Association between the Triglyceride-Glucose Index and Vitamin D Status in Type 2 Diabetes Mellitus
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection and Definition
2.3. Biochemical Parameter Detection
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Participants
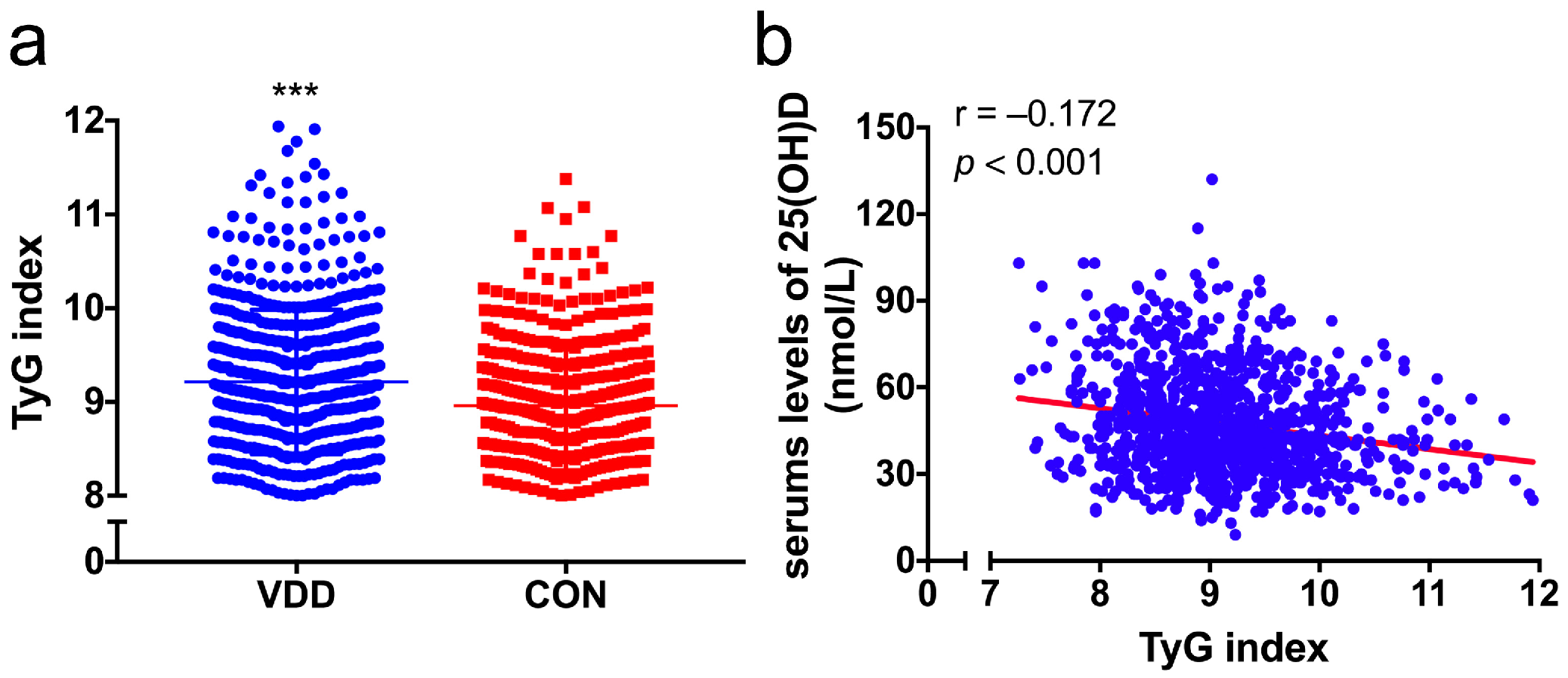
3.2. Correlation between the TyG Index and Vitamin D or Glycolipid-Metabolic Risk Factors
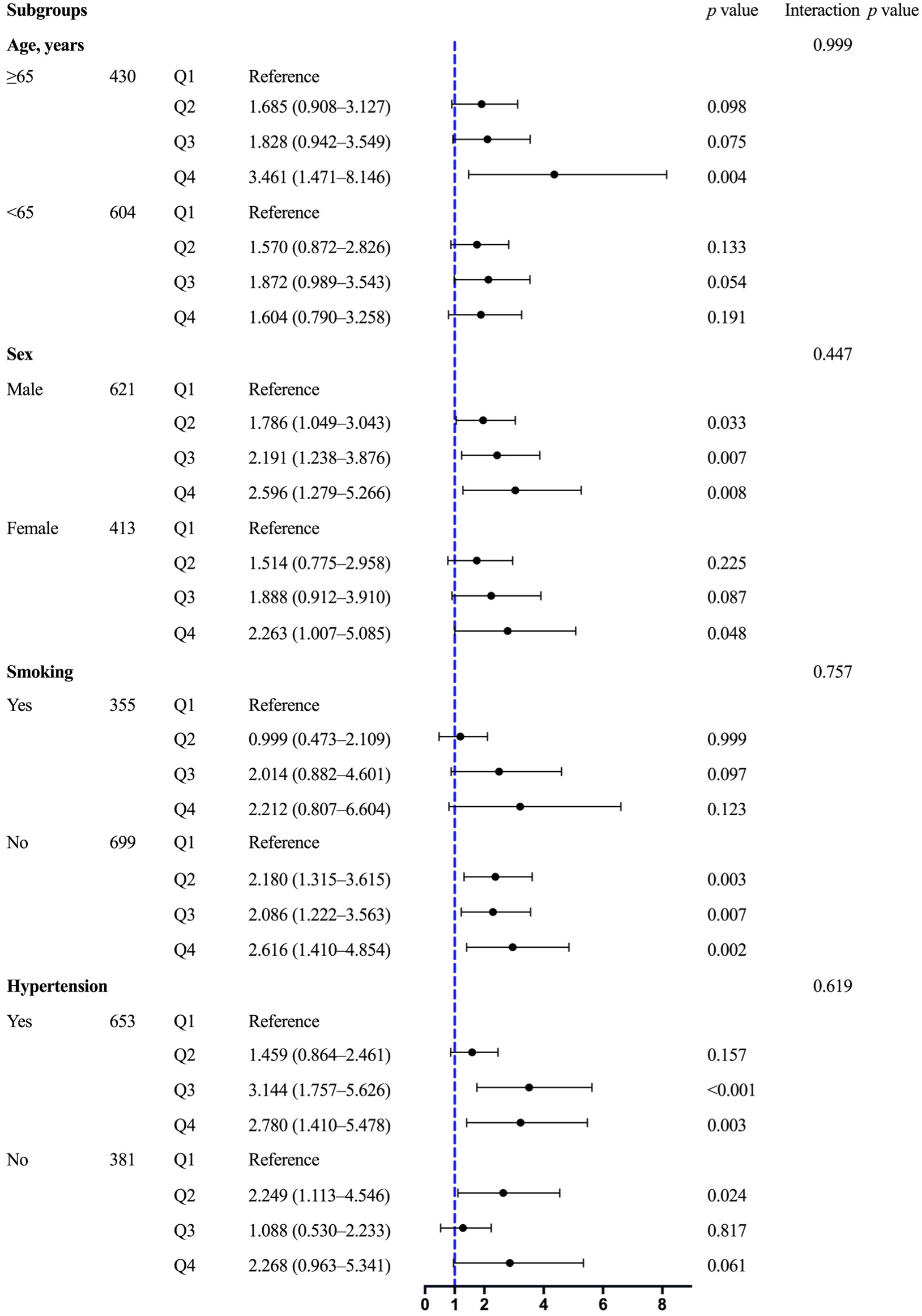
3.3. Association between the TyG Index and VDD
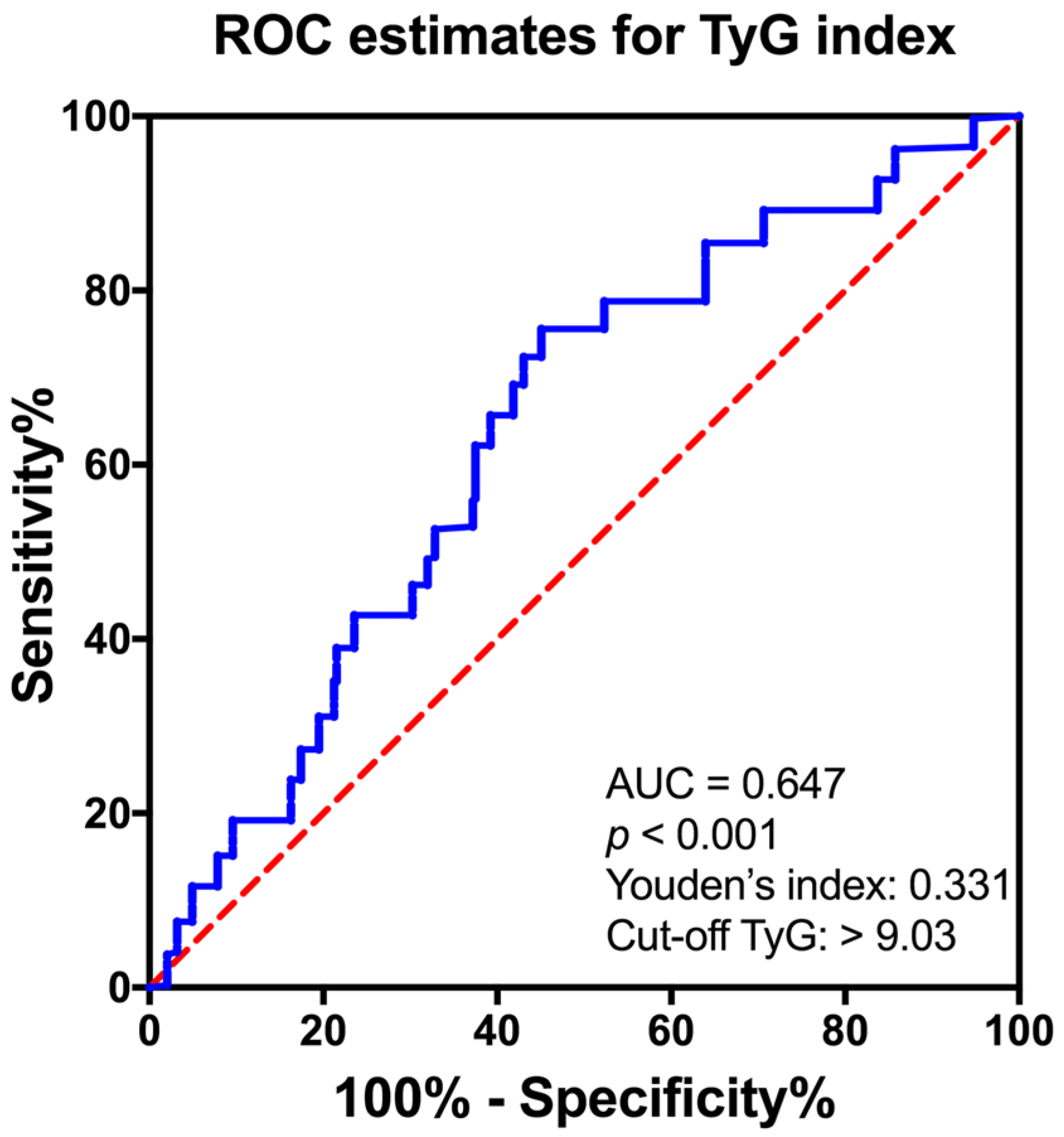
3.4. The Predictive Value of TyG Index for VDD Prevalence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Szymczak-Pajor, I.; Sliwinska, A. Analysis of Association between Vitamin D Deficiency and Insulin Resistance. Nutrients 2019, 11, 794. [Google Scholar] [CrossRef] [PubMed]
- Niroomand, M.; Fotouhi, A.; Irannejad, N.; Hosseinpanah, F. Does high-dose vitamin D supplementation impact insulin resistance and risk of development of diabetes in patients with pre-diabetes? A double-blind randomized clinical trial. Diabetes Res. Clin. Pract. 2019, 148, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Yoshihara, E.; He, N.; Hah, N.; Fan, W.; Pinto, A.F.M.; Huddy, T.; Wang, Y.; Ross, B.; Estepa, G.; et al. Vitamin D Switches BAF Complexes to Protect beta Cells. Cell 2018, 173, 1135–1149.e15. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Kim, G.H.; Park, S.I.; Lim, J.H. Vitamin D Rescues Pancreatic beta Cell Dysfunction due to Iron Overload via Elevation of the Vitamin D Receptor and Maintenance of Ca(2+) Homeostasis. Mol. Nutr. Food Res. 2021, 65, e2000772. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- DeLuca, H.F. Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutr. 2004, 80 (Suppl. S6), 1689S–1696S. [Google Scholar] [CrossRef]
- Bouillon, R.; Bischoff-Ferrari, H.; Willett, W. Vitamin D and health: Perspectives from mice and man. J. Bone Miner. Res. 2008, 23, 974–979. [Google Scholar] [CrossRef]
- Nagpal, S.; Na, S.; Rathnachalam, R. Noncalcemic actions of vitamin D receptor ligands. Endocr. Rev. 2005, 26, 662–687. [Google Scholar] [CrossRef]
- Morro, M.; Vila, L.; Franckhauser, S.; Mallol, C.; Elias, G.; Ferre, T.; Molas, M.; Casana, E.; Rodo, J.; Pujol, A.; et al. Vitamin D Receptor Overexpression in beta-Cells Ameliorates Diabetes in Mice. Diabetes 2020, 69, 927–939. [Google Scholar] [CrossRef]
- Abbas, M.A. Physiological functions of Vitamin D in adipose tissue. J. Steroid Biochem. Mol. Biol. 2017, 165, 369–381. [Google Scholar] [CrossRef]
- Fan, Y.; Futawaka, K.; Koyama, R.; Fukuda, Y.; Hayashi, M.; Imamoto, M.; Miyawaki, T.; Kasahara, M.; Tagami, T.; Moriyama, K. Vitamin D3/VDR resists diet-induced obesity by modulating UCP3 expression in muscles. J. Biomed. Sci. 2016, 23, 56. [Google Scholar] [CrossRef] [PubMed]
- Scragg, R.; Sowers, M.; Bell, C. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care 2004, 27, 2813–2818. [Google Scholar] [CrossRef] [PubMed]
- Hurskainen, A.R.; Virtanen, J.K.; Tuomainen, T.P.; Nurmi, T.; Voutilainen, S. Association of serum 25-hydroxyvitamin D with type 2 diabetes and markers of insulin resistance in a general older population in Finland. Diabetes Metab. Res. Rev. 2012, 28, 418–423. [Google Scholar] [CrossRef]
- Afzal, S.; Bojesen, S.E.; Nordestgaard, B.G. Low 25-hydroxyvitamin D and risk of type 2 diabetes: A prospective cohort study and metaanalysis. Clin. Chem. 2013, 59, 381–391. [Google Scholar] [CrossRef]
- Gagnon, C.; Lu, Z.X.; Magliano, D.J.; Dunstan, D.W.; Shaw, J.E.; Zimmet, P.Z.; Sikaris, K.; Grantham, N.; Ebeling, P.R.; Daly, R.M. Serum 25-hydroxyvitamin D, calcium intake, and risk of type 2 diabetes after 5 years: Results from a national, population-based prospective study (the Australian Diabetes, Obesity and Lifestyle study). Diabetes Care 2011, 34, 1133–1138. [Google Scholar] [CrossRef]
- Pittas, A.G.; Sun, Q.; Manson, J.E.; Dawson-Hughes, B.; Hu, F.B. Plasma 25-hydroxyvitamin D concentration and risk of incident type 2 diabetes in women. Diabetes Care 2010, 33, 2021–2023. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Simental-Mendia, L.E.; Gonzalez-Ortiz, M.; Martinez-Abundis, E.; Ramos-Zavala, M.G.; Hernandez-Gonzalez, S.O.; Jacques-Camarena, O.; Rodriguez-Moran, M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J. Clin. Endocrinol. Metab. 2010, 95, 3347–3351. [Google Scholar] [CrossRef]
- Brito, A.D.M.; Hermsdorff, H.H.M.; Filgueiras, M.S.; Suhett, L.G.; Vieira-Ribeiro, S.A.; Franceschini, S.; Novaes, J.F. Predictive capacity of triglyceride-glucose (TyG) index for insulin resistance and cardiometabolic risk in children and adolescents: A systematic review. Crit. Rev. Food Sci. Nutr. 2021, 61, 2783–2792. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, B.; Liu, Y.; Sun, X.; Luo, X.; Wang, C.; Li, L.; Zhang, L.; Ren, Y.; Zhao, Y.; et al. Cumulative increased risk of incident type 2 diabetes mellitus with increasing triglyceride glucose index in normal-weight people: The Rural Chinese Cohort Study. Cardiovasc. Diabetol. 2017, 16, 30. [Google Scholar] [CrossRef]
- Xuan, X.; Hamaguchi, M.; Cao, Q.; Okamura, T.; Hashimoto, Y.; Obora, A.; Kojima, T.; Fukui, M.; Yuan, G.; Guo, Z.; et al. U-shaped association between the triglyceride-glucose index and the risk of incident diabetes in people with normal glycemic level: A population-base longitudinal cohort study. Clin. Nutr. 2021, 40, 1555–1561. [Google Scholar] [CrossRef]
- Zou, S.; Yang, C.; Shen, R.; Wei, X.; Gong, J.; Pan, Y.; Lv, Y.; Xu, Y. Association Between the Triglyceride-Glucose Index and the Incidence of Diabetes in People With Different Phenotypes of Obesity: A Retrospective Study. Front. Endocrinol. 2021, 12, 784616. [Google Scholar] [CrossRef]
- Chen, C.L.; Liu, L.; Lo, K.; Huang, J.Y.; Yu, Y.L.; Huang, Y.Q.; Feng, Y.Q. Association Between Triglyceride Glucose Index and Risk of New-Onset Diabetes Among Chinese Adults: Findings From the China Health and Retirement Longitudinal Study. Front. Cardiovasc. Med. 2020, 7, 610322. [Google Scholar] [CrossRef]
- Ma, X.; Dong, L.; Shao, Q.; Cheng, Y.; Lv, S.; Sun, Y.; Shen, H.; Wang, Z.; Zhou, Y.; Liu, X. Triglyceride glucose index for predicting cardiovascular outcomes after percutaneous coronary intervention in patients with type 2 diabetes mellitus and acute coronary syndrome. Cardiovasc. Diabetol. 2020, 19, 31. [Google Scholar] [CrossRef]
- Pan, Y.; Zhong, S.; Zhou, K.; Tian, Z.; Chen, F.; Liu, Z.; Geng, Z.; Li, S.; Huang, R.; Wang, H.; et al. Association between Diabetes Complications and the Triglyceride-Glucose Index in Hospitalized Patients with Type 2 Diabetes. J. Diabetes Res. 2021, 2021, 8757996. [Google Scholar] [CrossRef]
- Jia, Y.; Song, T.; Li, Z.; Zhou, L.; Chen, S. The Relationship Between Triglyceride Glucose Index and Vitamin D in Type 2 Diabetes Mellitus. Diabetes Metab. Syndr. Obes. 2022, 15, 511–525. [Google Scholar] [CrossRef]
- American Diabetes A. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42 (Suppl. 1), S13–S28. [Google Scholar] [CrossRef]
- Zhou, B.F.; Cooperative Meta-Analysis Group of the Working Group on Obesity in China. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults—Study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed. Environ. Sci. 2002, 15, 83–96. [Google Scholar]
- Carey, R.M.; Whelton, P.K.; 2017 ACC/AHA Hypertension Guideline Writing Committee. Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension Guideline. Ann. Intern. Med. 2018, 168, 351–358. [Google Scholar] [CrossRef]
- Word Helth Organization. Guidelines for Controlling and Monitoring the Tobacco Epidemic; World Health Organization: Geneva, Switzerland, 1998. [Google Scholar]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef]
- Ning, Z.; Song, S.; Miao, L.; Zhang, P.; Wang, X.; Liu, J.; Hu, Y.; Xu, Y.; Zhao, T.; Liang, Y.; et al. High prevalence of vitamin D deficiency in urban health checkup population. Clin. Nutr. 2016, 35, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.K.; Zhang, Z.; Ke, Y.H.; He, J.W.; Fu, W.Z.; Zhang, C.Q.; Zhang, Z.L. High prevalence of vitamin D insufficiency in China: Relationship with the levels of parathyroid hormone and markers of bone turnover. PLoS ONE 2012, 7, e47264. [Google Scholar] [CrossRef] [PubMed]
- Zhen, D.; Liu, L.; Guan, C.; Zhao, N.; Tang, X. High prevalence of vitamin D deficiency among middle-aged and elderly individuals in northwestern China: Its relationship to osteoporosis and lifestyle factors. Bone 2015, 71, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.Y.; Tian, F.; Lin, Q.Z.; Du, X.; Zhang, S.L.; Gui, Y.J.; Guo, L.L.; Xu, J.; Zhu, L.Y.; Wen, T.; et al. Comparison of remnant cholesterol levels estimated by calculated and measured LDL-C levels in Chinese patients with coronary heart disease. Clin. Chim. Acta 2020, 500, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhang, S.; Xu, J.; Xiang, Q.; Tian, F.; Li, X.; Guo, L.; Zhu, L.; Qu, P.; Fu, Y.; et al. Diagnostic value of monocyte to high-density lipoprotein ratio in acute aortic dissection in a Chinese han population. Expert Rev. Mol. Diagn. 2020, 20, 1243–1252. [Google Scholar] [CrossRef]
- Levey, A.S.; Coresh, J.; Greene, T.; Stevens, L.A.; Zhang, Y.L.; Hendriksen, S.; Kusek, J.W.; Van Lente, F.; Chronic Kidney Disease Epidemiology C. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann. Intern. Med. 2006, 145, 247–254. [Google Scholar] [CrossRef]
- Kennel, K.A.; Drake, M.T.; Hurley, D.L. Vitamin D deficiency in adults: When to test and how to treat. Mayo Clin. Proc. 2010, 85, 752–757. [Google Scholar] [CrossRef]
- Malabanan, A.; Veronikis, I.E.; Holick, M.F. Redefining vitamin D insufficiency. Lancet 1998, 351, 805–806. [Google Scholar] [CrossRef]
- van Schoor, N.; Lips, P. Global Overview of Vitamin D Status. Endocrinol. Metab. Clin. N. Am. 2017, 46, 845–870. [Google Scholar] [CrossRef]
- Yu, S.; Fang, H.; Han, J.; Cheng, X.; Xia, L.; Li, S.; Liu, M.; Tao, Z.; Wang, L.; Hou, L.; et al. The high prevalence of hypovitaminosis D in China: A multicenter vitamin D status survey. Medicine 2015, 94, e585. [Google Scholar] [CrossRef]
- Knekt, P.; Laaksonen, M.; Mattila, C.; Harkanen, T.; Marniemi, J.; Heliovaara, M.; Rissanen, H.; Montonen, J.; Reunanen, A. Serum vitamin D and subsequent occurrence of type 2 diabetes. Epidemiology 2008, 19, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Forouhi, N.G.; Ye, Z.; Rickard, A.P.; Khaw, K.T.; Luben, R.; Langenberg, C.; Wareham, N.J. Circulating 25-hydroxyvitamin D concentration and the risk of type 2 diabetes: Results from the European Prospective Investigation into Cancer (EPIC)-Norfolk cohort and updated meta-analysis of prospective studies. Diabetologia 2012, 55, 2173–2182. [Google Scholar] [CrossRef]
- Gonzalez-Molero, I.; Rojo-Martinez, G.; Morcillo, S.; Gutierrez-Repiso, C.; Rubio-Martin, E.; Almaraz, M.C.; Olveira, G.; Soriguer, F. Vitamin D and incidence of diabetes: A prospective cohort study. Clin. Nutr. 2012, 31, 571–573. [Google Scholar] [CrossRef]
- Xiao, Y.; Wei, L.; Xiong, X.; Yang, M.; Sun, L. Association Between Vitamin D Status and Diabetic Complications in Patients With Type 2 Diabetes Mellitus: A Cross-Sectional Study in Hunan China. Front. Endocrinol. 2020, 11, 564738. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, T.W.; Vaisanen, S.; Frank, C.; Molnar, F.; Sinkkonen, L.; Carlberg, C. The human peroxisome proliferator-activated receptor delta gene is a primary target of 1alpha,25-dihydroxyvitamin D3 and its nuclear receptor. J. Mol. Biol. 2005, 349, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Gulseth, H.L.; Wium, C.; Angel, K.; Eriksen, E.F.; Birkeland, K.I. Effects of Vitamin D Supplementation on Insulin Sensitivity and Insulin Secretion in Subjects With Type 2 Diabetes and Vitamin D Deficiency: A Randomized Controlled Trial. Diabetes Care 2017, 40, 872–878. [Google Scholar] [CrossRef]
- Pittas, A.G.; Jorde, R.; Kawahara, T.; Dawson-Hughes, B. Vitamin D Supplementation for Prevention of Type 2 Diabetes Mellitus: To D or Not to D? J. Clin. Endocrinol. Metab. 2020, 105, 3721–3733. [Google Scholar] [CrossRef]
- Krul-Poel, Y.H.; Ter Wee, M.M.; Lips, P.; Simsek, S. MANAGEMENT OF ENDOCRINE DISEASE: The effect of vitamin D supplementation on glycaemic control in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Eur. J. Endocrinol. 2017, 176, R1–R14. [Google Scholar] [CrossRef]
- Faerch, K.; Vaag, A.; Holst, J.J.; Hansen, T.; Jorgensen, T.; Borch-Johnsen, K. Natural history of insulin sensitivity and insulin secretion in the progression from normal glucose tolerance to impaired fasting glycemia and impaired glucose tolerance: The Inter99 study. Diabetes Care 2009, 32, 439–444. [Google Scholar] [CrossRef]
- Knowles, J.W.; Assimes, T.L.; Tsao, P.S.; Natali, A.; Mari, A.; Quertermous, T.; Reaven, G.M.; Abbasi, F. Measurement of insulin-mediated glucose uptake: Direct comparison of the modified insulin suppression test and the euglycemic, hyperinsulinemic clamp. Metabolism 2013, 62, 548–553. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Park, J.S.; Ahn, Y. The Cut-off Values of Triglycerides and Glucose Index for Metabolic Syndrome in American and Korean Adolescents. J. Korean Med. Sci. 2017, 32, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Yu, S.; Chi, C.; Fan, X.; Tang, J.; Ji, H.; Teliewubai, J.; Zhang, Y.; Xu, Y. Association between macro- and microvascular damage and the triglyceride glucose index in community-dwelling elderly individuals: The Northern Shanghai Study. Cardiovasc. Diabetol. 2019, 18, 95. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Guo, Y.; Ruan, G.Y.; Peng, R.; Li, X.P. Estrogen lowers triglyceride via regulating hepatic APOA5 expression. Lipids Health Dis. 2017, 16, 72. [Google Scholar] [CrossRef]
- Guo, W.; Fu, J.; Chen, X.; Gao, B.; Fu, Z.; Fan, H.; Cui, Q.; Zhu, X.; Zhao, Y.; Yang, T.; et al. The effects of estrogen on serum level and hepatocyte expression of PCSK9. Metabolism 2015, 64, 554–560. [Google Scholar] [CrossRef]
- Dhas, Y.; Banerjee, J.; Damle, G.; Mishra, N. Association of vitamin D deficiency with insulin resistance in middle-aged type 2 diabetics. Clin. Chim. Acta 2019, 492, 95–101. [Google Scholar] [CrossRef]
- Mustafa, A.; Shekhar, C. Association between serum 25-hydroxyvitamin-D and Triglycerides-Glucose index among Indian adolescents. BMC Nutr. 2022, 8, 69. [Google Scholar] [CrossRef]


| All (n = 1034) | VDD (n = 632) | CON (n = 402) | p Value | |
|---|---|---|---|---|
| Age, years | 62.0 ± 11.14 | 61.9 ± 11.38 | 62.2 ± 10.77 | 0.66 |
| Male, n (%) | 621 (60.1) | 376 (59.5) | 256 (40.5) | 0.64 |
| Smoking, n (%) | 335 (32.4) | 217 (34.4) | 118 (29.4) | 0.08 |
| Obesity, n (%) | 134 (13.0) | 96 (15.2) | 39 (9.7) | 0.01 |
| Hypertension, n (%) | 653 (63.2) | 415 (65.7) | 238 (59.2) | 0.04 |
| Insulin therapy, n (%) | 568 (54.9) | 382 (60.4) | 186 (46.3) | <0.001 |
| Hypoglycemic agents, n (%) | 916 (88.6) | 546 (86.4) | 368 (91.5) | 0.01 |
| Statin therapy, n (%) | 757 (73.2) | 469 (74.2) | 288 (71.6) | 0.36 |
| SBP, mmHg | 135.79 ± 20.40 | 137.16 ± 20.73 | 133.64 ± 19.71 | 0.007 |
| DBP, mmHg | 80.76 ± 11.83 | 80.86 ± 12.06 | 80.60 ± 11.47 | 0.73 |
| BMI, kg/m2 | 24.28 ± 3.35 | 24.46 ± 3.38 | 24.00 ± 3.30 | 0.03 |
| Fasting glucose, mmol/L | 7.02 (5.80–8.64) | 6.96 (5.76–8.75) | 7.00 (5.90–8.84) | 0.05 |
| HbA1c, % | 8.38 (6.93–10.20) | 8.72 (7.11–10.66) | 8.12 (6.89–9.70) | 0.005 |
| C-peptide, pmol/L | 372.00 (224.00–574.93) | 337.50 (208.20–572.00) | 358.40 (223.00–549.00) | 0.595 |
| TC, mmol/L | 4.40 ± 1.21 | 4.53 ± 1.29 | 4.19 ± 1.03 | <0.001 |
| TG, mmol/L | 1.50 (1.02–2.23) | 1.67 (1.06–2.38) | 1.32 (0.91–1.92) | <0.001 |
| LDL-C, mmol/L | 2.78 ± 1.05 | 2.89 ± 1.10 | 2.60 ± 0.93 | <0.001 |
| HDL-C, mmol/L | 1.08 ± 0.31 | 1.07 ± 0.31 | 1.09 ± 0.30 | 0.34 |
| Albumin, g/L | 37.81 ± 4.52 | 37.39 ± 4.76 | 38.47 ± 4.05 | <0.001 |
| UA, μmol/L | 333.29 ± 108.05 | 337.20 ± 117.28 | 327.16 ± 91.54 | 0.14 |
| BUN, mmol/L | 6.48 (5.20–8.00) | 6.67 (5.20–8.10) | 6.40 (5.28–7.68) | 0.25 |
| Scr, μmol/L | 75.00 (60.40–98.00) | 76.00 (61.00–105.00) | 76.00 (63.00–96.10) | 0.92 |
| eGFR, ml/min per 1.73 m2 | 84.04 (59.90–102.18) | 82.82 (56.03–103.13) | 85.46 (65.40–100.26) | 0.40 |
| UACR, mg/g | 18.00 (5.25–147.00) | 35.10 (7.00–309.60) | 12.10 (4.30–82.50) | <0.001 |
| 25(OH)D, nmol/L | 44.00 (34.00–59.00) | 37.00 (31.00–43.00) | 63.00 (56.00–71.00) | <0.001 |
| TyG index | 9.11 ± 0.75 | 9.21 ± 0.77 | 8.96 ± 0.69 | <0.001 |
| r | p Value | |
|---|---|---|
| HbA1c, % | 0.300 | <0.001 |
| C-peptide, pmol/L | 0.201 | <0.001 |
| TC, mmol/L | 0.353 | <0.001 |
| LDL-C, mmol/L | 0.348 | <0.001 |
| HDL-C, mmol/L | −0.368 | <0.001 |
| BMI, kg/m2 | 0.241 | <0.001 |
| SBP, mmHg | 0.028 | 0.370 |
| DBP, mmHg | 0.162 | 0.001 |
| Unadjusted | Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Q1 | Reference | Reference | Reference | Reference | ||||
| Q2 | 1.486 (1.044–2.114) | 0.028 | 1.561 (1.087–2.242) | 0.016 | 1.747 (1.204–2.535) | 0.003 | 1.708 (1.132–2.576) | 0.011 |
| Q3 | 1.921 (1.348–2.739) | <0.001 | 2.011 (1.394–2.902) | <0.001 | 2.162 (1.480–3.158) | <0.001 | 2.041 (1.315–3.169) | 0.001 |
| Q4 | 2.625 (1.822–3.782) | <0.001 | 2.709 (1.849–3.969) | <0.001 | 2.894 (1.940–4.319) | <0.001 | 2.543 (1.520–4.253) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, Q.; Xu, H.; Zhan, J.; Lu, S.; Li, S.; Wang, Y.; Wang, Y.; He, J.; Ni, Y.; Li, L.; et al. Association between the Triglyceride-Glucose Index and Vitamin D Status in Type 2 Diabetes Mellitus. Nutrients 2023, 15, 639. https://doi.org/10.3390/nu15030639
Xiang Q, Xu H, Zhan J, Lu S, Li S, Wang Y, Wang Y, He J, Ni Y, Li L, et al. Association between the Triglyceride-Glucose Index and Vitamin D Status in Type 2 Diabetes Mellitus. Nutrients. 2023; 15(3):639. https://doi.org/10.3390/nu15030639
Chicago/Turabian StyleXiang, Qunyan, Hui Xu, Junkun Zhan, Shuzhen Lu, Shuang Li, Yanjiao Wang, Yi Wang, Jieyu He, Yuqing Ni, Linsen Li, and et al. 2023. "Association between the Triglyceride-Glucose Index and Vitamin D Status in Type 2 Diabetes Mellitus" Nutrients 15, no. 3: 639. https://doi.org/10.3390/nu15030639
APA StyleXiang, Q., Xu, H., Zhan, J., Lu, S., Li, S., Wang, Y., Wang, Y., He, J., Ni, Y., Li, L., Liu, Y., & Liu, Y. (2023). Association between the Triglyceride-Glucose Index and Vitamin D Status in Type 2 Diabetes Mellitus. Nutrients, 15(3), 639. https://doi.org/10.3390/nu15030639






