miRNAs and Alzheimer’s Disease: Exploring the Role of Inflammation and Vitamin E in an Old-Age Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects and Study Design
2.1.1. Healthy Control (HC)
2.1.2. Alzheimer’s Disease (AD)
2.2. Cognitive, Functional, and Neuropsychological Assessment
2.3. Blood Sample
2.4. Vitamin E
2.5. Inflammatory Molecules
2.6. miRNAs
2.7. Statistical Analysis
3. Results
3.1. Sample Characteristics
3.2. Vitamin E
3.3. Cytokines
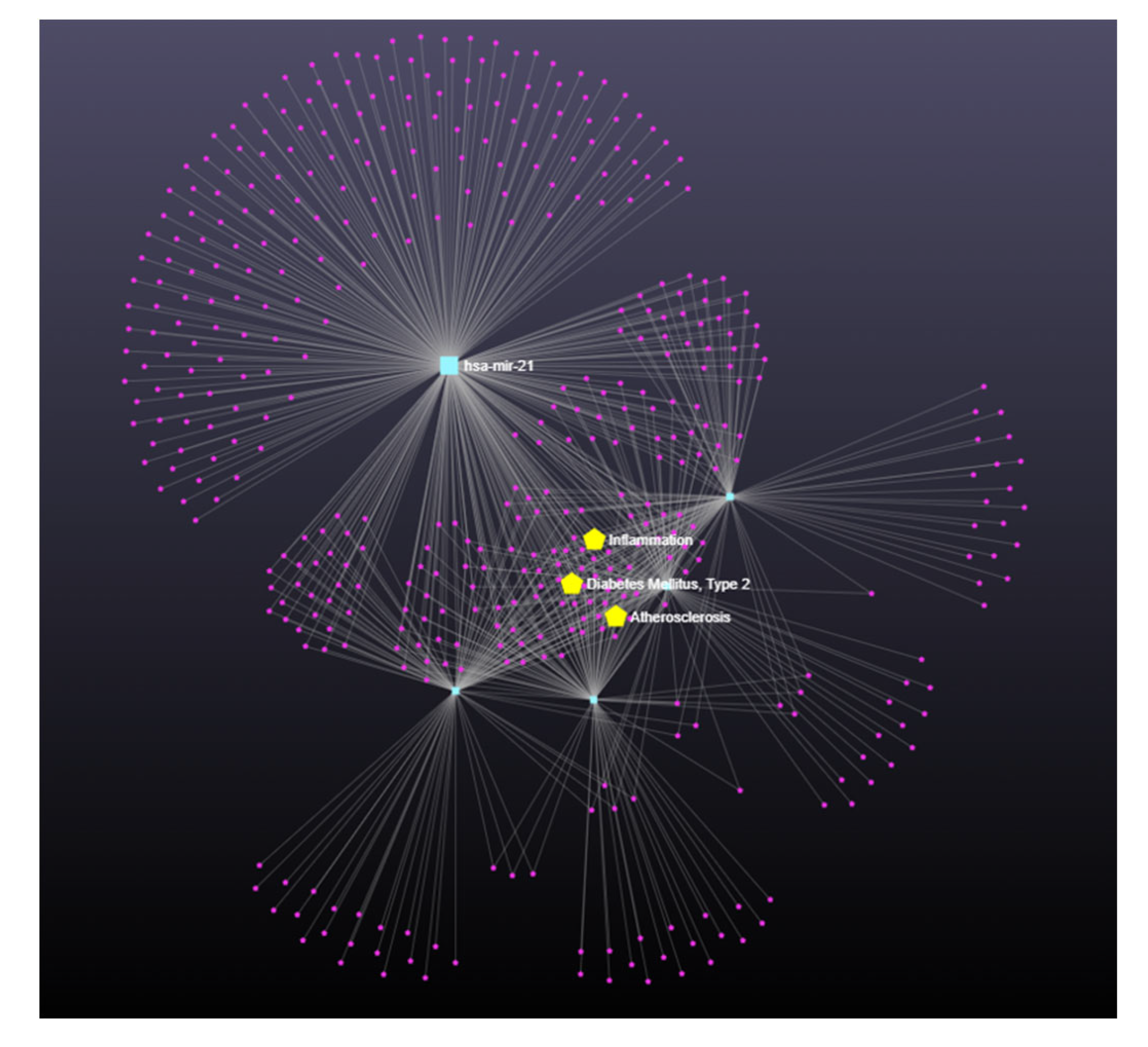
3.4. Exosomal miRNAs
3.5. miRNA-122, Alpha-Tocopherol, and AD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dubois, B.; Hampel, H.; Feldman, H.H.; Scheltens, P.; Aisen, P.; Andrieu, S.; Bakardjian, H.; Benali, H.; Bertram, L.; Blennow, K.; et al. Preclinical Alzheimer’s Disease: Definition, Natural History, and Diagnostic Criteria. Alzheimers Dement. 2016, 12, 292. [Google Scholar] [CrossRef] [PubMed]
- Ayodele, T.; Rogaeva, E.; Kurup, J.T.; Beecham, G.; Reitz, C. Early-Onset Alzheimer’s Disease: What Is Missing in Research? Curr. Neurol. Neurosci. Rep. 2021, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; McKeehan, N.; Fillit, H.M. Translating the Biology of Aging into Novel Therapeutics for Alzheimer Disease. Neurology 2019, 92, 84–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sultana, R.; Butterfield, D.A. Oxidative Modification of Brain Proteins in Alzheimer’s Disease: Perspective on Future Studies Based on Results of Redox Proteomics Studies. J. Alzheimers Dis. 2013, 33, S243–S251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mecocci, P.; Boccardi, V.; Cecchetti, R.; Bastiani, P.; Scamosci, M.; Ruggiero, C.; Baroni, M. A Long Journey into Aging, Brain Aging, and Alzheimer’s Disease Following the Oxidative Stress Tracks. J. Alzheimers Dis. 2018, 62, 1319–1335. [Google Scholar] [CrossRef] [Green Version]
- Allan Butterfield, D.; Howard, B.; Yatin, S.; Koppal, T.; Drake, J.; Hensley, K.; Aksenov, M.; Aksenova, M.; Subramaniam, R.; Varadarajan, S.; et al. Elevated Oxidative Stress in Models of Normal Brain Aging and Alzheimer’s Disease. Life Sci. 1999, 65, 1883–1892. [Google Scholar] [CrossRef]
- Wang, W.Y.; Tan, M.S.; Yu, J.T.; Tan, L. Role of Pro-Inflammatory Cytokines Released from Microglia in Alzheimer’s Disease. Ann. Transl. Med. 2015, 3, 136. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822. [Google Scholar] [CrossRef]
- Leung, R.; Proitsi, P.; Simmons, A.; Lunnon, K.; Güntert, A.; Kronenberg, D.; Pritchard, M.; Tsolaki, M.; Mecocci, P.; Kloszewska, I.; et al. Inflammatory Proteins in Plasma Are Associated with Severity of Alzheimer’s Disease. PLoS ONE 2013, 8, 64971. [Google Scholar] [CrossRef] [Green Version]
- Nelson, L.; Tabet, N. Slowing the Progression of Alzheimer’s Disease; What Works? Ageing Res. Rev. 2015, 23, 193–209. [Google Scholar] [CrossRef]
- van der Beek, E.M.; Kamphuis, P.J.G.H. The Potential Role of Nutritional Components in the Management of Alzheimer’s Disease. Eur. J. Pharmacol. 2008, 585, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Kishida, R.; Yamagishi, K.; Maruyama, K.; Okada, C.; Tanaka, M.; Ikeda, A.; Hayama-Terada, M.; Shimizu, Y.; Muraki, I.; Umesawa, M.; et al. Dietary Intake of Beans and Risk of Disabling Dementia: The Circulatory Risk in Communities Study (CIRCS). Eur. J. Clin. Nutr. 2023, 77, 65–70. [Google Scholar] [CrossRef]
- Ferrero, G.; Carpi, S.; Polini, B.; Pardini, B.; Nieri, P.; Impeduglia, A.; Grioni, S.; Tarallo, S.; Naccarati, A. Intake of Natural Compounds and Circulating Microrna Expression Levels: Their Relationship Investigated in Healthy Subjects with Different Dietary Habits. Front. Pharmacol. 2021, 11, 2214. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Zhu, M.; Kong, C.; Pang, Y.; Zhang, H.; Qiu, Q.; Wei, C.; Tang, Y.; Wang, Q.; Li, Y.; et al. Blood Neuro-Exosomal Synaptic Proteins Predict Alzheimer’s Disease at the Asymptomatic Stage. Alzheimers Dement. 2021, 17, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Zhu, M.; Yang, J.; Pang, Y.; Wang, Q.; Li, T.T.; Li, F.; Wang, Q.; Li, Y.; Wei, Y. Exosomal MicroRNA-Based Predictive Model for Preclinical Alzheimer’s Disease: A Multicenter Study. Biol. Psychiatry 2022, 92, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wang, L. Inflamma-MicroRNAs in Alzheimer’s Disease: From Disease Pathogenesis to Therapeutic Potentials. Front. Cell. Neurosci. 2021, 15, 785433. [Google Scholar] [CrossRef]
- Giuliani, A.; Gaetani, S.; Sorgentoni, G.; Agarbati, S.; Laggetta, M.; Matacchione, G.; Gobbi, M.; Rossi, T.; Galeazzi, R.; Piccinini, G.; et al. Circulating Inflamma-MiRs as Potential Biomarkers of Cognitive Impairment in Patients Affected by Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 647015. [Google Scholar] [CrossRef]
- Konovalova, J.; Gerasymchuk, D.; Parkkinen, I.; Chmielarz, P.; Domanskyi, A. Interplay between MicroRNAs and Oxidative Stress in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 6055. [Google Scholar] [CrossRef] [Green Version]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The Diagnosis of Dementia Due to Alzheimer’s Disease: Recommendations from the National Institute on Aging-Alzheimer’s Association Workgroups on Diagnostic Guidelines for Alzheimer’s Disease. Alzheimers Dement. 2011, 7, 263. [Google Scholar] [CrossRef] [Green Version]
- Boccardi, V.; Paolacci, L.; Remondini, D.; Giampieri, E.; Poli, G.; Curti, N.; Cecchetti, R.; Villa, A.; Ruggiero, C.; Brancorsini, S.; et al. Cognitive Decline and Alzheimer’s Disease in Old Age: A Sex-Specific Cytokinome Signature. J. Alzheimers Dis. 2019, 72, 911–918. [Google Scholar] [CrossRef]
- Mangialasche, F.; Westman, E.; Kivipelto, M.; Muehlboeck, J.S.; Cecchetti, R.; Baglioni, M.; Tarducci, R.; Gobbi, G.; Floridi, P.; Soininen, H.; et al. Classification and Prediction of Clinical Diagnosis of Alzheimer’s Disease Based on MRI and Plasma Measures of α-/γ-Tocotrienols and γ-Tocopherol. J. Intern. Med. 2013, 273, 602–621. [Google Scholar] [CrossRef]
- Poudel, P.; Park, S. Recent Advances in the Treatment of Alzheimer’s Disease Using Nanoparticle-Based Drug Delivery Systems. Pharmaceutics 2022, 14, 835. [Google Scholar] [CrossRef] [PubMed]
- Bonda, D.J.; Wang, X.; Lee, H.G.; Smith, M.A.; Perry, G.; Zhu, X. Neuronal Failure in Alzheimer’s Disease: A View through the Oxidative Stress Looking-Glass. Neurosci. Bull. 2014, 30, 243–252. [Google Scholar] [CrossRef]
- Sinyor, B.; Mineo, J.; Ochner, C. Alzheimer’s Disease, Inflammation, and the Role of Antioxidants. J. Alzheimers Dis. Rep. 2020, 4, 175. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, G.; Maclean, A.G.; Philipp, M.T. Cytokines and Chemokines at the Crossroads of Neuroinflammation, Neurodegeneration, and Neuropathic Pain. Mediat. Inflamm. 2013, 2013, 480739. [Google Scholar] [CrossRef] [Green Version]
- Gugliandolo, A.; Bramanti, P.; Mazzon, E. Role of Vitamin E in the Treatment of Alzheimer’s Disease: Evidence from Animal Models. Int. J. Mol. Sci. 2017, 18, 2504. [Google Scholar] [CrossRef] [Green Version]
- Browne, D.; McGuinness, B.; Woodside, J.v.; McKay, G.J. Vitamin E and Alzheimer’s Disease: What Do We Know so Far? Clin. Interv. Aging 2019, 14, 1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galli, F.; Azzi, A.; Birringer, M.; Cook-Mills, J.M.; Eggersdorfer, M.; Frank, J.; Cruciani, G.; Lorkowski, S.; Özer, N.K. Vitamin E: Emerging Aspects and New Directions. Free Radic. Biol. Med. 2017, 102, 16–36. [Google Scholar] [CrossRef]
- Nishida, Y.; Yokota, T.; Takahashi, T.; Uchihara, T.; Jishage, K.-i.; Mizusawa, H. Deletion of Vitamin E Enhances Phenotype of Alzheimer Disease Model Mouse. Biochem. Biophys. Res. Commun. 2006, 350, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.; Yao, Y.; Uryu, K.; Yang, H.; Lee, V.M.Y.; Trojanowski, J.Q.; Praticò, D. Early Vitamin E Supplementation in Young but Not Aged Mice Reduces Abeta Levels and Amyloid Deposition in a Transgenic Model of Alzheimer’s Disease. FASEB J. 2004, 18, 323–325. [Google Scholar] [CrossRef]
- Devore, E.E.; Grodstein, F.; van Rooij, F.J.A.; Hofman, A.; Stampfer, M.J.; Witteman, J.C.M.; Breteler, M.M.B. Dietary Antioxidants and Long-Term Risk of Dementia. Arch. Neurol. 2010, 67, 819–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Wilson, R.S. Vitamin E and Cognitive Decline in Older Persons. Arch. Neurol. 2002, 59, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Quintanilha, B.J.; Reis, B.Z.; Silva Duarte, G.B.; Cozzolino, S.M.F.; Rogero, M.M. Nutrimiromics: Role of MicroRNAs and Nutrition in Modulating Inflammation and Chronic Diseases. Nutrients 2017, 9, 1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene Regulation by Long Non-Coding RNAs and Its Biological Functions. Nat. Rev. Mol. Cell Biol. 2020, 22, 96–118. [Google Scholar] [CrossRef]
- Zhao, Y.; Jaber, V.; Alexandrov, P.N.; Vergallo, A.; Lista, S.; Hampel, H.; Lukiw, W.J. MicroRNA-Based Biomarkers in Alzheimer’s Disease (AD). Front. Neurosci. 2020, 14, 585432. [Google Scholar] [CrossRef]
- Bai, X.; Bian, Z. MicroRNA-21 Is a Versatile Regulator and Potential Treatment Target in Central Nervous System Disorders. Front. Mol. Neurosci. 2022, 15, 842288. [Google Scholar] [CrossRef]
- Cui, G.H.; Wu, J.; Mou, F.F.; Xie, W.H.; Wang, F.B.; Wang, Q.L.; Fang, J.; Xu, Y.W.; Dong, Y.R.; Liu, J.R.; et al. Exosomes Derived from Hypoxia-Preconditioned Mesenchymal Stromal Cells Ameliorate Cognitive Decline by Rescuing Synaptic Dysfunction and Regulating Inflammatory Responses in APP/PS1 Mice. FASEB J. 2018, 32, 654–668. [Google Scholar] [CrossRef] [Green Version]
- Souza, V.C.; Morais, G.S.; Henriques, A.D.; Machado-Silva, W.; Perez, D.I.V.; Brito, C.J.; Camargos, E.F.; Moraes, C.F.; Nóbrega, O.T. Whole-Blood Levels of MicroRNA-9 Are Decreased in Patients With Late-Onset Alzheimer Disease. Am. J. Alzheimers Dis. Other Demen. 2020, 35, 1533317520911573. [Google Scholar] [CrossRef]
- Jahangard, Y.; Monfared, H.; Moradi, A.; Zare, M.; Mirnajafi-Zadeh, J.; Mowla, S.J. Therapeutic Effects of Transplanted Exosomes Containing MiR-29b to a Rat Model of Alzheimer’s Disease. Front. Neurosci. 2020, 14, 564. [Google Scholar] [CrossRef]
- Yuva-Aydemir, Y.; Simkin, A.; Gascon, E.; Gao, F.-B. MicroRNA-9: Functional Evolution of a Conserved Small Regulatory RNA. RNA Biol. 2011, 8, 557–564. [Google Scholar] [CrossRef] [Green Version]
- Walgrave, H.; Zhou, L.; de Strooper, B.; Salta, E. The Promise of MicroRNA-Based Therapies in Alzheimer’s Disease: Challenges and Perspectives. Mol. Neurodegener. 2021, 16, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Salta, E.; Sierksma, A.; vanden Eynden, E.; de Strooper, B. MiR-132 Loss de-Represses ITPKB and Aggravates Amyloid and TAU Pathology in Alzheimer’s Brain. EMBO Mol. Med. 2016, 8, 1005–1018. [Google Scholar] [CrossRef] [PubMed]
- Tarkowski, E.; Wallin, A.; Regland, B.; Blennow, K.; Tarkowski, A. Local and Systemic GM-CSF Increase in Alzheimer’s Disease and Vascular Dementia. Acta Neurol. Scand. 2001, 103, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Griffin, W.S.T.; Nicoll, J.A.R.; Grimaldi, L.M.E.; Sheng, J.G.; Mrak, R.E. The Pervasiveness of Interleukin-1 in Alzheimer Pathogenesis: A Role for Specific Polymorphisms in Disease Risk. Exp. Gerontol. 2000, 35, 481. [Google Scholar] [CrossRef] [Green Version]
- Doroszkiewicz, J.; Kulczynska-Przybik, A.; Dulewicz, M.; Borawska, R.; Krawiec, A.; Slowik, A.; Mroczko, B. The Cerebrospinal Fluid Interleukin 8 (IL-8) Concentration in Alzheimer’s Disease (AD). Alzheimer’s Dement. 2021, 17, e051317. [Google Scholar] [CrossRef]
- Tripathy, D.; Thirumangalakudi, L.; Grammas, P. Expression of Macrophage Inflammatory Protein 1-Alpha Is Elevated in Alzheimer’s Vessels and Is Regulated by Oxidative Stress. J. Alzheimers Dis. 2007, 11, 447–455. [Google Scholar] [CrossRef]
- Gaedicke, S.; Zhang, X.; Schmelzer, C.; Lou, Y.; Doering, F.; Frank, J.; Rimbach, G. Vitamin E Dependent MicroRNA Regulation in Rat Liver. FEBS Lett. 2008, 582, 3542–3546. [Google Scholar] [CrossRef] [Green Version]
- Tahamtan, A.; Teymoori-Rad, M.; Nakstad, B.; Salimi, V. Anti-Inflammatory MicroRNAs and Their Potential for Inflammatory Diseases Treatment. Front. Immunol. 2018, 9, 1377. [Google Scholar] [CrossRef] [Green Version]
- Esau, C.; Davis, S.; Murray, S.F.; Yu, X.X.; Pandey, S.K.; Pear, M.; Watts, L.; Booten, S.L.; Graham, M.; McKay, R.; et al. MiR-122 Regulation of Lipid Metabolism Revealed by in Vivo Antisense Targeting. Cell Metab. 2006, 3, 87–98. [Google Scholar] [CrossRef] [Green Version]
- Maciejczyk, M.; Żebrowska, E.; Chabowski, A. Insulin Resistance and Oxidative Stress in the Brain: What’s New? Int. J. Mol. Sci. 2019, 20, 874. [Google Scholar] [CrossRef] [Green Version]
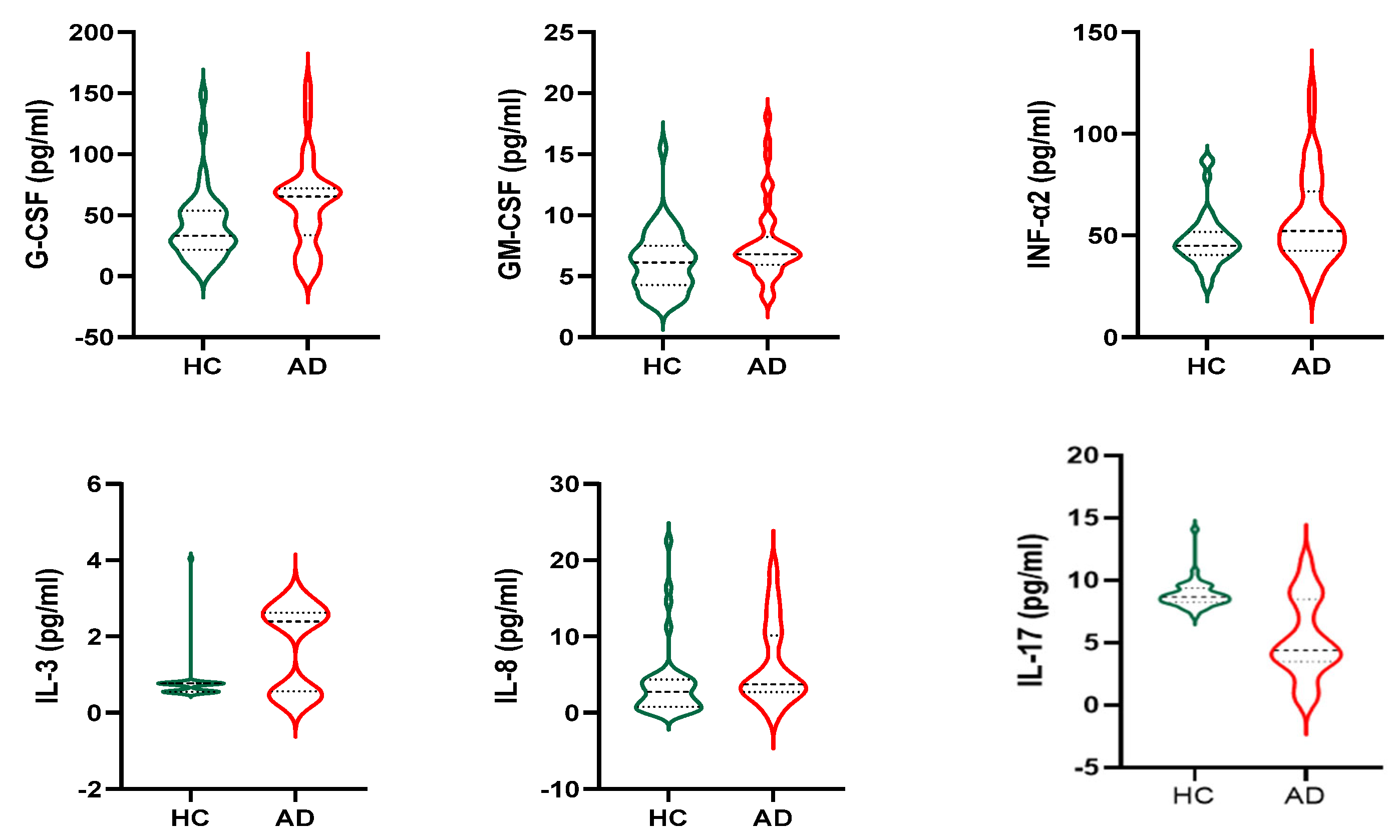
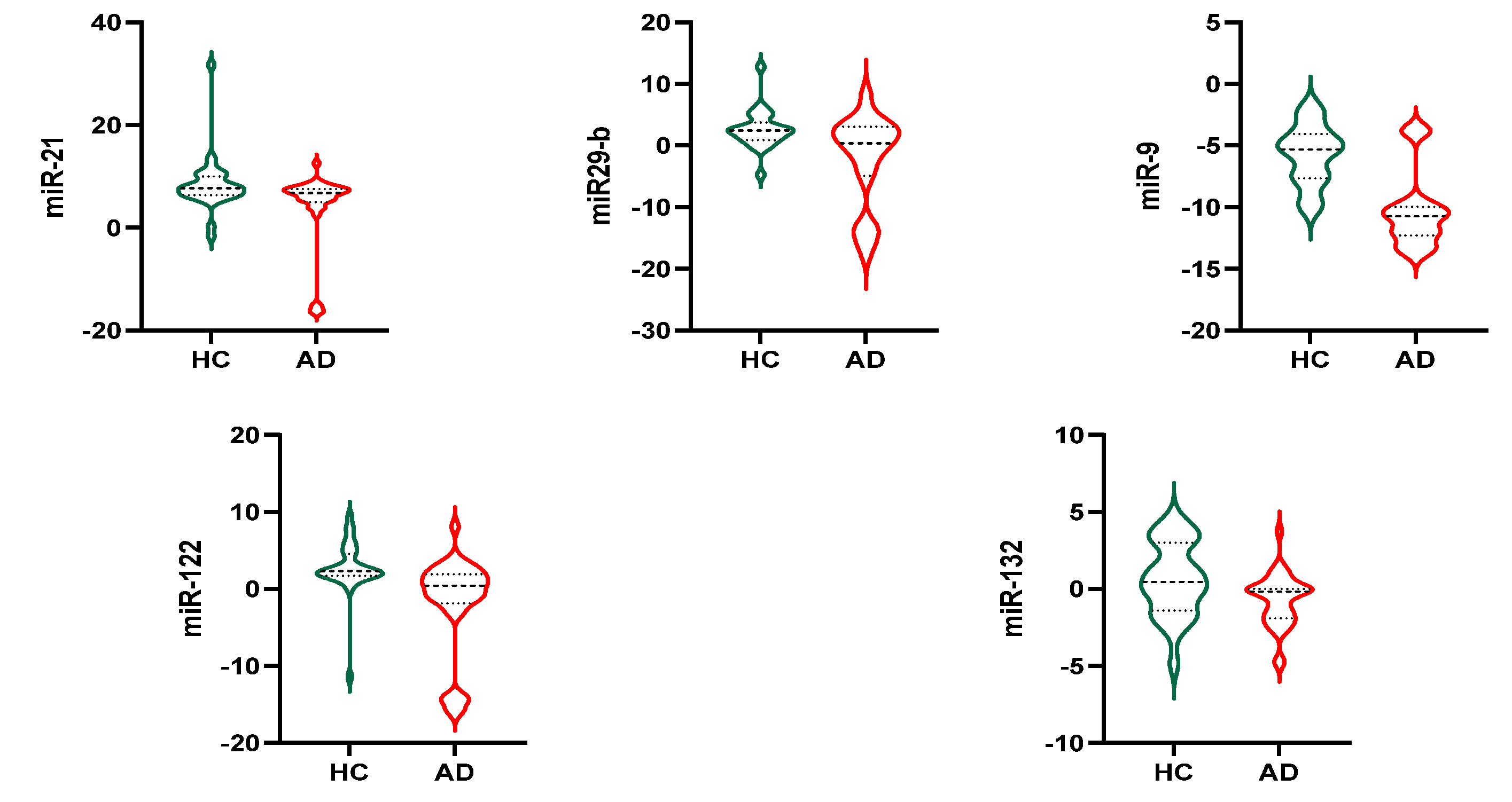
| Total | HC | AD | p | |
|---|---|---|---|---|
| N | 80 | 40 | 40 | |
| F/M (n) | 45/35 | 18/22 | 27/13 | 0.043 * |
| Age (years) | 77.58 ± 3.86 | 76.33 ± 3.61 | 78.83 ± 3.74 | 0.003 |
| MMSE | 21.33 ± 8.32 | 28.83 ± 1.50 | 13.43 ± 4.13 | <0.0001 |
| GDS | 5.03 ± 3.23 | 4.92 ± 2.86 | 5.16 ± 3.67 | 0.765 |
| ADL | 4.50 ± 1.56 | 5.53 ± 0.78 | 3.48 ± 1.48 | <0.0001 |
| IADL | 3.83 ± 2.96 | 6.35 ± 1.79 | 1.30 ± 1.24 | <0.0001 |
| Glycemia (mg/dL) | 100.5 ± 23.3 | 101.8 ± 24.6 | 99.1 ± 22.2 | 0.630 |
| Creatinine (mg/dL) | 1.03 ± 0.17 | 1.06 ± 0.23 | 1.0 ± 0.11 | 0.196 |
| TC (mg/dL) | 197.4 ± 39.9 | 192.8 ± 35.7 | 202.3 ± 43.8 | 0.294 |
| HDL-C(mg/dL) | 57.2 ± 15.9 | 57.2 ± 16.9 | 57.1 ± 15.0 | 0.986 |
| LDL-C (mg/dL) | 118.4 ± 35.2 | 113.3 ± 33.5 | 123.8 ± 36.6 | 0.218 |
| Triglycerides (mg/dL) | 112.1 ± 49.5 | 118.8 ± 48.5 | 125.5 ± 50.9 | 0.546 |
| Partial Correlations | ||
|---|---|---|
| Correlation Coefficient (r) | p-Value | |
| miR-122/GM-CSF | 0.389 | 0.019 |
| miR-122/INF-α2 | 0.375 | 0.024 |
| miR-122/IL-1α | 0.360 | 0.031 |
| miR-122/IL-8 | 0.375 | 0.024 |
| miR-122/MIP-1β | 0.354 | 0.034 |
| Model 1 | ||||
| B | OR | IC 95% | p | |
| Age | 0.182 | 1.199 | 1.029–1.398 | 0.020 |
| Gender | 0.493 | 1.637 | 0.578–4.634 | 0.353 |
| α-tocopherol | −0.329 | 0.720 | 0.579–0.895 | 0.003 |
| Model 2 | ||||
| B | OR | IC 95% | p | |
| Age | 0.175 | 1.191 | 0.989–1.434 | 0.065 |
| Gender | 0.047 | 1.048 | 0.326–3.363 | 0.938 |
| α-tocopherol | −0.252 | 0.777 | 0.617–0.980 | 0.033 |
| MiR-122 | −0.215 | 0.806 | 0.654–0.995 | 0.045 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boccardi, V.; Poli, G.; Cecchetti, R.; Bastiani, P.; Scamosci, M.; Febo, M.; Mazzon, E.; Bruscoli, S.; Brancorsini, S.; Mecocci, P. miRNAs and Alzheimer’s Disease: Exploring the Role of Inflammation and Vitamin E in an Old-Age Population. Nutrients 2023, 15, 634. https://doi.org/10.3390/nu15030634
Boccardi V, Poli G, Cecchetti R, Bastiani P, Scamosci M, Febo M, Mazzon E, Bruscoli S, Brancorsini S, Mecocci P. miRNAs and Alzheimer’s Disease: Exploring the Role of Inflammation and Vitamin E in an Old-Age Population. Nutrients. 2023; 15(3):634. https://doi.org/10.3390/nu15030634
Chicago/Turabian StyleBoccardi, Virginia, Giulia Poli, Roberta Cecchetti, Patrizia Bastiani, Michela Scamosci, Marta Febo, Emanuela Mazzon, Stefano Bruscoli, Stefano Brancorsini, and Patrizia Mecocci. 2023. "miRNAs and Alzheimer’s Disease: Exploring the Role of Inflammation and Vitamin E in an Old-Age Population" Nutrients 15, no. 3: 634. https://doi.org/10.3390/nu15030634
APA StyleBoccardi, V., Poli, G., Cecchetti, R., Bastiani, P., Scamosci, M., Febo, M., Mazzon, E., Bruscoli, S., Brancorsini, S., & Mecocci, P. (2023). miRNAs and Alzheimer’s Disease: Exploring the Role of Inflammation and Vitamin E in an Old-Age Population. Nutrients, 15(3), 634. https://doi.org/10.3390/nu15030634









