Metabolic Syndrome and Its Components Are Associated with New-Onset Hyperuricemia in a Large Taiwanese Population Follow-Up Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Declaration
2.2. TWB
2.3. Definition of New-Onset Hyperuricemia
2.4. Definition of MetS
2.5. Study Participants
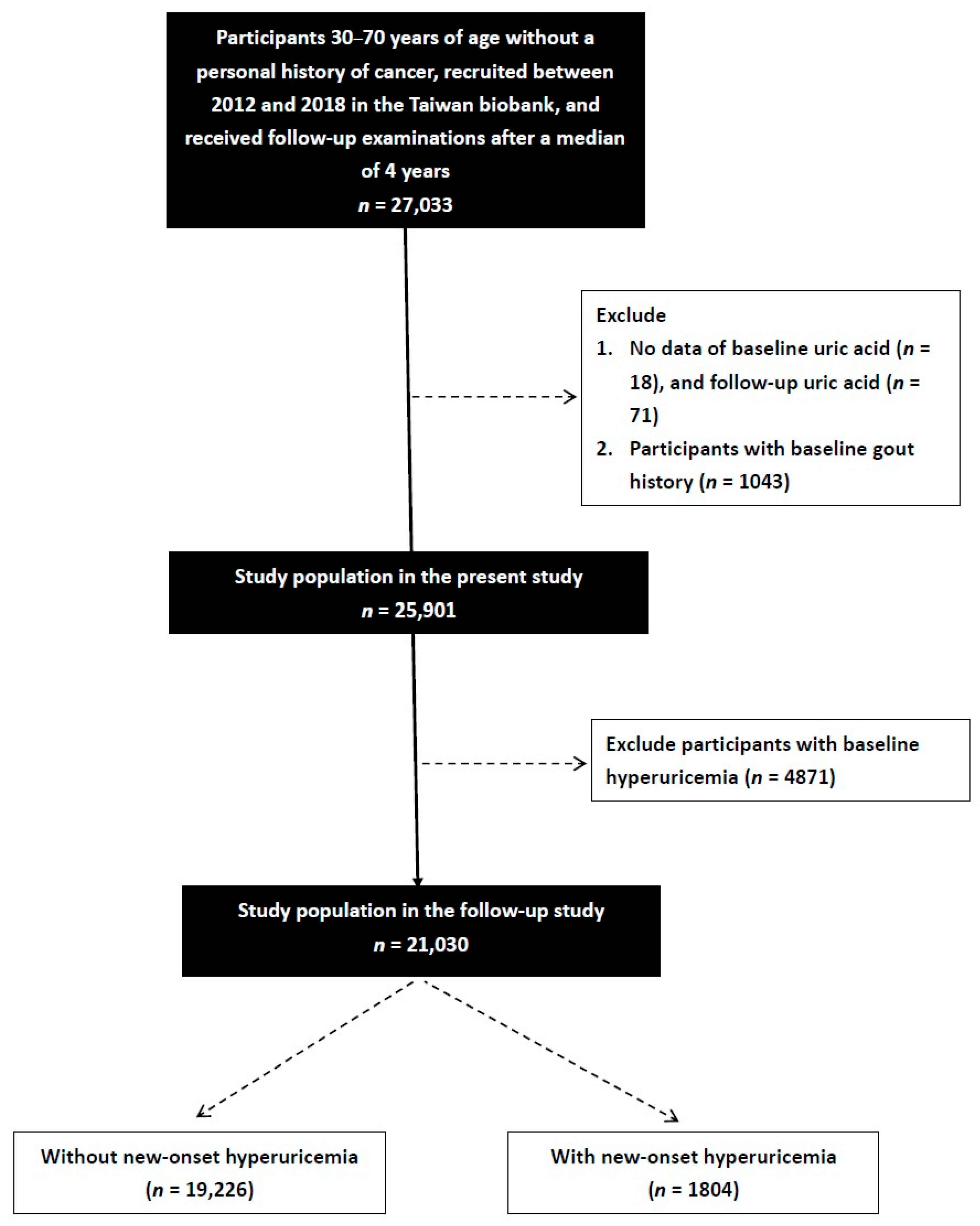
2.6. Study Design
2.7. Statistical Analysis
3. Results
3.1. Comparison of the Participants Who Did and Did Not Develop New-Onset Hyperuricemia
3.2. Association of MetS and New-Onset Hyperuricemia
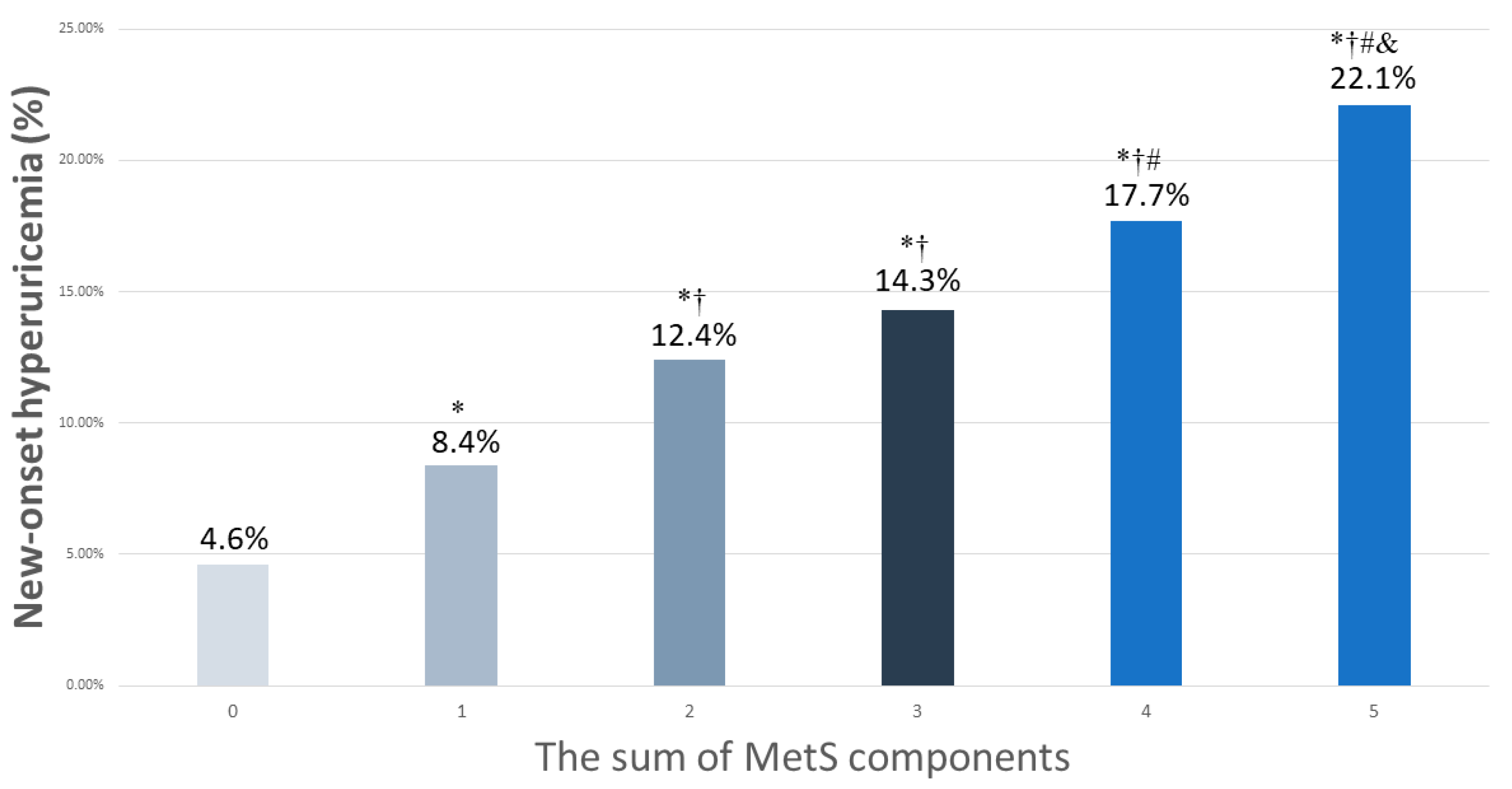
3.3. Associations among MetS Components with New-Onset Hyperuricemia
3.4. Associations among the MetS Components with New-Onset Hyperuricemia
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, L.; Zhang, Y.; Zeng, C. Update on the epidemiology, genetics, and therapeutic options of hyperuricemia. Am. J. Transl. Res. 2020, 12, 3167–3181. [Google Scholar] [PubMed]
- Yu, Q.; Shen, H.-C.; Hu, Y.-C.; Chen, Y.-F.; Tung, T.-H. Prevalence and Metabolic Factors of Hyperuricemia in an Elderly Agricultural and Fishing Population in Taiwan. Arch. Rheumatol. 2017, 32, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2015, 213, 8–14. [Google Scholar] [CrossRef]
- Stewart, D.J.; Langlois, V.; Noone, D. Hyperuricemia and Hypertension: Links and Risks. Integr. Blood Press. Control 2019, 12, 43–62. [Google Scholar] [CrossRef]
- Kuwabara, M.; Niwa, K.; Nishihara, S.; Nishi, Y.; Takahashi, O.; Kario, K.; Yamamoto, K.; Yamashita, T.; Hisatome, I. Hyperuricemia is an independent competing risk factor for atrial fibrillation. Int. J. Cardiol. 2016, 231, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Day, R.O.; Graham, G.G.; Hicks, M.; McLachlan, A.J.; Stocker, S.; Williams, K.M. Clinical Pharmacokinetics and Pharmacodynamics of Allopurinol and Oxypurinol. Clin. Pharmacokinet. 2007, 46, 623–644. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, M.; Kuwabara, R.; Niwa, K.; Hisatome, I.; Smits, G.; Roncal-Jimenez, C.A.; MacLean, P.S.; Yracheta, J.M.; Ohno, M.; Lanaspa, M.A.; et al. Different Risk for Hypertension, Diabetes, Dyslipidemia, and Hyperuricemia According to Level of Body Mass Index in Japanese and American Subjects. Nutrients 2018, 10, 1011. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Li, L.; Cui, J.; Yin, F.; Yang, F.; Yuan, D.-M.; Xin, H.-L.; Zhang, L.; Gao, W.-G.; Sun, J.-P. Associations between anthropometric parameters (body mass index, waist circumference and waist to hip ratio) and newly diagnosed hyperuricemia in adults in Qingdao, China: A cross-sectional study. Asia Pac. J. Clin. Nutr. 2020, 29, 763–770. [Google Scholar]
- Cicero, A.F.G.; Fogacci, F.; Giovannini, M.; Grandi, E.; D’Addato, S.; Borghi, C. Interaction between low-density lipoprotein-cholesterolaemia, serum uric level and incident hypertension: Data from the Brisighella Heart Study. J. Hypertens. 2019, 37, 728–731. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Giovannini, M.; Grandi, E.; Rosticci, M.; D’addato, S.; Borghi, C. Serum uric acid predicts incident metabolic syndrome in the elderly in an analysis of the Brisighella Heart Study. Sci. Rep. 2018, 8, 11529. [Google Scholar] [CrossRef]
- Ford, E.S.; Giles, W.H.; Dietz, W.H. Prevalence of the Metabolic Syndrome among US Adults: Findings from the third National Health and Nutrition Examination Survey. JAMA 2002, 287, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Hwang, L.-C.; Bai, C.-H.; Chen, C.-J. Prevalence of Obesity and Metabolic Syndrome in Taiwan. J. Formos. Med. Assoc. 2006, 105, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Klein, B.E.; Klein, R.; Lee, K.E. Components of the Metabolic Syndrome and Risk of Cardiovascular Disease and Diabetes in Beaver Dam. Diabetes Care 2002, 25, 1790–1794. [Google Scholar] [CrossRef] [PubMed]
- Gami, A.S.; Witt, B.J.; Howard, D.E.; Erwin, P.J.; Gami, L.A.; Somers, V.K.; Montori, V.M. Metabolic Syndrome and Risk of Incident Cardiovascular Events and Death: A Systematic Review and Meta-Analysis of Longitudinal Studies. J. Am. Coll. Cardiol. 2007, 49, 403–414. [Google Scholar] [CrossRef]
- Chen, J.; Muntner, P.; Hamm, L.L.; Jones, D.W.; Batuman, V.; Fonseca, V.; Whelton, P.K.; He, J. The Metabolic Syndrome and Chronic Kidney Disease in U.S. Adults. Ann. Intern. Med. 2004, 140, 167–174. [Google Scholar] [CrossRef]
- Pasquali, R.; Gambineri, A.; Anconetani, B.; Vicennati, V.; Colitta, D.; Caramelli, E.; Casimirri, F.; Morselli/labate, A.M. The natural history of the metabolic syndrome in young women with the polycystic ovary syndrome and the effect of long-term oestrogen-progestagen treatment. Clin. Endocrinol. 1999, 50, 517–527. [Google Scholar] [CrossRef]
- Ip, M.S.M.; Lam, B.; Ng, M.M.T.; Lam, W.K.; Tsang, K.W.T.; Lam, K.S.L. Obstructive Sleep Apnea Is Independently Associated with Insulin Resistance. Am. J. Respir. Crit. Care Med. 2002, 165, 670–676. [Google Scholar] [CrossRef]
- Huang, G.; Xu, J.; Zhang, T.; Cai, L.; Liu, H.; Yu, X.; Wu, J. Hyperuricemia is associated with metabolic syndrome in the community very elderly in Chengdu. Sci. Rep. 2020, 10, 8678. [Google Scholar] [CrossRef]
- Ali, N.; Miah, R.; Hasan, M.; Barman, Z.; Mou, A.D.; Hafsa, J.M.; Das Trisha, A.; Hasan, A.; Islam, F. Association between serum uric acid and metabolic syndrome: A cross-sectional study in Bangladeshi adults. Sci. Rep. 2020, 10, 1–7. [Google Scholar] [CrossRef]
- Sautin, Y.; Nakagawa, T.; Zharikov, S.; Johnson, R.J. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am. J. Physiol. Physiol. 2007, 293, C584–C596. [Google Scholar] [CrossRef]
- Chen, C.-H.; Yang, J.-H.; Chiang, C.W.K.; Hsiung, C.-N.; Wu, P.-E.; Chang, L.-C.; Chu, H.-W.; Chang, J.; Song, I.W.; Yang, S.-L.; et al. Population structure of Han Chinese in the modern Taiwanese population based on 10,000 participants in the Taiwan Biobank project. Hum. Mol. Genet. 2016, 25, 5321–5331. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.-T.; Hung, T.-H.; Yeh, C.-K. Taiwan Regulation of Biobanks. J. Law Med. Ethics A J. Am. Soc. Law Med. Ethics 2015, 43, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A More Accurate Method to Estimate Glomerular Filtration Rate from Serum Creatinine: A New Prediction Equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Education. Physical Fitness 333 Plan. 1999.
- Isomaa, B.; Henricsson, M.; Almgren, P.; Tuomi, T.; Taskinen, M.-R.; Groop, L. The metabolic syndrome influences the risk of chronic complications in patients with Type II diabetes. Diabetologia 2001, 44, 1148–1154. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.-E.; Ma, S.; Wai, D.; Chew, S.-K.; Tai, E.-S. Can We Apply the National Cholesterol Education Program Adult Treatment Panel Definition of the Metabolic Syndrome to Asians? Diabetes Care 2004, 27, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-H.; Ma, Q.-H.; Xu, Y.; Chen, X.; Pan, C.-W. Metabolic Syndrome and 5-Year Incident Hyperuricemia among Older Chinese Adults: A Community-Based Cohort Study. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 4191–4200. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-Y.; Fu, Y.-P.; Zhou, M. The bidirectional relationship between metabolic syndrome and hyperuricemia in China: A longitudinal study from CHARLS. Endocrine 2022, 76, 62–69. [Google Scholar] [CrossRef]
- Battelli, M.G.; Bortolotti, M.; Polito, L.; Bolognesi, A. The role of xanthine oxidoreductase and uric acid in metabolic syndrome. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2018, 1864, 2557–2565. [Google Scholar] [CrossRef]
- Palmer, T.M.; Nordestgaard, B.G.; Benn, M.; Tybjaerg-Hansen, A.; Smith, G.D.; Lawlor, D.A.; Timpson, N.J. Association of plasma uric acid with ischaemic heart disease and blood pressure: Mendelian randomisation analysis of two large cohorts. BMJ 2013, 347, f4262. [Google Scholar] [CrossRef]
- Takiue, Y.; Hosoyamada, M.; Kimura, M.; Saito, H. The Effect of Female Hormones Upon Urate Transport Systems in the Mouse Kidney. Nucleosides Nucleotides Nucleic Acids 2011, 30, 113–119. [Google Scholar] [CrossRef]
- Matsuura, F.; Yamashita, S.; Nakamura, T.; Nishida, M.; Nozaki, S.; Funahashi, T.; Matsuzawa, Y. Effect of visceral fat accumulation on uric acid metabolism in male obese subjects: Visceral fat obesity is linked more closely to overproduction of uric acid than subcutaneous fat obesity. Metabolism 1998, 47, 929–933. [Google Scholar] [CrossRef]
- Fruehwald-Schultes, B.; Peters, A.; Kern, W.; Beyer, J.; Pfützner, A. Serum leptin is associated with serum uric acid concentrations in humans. Metabolism 1999, 48, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wei, F.; Chen, C.; Cai, C.; Zhang, K.; Sun, N.; Tian, J.; Shi, W.; Zhang, M.; Zang, Y.; et al. Higher triglyceride level predicts hyperuricemia: A prospective study of 6-year follow-up. J. Clin. Lipidol. 2017, 12, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Chang, Y.; Zhang, Y.; Kim, S.-G.; Cho, J.; Son, H.J.; Shin, H.; Guallar, E. A Cohort Study of Hyperuricemia in Middle-aged South Korean Men. Am. J. Epidemiol. 2011, 175, 133–143. [Google Scholar] [CrossRef]
- Gao, Z.; Zuo, M.; Han, F.; Yuan, X.; Sun, M.; Li, X.; Liu, R.; Jiang, W.; Zhang, L.; Chang, B.; et al. Renal impairment markers in type 2 diabetes patients with different types of hyperuricemia. J. Diabetes Investig. 2018, 10, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Shao, X.; Zhou, S.; Cui, Z.; Liu, H.; Wang, T.; Fan, X.; Yu, P. Triglyceride-glucose index is significantly associated with the risk of hyperuricemia in patients with diabetic kidney disease. Sci. Rep. 2022, 12, 19988. [Google Scholar] [CrossRef] [PubMed]
- Onat, A.; Can, G.; Örnek, E.; Altay, S.; Yüksel, M.; Ademoğlu, E. Elevated serum uric acid in nondiabetic people mark pro-inflammatory state and HDL dysfunction and independently predicts coronary disease. Clin. Rheumatol. 2013, 32, 1767–1775. [Google Scholar] [CrossRef]
- Peng, T.-C.; Wang, C.-C.; Kao, T.-W.; Chan, J.Y.-H.; Yang, Y.-H.; Chang, Y.-W.; Chen, W.-L. Relationship between Hyperuricemia and Lipid Profiles in US Adults. BioMed Res. Int. 2015, 2015, 127596. [Google Scholar] [CrossRef]
- Vekic, J.; Jelic-Ivanovic, Z.; Spasojevic-Kalimanovska, V.; Memon, L.; Zeljkovic, A.; Bogavac-Stanojevic, N.; Spasic, S. High serum uric acid and low-grade inflammation are associated with smaller LDL and HDL particles. Atherosclerosis 2009, 203, 236–242. [Google Scholar] [CrossRef]
- Vekic, J.; Kotur-Stevuljevic, J.; Jelić-Ivanović, Z.; Spasic, S.; Spasojević-Kalimanovska, V.; Topić, A.; Zeljkovic, A.; Stefanovic, A.; Žunić, G. Association of oxidative stress and PON1 with LDL and HDL particle size in middle-aged subjects. Eur. J. Clin. Investig. 2007, 37, 715–723. [Google Scholar] [CrossRef]
- Yoo, T.W.; Sung, K.C.; Shin, H.S.; Kim, B.J.; Kim, B.S.; Kang, J.H.; Lee, M.H.; Park, J.R.; Kim, H.; Rhee, E.J.; et al. Relationship between Serum Uric Acid Concentration and Insulin Resistance and Metabolic Syndrome. Circ. J. 2005, 69, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-Y.; Zhu, W.-H.; Chen, Z.-W.; Dai, H.-L.; Ren, J.-J.; Chen, J.-H.; Chen, L.-Q.; Fang, L.-Z. Relationship between hyperuricemia and metabolic syndrome. J. Zhejiang Univ. B 2007, 8, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.; Kang, H.; Greffin, S.; Rosa, M.G.; Lugon, J. Serum uric acid and disorders of glucose metabolism: The role of glycosuria. Braz. J. Med. Biol. Res. 2014, 47, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Vallon, V.; Thomson, S.C. Renal Function in Diabetic Disease Models: The Tubular System in the Pathophysiology of the Diabetic Kidney. Annu. Rev. Physiol. 2012, 74, 351–375. [Google Scholar] [CrossRef]
- Ito, O.; Kondo, Y.; Takahashi, N.; Omata, K.; Abe, K. Role of calcium in insulin-stimulated NaC1 transport in medullary thick ascending limb. Am. J. Physiol. Physiol. 1995, 269, F236–F241. [Google Scholar] [CrossRef]
- Han, T.; Lan, L.; Qu, R.; Xu, Q.; Jiang, R.; Na, L.; Sun, C. Temporal Relationship Between Hyperuricemia and Insulin Resistance and Its Impact on Future Risk of Hypertension. Hypertension 2017, 70, 703–711. [Google Scholar] [CrossRef]
- Cicero, A.F.; Salvi, P.; D’Addato, S.; Rosticci, M.; Borghi, C. Association between serum uric acid, hypertension, vascular stiffness and subclinical atherosclerosis: Data from the Brisighella Heart Study. J. Hypertens. 2014, 32, 57–64. [Google Scholar] [CrossRef]
- Bombelli, M.; Ronchi, I.; Volpe, M.; Facchetti, R.; Carugo, S.; Dell’oro, R.; Cuspidi, C.; Grassi, G.; Mancia, G. Prognostic value of serum uric acid: New-onset in and out-of-office hypertension and long-term mortality. J. Hypertens. 2014, 32, 1237–1244. [Google Scholar] [CrossRef]
- Mishima, M.; Hamada, T.; Maharani, N.; Ikeda, N.; Onohara, T.; Notsu, T.; Ninomiya, H.; Miyazaki, S.; Mizuta, E.; Sugihara, S.; et al. Effects of Uric Acid on the NO Production of HUVECs and its Restoration by Urate Lowering Agents. Drug Res. 2016, 66, 270–274. [Google Scholar] [CrossRef]
- Sánchez-Lozada, L.G.; Soto, V.; Tapia, E.; Avila-Casado, C.; Sautin, Y.Y.; Nakagawa, T.; Franco, M.; Rodríguez-Iturbe, B.; Johnson, R.J. Role of oxidative stress in the renal abnormalities induced by experimental hyperuricemia. Am. J. Physiol.-Ren. Physiol. 2008, 295, F1134–F1141. [Google Scholar] [CrossRef]
- Kanellis, J.; Watanabe, S.; Li, J.H.; Kang, D.H.; Li, P.; Nakagawa, T.; Wamsley, A.; Sheikh-Hamad, D.; Lan, H.Y.; Feng, L.; et al. Uric Acid Stimulates Monocyte Chemoattractant Protein-1 Production in Vascular Smooth Muscle Cells Via Mitogen-Activated Protein Kinase and Cyclooxygenase-2. Hypertension 2003, 41, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- McAdams-DeMarco, M.A.; Law, A.; Maynard, J.W.; Coresh, J.; Baer, A.N. Risk factors for incident hyperuricemia during mid-adulthood in African American and White men and women enrolled in the ARIC cohort study. BMC Musculoskelet. Disord. 2013, 14, 347. [Google Scholar] [CrossRef] [PubMed]
- Messerli, F.H.; Frohlich, E.D.; Dreslinski, G.R.; Suarez, D.H.; Aristimuno, G.G. Serum Uric Acid in Essential Hypertension: An Indicator of Renal Vascular Involvement. Ann. Intern. Med. 1980, 93, 817–821. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | New-Onset Hyperuricemia (-) (n = 19,226) | New-Onset Hyperuricemia (+) (n = 1804) | p |
|---|---|---|---|
| Age (year) | 50.7 ± 10.3 | 52.6 ± 10.1 | <0.001 |
| Male (%) | 29.0 | 39.7 | <0.001 |
| DM (%) | 4.4 | 7.3 | <0.001 |
| Hypertension (%) | 9.6 | 17.5 | <0.001 |
| Smoking history (%) | 21.6 | 28.5 | <0.001 |
| Alcohol history (%) | 2.0 | 4.9 | <0.001 |
| Regular exercise habits (%) | 48.4 | 48.7 | 0.763 |
| Systolic BP (mmHg) | 115.4 ± 17.3 | 121.7 ± 17.9 | <0.001 |
| Diastolic BP (mmHg) | 71.0 ± 10.6 | 74.7 ± 10.7 | <0.001 |
| Body height (cm) | 160.5 ± 7.8 | 161.2 ± 8.2 | <0.001 |
| Body weight (kg) | 60.3 ± 10.6 | 65.5 ± 11.1 | <0.001 |
| Waist circumference (cm) | 81.2 ± 9.1 | 85.8 ± 9.0 | <0.001 |
| Hip circumference (cm) | 94.7 ± 6.3 | 97.1 ± 6.7 | <0.001 |
| BMI (kg/m2) | 23.3 ± 3.2 | 25.1 ± 3.4 | |
| Laboratory parameters | |||
| Uric acid (mg/dL) | 4.9 ± 1.0 | 5.8 ± 0.8 | <0.001 |
| Fasting glucose (mg/dL) | 95.0 ± 19.7 | 99.3 ± 25.2 | <0.001 |
| Hemoglobin (g/dL) | 13.5 ± 1.5 | 13.9 ± 1.5 | <0.001 |
| Triglyceride (mg/dL) | 101.2 ± 72.2 | 129.4 ± 81.0 | <0.001 |
| Total cholesterol (mg/dL) | 193.8 ± 34.7 | 196.6 ± 35.4 | 0.001 |
| HDL cholesterol (mg/dL) | 56.3 ± 13.3 | 51.5 ± 12.3 | <0.001 |
| LDL cholesterol (mg/dL) | 119.8 ± 31.0 | 124.2 ± 31.2 | <0.001 |
| eGFR (mL/min/1.73 m2) | 113.5 ± 24.7 | 105.0 ± 24.0 | <0.001 |
| MetS (%) | 11.5 | 22.7 | <0.001 |
| MetS numbers | 1.05 ± 1.12 | 1.62 ± 1.24 | <0.001 |
| MetS component | |||
| Abdominal obesity (%) | 37.4 | 52.4 | <0.001 |
| Hypertriglyceridemia (%) | 14.0 | 27.1 | <0.001 |
| Low HDL cholesterol (%) | 22.6 | 33.1 | <0.001 |
| Hyperglycemia (%) | 8.7 | 13.0 | <0.001 |
| High blood pressure (%) | 22.0 | 36.0 | <0.001 |
| Variables | Age and Sex Adjusted | Multivariable * | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | p | Odds Ratio (95% CI) | p | |
| Age (per 1 year) | 1.013 (1.008–1.018) | < 0.001 | 1.008 (1.003–1.014) | 0.003 |
| Female (vs. male) | 0.643 (0.582–0.711) | < 0.001 | 4.697 (3.900–5.657) | <0.001 |
| Smoking history | - | - | 1.047 (0.909–1.205) | 0.525 |
| Alcohol history | - | - | 1.804 (1.382–2.355) | <0.001 |
| Uric acid (per 1 mg/dL) | - | - | 5.337 (4.847–5.877) | <0.001 |
| Hemoglobin (per 1 g/dL) | - | - | 0.994 (0.950–1.041) | 0.809 |
| Total cholesterol (per 10 mg/dL) | - | - | 0.996 (0.993–0.999) | 0.015 |
| LDL-cholesterol (per 1 mg/dL) | - | - | 1.004 (1.001–1.008) | 0.021 |
| eGFR (per 1 mL/min/1.73 m2) | - | - | 0.996 (0.994–0.999) | 0.003 |
| MetS | 2.086 (1.849–2.354) | < 0.001 | 1.493 (1.312–1.700) | <0.001 |
| The Sum of MetS Components | Age and Sex Adjusted | Multivariable * | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | p | Odds Ratio (95% CI) | p | |
| MetS number: 0 | Reference | Reference | ||
| MetS number: 1 | 1.853 (1.614–2.126) | <0.001 | 1.413(1.222–1.634) | <0.001 |
| MetS number: 2 | 2.815 (2.438–3.249) | <0.001 | 1.918(1.647–2.233) | <0.001 |
| MetS number: 3 | 3.262 (2.750–3.869) | <0.001 | 1.915(1.597–2.296) | <0.001 |
| MetS number: 4 | 4.182 (3.326–5.258) | <0.001 | 2.428(1.901–3.100) | <0.001 |
| MetS number: 5 | 5.357 (3.533–8.121) | <0.001 | 3.593(2.281–5.658) | <0.001 |
| Mets Components | Age and Sex Adjusted | Multivariable * | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | p | Odds Ratio (95% CI) | p | |
| Abdominal obesity | 1.353 (1.304–1.404) | <0.001 | 1.180 (1.134–1.229) | <0.001 |
| Hypertriglyceridemia | 1.427 (1.369–1.488) | <0.001 | 1.293 (1.233–1.355) | <0.001 |
| Low HDL cholesterol | 1.303 (1.254–1.354) | <0.001 | 1.185 (1.135–1.236) | <0.001 |
| Hyperglycemia | 1.126 (1.070–1.185) | <0.001 | 1.136 (1.075–1.201) | <0.001 |
| High blood pressure | 1.251 (1.202–1.303) | <0.001 | 1.167 (1.118–1.217) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, Y.-C.; Liu, Y.-H.; Chen, S.-C.; Su, H.-M. Metabolic Syndrome and Its Components Are Associated with New-Onset Hyperuricemia in a Large Taiwanese Population Follow-Up Study. Nutrients 2023, 15, 1083. https://doi.org/10.3390/nu15051083
Tu Y-C, Liu Y-H, Chen S-C, Su H-M. Metabolic Syndrome and Its Components Are Associated with New-Onset Hyperuricemia in a Large Taiwanese Population Follow-Up Study. Nutrients. 2023; 15(5):1083. https://doi.org/10.3390/nu15051083
Chicago/Turabian StyleTu, Yen-Chieh, Yi-Hsueh Liu, Szu-Chia Chen, and Ho-Ming Su. 2023. "Metabolic Syndrome and Its Components Are Associated with New-Onset Hyperuricemia in a Large Taiwanese Population Follow-Up Study" Nutrients 15, no. 5: 1083. https://doi.org/10.3390/nu15051083
APA StyleTu, Y.-C., Liu, Y.-H., Chen, S.-C., & Su, H.-M. (2023). Metabolic Syndrome and Its Components Are Associated with New-Onset Hyperuricemia in a Large Taiwanese Population Follow-Up Study. Nutrients, 15(5), 1083. https://doi.org/10.3390/nu15051083







