Osteoporosis and Celiac Disease: Updates and Hidden Pitfalls
Abstract
1. Celiac Disease: An Introduction
2. Methods
3. Pathologic Bone Alterations in Celiac Disease
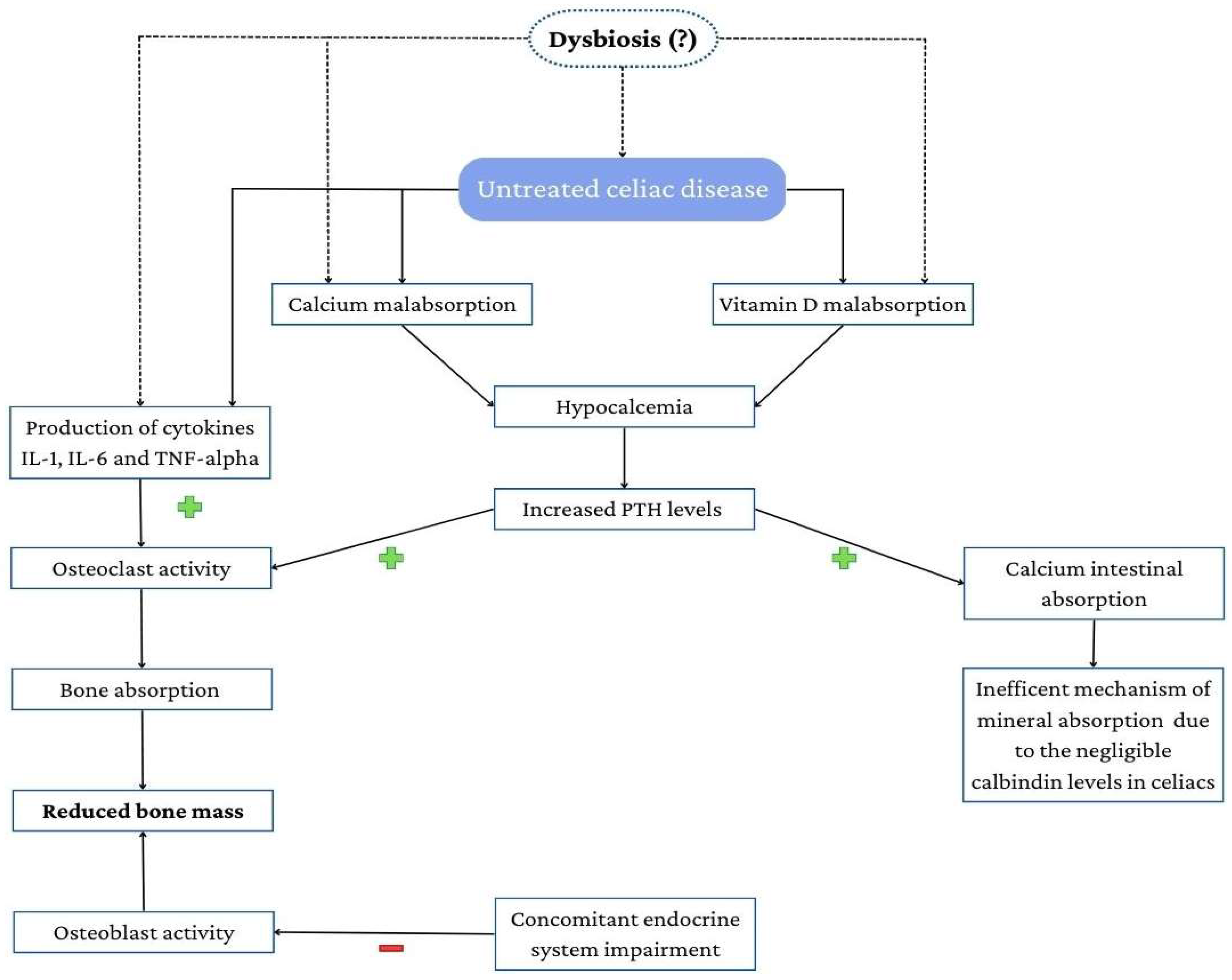
3.1. Mechanisms Underlying Osteoporosis in Celiac Disease
3.1.1. Calcium Homeostasis
3.1.2. The Role of Vitamin D
3.2. Sex Differences
3.3. Endocrine Axis
3.4. The Emerging Role of Gut Microbiota
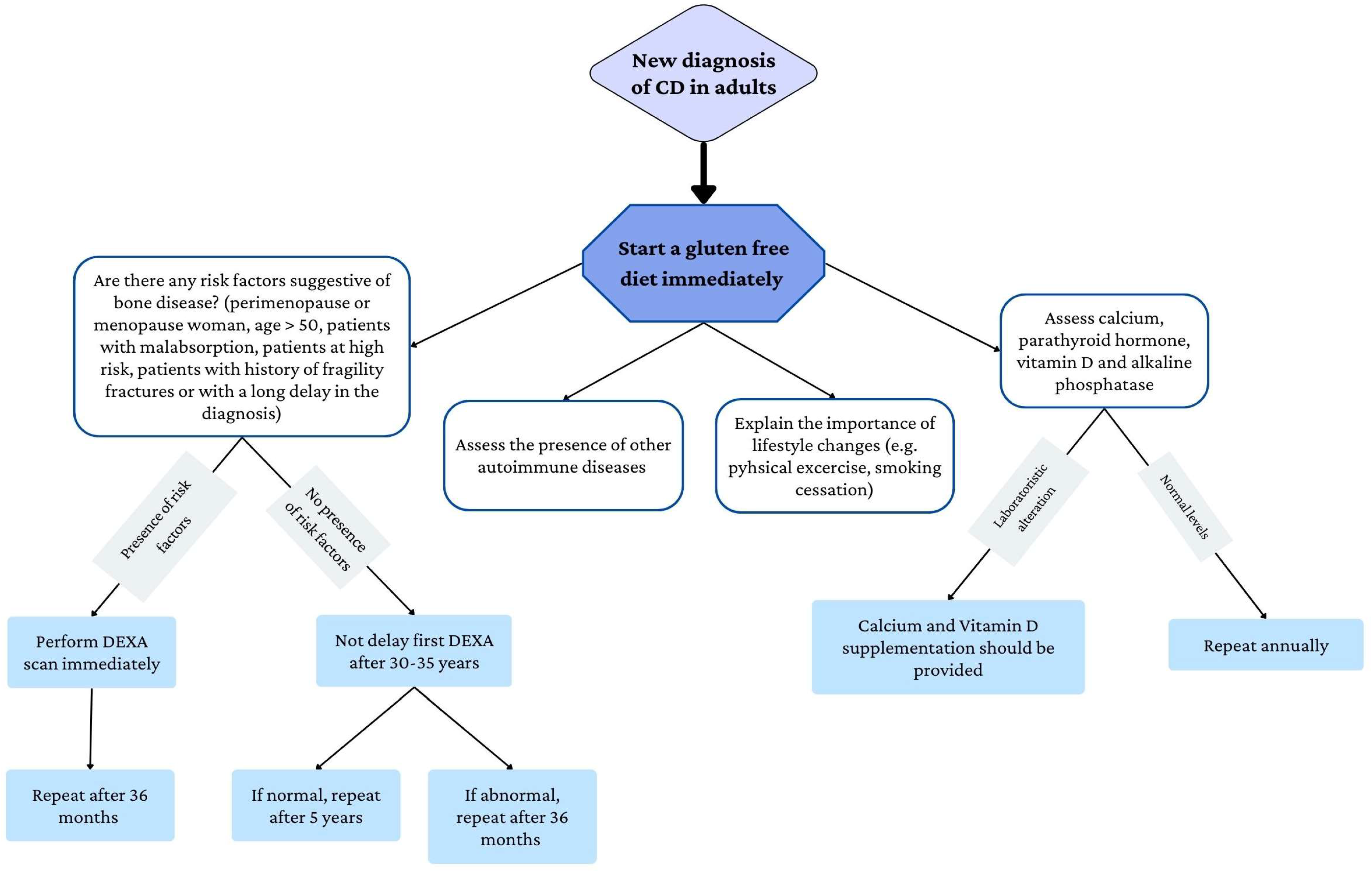
4. Management of Bone Disease
5. Pharmacological Treatment
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caio, G.; Volta, U.; Sapone, A.; Leffler, D.A.; De Giorgio, R.; Catassi, C.; Fasano, A. Celiac disease: A comprehensive current review. BMC Med. 2019, 17, 142. [Google Scholar] [CrossRef]
- Jelínková, L.; Tučková, L.; Cinová, J.; Flegelová, Z.; Tlaskalová-Hogenová, H. Gliadin stimulates human monocytes to production of IL-8 and TNF-α through a mechanism involving NF-κB. FEBS Lett. 2004, 571, 81–85. [Google Scholar] [CrossRef]
- Shan, L.; Molberg, Ø.; Parrot, I.; Hausch, F.; Filiz, F.; Gray, G.M.; Sollid, L.M.; Khosla, C. Structural Basis for Gluten Intolerance in Celiac Sprue. Science 2002, 297, 2275–2279. [Google Scholar] [CrossRef]
- Picarelli, A. 31-43 Amino Acid Sequence of the a-Gliadin Induces Anti-Endomysial Antibody Production during in Vitro Challenge. Scand. J. Gastroenterol. 1999, 34, 1099–1102. [Google Scholar] [CrossRef]
- Lionetti, E.; Catassi, C. New Clues in Celiac Disease Epidemiology, Pathogenesis, Clinical Manifestations, and Treatment. Int. Rev. Immunol. 2011, 30, 219–231. [Google Scholar] [CrossRef]
- Singh, P.; Arora, A.; Strand, T.A.; Leffler, D.A.; Catassi, C.; Green, P.H.; Kelly, C.P.; Ahuja, V.; Makharia, G.K. Global Prevalence of Celiac Disease: Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2018, 16, 823–836.e2. [Google Scholar] [CrossRef]
- King, J.A.; Jeong, J.; Underwood, F.E.; Quan, J.; Panaccione, N.; Windsor, J.W.; Coward, S.; Debruyn, J.; Ronksley, P.E.; Shaheen, A.-A.; et al. Incidence of Celiac Disease Is Increasing Over Time: A Systematic Review and Meta-analysis. Am. J. Gastroenterol. 2020, 115, 507–525. [Google Scholar] [CrossRef]
- Lebwohl, B.; Sanders, D.S.; Green, P.H.R. Coeliac disease. Lancet 2018, 391, 70–81. [Google Scholar] [CrossRef]
- Okada, H.; Kuhn, C.; Feillet, H.; Bach, J. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: An update. Clin. Exp. Immunol. 2010, 160, 1–9. [Google Scholar] [CrossRef]
- De Punder, K.; Pruimboom, L. The Dietary Intake of Wheat and other Cereal Grains and Their Role in Inflammation. Nutrients 2013, 5, 771–787. [Google Scholar] [CrossRef]
- Fasano, A. All disease begins in the (leaky) gut: Role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Research 2020, 9, 69. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Leffler, D.A.; Bai, J.C.; Biagi, F.; Fasano, A.; Green, P.H.R.; Hadjivassiliou, M.; Kaukinen, K.; Kelly, C.P.; Leonard, J.N.; et al. The Oslo definitions for coeliac disease and related terms. Gut 2013, 62, 43–52. [Google Scholar] [CrossRef]
- Volta, U.; Caio, G.; Giancola, F.; Rhoden, K.J.; Ruggeri, E.; Boschetti, E.; Stanghellini, V.; De Giorgio, R. Features and Progression of Potential Celiac Disease in Adults. Clin. Gastroenterol. Hepatol. 2016, 14, 686–693.e1. [Google Scholar] [CrossRef]
- Majsiak, E.; Cukrowska, B.; Choina, M.; Bielawski, K.; Cielecka-Kuszyk, J.; Konopka, E.; Wysokiński, M.; Bierła, J.B. Evaluation of the Usefulness of a Serological Test for Diagnosis of Celiac Disease Simultaneously Detecting Specific Antibodies and Total IgA. Nutrients 2022, 15, 202. [Google Scholar] [CrossRef]
- Al-Toma, A.; Volta, U.; Auricchio, R.; Castillejo, G.; Sanders, D.S.; Cellier, C.; Mulder, C.J.; Lundin, K.E.A. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United Eur. Gastroenterol. J. 2019, 7, 583–613. [Google Scholar] [CrossRef]
- Volta, U.; Fabbri, A.; Parisi, C.; Piscaglia, M.; Caio, G.; Tovoli, F.; Fiorini, E. Old and new serological tests for celiac disease screening. Expert Rev. Gastroenterol. Hepatol. 2010, 4, 31–35. [Google Scholar] [CrossRef]
- Catassi, C.; Fasano, A. Celiac Disease Diagnosis: Simple Rules Are Better Than Complicated Algorithms. Am. J. Med. 2010, 123, 691–693. [Google Scholar] [CrossRef]
- Jericho, H.; Sansotta, N.; Guandalini, S. Extraintestinal Manifestations of Celiac Disease: Effectiveness of the Gluten-Free Diet. J. Craniofacial Surg. 2017, 65, 75–79. [Google Scholar] [CrossRef]
- Larussa, T.; Suraci, E.; Nazionale, I.; Abenavoli, L.; Imeneo, M.; Luzza, F. Bone Mineralization in Celiac Disease. Gastroenterol. Res. Pr. 2012, 2012, e198025. [Google Scholar] [CrossRef]
- Duerksen, D.; Lix, L.; Johansson, H.; McCloskey, E.; Harvey, N.; Kanis, J.; Leslie, W. Fracture risk assessment in celiac disease: A registry-based cohort study. Osteoporos. Int. 2021, 32, 93–99. [Google Scholar] [CrossRef]
- Wolf, R.L.; Stone, K.L.; Cauley, J.A. Update on the epidemiology of osteoporosis. Curr. Rheumatol. Rep. 2000, 2, 74–86. [Google Scholar] [CrossRef]
- Iqbal, M.M. Osteoporosis: Epidemiology, diagnosis, and treatment. South. Med, J. 2000, 93, 2–19. [Google Scholar] [CrossRef]
- Mirza, F.; Canalis, E. Secondary osteoporosis: Pathophysiology and management. Eur. J. Endocrinol. 2015, 173, R131–R151. [Google Scholar] [CrossRef]
- Corazza, G.R.; Di Stefano, M.; Mauriño, E.; Bai, J.C. Bones in coeliac disease: Diagnosis and treatment. Best Pract. Res. Clin. Gastroenterol. 2005, 19, 453–465. [Google Scholar] [CrossRef]
- Mosca, C.; Thorsteinsdottir, F.; Abrahamsen, B.; Rumessen, J.J.; Händel, M.N. Newly Diagnosed Celiac Disease and Bone Health in Young Adults: A Systematic Literature Review. Calcif. Tissue Int. 2022, 110, 641–648. [Google Scholar] [CrossRef]
- Roldan, G.A.; Jamot, S.; Kopec, K.; Charoen, A.; Leffler, D.; Feller, E.R.; Shah, S.A. Celiac Disease Presenting in a Community-Based Gastroenterology Practice: Obesity and Bone Disease Are Common. Dig. Dis. Sci. 2022, 1–7. [Google Scholar] [CrossRef]
- Stenson, W.F.; Newberry, R.; Lorenz, R.; Baldus, C.; Civitelli, R. Increased Prevalence of Celiac Disease and Need for Routine Screening Among Patients With Osteoporosis. Arch. Intern. Med. 2005, 165, 393–399. [Google Scholar] [CrossRef]
- Mather, K.J.; Meddings, J.B.; Beck, P.L.; Scott, R.B.; Hanley, D.A. Prevalence of IgA-antiendomysial antibody in asymptomatic low bone mineral density. Am. J. Gastroenterol. 2001, 96, 120–125. [Google Scholar] [CrossRef]
- Karakan, T. Prevalence of IgA-antiendomysial antibody in a patient cohort with idiopathic low bone mineral density. World J. Gastroenterol. 2007, 13, 2978–2982. [Google Scholar] [CrossRef]
- Bommu, V.J.L.; Mirza, L. Osteoporosis Can Be the Sole Presentation in Celiac Disease. Cureus 2021, 13, e20602. [Google Scholar] [CrossRef]
- Walker, M.D.; Williams, J.; Lewis, S.K.; Bai, J.C.; Lebwohl, B.; Green, P.H. Measurement of Forearm Bone Density by Dual Energy X-Ray Absorptiometry Increases the Prevalence of Osteoporosis in Men With Celiac Disease. Clin. Gastroenterol. Hepatol. 2020, 18, 99–106. [Google Scholar] [CrossRef]
- Capriles, V.; Martini, L.A.; Areas, J.A.G. Metabolic osteopathy in celiac disease: Importance of a gluten-free diet. Nutr. Rev. 2009, 67, 599–606. [Google Scholar] [CrossRef]
- Krupa-Kozak, U. Pathologic bone alterations in celiac disease: Etiology, epidemiology, and treatment. Nutrition 2014, 30, 16–24. [Google Scholar] [CrossRef]
- Selby, P.L.; Davies, M.; Adams, J.E.; Mawer, E.B. Bone Loss in Celiac Disease Is Related to Secondary Hyperparathyroidism. J. Bone Miner. Res. 1999, 14, 652–657. [Google Scholar] [CrossRef]
- Molteni, N.; Bardella, M.T.; Vezzoli, G.; Pozzoli, E.; Bianchi, P. Intestinal calcium absorption as shown by stable strontium test in celiac disease before and after gluten-free diet. Am. J. Gastroenterol. 1995, 90, 2025–2028. [Google Scholar]
- Kelsey, J.L. Risk factors for osteoporosis and associated fractures. Public Health Rep. 1989, 104, 14–20. [Google Scholar]
- Nordin, B.E.C.; Morris, H.A.; Need, A.G.; Durbridge, T.C.; Horowitz, M.; Cleghorn, D.B. Osteoporosis and calcium. Aust. New Zealand J. Med. 1991, 21, 275–279. [Google Scholar] [CrossRef]
- Zanchi, C.; Di Leo, G.; Ronfani, L.; Martelossi, S.; Not, T.; Ventura, A. Bone Metabolism in Celiac Disease. J. Pediatr. 2008, 153, 262–265. [Google Scholar] [CrossRef]
- Caruso, R.; Pallone, F.; Stasi, E.; Romeo, S.; Monteleone, G. Appropriate nutrient supplementation in celiac disease. Ann. Med. 2013, 45, 522–531. [Google Scholar] [CrossRef]
- Krupa-Kozak, U.; Drabińska, N. Calcium in Gluten-Free Life: Health-Related and Nutritional Implications. Foods 2016, 5, 51. [Google Scholar] [CrossRef]
- Vogelsang, E.K.S.H. Calcaneal Ultrasound Attenuation and Vitamin-D-Receptor Genotypes in Celiac Disease. Scand. J. Gastroenterol. 2000, 35, 172–176. [Google Scholar] [CrossRef]
- Ao, T.; Kikuta, J.; Ishii, M. The Effects of Vitamin D on Immune System and Inflammatory Diseases. Biomolecules 2021, 11, 1624. [Google Scholar] [CrossRef]
- Martens, P.-J.; Gysemans, C.; Verstuyf, A.; Mathieu, C. Vitamin D’s Effect on Immune Function. Nutrients 2020, 12, 1248. [Google Scholar] [CrossRef]
- Giustina, A.; Adler, R.A.; Binkley, N.; Bollerslev, J.; Bouillon, R.; Dawson-Hughes, B.; Ebeling, P.R.; Feldman, D.; Formenti, A.M.; Lazaretti-Castro, M.; et al. Consensus statement from 2nd International Conference on Controversies in Vitamin D. Rev. Endocr. Metab. Disord. 2020, 21, 89–116. [Google Scholar] [CrossRef]
- Seth, G.A.A.; Kumar, P.; Jain, A. Prevalence and management of vitamin D deficiency in children with newly diagnosed coeliac disease: Cohort study. Ann. Trop. Paediatr. 2021, 41, 247–252. [Google Scholar] [CrossRef]
- Carlos, M.; Diana, G.; Roberto, M.; Horacio, V.; Maria, P.L.; Eduardo, M.; Sonia, N.; Silvia, P.; Edgardo, S.; Luis, A.B.; et al. Effect of treatment on bone mass, mineral metabolism, and body composition in untreated celiac disease patients. Am. J. Gastroenterol. 1997, 92, 313–318. [Google Scholar]
- Barera, G.; Maruca, K.; Sgaramella, P.; Di Stefano, M.; Mora, S. Short-term, low dose vitamin D supplementation in young patients with celiac disease: A pilot study. Eur. J. Gastroenterol. Hepatol. 2020, 32, 663–664. [Google Scholar] [CrossRef]
- Lo, C.W.; Paris, P.W.; Clemens, T.L.; Nolan, J.; Holick, M.F. Vitamin D absorption in healthy subjects and in patients with intestinal malabsorption syndromes. Am. J. Clin. Nutr. 1985, 42, 644–649. [Google Scholar] [CrossRef]
- Vogelsang, H.; Schöfl, R.; Tillinger, W.; Ferenci, P.; Gangl, A. 25-hydroxyvitamin D absorption in patients with Crohn’s disease and with pancreatic insufficiency. Wien. Klin. Wochenschr. 1997, 109, 678–682. [Google Scholar]
- Margulies, S.L.; Kurian, D.; Elliott, M.S.; Han, Z. Vitamin D deficiency in patients with intestinal malabsorption syndromes - think in and outside the gut: Malabsorption and vitamin D deficiency. J. Dig. Dis. 2015, 16, 617–633. [Google Scholar] [CrossRef]
- Ganji, R.; Moghbeli, M.; Sadeghi, R.; Bayat, G.; Ganji, A. Prevalence of osteoporosis and osteopenia in men and premenopausal women with celiac disease: A systematic review. Nutr. J. 2019, 18, 9. [Google Scholar] [CrossRef]
- Meyer, D.; Stavropolous, S.; Diamond, B.; Shane, E.; Green, P.H. Osteoporosis in a North American adult population with celiac disease1. Am. J. Gastroenterol. 2001, 96, 112–119. [Google Scholar] [CrossRef]
- Bayer, M.; Stepan, J.J.; Sedlackova, M.; Wergedal, J.E.; Kutilek, S. Spinal Bone Mineral Density in Children with Celiac Disease. J. Clin. Densitom. 1998, 1, 129–136. [Google Scholar] [CrossRef]
- Karlamangla, A.S.; Burnett-Bowie, S.-A.; Crandall, C.J. Bone Health During the Menopause Transition and Beyond. Obstet. Gynecol. Clin. North Am. 2018, 45, 695–708. [Google Scholar] [CrossRef]
- Stazi, A.V.; Trecca, A.; Trinti, B. Osteoporosis in celiac disease and in endocrine and reproductive disorders. World J. Gastroenterol. 2008, 14, 498–505. [Google Scholar] [CrossRef]
- Singh, A.D.; Singh, P.; Farooqui, N.; Strand, T.; Ahuja, V.; Makharia, G.K. Prevalence of celiac disease in patients with short stature: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2021, 36, 44–54. [Google Scholar] [CrossRef]
- Meazza, C.; Pagani, S.; Gertosio, C.; Bozzola, E.; Bozzola, M. Celiac disease and short stature in children. Expert Rev. Endocrinol. Metab. 2014, 9, 535–542. [Google Scholar] [CrossRef]
- Pärnänen, A.; Kaukinen, K.; Helakorpi, S.; Uutela, A.; Lähdeaho, M.-L.; Huhtala, H.; Collin, P.; Mäki, M.; Kurppa, K. Symptom-detected and screen-detected celiac disease and adult height: A large cohort study. Eur. J. Gastroenterol. Hepatol. 2012, 24, 1066–1070. [Google Scholar] [CrossRef]
- Luciano, A.; Bolognani, M.; Di Falco, A.; Trabucchi, C.; Bonetti, P.; Castellarin, A. Catch-up growth and final height in celiac disease. Pediatr. Medica Chir. 2002, 24, 9–12. [Google Scholar]
- Weiss, B.; Skourikhin, Y.; Modan-Moses, D.; Broide, E.; Fradkin, A.; Bujanover, Y. Is Adult Height of Patients With Celiac Disease Influenced by Delayed Diagnosis. Am. J. Gastroenterol. 2008, 103, 1770–1774. [Google Scholar] [CrossRef]
- Sonti, R.; Lebwohl, B.; Lewis, S.K.; Abu Daya, H.; Klavan, H.; Aguilar, K.; Green, P.H. Men with celiac disease are shorter than their peers in the general population. Eur. J. Gastroenterol. Hepatol. 2013, 25, 1033–1037. [Google Scholar] [CrossRef]
- Kahaly, G.J.; Frommer, L.; Schuppan, D. Celiac disease and endocrine autoimmunity—The genetic link. Autoimmun. Rev. 2018, 17, 1169–1175. [Google Scholar] [CrossRef]
- Giovenale, D.; Meazza, C.; Cardinale, G.M.; Sposito, M.; Mastrangelo, C.; Messini, B.; Citro, G.; Delvecchio, M.; Di Maio, S.; Bozzola, M. The Prevalence of Growth Hormone Deficiency and Celiac Disease in Short Children. Clin. Med. Res. 2006, 4, 180–183. [Google Scholar] [CrossRef]
- A retrospective evaluation of the association of celiac disease and growth hormone deficiency: More than a casual association? Minerva Endocrinol. 2017, 42, 24–29. [CrossRef]
- Delvecchio, M.; De Bellis, A.; Francavilla, R.; Rutigliano, V.; Predieri, B.; Indrio, F.; De Venuto, D.; Sinisi, A.A.; Bizzarro, A.; Bellastella, A.; et al. Anti-Pituitary Antibodies in Children With Newly Diagnosed Celiac Disease: A Novel Finding Contributing to Linear-Growth Impairment. Am. J. Gastroenterol. 2010, 105, 691–696. [Google Scholar] [CrossRef]
- Giovenale, D.; Meazza, C.; Cardinale, G.M.; Farinelli, E.; Mastrangelo, C.; Messini, B.; Citro, G.; Del Vecchio, M.; Di Maio, S.; Possenti, I.; et al. Growth Hormone Treatment in Prepubertal Children With Celiac Disease and Growth Hormone Deficiency. J. Craniofacial Surg. 2007, 45, 433–437. [Google Scholar] [CrossRef]
- Qutub, L.M.; Saadah, O.I. Prevalence of combined growth hormone deficiency and celiac disease among Saudi Arabian children with short stature: A tertiary care center experience. Chin. Med. J. 2020, 133, 729–731. [Google Scholar] [CrossRef]
- Elfström, P.; Montgomery, S.; Kämpe, O.; Ekbom, A.; Ludvigsson, J. Risk of Thyroid Disease in Individuals with Celiac Disease. J. Clin. Endocrinol. Metab. 2008, 93, 3915–3921. [Google Scholar] [CrossRef]
- Ansaldi, N.; Palmas, T.; Corrias, A.; Barbato, M.; Dʼaltiglia, M.R.; Campanozzi, A.; Baldassarre, M.; Rea, F.; Pluvio, R.; Bonamico, M.; et al. Autoimmune Thyroid Disease and Celiac Disease in Children. J. Craniofacial Surg. 2003, 37, 63–66. [Google Scholar] [CrossRef]
- Reifen, R.; Buskila, D.; Maislos, M.; Press, J.; Lerner, A. Serum prolactin in coeliac disease: A marker for disease activity. Arch. Dis. Child. 1997, 77, 155–157. [Google Scholar] [CrossRef]
- di Filippo, L.; Doga, M.; Resmini, E.; Giustina, A. Hyperprolactinemia and bone. Pituitary 2020, 23, 314–321. [Google Scholar] [CrossRef]
- Hermanns, U.; Hafez, E.S.E. Prolactin and Male Reproduction. Arch. Androl. 1981, 6, 95–125. [Google Scholar] [CrossRef]
- Capozzi, A.; Scambia, G.; Pontecorvi, A.; Lello, S. Hyperprolactinemia: Pathophysiology and therapeutic approach. Gynecol. Endocrinol. 2015, 31, 506–510. [Google Scholar] [CrossRef]
- Lee, Y.K.; Mazmanian, S.K. Has the Microbiota Played a Critical Role in the Evolution of the Adaptive Immune System? Science 2010, 330, 1768–1773. [Google Scholar] [CrossRef]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef]
- Seely, K.D.; Kotelko, C.A.; Douglas, H.; Bealer, B.; Brooks, A.E. The Human Gut Microbiota: A Key Mediator of Osteoporosis and Osteogenesis. Int. J. Mol. Sci. 2021, 22, 9452. [Google Scholar] [CrossRef]
- Carroccio, A.; Soresi, M.; D’Alcamo, A.; Sciumè, C.; Iacono, G.; Geraci, G.; Brusca, I.; Seidita, A.; Adragna, F.; Carta, M.; et al. Risk of low bone mineral density and low body mass index in patients with non-celiac wheat-sensitivity: A prospective observation study. BMC Med. 2014, 12, 230. [Google Scholar] [CrossRef]
- Caio, G.; Riegler, G.; Patturelli, M.; Facchiano, A.; De Magistris, L.; Sapone, A. Pathophysiology of non-celiac gluten sensitivity: Where are we now? Minerva Gastroenterol. Dietol. 2017, 63, 16–21. [Google Scholar] [CrossRef]
- Collins, F.L.; Rios-Arce, N.D.; Schepper, J.D.; Parameswaran, N.; McCabe, L.R. The Potential of Probiotics as a Therapy for Osteoporosis. Microbiol. Spectr. 2017, 5, 213–233. [Google Scholar] [CrossRef]
- McCabe, L.R.; Irwin, R.; Schaefer, L.; Britton, R.A. Probiotic use decreases intestinal inflammation and increases bone density in healthy male but not female mice. J. Cell. Physiol. 2013, 228, 1793–1798. [Google Scholar] [CrossRef]
- Rodrigues, F.C.; Castro, A.S.B.; Rodrigues, V.C.; Fernandes, S.A.; Fontes, E.A.F.; de Oliveira, T.T.; Martino, H.S.D.; Ferreira, C.L.D.L.F. Yacon Flour and Bifidobacterium longum Modulate Bone Health in Rats. J. Med. Food 2012, 15, 664–670. [Google Scholar] [CrossRef]
- Kruger, M.C.; Fear, A.; Chua, W.-H.; Plimmer, G.G.; Schollum, L.M. The effect of Lactobacillus rhamnosus HN001 on mineral absorption and bone health in growing male and ovariectomised female rats. Dairy Sci. Technol. 2009, 89, 219–231. [Google Scholar] [CrossRef]
- Ohlsson, C.; Engdahl, C.; Fåk, F.; Andersson, A.; Windahl, S.H.; Farman, H.H.; Movérare-Skrtic, S.; Islander, U.; Sjögren, K. Probiotics Protect Mice from Ovariectomy-Induced Cortical Bone Loss. PLoS ONE 2014, 9, e92368. [Google Scholar] [CrossRef]
- Rizzoli, R.; Biver, E. Are Probiotics the New Calcium and Vitamin D for Bone Health? Curr. Osteoporos. Rep. 2020, 18, 273–284. [Google Scholar] [CrossRef]
- de Sire, A.; de Sire, R.; Curci, C.; Castiglione, F.; Wahli, W. Role of Dietary Supplements and Probiotics in Modulating Microbiota and Bone Health: The Gut-Bone Axis. Cells 2022, 11, 743. [Google Scholar] [CrossRef]
- Lei, M.; Hua, L.-M.; Wang, D.-W. The effect of probiotic treatment on elderly patients with distal radius fracture: A prospective double-blind, placebo-controlled randomised clinical trial. Benef. Microbes 2016, 7, 631–637. [Google Scholar] [CrossRef]
- Nadal, I.; Donant, E.; Ribes-Koninckx, C.; Calabuig, M.; Sanz, Y. Imbalance in the composition of the duodenal microbiota of children with coeliac disease. J. Med. Microbiol. 2007, 56, 1669–1674. [Google Scholar] [CrossRef]
- Chibbar, R.; Dieleman, L.A. The Gut Microbiota in Celiac Disease and Probiotics. Nutrients 2019, 11, 2375. [Google Scholar] [CrossRef]
- Seiler, C.L.; Kiflen, M.; Stefanolo, J.P.; Bai, J.C.; Bercik, P.; Kelly, C.P.; Verdu, E.F.; Moayyedi, P.; Pinto-Sanchez, M.I. Probiotics for Celiac Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am. J. Gastroenterol. 2020, 115, 1584–1595. [Google Scholar] [CrossRef]
- Marasco, G.; Cirota, G.G.; Rossini, B.; Lungaro, L.; Di Biase, A.R.; Colecchia, A.; Volta, U.; de Giorgio, R.; Festi, D.; Caio, G. Probiotics, Prebiotics and Other Dietary Supplements for Gut Microbiota Modulation in Celiac Disease Patients. Nutrients 2020, 12, 2674. [Google Scholar] [CrossRef]
- Casella, S.; Zanini, B.; Lanzarotto, F.; Villanacci, V.; Ricci, C.; Lanzini, A. Celiac Disease in Elderly Adults: Clinical, Serological, and Histological Characteristics and the Effect of a Gluten-Free Diet. J. Am. Geriatr. Soc. 2012, 60, 1064–1069. [Google Scholar] [CrossRef]
- Valdimarsson, T.; Lofman, O.; Toss, G.; Strom, M. Reversal of osteopenia with diet in adult coeliac disease. Gut 1996, 38, 322–327. [Google Scholar] [CrossRef]
- Móra, S.; Weber, G.; Barera, G.; Bellini, A.; Pasolini, D.; Prinster, C.; Bianchi, C.; Chiumello, G. Effect of gluten-free diet on bone mineral content in growing patients with celiac disease. Am. J. Clin. Nutr. 1993, 57, 224–228. [Google Scholar] [CrossRef]
- Verma, A.; Lata, K.; Khanna, A.; Singh, R.; Sachdeva, A.; Jindal, P.; Yadav, S. Study of effect of gluten-free diet on vitamin D levels and bone mineral density in celiac disease patients. J. Fam. Med. Prim. Care 2022, 11, 603. [Google Scholar] [CrossRef]
- Tannenbaum, C.; Clark, J.; Schwartzman, K.; Wallenstein, S.; Lapinski, R.; Meier, D.; Luckey, M. Yield of Laboratory Testing to Identify Secondary Contributors to Osteoporosis in Otherwise Healthy Women. J. Clin. Endocrinol. Metab. 2002, 87, 4431–4437. [Google Scholar] [CrossRef]
- Duerksen, D.; Pinto-Sanchez, M.I.; Anca, A.; Schnetzler, J.; Case, S.; Zelin, J.; Smallwood, A.; Turner, J.; Verdú, E.; Butzner, J.D.; et al. Management of bone health in patients with celiac disease. Can. Fam. Physician 2018, 64, 433–438. [Google Scholar]
- Zingone, F.; Maimaris, S.; Auricchio, R.; Caio, G.P.I.; Carroccio, A.; Elli, L.; Galliani, E.; Montagnani, M.; Valiante, F.; Biagi, F. Guidelines of the Italian societies of gastroenterology on the diagnosis and management of coeliac disease and dermatitis herpetiformis. Dig. Liver Dis. 2022, 54, 1304–1319. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Bai, J.C.; Biagi, F.; Card, T.R.; Ciacci, C.; Ciclitira, P.J.; Green, P.H.R.; Hadjivassiliou, M.; Holdoway, A.; van Heel, D.A.; et al. Diagnosis and management of adult coeliac disease: Guidelines from the British Society of Gastroenterology. Gut 2014, 63, 1210–1228. [Google Scholar] [CrossRef]
- Scott, E.M.; Gaywood, I.; Scott, B.B. Guidelines for osteoporosis in coeliac disease and inflammatory bowel disease. Gut 2000, 46, I1–I8. [Google Scholar] [CrossRef]
- Nuti, R.; Brandi, M.L.; Checchia, G.; Di Munno, O.; Dominguez, L.; Falaschi, P.; Fiore, C.E.; Iolascon, G.; Maggi, S.; Michieli, R.; et al. Guidelines for the management of osteoporosis and fragility fractures. Intern. Emerg. Med. 2019, 14, 85–102. [Google Scholar] [CrossRef]
- Al-Toma, A.; Herman, A.; Lems, W.F.; Mulder, C.J.J. The Dietary and Non-Dietary Management of Osteoporosis in Adult-Onset Celiac Disease: Current Status and Practical Guidance. Nutrients 2022, 14, 4554. [Google Scholar] [CrossRef]
- Kanis, J.A.; Cooper, C.; Rizzoli, R.; Reginster, J.-Y. European Guidance for the Diagnosis and Management of Osteoporosis in Postmenopausal Women. Osteoporos. Int. 2019, 30, 3–44. [Google Scholar] [CrossRef]
- Galindo-Zavala, R.; Bou-Torrent, R.; Magallares-López, B.; Mir-Perelló, C.; Palmou-Fontana, N.; Sevilla-Pérez, B.; Ildefonso, M.M.-S.; González-Fernández, M.I.; Román-Pascual, A.; Alcañiz-Rodríguez, P.; et al. Expert panel consensus recommendations for diagnosis and treatment of secondary osteoporosis in children. Pediatr. Rheumatol. 2020, 18, 20. [Google Scholar] [CrossRef]
- Cosman, F.; Crittenden, D.B.; Adachi, J.D.; Binkley, N.; Czerwinski, E.; Ferrari, S.; Hofbauer, L.C.; Lau, E.; Lewiecki, E.M.; Miyauchi, A.; et al. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 2016, 375, 1532–1543. [Google Scholar] [CrossRef]
- Vescini, F.; Chiodini, I.; Falchetti, A.; Palermo, A.; Salcuni, A.S.; Bonadonna, S.; De Geronimo, V.; Cesareo, R.; Giovanelli, L.; Brigo, M.; et al. Management of Osteoporosis in Men: A Narrative Review. Int. J. Mol. Sci. 2021, 22, 13640. [Google Scholar] [CrossRef]
- Brent, M.B. Abaloparatide: A review of preclinical and clinical studies. Eur. J. Pharmacol. 2021, 909, 174409. [Google Scholar] [CrossRef]
- Ferrari, S.L.; For the IOF Committee of Scientific Advisors Working Group on Osteoporosis Pathophysiology; Bianchi, M.L.; Eisman, J.A.; Foldes, A.J.; Adami, S.; Wahl, D.A.; Stepan, J.; De Vernejoul, M.-C.; Kaufman, J.-M. Osteoporosis in young adults: Pathophysiology, diagnosis, and management. Osteoporos. Int. 2012, 23, 2735–2748. [Google Scholar] [CrossRef]
- Lewiecki, E.M.; Bilezikian, J.P.; Binkley, N.; Hans, D.; Krueger, D.; Miller, P.D.; Oates, M.; Shane, E. Update on osteoporosis from the 2014 Santa Fe Bone symposium. Endocr. Res. 2015, 40, 106–119. [Google Scholar] [CrossRef]
- Kumar, M.; Rastogi, A.; Bhadada, S.; Bhansali, A.; Vaiphei, K.; Kochhar, R. Effect of zoledronic acid on bone mineral density in patients of celiac disease: A prospective, randomized, pilot study. Indian J. Med. Res. 2013, 138, 882–887. [Google Scholar]
- Fouda, M.A.; Khan, A.A.; Sultan, M.S.; Rios, L.P.; McAssey, K.; Armstrong, D. Evaluation and Management of Skeletal Health in Celiac Disease: Position Statement. Can. J. Gastroenterol. 2012, 26, 819–829. [Google Scholar] [CrossRef]
| Pharmacological Therapeutics for Osteoporosis | |||
|---|---|---|---|
| Medication | Mechanism of Action | Target Groups | |
| Biphosphonates: | Alendronate | Bisphosphonates have an affinity with hydroxyapatite, and they inhibit farnesyl pyrophosphate synthase in osteoclast. They induce osteoclasts’ apoptosis, inhibiting bone resorption and increasing BMD [102]. | Women, men, off-label use in children [103] |
| Risendronate | |||
| Ibandronate | |||
| Pamidronate | |||
| Zolendronate | |||
| Monoclonal antibodies: | Denosumab | Monoclonal antibody to the RANKL, a major regulator of bone resorption. | Women and men |
| Romosozumab | Monoclonal antibody to sclerosin, a blocking protein to the WNT bone signaling pathway. A strong anabolic drug which improves bone formation and suppresses resorption [104]. | Post-menopausal women only | |
| Anabolic agent: | Teriparatide | It is a portion [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34] of human PTH. Intermittent exposure to PTH will activate osteoblasts more than osteoclasts. Thus, once-daily injections of teriparatide have been shown to reduce significantly the risk of vertebral fractures and also non-vertebral fractures [102]. | Women and men [105] |
| Abaloparatide | It is a parathyroid hormone 1 receptor (PTHrP) analogue; it selectively activates the PTH1 receptor, a G protein-coupled receptor (GPCR) expressed in the osteoblasts and osteocytes [106]. | Post-menopausal women only | |
| Estrogen-related drugs: | Raloxifene | Selective estrogen receptor modulators (SERMs), hence mixed agonist and antagonist of the estrogen receptor in different tissues. In bone, they have an estrogenic (protective) activity. | Post-menopausal women only [102] |
| Bazedoxifene/ conjugated estrogens | |||
| Hormone replacement therapy | It mimics the protective effects of estrogens and progesterone. It prevents bone loss in post-menopausal women by inhibiting osteoclast-driven bone resorption and reducing the rate of bone remodeling [102]. | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lungaro, L.; Manza, F.; Costanzini, A.; Barbalinardo, M.; Gentili, D.; Caputo, F.; Guarino, M.; Zoli, G.; Volta, U.; De Giorgio, R.; et al. Osteoporosis and Celiac Disease: Updates and Hidden Pitfalls. Nutrients 2023, 15, 1089. https://doi.org/10.3390/nu15051089
Lungaro L, Manza F, Costanzini A, Barbalinardo M, Gentili D, Caputo F, Guarino M, Zoli G, Volta U, De Giorgio R, et al. Osteoporosis and Celiac Disease: Updates and Hidden Pitfalls. Nutrients. 2023; 15(5):1089. https://doi.org/10.3390/nu15051089
Chicago/Turabian StyleLungaro, Lisa, Francesca Manza, Anna Costanzini, Marianna Barbalinardo, Denis Gentili, Fabio Caputo, Matteo Guarino, Giorgio Zoli, Umberto Volta, Roberto De Giorgio, and et al. 2023. "Osteoporosis and Celiac Disease: Updates and Hidden Pitfalls" Nutrients 15, no. 5: 1089. https://doi.org/10.3390/nu15051089
APA StyleLungaro, L., Manza, F., Costanzini, A., Barbalinardo, M., Gentili, D., Caputo, F., Guarino, M., Zoli, G., Volta, U., De Giorgio, R., & Caio, G. (2023). Osteoporosis and Celiac Disease: Updates and Hidden Pitfalls. Nutrients, 15(5), 1089. https://doi.org/10.3390/nu15051089








