Plasma LEAP-2 Following a Low-Calorie Diet with or without Interval Exercise in Women with Obesity
Abstract
1. Introduction
2. Methods
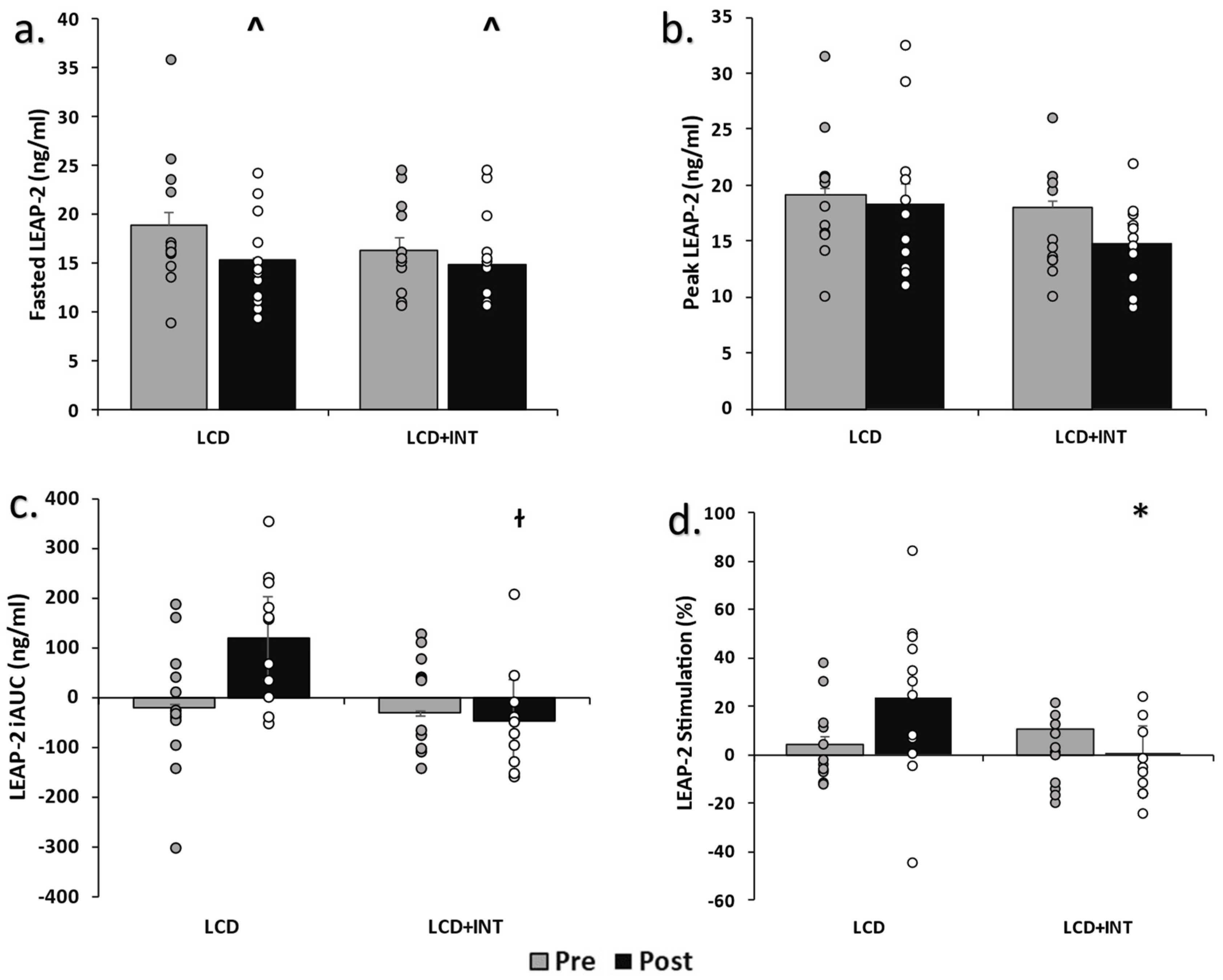
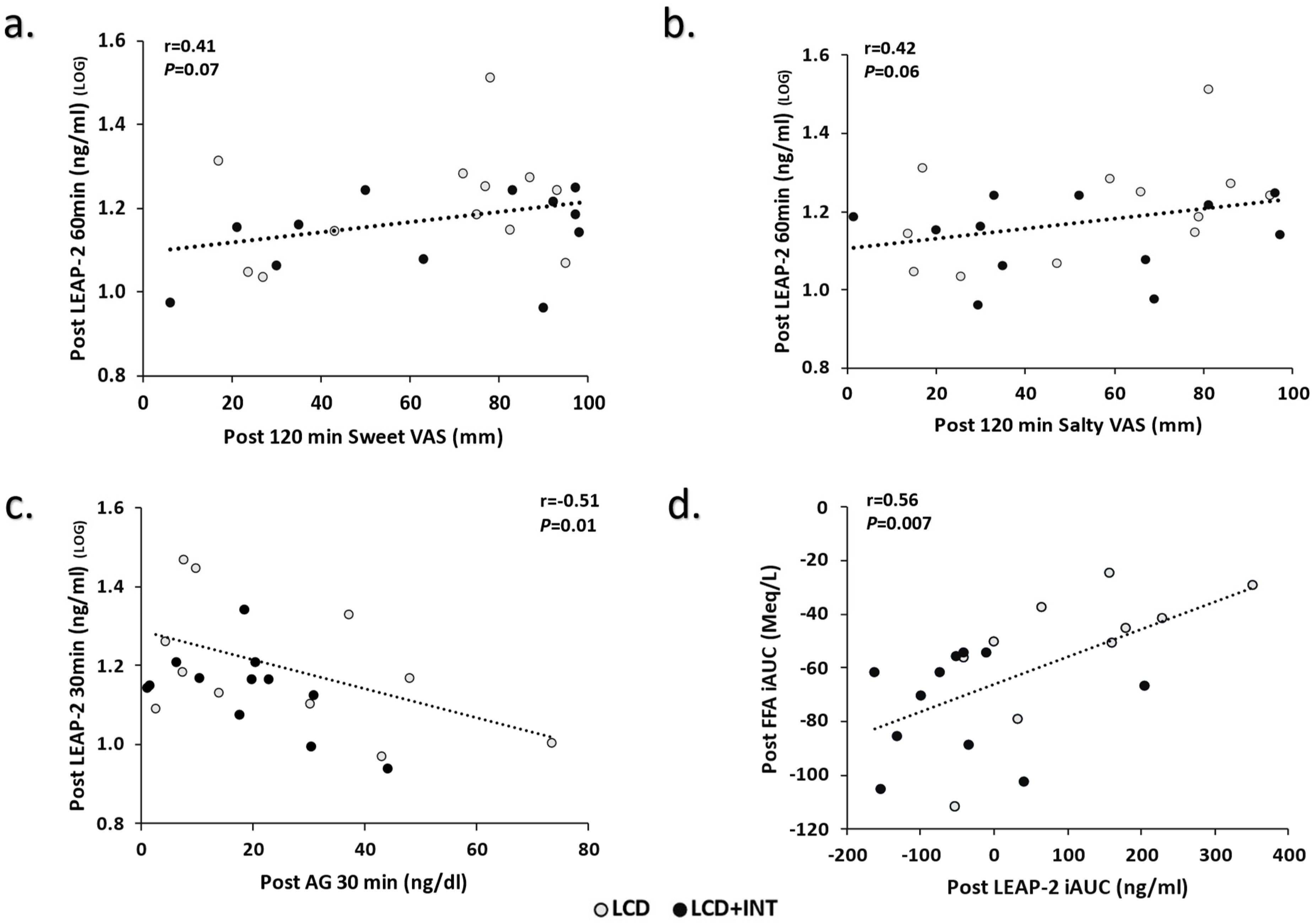
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cawley, J.; Biener, A.; Meyerhoefer, C.; Ding, Y.; Zvenyach, T.; Smolarz, B.G.; Ramasamy, A. Direct medical costs of obesity in the United States and the most populous states. J. Manag. Care Spec. Pharm. 2021, 27, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Deschaine, S.L.; Leggio, L. From “Hunger Hormone” to “It’s Complicated”: Ghrelin Beyond Feeding Control. Physiology 2022, 37, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liszt, K.I.; Deloose, E.; Canovai, E.; Thijs, T.; Farré, R.; Ceulemans, L.J.; Lannoo, M.; Tack, J.; Depoortere, I. Obesity alters adrenergic and chemosensory signaling pathways that regulate ghrelin secretion in the human gut. FASEB J. 2019, 33, 4907–4920. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, C.A.; Jensen, M.S.; Holm, S.; Gasbjerg, L.S.; Byberg, S.; Skov-Jeppesen, K.; Hartmann, B.; Holst, J.J.; Dela, F.; Vilsbøll, T.; et al. LEAP2 reduces postprandial glucose excursions and ad libitum food intake in healthy men. Cell. Rep. Med. 2022, 3, 100582. [Google Scholar] [CrossRef] [PubMed]
- Shankar, K.; Metzger, N.P.; Singh, O.; Mani, B.K.; Osborne-Lawrence, S.; Varshney, S.; Gupta, D.; Ogden, S.B.; Takemi, S.; Richard, C.P.; et al. LEAP2 deletion in mice enhances ghrelin’s actions as an orexigen and growth hormone secretagogue. Mol. Metab. 2021, 53, 101327. [Google Scholar] [CrossRef]
- Lu, X.; Huang, L.; Huang, Z.; Feng, D.; Clark, R.J.; Chen, C. LEAP-2: An Emerging Endogenous Ghrelin Receptor Antagonist in the Pathophysiology of Obesity. Front. Endocrinol. 2021, 12, 717544. [Google Scholar] [CrossRef]
- Fernandez, G.; Cabral, A.; De Francesco, P.N.; Uriarte, M.; Reynaldo, M.; Castrogiovanni, D.; Zubiría, G.; Giovambattista, A.; Cantel, S.; Denoyelle, S.; et al. GHSR controls food deprivation-induced activation of CRF neurons of the hypothalamic paraventricular nucleus in a LEAP2-dependent manner. Cell. Mol. Life Sci. 2022, 79, 1–18. [Google Scholar] [CrossRef]
- Ge, X.; Yang, H.; Bednarek, M.A.; Galon-Tilleman, H.; Chen, P.; Chen, M.; Lichtman, J.S.; Wang, Y.; Dalmas, O.; Yin, Y.; et al. LEAP2 Is an Endogenous Antagonist of the Ghrelin Receptor. Cell Metab. 2018, 27, 461–469.e6. [Google Scholar] [CrossRef]
- Islam, M.N.; Mita, Y.; Maruyama, K.; Tanida, R.; Zhang, W.; Sakoda, H.; Nakazato, M. Liver-expressed antimicrobial peptide 2 antagonizes the effect of ghrelin in rodents. J. Endocrinol. 2020, 244, 13–23. [Google Scholar] [CrossRef]
- Hagemann, C.A.; Zhang, C.; Hansen, H.H.; Jorsal, T.; Rigbolt, K.T.G.; Madsen, M.R.; Bergmann, N.C.; Heimbürger, S.M.N.; Falkenhahn, M.; Theis, S.; et al. Identification and Metabolic Profiling of a Novel Human Gut-derived LEAP2 Fragment. J. Clin. Endocrinol. Metab. 2021, 106, E966–E981. [Google Scholar] [CrossRef]
- Mani, B.K.; Puzziferri, N.; He, Z.; Rodriguez, J.A.; Osborne-Lawrence, S.; Metzger, N.P.; Chhina, N.; Gaylinn, B.; Thorner, M.O.; Thomas, E.L.; et al. LEAP2 changes with body mass and food intake in humans and mice. J. Clin. Invest. 2019, 129, 3909–3923. [Google Scholar] [CrossRef]
- Nunez-Salces, M.; Li, H.; Feinle-Bisset, C.; Young, R.L.; Page, A.J. Nutrient-sensing components of the mouse stomach and the gastric ghrelin cell. Neurogastroenterol. Motil. 2020, 32, e13944. [Google Scholar] [CrossRef] [PubMed]
- Holm, S.; Husted, A.S.; Skov, L.J.; Morville, T.H.; Hagemann, C.A.; Jorsal, T.; Dall, M.; Jakobsen, A.; Klein, A.B.; Treebak, J.T.; et al. Beta-Hydroxybutyrate Suppresses Hepatic Production of the Ghrelin Receptor Antagonist LEAP2. Endocrinology 2022, 163, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Malin, S.K.; Heiston, E.M.; Gilbertson, N.M.; Eichner, N.Z.M. Short-term interval exercise suppresses acylated ghrelin and hunger during caloric restriction in women with obesity. Physiol. Behav. 2020, 223, 112978. [Google Scholar] [CrossRef]
- Howe, S.M.; Hand, T.M.; Manore, M.M. Exercise-trained men and women: Role of exercise and diet on appetite and energy intake. Nutrients 2014, 6, 4935–4960. [Google Scholar] [CrossRef] [PubMed]
- Heiston, E.M.; Eichner, N.Z.M.; Gilbertson, N.M.; Gaitan, J.; Kranz, S.; Weltman, A.; Malin, S.K. Two weeks of exercise training intensity on appetite regulation in obese adults with prediabetes. J. Appl. Physiol. 2019, 126, 746–754. [Google Scholar] [CrossRef]
- Gilbertson, N.M.; Eichner, N.Z.; Heiston, E.M.; Gaitan, J.; Francois, M.E.; Mehaffey, J.H.; Hassinger, T.E.; Hallowell, P.T.; Weltman, A.L.; Malin, S.K. A low-calorie diet with or without interval exercise training improves adiposopathy in obese women. Appl. Physiol. Nutr. Metab. 2019, 44, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Francois, M.E.; Gilbertson, N.M.; Eichner, N.Z.M.; Heiston, E.M.; Fabris, C.; Breton, M.; Mehaffey, J.H.; Hassinger, T.; Hallowell, P.T.; Malin, S.K. Combining Short-Term Interval Training with Caloric Restriction Improves ß-Cell Function in Obese Adults. Nutrients 2018, 10, 717. [Google Scholar] [CrossRef]
- Lugilde, J.; Casado, S.; Beiroa, D.; Cuñarro, J.; Garcia-Lavandeira, M.; Álvarez, C.V.; Nogueiras, R.; Diéguez, C.; Tovar, S. LEAP-2 Counteracts Ghrelin-Induced Food Intake in a Nutrient, Growth Hormone and Age Independent Manner. Cells 2022, 11, 324. [Google Scholar] [CrossRef]
- Li, J.; Huang, P.; Xiong, J.; Liang, X.; Li, M.; Ke, H.; Chen, C.; Han, Y.; Huang, Y.; Zhou, Y.; et al. Serum levels of ghrelin and LEAP2 in patients with type 2 diabetes mellitus: Correlation with circulating glucose and lipids. Endocr. Connect. 2022, 11, e220012. [Google Scholar] [CrossRef] [PubMed]
- King, J.; Wasse, L.K.; Ewens, J.; Crystallis, K.; Emmanuel, J.; Batterham, R.; Stensel, D. Differential acylated ghrelin, peptide YY3-36, appetite, and food intake responses to equivalent energy deficits created by exercise and food restriction. J. Clin. Endocrinol. Metab. 2011, 96, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Hazell, T.J.; Islam, H.; Townsend, L.K.; Schmale, M.S.; Copeland, J.L. Effects of exercise intensity on plasma concentrations of appetite-regulating hormones: Potential mechanisms. Appetite 2016, 98, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Holliday, A.; Blannin, A. Appetite, food intake and gut hormone responses to intense aerobic exercise of different duration. J. Endocrinol. 2017, 235, 193–205. [Google Scholar] [CrossRef]
- Slam, H.; Townsend, L.K.; McKie, G.L.; Medeiros, P.J.; Gurd, B.J.; Hazell, T.J. Potential involvement of lactate and interleukin-6 in the appetite-regulatory hormonal response to an acute exercise bout. J. Appl. Physiol. 2017, 123, 614–623. [Google Scholar]
- Pepino, M.Y.; Finkbeiner, S.; Beauchamp, G.K.; Mennella, J.A. Obese women have lower monosodium glutamate taste sensitivity and prefer higher concentrations than do normal-weight women. Obesity 2010, 18, 959–965. [Google Scholar] [CrossRef]
- Noel, C.A.; Sugrue, M.; Dando, R. Participants with pharmacologically impaired taste function seek out more intense, higher calorie stimuli. Appetite 2017, 117, 74–81. [Google Scholar] [CrossRef]
- Noel, C.A.; Cassano, P.A.; Dando, R. College-Aged Males Experience Attenuated Sweet and Salty Taste with Modest Weight Gain. J. Nutr. 2017, 147, 1885–1891. [Google Scholar] [CrossRef]
- Chu, G.; Peng, H.; Yu, N.; Zhang, Y.; In, X.; Lu, Y. Involvement of POMC neurons in LEAP2 regulation of food intake and body weight. Front. Endocrinol. 2022, 13, 932761. [Google Scholar] [CrossRef]
- Barrile, F.; M’Kadmi, C.; De Francesco, P.N.; Cabral, A.; Romero, G.G.; Mustafa, E.R.; Cantel, S.; Damian, M.; Mary, S.; Denoyelle, S.; et al. Development of a novel fluorescent ligand growth hormone secretagogue receptor based on the N-Terminal Leap2 region. Mol. Cell. Endocrinol. 2019, 498, 110573. [Google Scholar] [CrossRef]
| LCD | LCD+INT | T | G × T | |||
|---|---|---|---|---|---|---|
| N | 13 | 12 | - | - | ||
| Age (y) | 46.2 ± 3.5 | 51.3 ± 3.8 | - | - | ||
| Body Composition and Fitness | Pre | Δ | Pre | Δ | ||
| Weight (kg) | 102.2 ± 4.6 | −2.5 ± 0.4 | 105.7 ± 6.0 | −1.5 ± 0.4 | <0.001 | 0.07 |
| Body Mass Index (kg/m2) | 37.5 ± 1.5 | −0.8 ± 0.1 | 37.7 ± 2.1 | −0.5 ± 0.1 | <0.001 | 0.04 |
| Waist Circumference (cm) | 114.1 ± 3.5 | 0.5 ± 3.8 | 114.1 ± 4.3 | −1.7 ± 1.3 | 0.46 | 0.19 |
| Fat Free Mass (kg) | 52.7 ± 2.0 | −0.9 ± 0.3 | 53.7 ± 2.5 | −0.7 ± 0.4 | 0.005 | 0.75 |
| Fat Mass (kg) | 49.0 ± 3.3 | −1.2 ± 0.2 | 51.6 ± 4.1 | −0.4 ± 0.3 | <0.001 | 0.03 |
| VO2peak (mL/kg/min) | 18.9 ± 1.2 | −0.6 ± 0.5 | 17.6 ± 1.1 | 0.7 ± 0.5 | 0.55 | 0.04 |
| Dietary Intake | ||||||
| Calories (kcal/day) | 1939 ± 193.0 | −529.6 ± 194.7 | 2287.8 ± 195.0 | −707.6 ± 209.9 | <0.001 | 0.56 |
| Relative Calories (kcals/kg/day) | 19.6 ± 2.2 | −5.2 ± 1.9 | 22.4 ± 2.4 | −6.7 ± 2.2 | <0.001 | 0.61 |
| Carbohydrate (g/day) | 228.3 ± 22.8 | −36.3 ± 24.7 | 266.1 ± 32.8 | −62.2 ± 30.3 | 0.03 | 0.62 |
| Relative Carbohydrates (g/kg/day) | 2.3 ± 0.0 | −0.3 ± 0.2 | 2.6 ± 0.3 | −0.5 ± 0.3 | 0.04 | 0.56 |
| Fat (g/day) | 80.9 ± 8.9 | −38.3 ± 8.2 | 96.7 ± 7.7 | −42.9 ± 8.3 | <0.001 | 0.70 |
| Relative Fat (g/kg/day) | 0.8 ± 0.1 | −0.3 ± 0.1 | 0.9 ± 0.1 | −0.4 ± 0.1 | <0.001 | 0.91 |
| Protein (g/day) | 76.5 ± 9.5 | −12.6 ± 8.3 | 91.4 ± 7.1 | −22.2 ± 9.0 | 0.009 | 0.44 |
| Relative Protein (g/kg/day) | 0.7 ± 0.1 | −0.1 ± 0.0 | 0.9 ± 0.1 | −0.2 ± 0.1 | 0.01 | 0.48 |
| LCD | LCD+INT | T | G × T | |||
|---|---|---|---|---|---|---|
| N | 13 | 12 | - | - | ||
| Blood Lipids | Pre | Δ | Pre | Δ | ||
| Cholesterol (mg/dL) | 204.2 ± 17.1 | −29.4 ± 5.8 | 192.2 ± 11.1 | −23.2 ± 6.5 | <0.001 | 0.48 |
| Triglycerides (mg/dL) | 118.8 ± 15.7 | −17.6 ± 9.8 | 109.5 ± 13.7 | −33.9 ± 10.6 | 0.002 | 0.27 |
| LDL (mg/dL) | 137.0 ± 14.9 | −23.3 ± 6.0 | 123.4 ± 9.9 | −15.7 ± 5.1 | <0.001 | 0.35 |
| HDL (mg/dL) | 47.4 ± 2.7 | −3.3 ± 1.2 | 50.6 ± 4.6 | −1.8 ± 3.4 | 0.16 | 0.68 |
| Cholesterol/HDL (mg/dL) | 4.4 ± 0.3 | −0.3 ± 0.2 | 3.9 ± 0.2 | −0.3 ± 0.2 | 0.01 | 0.93 |
| LDL/HDL (mg/dL) | 2.9 ± 0.3 | −0.3 ± 0.2 | 2.5 ± 0.2 | −0.2 ± 0.1 | 0.03 | 0.63 |
| Fasted Free Fatty Acids (mM) | 0.5 ± 0.1 | 0.0 ± 0.1 | 0.5 ± 0.0 | 0.1 ± 0.0 | 0.04 | 0.17 |
| Free Fatty Acids iAUC180 (mM) | −60.5 ± 5.4 | 2.7 ± 6.7 | −60.6 ± 4.6 | −13.3 ± 7.0 | 0.30 | 0.11 |
| Glucose Metabolism | ||||||
| Fasted Glucose (mg/dL) | 96.6 ± 1.9 | −3.3 ± 2.2 | 99.3 ± 1.7 | −2.3 ± 1.8 | 0.06 | 0.83 |
| Glucose iAUC180 (mg/dL) | 3537.1 ± 981.7 | −544.1 ± 858.1 | 4214.6 ± 969.3 | 469.7 ± 527.3 | 0.94 | 0.33 |
| Fasted Insulin (µU/mL) | 19.2 ± 3.9 | −3.2 ± 2.9 | 17.8 ± 4.1 | 1.5 ± 4.3 | 0.73 | 0.37 |
| Insulin iAUC180 (µU/mL) | 14,067.6 ± 1714.2 | −3783.0 ± 111.1 | 14,687.3 ± 3527.5 | −1998.3 ± 1154.7 | 0.003 | 0.23 |
| Fasted C-peptide (ng/mL) | 2.6 ± 0.3 | −0.2 ± 0.2 | 2.3 ± 0.3 | −0.3 ± 0.1 | 0.06 | 0.83 |
| C-peptide iAUC180 (ng/mL) | 1119.4 ± 95.2 | −102.7 ± 82.7 | 1033.6 ± 90.3 | 10.02 ± 46.2 | 0.07 | 0.13 |
| LCD | LCD+INT | T | G × T | |||
|---|---|---|---|---|---|---|
| N | 13 | 12 | - | - | ||
| Appetite | Pre | Δ | Pre | Δ | ||
| Fasted Sweet (mm) | 46.1 ± 7.2 | 17.0 ± 11.6 | 49.9 ± 7.3 | 7.4 ± 6.9 | 0.09 | 0.49 |
| 120 min Sweet (mm) | 62.4 ± 5.9 | 4.3 ± 8.4 | 63.7 ± 8.3 | −0.2 ± 4.6 | 0.68 | 0.64 |
| Fasted Salty (mm) | 52.0 ± 6.8 | −6.1 ± 12.7 | 62.3 ± 7.6 | 2.7 ± 7.5 | 0.82 | 0.56 |
| 120 min Salty (mm) | 58.1 ± 5.4 | −3.1 ± 10.0 | 63.2 ± 7.3 | −12.3 ± 8.8 | 0.26 | 0.50 |
| Fasted Savory (mm) | 45.6 ± 5.7 | −6.2 ± 11.9 | 48.6 ± 8.9 | 1.2 ± 11.6 | 0.76 | 0.65 |
| 120 min Savory (mm) | 44.0 ± 6.3 | −5.1 ± 6.6 | 49.1 ± 8.6 | −10.7 ± 11.8 | 0.24 | 0.67 |
| Fasted Fatty (mm) | 48.6 ± 7.3 | 7.3 ± 10.8 | 58.7 ± 7.1 | 7.6 ± 4.7 | 0.23 | 0.98 |
| 120 min Fatty (mm) | 46.5 ± 5.7 | 2.4 ± 8.6 | 63.2 ± 6.7 | −4.9 ± 5.5 | 0.81 | 0.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ragland, T.J.; Malin, S.K. Plasma LEAP-2 Following a Low-Calorie Diet with or without Interval Exercise in Women with Obesity. Nutrients 2023, 15, 655. https://doi.org/10.3390/nu15030655
Ragland TJ, Malin SK. Plasma LEAP-2 Following a Low-Calorie Diet with or without Interval Exercise in Women with Obesity. Nutrients. 2023; 15(3):655. https://doi.org/10.3390/nu15030655
Chicago/Turabian StyleRagland, Tristan J., and Steven K. Malin. 2023. "Plasma LEAP-2 Following a Low-Calorie Diet with or without Interval Exercise in Women with Obesity" Nutrients 15, no. 3: 655. https://doi.org/10.3390/nu15030655
APA StyleRagland, T. J., & Malin, S. K. (2023). Plasma LEAP-2 Following a Low-Calorie Diet with or without Interval Exercise in Women with Obesity. Nutrients, 15(3), 655. https://doi.org/10.3390/nu15030655







