Habits, and Clinical Aspects. Nutrients, 15(5), 1114. https://doi.org/10.3390/nu15051114








