Arm Circumference, Arm-to-Waist Ratio in Relation to Cardiovascular and All-Cause Mortality among Patients with Diabetes Mellitus
Abstract
1. Introduction
2. Materials and Methods
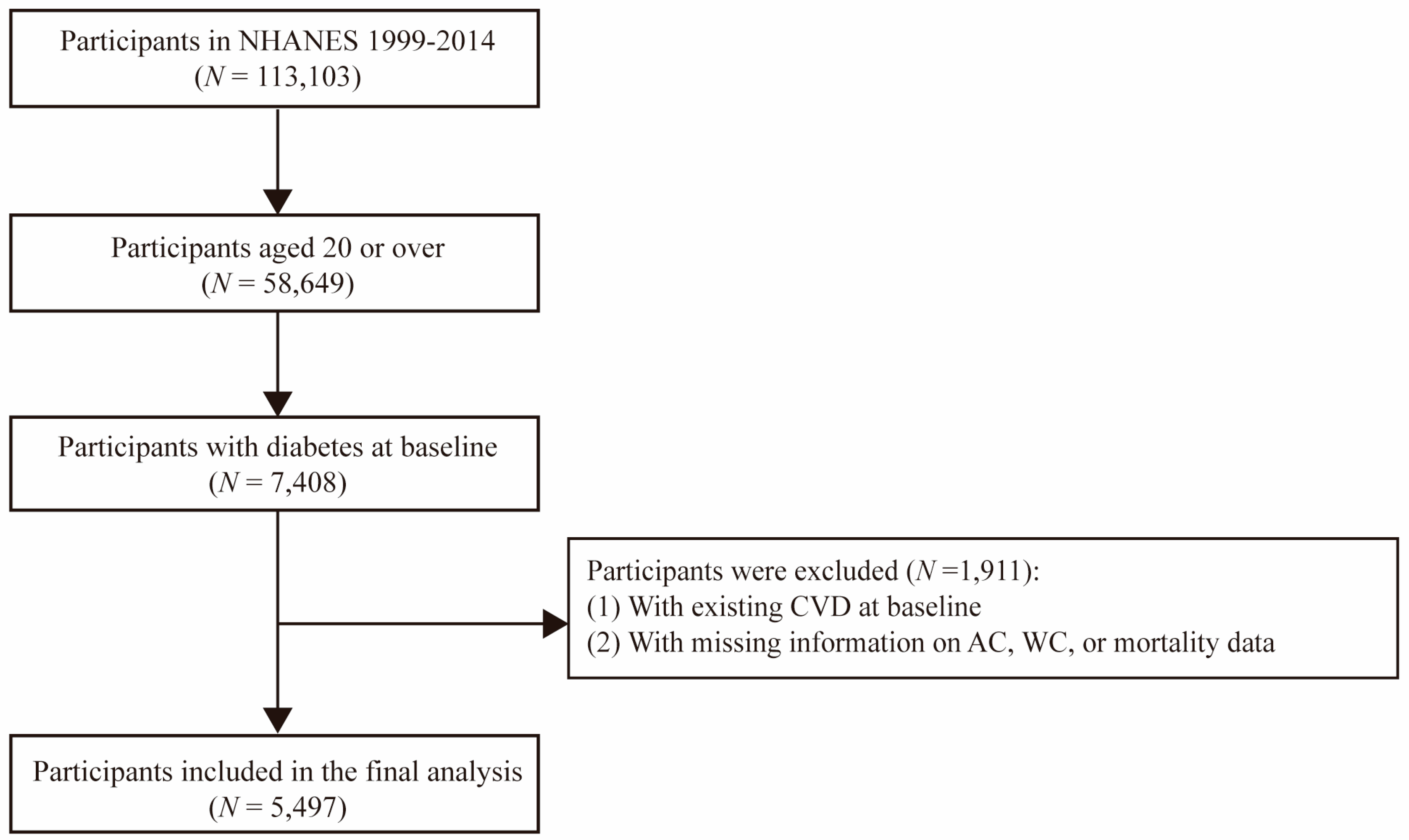
2.1. Study Population
2.2. Exposure Measurement
2.3. Covariate Assessment
2.4. Outcome Ascertainment
2.5. Statistical Analysis
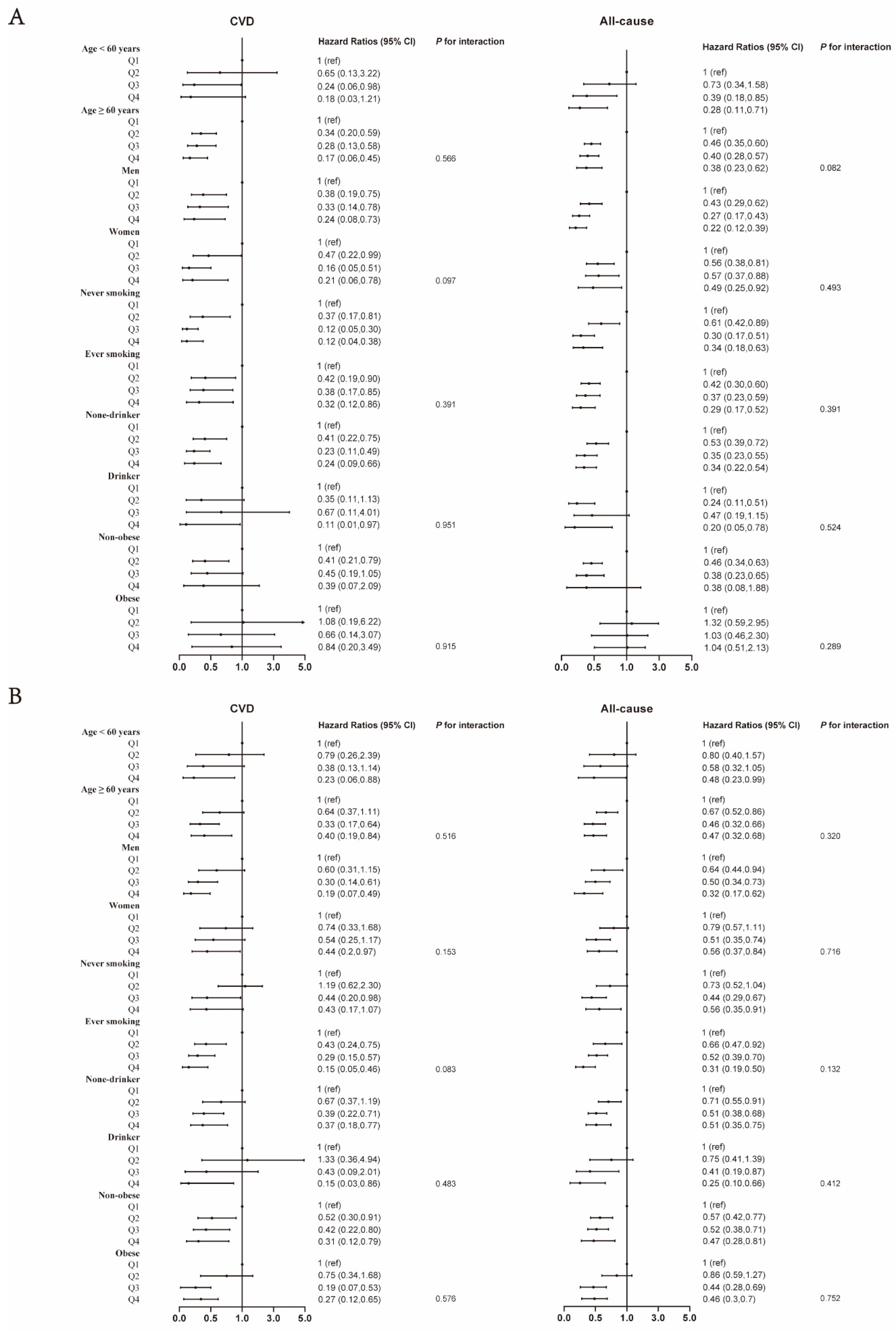
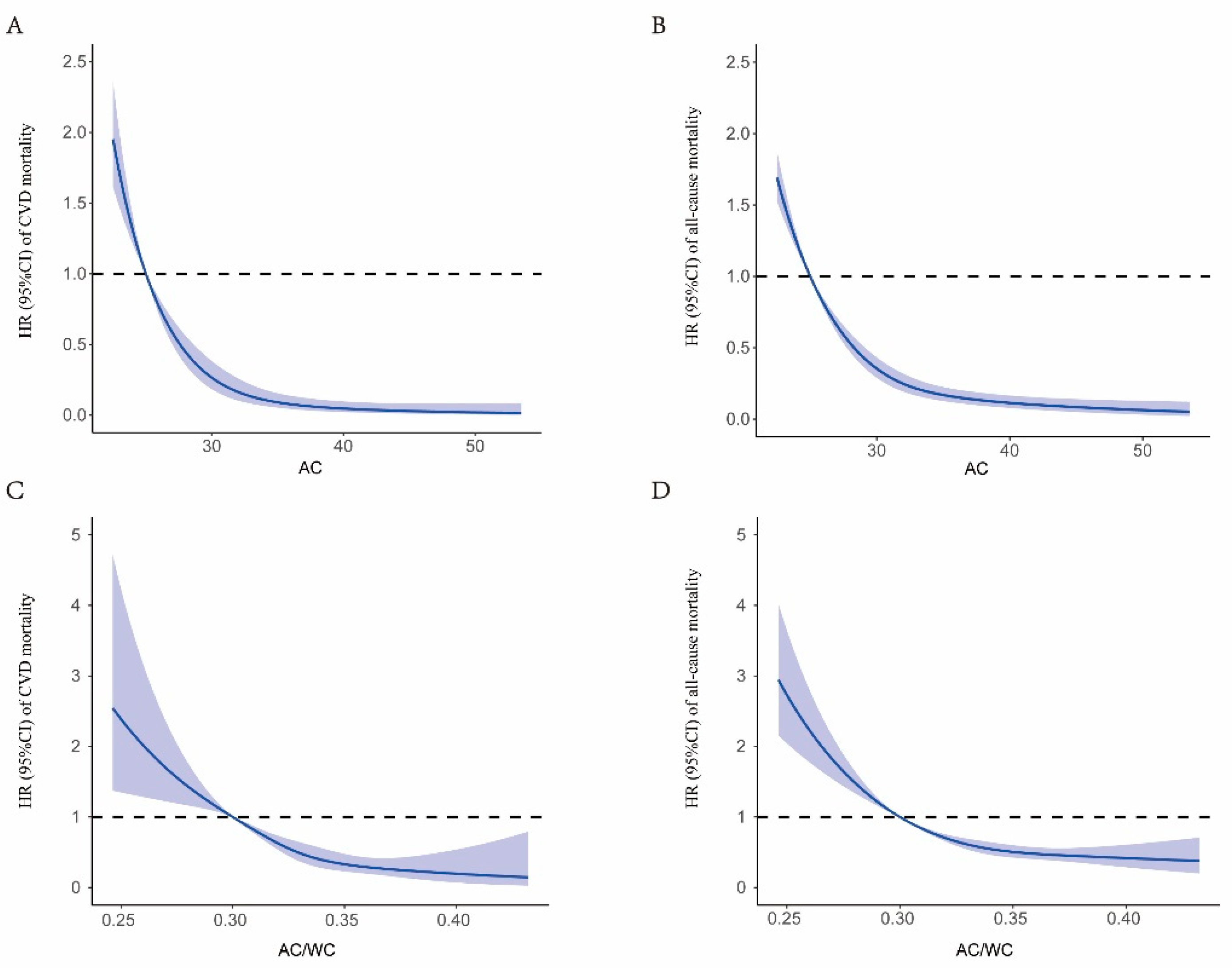
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schlesinger, S.; Neuenschwander, M.; Barbaresko, J.; Lang, A.; Maalmi, H.; Rathmann, W.; Roden, M.; Herder, C. Prediabetes and risk of mortality, diabetes-related complications and comorbidities: Umbrella review of meta-analyses of prospective studies. Diabetologia 2022, 65, 275–285. [Google Scholar] [CrossRef]
- WHO. Diabetes. 2023. Available online: https://www.who.int/health-topics/diabetes#tab=tab_1 (accessed on 28 January 2023).
- Bommer, C.; Heesemann, E.; Sagalova, V.; Manne-Goehler, J.; Atun, R.; Barnighausen, T.; Vollmer, S. The global economic burden of diabetes in adults aged 20–79 years: A cost-of-illness study. Lancet Diabetes Endocrinol. 2017, 5, 423–430. [Google Scholar] [CrossRef]
- Bommer, C.; Sagalova, V.; Heesemann, E.; Manne-Goehler, J.; Atun, R.; Barnighausen, T.; Davies, J.; Vollmer, S. Global Economic Burden of Diabetes in Adults: Projections From 2015 to 2030. Diabetes Care 2018, 41, 963–970. [Google Scholar] [CrossRef]
- Qiao, Q.; Nyamdorj, R. Is the association of type II diabetes with waist circumference or waist-to-hip ratio stronger than that with body mass index? Eur. J. Clin. Nutr. 2010, 64, 30–34. [Google Scholar] [CrossRef]
- Hou, X.; Chen, S.; Hu, G.; Chen, P.; Wu, J.; Ma, X.; Yang, Z.; Yang, W.; Jia, W. Stronger associations of waist circumference and waist-to-height ratio with diabetes than BMI in Chinese adults. Diabetes Res. Clin. Pract. 2019, 147, 9–18. [Google Scholar] [CrossRef]
- Wei, J.; Liu, X.; Xue, H.; Wang, Y.; Shi, Z. Comparisons of Visceral Adiposity Index, Body Shape Index, Body Mass Index and Waist Circumference and Their Associations with Diabetes Mellitus in Adults. Nutrients 2019, 11, 1580. [Google Scholar] [CrossRef]
- Grinker, J.A.; Tucker, K.L.; Vokonas, P.S.; Rush, D. Changes in patterns of fatness in adult men in relation to serum indices of cardiovascular risk: The Normative Aging Study. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 1369–1378. [Google Scholar] [CrossRef]
- ÇİÇEK, B.; Öztürk, A.; Mazicioğlu, M.M.; Inanc, N.; Kurtoğlu, S. A novel cut-off for abdominal obesity derived from various anthropometric indices to predict body composition: Arm fat area. Turk. J. Med. Sci. 2010, 4, 515–523. [Google Scholar] [CrossRef]
- Hou, Y.; Jia, X.; Xuan, L.; Zhu, W.; Deng, C.; Wang, L.; Zhao, Z.; Li, M.; Lu, J.; Xu, Y.; et al. Association between mid-upper arm circumference and cardiometabolic risk in Chinese population: A cross-sectional study. BMJ Open 2019, 9, e28904. [Google Scholar] [CrossRef]
- Zhu, Y.; Lin, Q.; Zhang, Y.; Deng, H.; Hu, X.; Yang, X.; Yao, B. Mid-upper arm circumference as a simple tool for identifying central obesity and insulin resistance in type 2 diabetes. PLoS ONE 2020, 15, e231308. [Google Scholar] [CrossRef]
- Devang, N.; Nandini, M.; Rao, S.; Adhikari, P. Mid Arm Circumference: An Alternate Anthropometric Index of Obesity in Type 2 Diabetes and Metabolic Syndrome. J. Adv. Med. Med. Res. 2016, 12, 1–8. [Google Scholar] [CrossRef]
- Wu, L.W.; Lin, Y.Y.; Kao, T.W.; Lin, C.M.; Wang, C.C.; Wang, G.C.; Peng, T.C.; Chen, W.L. Mid-Arm Circumference and All-Cause, Cardiovascular, and Cancer Mortality among Obese and Non-Obese US Adults: The National Health and Nutrition Examination Survey III. Sci. Rep. 2017, 7, 2302. [Google Scholar] [CrossRef]
- Abreo, A.P.; Bailey, S.R.; Abreo, K. Associations between calf, thigh, and arm circumference and cardiovascular and all-cause mortality in NHANES 1999-2004. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1410–1415. [Google Scholar] [CrossRef]
- Ness-Abramof, R.; Apovian, C.M. Waist circumference measurement in clinical practice. Nutr. Clin. Pract. 2008, 23, 397–404. [Google Scholar] [CrossRef]
- Despres, J.P. Intra-abdominal obesity: An untreated risk factor for Type 2 diabetes and cardiovascular disease. J. Endocrinol. Investig. 2006, 29 (Suppl. 3), 77–82. [Google Scholar]
- Katzmarzyk, P.T.; Janssen, I.; Ross, R.; Church, T.S.; Blair, S.N. The importance of waist circumference in the definition of metabolic syndrome: Prospective analyses of mortality in men. Diabetes Care 2006, 29, 404–409. [Google Scholar] [CrossRef]
- Janiszewski, P.M.; Janssen, I.; Ross, R. Does waist circumference predict diabetes and cardiovascular disease beyond commonly evaluated cardiometabolic risk factors? Diabetes Care 2007, 30, 3105–3109. [Google Scholar] [CrossRef]
- Yang, G.R.; Yuan, M.X.; Wan, G.; Zhang, X.L.; Fu, H.J.; Yuan, S.Y.; Zhu, L.X.; Xie, R.R.; Zhang, J.D.; Li, Y.L.; et al. Neck circumference and waist circumference associated with cardiovascular events in type 2 diabetes (Beijing Community Diabetes Study 23). Sci. Rep. 2021, 11, 9491. [Google Scholar] [CrossRef]
- Cameron, A.J.; Magliano, D.J.; Soderberg, S. A systematic review of the impact of including both waist and hip circumference in risk models for cardiovascular diseases, diabetes and mortality. Obes. Rev. 2013, 14, 86–94. [Google Scholar] [CrossRef]
- Han, T.S.; Al-Gindan, Y.Y.; Govan, L.; Hankey, C.R.; Lean, M. Associations of BMI, waist circumference, body fat, and skeletal muscle with type 2 diabetes in adults. Acta Diabetol. 2019, 56, 947–954. [Google Scholar] [CrossRef]
- Ahluwalia, N.; Dwyer, J.; Terry, A.; Moshfegh, A.; Johnson, C. Update on NHANES Dietary Data: Focus on Collection, Release, Analytical Considerations, and Uses to Inform Public Policy. Adv. Nutr. 2016, 7, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Dietary Guidelines Advisory Committee. Dietary Guidelines for Americans, 2015–2020, 8th ed.; Government Printing Office: Washington, DC, USA, 2015; p. 122.
- Zipf, G.; Chiappa, M.; Porter, K.S.; Ostchega, Y.; Lewis, B.G.; Dostal, J. National health and nutrition examination survey: Plan and operations, 1999–2010. Vital Health Stat 1 2013, 56, 1–37. [Google Scholar]
- Rasmussen, J.; Andersen, A.; Fisker, A.B.; Ravn, H.; Sodemann, M.; Rodrigues, A.; Benn, C.S.; Aaby, P. Mid-upper-arm-circumference and mid-upper-arm circumference z-score: The best predictor of mortality? Eur. J. Clin. Nutr. 2012, 66, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Mramba, L.; Ngari, M.; Mwangome, M.; Muchai, L.; Bauni, E.; Walker, A.S.; Gibb, D.M.; Fegan, G.; Berkley, J.A. A growth reference for mid upper arm circumference for age among school age children and adolescents, and validation for mortality: Growth curve construction and longitudinal cohort study. BMJ 2017, 358, j3423. [Google Scholar] [CrossRef]
- Schwinger, C.; Fadnes, L.T.; Van den Broeck, J. Using growth velocity to predict child mortality. Am. J. Clin. Nutr. 2016, 103, 801–807. [Google Scholar] [CrossRef]
- Tsai, A.C.; Lai, M.C.; Chang, T.L. Mid-arm and calf circumferences (MAC and CC) are better than body mass index (BMI) in predicting health status and mortality risk in institutionalized elderly Taiwanese. Arch. Gerontol. Geriatr. 2012, 54, 443–447. [Google Scholar] [CrossRef]
- Weng, C.H.; Tien, C.P.; Li, C.I.; L’Heureux, A.; Liu, C.S.; Lin, C.H.; Lin, C.C.; Lai, S.W.; Lai, M.M.; Lin, W.Y. Mid-upper arm circumference, calf circumference and mortality in Chinese long-term care facility residents: A prospective cohort study. BMJ Open 2018, 8, e20485. [Google Scholar] [CrossRef]
- Wijnhoven, H.A.; van Bokhorst-de, V.D.S.M.; Heymans, M.W.; de Vet, H.C.; Kruizenga, H.M.; Twisk, J.W.; Visser, M. Low mid-upper arm circumference, calf circumference, and body mass index and mortality in older persons. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 1107–1114. [Google Scholar] [CrossRef]
- Matjuda, E.N.; Engwa, G.A.; Letswalo, P.B.; Mungamba, M.M.; Sewani-Rusike, C.R.; Nkeh-Chungag, B.N. Association of Hypertension and Obesity with Risk Factors of Cardiovascular Diseases in Children Aged 6-9 Years Old in the Eastern Cape Province of South Africa. Children 2020, 7, 25. [Google Scholar] [CrossRef]
- Schneider, H.J.; Friedrich, N.; Klotsche, J.; Pieper, L.; Nauck, M.; John, U.; Dorr, M.; Felix, S.; Lehnert, H.; Pittrow, D.; et al. The predictive value of different measures of obesity for incident cardiovascular events and mortality. J. Clin. Endocrinol. Metab. 2010, 95, 1777–1785. [Google Scholar] [CrossRef]
- Erener, S. Diabetes, infection risk and COVID-19. Mol. Metab. 2020, 39, 101044. [Google Scholar] [CrossRef]
- Improta, C.A.; Nonaka, C.; Pereira, C.S.; Soares, M.; Macambira, S.G.; Souza, B. Exercise Training-Induced Changes in MicroRNAs: Beneficial Regulatory Effects in Hypertension, Type 2 Diabetes, and Obesity. Int. J. Mol. Sci. 2018, 19, 3608. [Google Scholar] [CrossRef]
- Oja, P.; Kelly, P.; Pedisic, Z.; Titze, S.; Bauman, A.; Foster, C.; Hamer, M.; Hillsdon, M.; Stamatakis, E. Associations of specific types of sports and exercise with all-cause and cardiovascular-disease mortality: A cohort study of 80 306 British adults. Br. J. Sports Med. 2017, 51, 812–817. [Google Scholar] [CrossRef]
- Nomura, T.; Kawae, T.; Kataoka, H.; Ikeda, Y. Assessment of lower extremity muscle mass, muscle strength, and exercise therapy in elderly patients with diabetes mellitus. Environ. Health Prev. Med. 2018, 23, 20. [Google Scholar] [CrossRef]
- Hayes, W.C.; Myers, E.R.; Morris, J.N.; Gerhart, T.N.; Yett, H.S.; Lipsitz, L.A. Impact near the hip dominates fracture risk in elderly nursing home residents who fall. Calcif. Tissue Int. 1993, 52, 192–198. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, R.; Ding, L.; Meng, Z.; Zhang, Q.; Shen, Y.; Hu, G.; Liu, M. Waist Circumference and its Changes Are More Strongly Associated with the Risk of Type 2 Diabetes than Body Mass Index and Changes in Body Weight in Chinese Adults. J. Nutr. 2020, 150, 1259–1265. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, S.M.; Han, K.D.; Jung, J.H.; Lee, S.S.; Oh, S.W.; Park, H.S.; Rhee, E.J.; Lee, W.Y.; Yoo, S.J. Waist Circumference and All-Cause Mortality Independent of Body Mass Index in Korean Population from the National Health Insurance Health Checkup 2009(-)2015. J. Clin. Med. 2019, 8, 72. [Google Scholar] [CrossRef]
- Jung, S.; Park, J.; Seo, Y.G. Relationship between arm-to-leg and limbs-to-trunk body composition ratio and cardiovascular disease risk factors. Sci. Rep. 2021, 11, 17414. [Google Scholar] [CrossRef]
- Steinberg, H.O.; Paradisi, G.; Cronin, J.; Crowde, K.; Hempfling, A.; Hook, G.; Baron, A.D. Type II diabetes abrogates sex differences in endothelial function in premenopausal women. Circulation 2000, 101, 2040–2046. [Google Scholar] [CrossRef]
- Han, T.S.; Sattar, N.; Williams, K.; Gonzalez-Villalpando, C.; Lean, M.E.; Haffner, S.M. Prospective study of C-reactive protein in relation to the development of diabetes and metabolic syndrome in the Mexico City Diabetes Study. Diabetes Care 2002, 25, 2016–2021. [Google Scholar] [CrossRef]
- Stapleton, P.A.; James, M.E.; Goodwill, A.G.; Frisbee, J.C. Obesity and vascular dysfunction. Pathophysiology 2008, 15, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, J.; Ren, Z.; Meng, W.; Tang, J.; Zhao, S.; Chi, C.; Xiong, J.; Teliewubai, J.; Maimaitiaili, R.; et al. Prognostic Value of Arm Circumference for Cardiac Damage and Major Adverse Cardiovascular Events: A Friend or a Foe? A 2-Year Follow-Up in the Northern Shanghai Study. Front. Cardiovasc. Med. 2022, 9, 816011. [Google Scholar] [CrossRef] [PubMed]
- Bates, C.J.; Hamer, M.; Mishra, G.D. A study of relationships between bone-related vitamins and minerals, related risk markers, and subsequent mortality in older British people: The National Diet and Nutrition Survey of People Aged 65 Years and Over. Osteoporos. Int. 2012, 23, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Ho, S.C.; Sham, A. Longitudinal changes in body mass index and body composition over 3 years and relationship to health outcomes in Hong Kong Chinese age 70 and older. J. Am. Geriatr. Soc. 2001, 49, 737–746. [Google Scholar] [CrossRef]
- Lo, K.; Huang, Y.Q.; Shen, G.; Huang, J.Y.; Liu, L.; Yu, Y.L.; Chen, C.L.; Feng, Y.Q. Effects of waist to height ratio, waist circumference, body mass index on the risk of chronic diseases, all-cause, cardiovascular and cancer mortality. Postgrad. Med. J. 2021, 97, 306–311. [Google Scholar] [CrossRef]
- Doehner, W.; Erdmann, E.; Cairns, R.; Clark, A.L.; Dormandy, J.A.; Ferrannini, E.; Anker, S.D. Inverse relation of body weight and weight change with mortality and morbidity in patients with type 2 diabetes and cardiovascular co-morbidity: An analysis of the PROactive study population. Int. J. Cardiol. 2012, 162, 20–26. [Google Scholar] [CrossRef]
- Hainer, V.; Aldhoon-Hainerova, I. Obesity paradox does exist. Diabetes Care 2013, 36 (Suppl. 2), S276–S281. [Google Scholar] [CrossRef]
- Alharbi, T.A.; Ryan, J.; Freak-Poli, R.; Gasevic, D.; Scali, J.; Ritchie, K.; Ancelin, M.L.; Owen, A.J. The Association of Weight Loss, Weight Status, and Abdominal Obesity with All-Cause Mortality in Older Adults. Gerontology 2022, 68, 1366–1374. [Google Scholar] [CrossRef]
| Characteristic | AC | AC/WC | ||||||
|---|---|---|---|---|---|---|---|---|
| Q1 <31.40 | Q2 31.40–34.49 | Q3 34.50–37.99 | Q4 ≥38.00 | Q1 <0.30 | Q2 0.30–0.32 | Q3 0.33–0.34 | Q4 ≥0.35 | |
| Number of participants | 1351 | 1385 | 1341 | 1420 | 1374 | 1375 | 1373 | 1375 |
| Age, years | ||||||||
| <40 | 85 (9.70) | 96 (9.10) | 142 (15.06) | 207 (16.17) | 83 (8.32) | 116 (11.24) | 136 (12.38) | 195 (19.27) |
| 40–59 | 334 (33.93) | 451 (40.94) | 518 (45.14) | 656 (56.67) | 331 (34.49) | 441 (40.46) | 564 (51.28) | 623 (53.93) |
| ≥60 | 932 (56.36) | 838 (49.96) | 681 (39.80) | 557 (27.16) | 960 (57.19) | 818 (48.30) | 673 (36.34) | 557 (26.80) |
| Gender | ||||||||
| Male | 641 (42.34) | 746 (53.16) | 743 (58.04) | 680 (50.42) | 762 (54.65) | 749 (56.16) | 740 (53.37) | 559 (41.54) |
| Female | 710 (57.66) | 639 (46.84) | 598 (41.96) | 740 (49.58) | 612 (45.35) | 626 (43.84) | 633 (46.63) | 816 (58.46) |
| Race/ethnicity | ||||||||
| Mexican American | 320 (9.53) | 362 (10.86) | 300 (8.65) | 191 (6.15) | 274 (7.58) | 348 (10.01) | 306 (9.43) | 245 (7.40) |
| Non-Hispanic white | 465 (55.32) | 478 (58.81) | 558 (68.38) | 522 (63.30) | 683 (73.75) | 538 (65.73) | 424 (55.44) | 378 (53.37) |
| Non-Hispanic black | 240 (10.76) | 312 (13.47) | 330 (13.36) | 563 (21.61) | 236 (9.12) | 282 (10.91) | 370 (15.54) | 557 (25.10) |
| Other | 326 (24.39) | 233 (16.86) | 153 (9.61) | 144 (8.94) | 181 (9.55) | 207 (13.35) | 273 (19.59) | 195 (14.12) |
| Education | ||||||||
| Less than high school | 590 (30.35) | 566 (28.11) | 460 (21.34) | 417 (20.39) | 563 (27.73) | 534 (26.03) | 485 (23.02) | 451 (21.52) |
| High school or equivalent | 301 (24.55) | 278 (22.44) | 309 (24.17) | 378 (29.83) | 317 (27.14) | 292 (24.78) | 322 (23.88) | 335 (26.30) |
| College or above | 456 (45.11) | 538 (49.45) | 570 (54.49) | 625 (49.77) | 491 (45.12) | 546 (49.19) | 564 (53.10) | 588 (52.18) |
| Alcohol drinking status | ||||||||
| Never drinking | 1050 (78.91) | 1078 (80.15) | 1082 (80.93) | 1170 (81.66) | 1118 (83.24) | 1079 (79.85) | 1086 (79.42) | 1097 (79.84) |
| Moderate drinking | 112 (10.64) | 138 (10.50) | 97 (9.13) | 100 (10.01) | 104 (10.16) | 113 (9.09) | 116 (10.73) | 114 (10.10) |
| Heavy drinking | 107 (10.45) | 104 (9.35) | 116 (9.94) | 108 (8.33) | 80 (6.60) | 118 (11.06) | 124 (9.85) | 113 (10.05) |
| Smoking status | ||||||||
| Never smoker | 660 (47.36) | 691 (49.13) | 644 (49.51) | 755 (54.66) | 607 (42.74) | 658 (49.48) | 680 (48.72) | 805 (60.51) |
| Former smoker | 440 (33.74) | 458 (34.14) | 469 (33.36) | 424 (27.67) | 517 (36.74) | 488 (34.79) | 429 (32.59) | 357 (23.98) |
| Current smoker | 251 (18.90) | 234 (16.73) | 224 (17.13) | 240 (17.68) | 250 (20.52) | 227 (15.73) | 260 (18.68) | 212 (15.50) |
| BMI, kg/m2 | 25.02 ± 3.08 | 29.23 ± 3.29 | 32.75 ± 3.60 | 40.50 ± 6.40 | 32.10 ± 7.32 | 31.47 ± 7.02 | 31.83 ± 6.83 | 32.51 ± 7.50 |
| Total cholesterol, mg/dL | 199.45 ± 52.37 | 197.05 ± 45.20 | 197.93 ± 52.40 | 191.67 ± 44.82 | 191.78 ± 49.49 | 196.88 ± 48.44 | 199.45 ± 50.48 | 197.77 ± 46.56 |
| HDL Cholesterol, mg/dL | 52.61 ± 15.51 | 48.40 ± 13.40 | 46.28 ± 12.80 | 45.45 ± 12.08 | 47.21 ± 13.42 | 47.29 ± 13.77 | 48.20 ± 13.90 | 49.94 ± 13.80 |
| Carbohydrate intake, gm | 213.06 ± 106.94 | 217.14 ± 102.17 | 233.19 ± 113.55 | 235.79 ± 125.54 | 218.27 ± 110.59 | 229.98 ± 110.64 | 230.70 ± 119.61 | 220.94 ± 110.38 |
| Dietary fiber intake, gm | 16.32 ± 9.96 | 16.19 ± 10.42 | 17.01 ± 11.11 | 15.59 ± 10.21 | 15.79 ± 9.91 | 16.59 ± 10.02 | 16.49 ± 10.47 | 16.19 ± 11.29 |
| Fasting Glucose, mmol/L | 8.93 ± 3.79 | 8.89 ± 3.80 | 9.11 ± 3.60 | 8.62 ± 3.09 | 8.99 ± 3.77 | 8.75 ± 3.43 | 8.93 ± 3.52 | 8.88 ± 3.62 |
| History of hypertension | ||||||||
| Yes | 733 (48.57) | 786 (55.39) | 832 (57.51) | 982 (66.93) | 896 (67.91) | 806 (54.80) | 818 (56.32) | 813 (53.11) |
| No | 617 (51.43) | 594 (44.61) | 504 (42.49) | 433 (33.07) | 473 (32.09) | 564 (45.20) | 552 (43.68) | 559 (46.89) |
| History of dyslipidemia | ||||||||
| Yes | 892 (64.75) | 956 (68.95) | 973 (74.53) | 993 (71.71) | 959 (71.44) | 990 (73.97) | 959 (69.85) | 906 (66.22) |
| No | 459 (35.25) | 429 (31.05) | 368 (25.47) | 427 (28.29) | 415 (28.56) | 385 (26.03) | 414 (30.15) | 469 (33.78) |
| History of cancer | ||||||||
| Yes | 189 (16.91) | 183 (15.09) | 154 (13.97) | 156 (11.79) | 221 (19.95) | 184 (14.56) | 160 (13.88) | 117 (8.78) |
| No | 1161 (83.09) | 1197 (84.91) | 1186 (86.03) | 1262 (88.21) | 1152 (80.05) | 1188 (85.44) | 1210 (86.12) | 1256 (91.22) |
| Self-reported health | ||||||||
| Very good to excellent | 238 (25.84) | 281 (30.42) | 270 (26.6) | 169 (14.72) | 223 (21.13) | 229 (23.28) | 244 (23.8) | 262 (27.14) |
| Good | 448 (40.05) | 464 (41) | 487 (45.53) | 497 (46.57) | 477 (43.36) | 480 (42.52) | 479 (43.17) | 460 (45.54) |
| Poor to fair | 463 (34.11) | 431 (28.58) | 386 (27.87) | 559 (38.7) | 498 (35.51) | 472 (34.2) | 455 (33.03) | 414 (27.32) |
| Characteristic | AC | AC/WC | ||||||
|---|---|---|---|---|---|---|---|---|
| Q1 <31.40 | Q2 31.40–34.49 | Q3 34.50–37.99 | Q4 ≥38.00 | Q1 <0.30 | Q2 0.30–0.32 | Q3 0.33–0.34 | Q4 ≥0.35 | |
| CVD | ||||||||
| Number of participants | 1351 | 1385 | 1341 | 1420 | 1374 | 1375 | 1373 | 1375 |
| Deaths/person-years | 121/9510 | 64/10,812 | 46/10,753 | 40/10,986 | 122/9459 | 75/10,306 | 44/10,664 | 30/11,631 |
| Unadjusted | 1[Reference] | 0.52 (0.29, 0.94) | 0.40 (0.23, 0.70) | 0.36 (0.21, 0.64) | 1[Reference] | 0.59 (0.34, 1.02) | 0.28 (0.16, 0.49) | 0.17 (0.09, 0.33) |
| Model 1 | 1[Reference] | 0.51 (0.29, 0.87) | 0.46 (0.27, 0.76) | 0.52 (0.31, 0.86) | 1[Reference] | 0.62 (0.37, 1.05) | 0.34 (0.19, 0.59) | 0.25 (0.13, 0.47) |
| Model 2 | 1[Reference] | 0.34 (0.20, 0.57) | 0.23 (0.12, 0.44) | 0.17 (0.07, 0.45) | 1[Reference] | 0.62 (0.37, 1.05) | 0.33 (0.19, 0.58) | 0.26 (0.14, 0.51) |
| Model 3 | 1[Reference] | 0.34 (0.20, 0.57) | 0.24 (0.13, 0.45) | 0.17 (0.07, 0.45) | 1[Reference] | 0.61 (0.37, 0.99) | 0.33 (0.19, 0.58) | 0.25 (0.13, 0.49) |
| Model 4 | 1[Reference] | 0.37 (0.22, 0.62) | 0.24 (0.12, 0.48) | 0.19 (0.07, 0.48) | 1[Reference] | 0.65 (0.38, 1.09) | 0.34 (0.20, 0.60) | 0.28 (0.15, 0.53) |
| All-cause mortality | ||||||||
| Number of participants | 1351 | 1385 | 1341 | 1420 | 1374 | 1375 | 1373 | 1375 |
| Deaths/person-years | 402/9510 | 288/10,812 | 217/10,753 | 186/10,986 | 437/9459 | 283/10,306 | 208/10,664 | 165/11,631 |
| Unadjusted | 1[Reference] | 0.61 (0.45, 0.81) | 0.46 (0.34, 0.63) | 0.40 (0.30, 0.52) | 1[Reference] | 0.63 (0.48, 0.82) | 0.39 (0.28, 0.55) | 0.27 (0.19, 0.39) |
| Model 1 | 1[Reference] | 0.60 (0.46, 0.79) | 0.52 (0.39, 0.69) | 0.55 (0.43, 0.72) | 1[Reference] | 0.67 (0.53, 0.85) | 0.48 (0.35, 0.65) | 0.39 (0.28, 0.54) |
| Model 2 | 1[Reference] | 0.47 (0.36, 0.62) | 0.34 (0.24, 0.48) | 0.29 (0.19, 0.43) | 1[Reference] | 0.69 (0.53, 0.88) | 0.48 (0.36, 0.65) | 0.43 (0.30, 0.60) |
| Model 3 | 1[Reference] | 0.46 (0.35, 0.61) | 0.35 (0.25, 0.49) | 0.29 (0.20, 0.44) | 1[Reference] | 0.67 (0.52, 0.86) | 0.48 (0.36, 0.64) | 0.42 (0.30, 0.59) |
| Model 4 | 1[Reference] | 0.47 (0.36, 0.61) | 0.35 (0.25, 0.49) | 0.29 (0.20, 0.43) | 1[Reference] | 0.69 (0.53, 0.88) | 0.49 (0.37, 0.64) | 0.43 (0.31, 0.62) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, X.; Yu, X.; Zhu, H.; Zhai, X.; Li, S.; Ma, W.; Ouyang, M.; Liu, K.; Eshak, E.S.; Cao, J. Arm Circumference, Arm-to-Waist Ratio in Relation to Cardiovascular and All-Cause Mortality among Patients with Diabetes Mellitus. Nutrients 2023, 15, 961. https://doi.org/10.3390/nu15040961
Xiao X, Yu X, Zhu H, Zhai X, Li S, Ma W, Ouyang M, Liu K, Eshak ES, Cao J. Arm Circumference, Arm-to-Waist Ratio in Relation to Cardiovascular and All-Cause Mortality among Patients with Diabetes Mellitus. Nutrients. 2023; 15(4):961. https://doi.org/10.3390/nu15040961
Chicago/Turabian StyleXiao, Xinyu, Xinyi Yu, Huiping Zhu, Xiaobing Zhai, Shiyang Li, Wenzhi Ma, Meishuo Ouyang, Keyang Liu, Ehab S. Eshak, and Jinhong Cao. 2023. "Arm Circumference, Arm-to-Waist Ratio in Relation to Cardiovascular and All-Cause Mortality among Patients with Diabetes Mellitus" Nutrients 15, no. 4: 961. https://doi.org/10.3390/nu15040961
APA StyleXiao, X., Yu, X., Zhu, H., Zhai, X., Li, S., Ma, W., Ouyang, M., Liu, K., Eshak, E. S., & Cao, J. (2023). Arm Circumference, Arm-to-Waist Ratio in Relation to Cardiovascular and All-Cause Mortality among Patients with Diabetes Mellitus. Nutrients, 15(4), 961. https://doi.org/10.3390/nu15040961






