miRNAs and Alzheimer’s Disease: Exploring the Role of Inflammation and Vitamin E in an Old-Age Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Study Design
2.1.1. Healthy Control (HC)
2.1.2. Alzheimer’s Disease (AD)
2.2. Cognitive, Functional, and Neuropsychological Assessment
2.3. Blood Sample
2.4. Vitamin E
2.5. Inflammatory Molecules
2.6. miRNAs
2.7. Statistical Analysis
3. Results
3.1. Sample Characteristics
3.2. Vitamin E
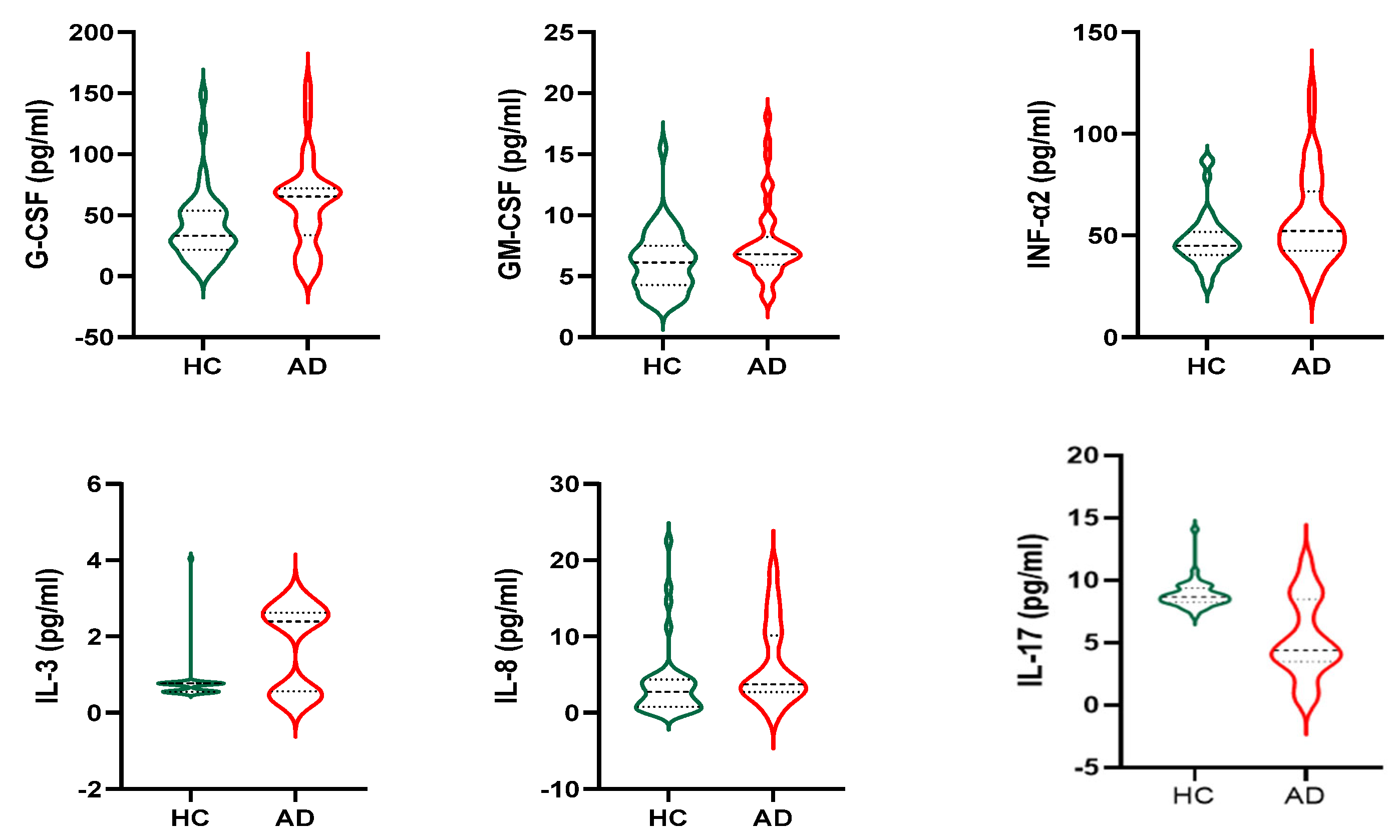
3.3. Cytokines
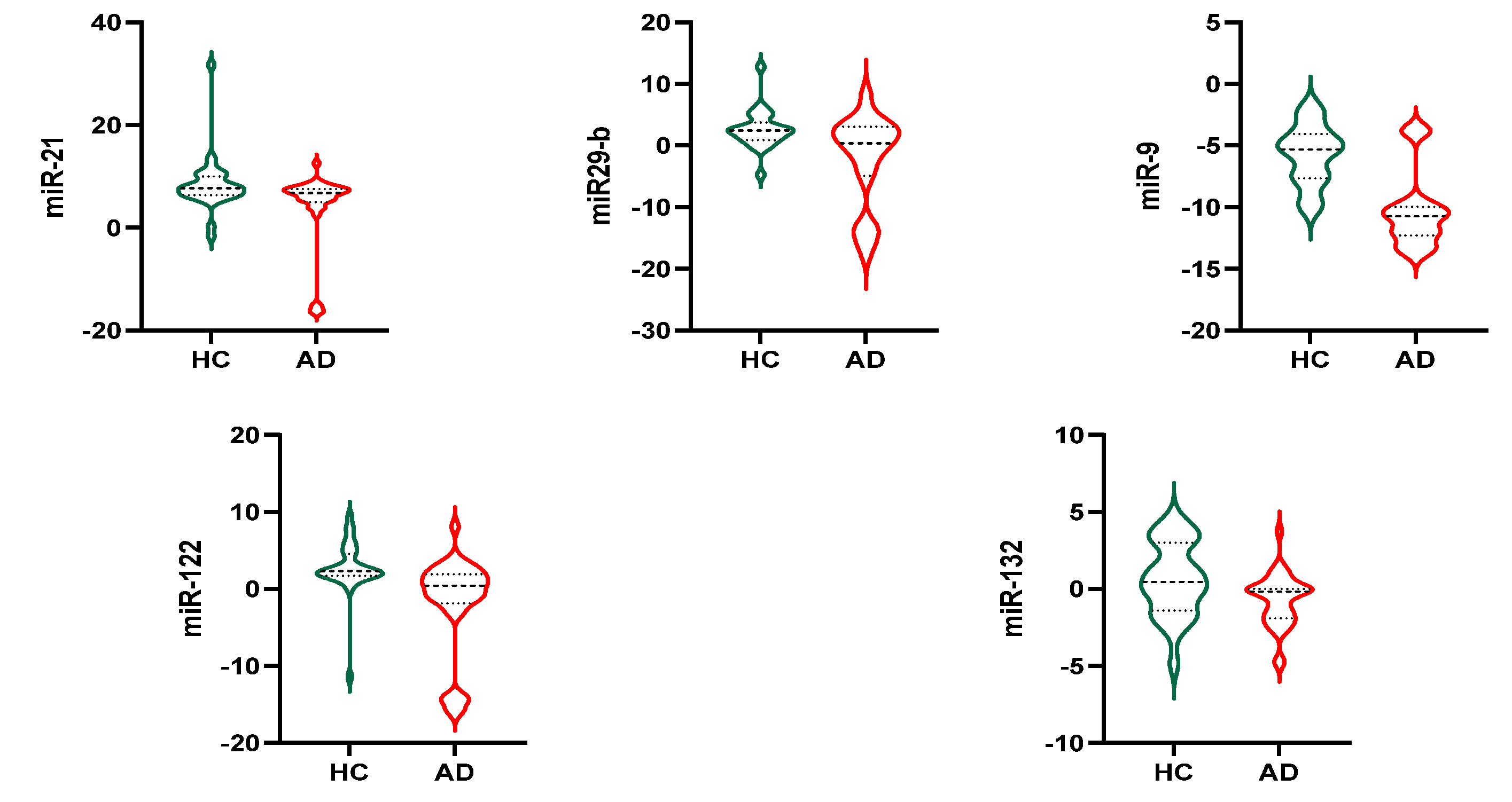
3.4. Exosomal miRNAs
3.5. miRNA-122, Alpha-Tocopherol, and AD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dubois, B.; Hampel, H.; Feldman, H.H.; Scheltens, P.; Aisen, P.; Andrieu, S.; Bakardjian, H.; Benali, H.; Bertram, L.; Blennow, K.; et al. Preclinical Alzheimer’s Disease: Definition, Natural History, and Diagnostic Criteria. Alzheimers Dement. 2016, 12, 292. [Google Scholar] [CrossRef] [PubMed]
- Ayodele, T.; Rogaeva, E.; Kurup, J.T.; Beecham, G.; Reitz, C. Early-Onset Alzheimer’s Disease: What Is Missing in Research? Curr. Neurol. Neurosci. Rep. 2021, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; McKeehan, N.; Fillit, H.M. Translating the Biology of Aging into Novel Therapeutics for Alzheimer Disease. Neurology 2019, 92, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Sultana, R.; Butterfield, D.A. Oxidative Modification of Brain Proteins in Alzheimer’s Disease: Perspective on Future Studies Based on Results of Redox Proteomics Studies. J. Alzheimers Dis. 2013, 33, S243–S251. [Google Scholar] [CrossRef] [PubMed]
- Mecocci, P.; Boccardi, V.; Cecchetti, R.; Bastiani, P.; Scamosci, M.; Ruggiero, C.; Baroni, M. A Long Journey into Aging, Brain Aging, and Alzheimer’s Disease Following the Oxidative Stress Tracks. J. Alzheimers Dis. 2018, 62, 1319–1335. [Google Scholar] [CrossRef]
- Allan Butterfield, D.; Howard, B.; Yatin, S.; Koppal, T.; Drake, J.; Hensley, K.; Aksenov, M.; Aksenova, M.; Subramaniam, R.; Varadarajan, S.; et al. Elevated Oxidative Stress in Models of Normal Brain Aging and Alzheimer’s Disease. Life Sci. 1999, 65, 1883–1892. [Google Scholar] [CrossRef]
- Wang, W.Y.; Tan, M.S.; Yu, J.T.; Tan, L. Role of Pro-Inflammatory Cytokines Released from Microglia in Alzheimer’s Disease. Ann. Transl. Med. 2015, 3, 136. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822. [Google Scholar] [CrossRef]
- Leung, R.; Proitsi, P.; Simmons, A.; Lunnon, K.; Güntert, A.; Kronenberg, D.; Pritchard, M.; Tsolaki, M.; Mecocci, P.; Kloszewska, I.; et al. Inflammatory Proteins in Plasma Are Associated with Severity of Alzheimer’s Disease. PLoS ONE 2013, 8, 64971. [Google Scholar] [CrossRef]
- Nelson, L.; Tabet, N. Slowing the Progression of Alzheimer’s Disease; What Works? Ageing Res. Rev. 2015, 23, 193–209. [Google Scholar] [CrossRef]
- van der Beek, E.M.; Kamphuis, P.J.G.H. The Potential Role of Nutritional Components in the Management of Alzheimer’s Disease. Eur. J. Pharmacol. 2008, 585, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Kishida, R.; Yamagishi, K.; Maruyama, K.; Okada, C.; Tanaka, M.; Ikeda, A.; Hayama-Terada, M.; Shimizu, Y.; Muraki, I.; Umesawa, M.; et al. Dietary Intake of Beans and Risk of Disabling Dementia: The Circulatory Risk in Communities Study (CIRCS). Eur. J. Clin. Nutr. 2023, 77, 65–70. [Google Scholar] [CrossRef]
- Ferrero, G.; Carpi, S.; Polini, B.; Pardini, B.; Nieri, P.; Impeduglia, A.; Grioni, S.; Tarallo, S.; Naccarati, A. Intake of Natural Compounds and Circulating Microrna Expression Levels: Their Relationship Investigated in Healthy Subjects with Different Dietary Habits. Front. Pharmacol. 2021, 11, 2214. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Zhu, M.; Kong, C.; Pang, Y.; Zhang, H.; Qiu, Q.; Wei, C.; Tang, Y.; Wang, Q.; Li, Y.; et al. Blood Neuro-Exosomal Synaptic Proteins Predict Alzheimer’s Disease at the Asymptomatic Stage. Alzheimers Dement. 2021, 17, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Zhu, M.; Yang, J.; Pang, Y.; Wang, Q.; Li, T.T.; Li, F.; Wang, Q.; Li, Y.; Wei, Y. Exosomal MicroRNA-Based Predictive Model for Preclinical Alzheimer’s Disease: A Multicenter Study. Biol. Psychiatry 2022, 92, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wang, L. Inflamma-MicroRNAs in Alzheimer’s Disease: From Disease Pathogenesis to Therapeutic Potentials. Front. Cell. Neurosci. 2021, 15, 785433. [Google Scholar] [CrossRef]
- Giuliani, A.; Gaetani, S.; Sorgentoni, G.; Agarbati, S.; Laggetta, M.; Matacchione, G.; Gobbi, M.; Rossi, T.; Galeazzi, R.; Piccinini, G.; et al. Circulating Inflamma-MiRs as Potential Biomarkers of Cognitive Impairment in Patients Affected by Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 647015. [Google Scholar] [CrossRef]
- Konovalova, J.; Gerasymchuk, D.; Parkkinen, I.; Chmielarz, P.; Domanskyi, A. Interplay between MicroRNAs and Oxidative Stress in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 6055. [Google Scholar] [CrossRef]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The Diagnosis of Dementia Due to Alzheimer’s Disease: Recommendations from the National Institute on Aging-Alzheimer’s Association Workgroups on Diagnostic Guidelines for Alzheimer’s Disease. Alzheimers Dement. 2011, 7, 263. [Google Scholar] [CrossRef]
- Boccardi, V.; Paolacci, L.; Remondini, D.; Giampieri, E.; Poli, G.; Curti, N.; Cecchetti, R.; Villa, A.; Ruggiero, C.; Brancorsini, S.; et al. Cognitive Decline and Alzheimer’s Disease in Old Age: A Sex-Specific Cytokinome Signature. J. Alzheimers Dis. 2019, 72, 911–918. [Google Scholar] [CrossRef]
- Mangialasche, F.; Westman, E.; Kivipelto, M.; Muehlboeck, J.S.; Cecchetti, R.; Baglioni, M.; Tarducci, R.; Gobbi, G.; Floridi, P.; Soininen, H.; et al. Classification and Prediction of Clinical Diagnosis of Alzheimer’s Disease Based on MRI and Plasma Measures of α-/γ-Tocotrienols and γ-Tocopherol. J. Intern. Med. 2013, 273, 602–621. [Google Scholar] [CrossRef]
- Poudel, P.; Park, S. Recent Advances in the Treatment of Alzheimer’s Disease Using Nanoparticle-Based Drug Delivery Systems. Pharmaceutics 2022, 14, 835. [Google Scholar] [CrossRef] [PubMed]
- Bonda, D.J.; Wang, X.; Lee, H.G.; Smith, M.A.; Perry, G.; Zhu, X. Neuronal Failure in Alzheimer’s Disease: A View through the Oxidative Stress Looking-Glass. Neurosci. Bull. 2014, 30, 243–252. [Google Scholar] [CrossRef]
- Sinyor, B.; Mineo, J.; Ochner, C. Alzheimer’s Disease, Inflammation, and the Role of Antioxidants. J. Alzheimers Dis. Rep. 2020, 4, 175. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, G.; Maclean, A.G.; Philipp, M.T. Cytokines and Chemokines at the Crossroads of Neuroinflammation, Neurodegeneration, and Neuropathic Pain. Mediat. Inflamm. 2013, 2013, 480739. [Google Scholar] [CrossRef]
- Gugliandolo, A.; Bramanti, P.; Mazzon, E. Role of Vitamin E in the Treatment of Alzheimer’s Disease: Evidence from Animal Models. Int. J. Mol. Sci. 2017, 18, 2504. [Google Scholar] [CrossRef]
- Browne, D.; McGuinness, B.; Woodside, J.v.; McKay, G.J. Vitamin E and Alzheimer’s Disease: What Do We Know so Far? Clin. Interv. Aging 2019, 14, 1303. [Google Scholar] [CrossRef] [PubMed]
- Galli, F.; Azzi, A.; Birringer, M.; Cook-Mills, J.M.; Eggersdorfer, M.; Frank, J.; Cruciani, G.; Lorkowski, S.; Özer, N.K. Vitamin E: Emerging Aspects and New Directions. Free Radic. Biol. Med. 2017, 102, 16–36. [Google Scholar] [CrossRef]
- Nishida, Y.; Yokota, T.; Takahashi, T.; Uchihara, T.; Jishage, K.-i.; Mizusawa, H. Deletion of Vitamin E Enhances Phenotype of Alzheimer Disease Model Mouse. Biochem. Biophys. Res. Commun. 2006, 350, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.; Yao, Y.; Uryu, K.; Yang, H.; Lee, V.M.Y.; Trojanowski, J.Q.; Praticò, D. Early Vitamin E Supplementation in Young but Not Aged Mice Reduces Abeta Levels and Amyloid Deposition in a Transgenic Model of Alzheimer’s Disease. FASEB J. 2004, 18, 323–325. [Google Scholar] [CrossRef]
- Devore, E.E.; Grodstein, F.; van Rooij, F.J.A.; Hofman, A.; Stampfer, M.J.; Witteman, J.C.M.; Breteler, M.M.B. Dietary Antioxidants and Long-Term Risk of Dementia. Arch. Neurol. 2010, 67, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Wilson, R.S. Vitamin E and Cognitive Decline in Older Persons. Arch. Neurol. 2002, 59, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Quintanilha, B.J.; Reis, B.Z.; Silva Duarte, G.B.; Cozzolino, S.M.F.; Rogero, M.M. Nutrimiromics: Role of MicroRNAs and Nutrition in Modulating Inflammation and Chronic Diseases. Nutrients 2017, 9, 1168. [Google Scholar] [CrossRef] [PubMed]
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene Regulation by Long Non-Coding RNAs and Its Biological Functions. Nat. Rev. Mol. Cell Biol. 2020, 22, 96–118. [Google Scholar] [CrossRef]
- Zhao, Y.; Jaber, V.; Alexandrov, P.N.; Vergallo, A.; Lista, S.; Hampel, H.; Lukiw, W.J. MicroRNA-Based Biomarkers in Alzheimer’s Disease (AD). Front. Neurosci. 2020, 14, 585432. [Google Scholar] [CrossRef]
- Bai, X.; Bian, Z. MicroRNA-21 Is a Versatile Regulator and Potential Treatment Target in Central Nervous System Disorders. Front. Mol. Neurosci. 2022, 15, 842288. [Google Scholar] [CrossRef]
- Cui, G.H.; Wu, J.; Mou, F.F.; Xie, W.H.; Wang, F.B.; Wang, Q.L.; Fang, J.; Xu, Y.W.; Dong, Y.R.; Liu, J.R.; et al. Exosomes Derived from Hypoxia-Preconditioned Mesenchymal Stromal Cells Ameliorate Cognitive Decline by Rescuing Synaptic Dysfunction and Regulating Inflammatory Responses in APP/PS1 Mice. FASEB J. 2018, 32, 654–668. [Google Scholar] [CrossRef]
- Souza, V.C.; Morais, G.S.; Henriques, A.D.; Machado-Silva, W.; Perez, D.I.V.; Brito, C.J.; Camargos, E.F.; Moraes, C.F.; Nóbrega, O.T. Whole-Blood Levels of MicroRNA-9 Are Decreased in Patients With Late-Onset Alzheimer Disease. Am. J. Alzheimers Dis. Other Demen. 2020, 35, 1533317520911573. [Google Scholar] [CrossRef]
- Jahangard, Y.; Monfared, H.; Moradi, A.; Zare, M.; Mirnajafi-Zadeh, J.; Mowla, S.J. Therapeutic Effects of Transplanted Exosomes Containing MiR-29b to a Rat Model of Alzheimer’s Disease. Front. Neurosci. 2020, 14, 564. [Google Scholar] [CrossRef]
- Yuva-Aydemir, Y.; Simkin, A.; Gascon, E.; Gao, F.-B. MicroRNA-9: Functional Evolution of a Conserved Small Regulatory RNA. RNA Biol. 2011, 8, 557–564. [Google Scholar] [CrossRef]
- Walgrave, H.; Zhou, L.; de Strooper, B.; Salta, E. The Promise of MicroRNA-Based Therapies in Alzheimer’s Disease: Challenges and Perspectives. Mol. Neurodegener. 2021, 16, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Salta, E.; Sierksma, A.; vanden Eynden, E.; de Strooper, B. MiR-132 Loss de-Represses ITPKB and Aggravates Amyloid and TAU Pathology in Alzheimer’s Brain. EMBO Mol. Med. 2016, 8, 1005–1018. [Google Scholar] [CrossRef] [PubMed]
- Tarkowski, E.; Wallin, A.; Regland, B.; Blennow, K.; Tarkowski, A. Local and Systemic GM-CSF Increase in Alzheimer’s Disease and Vascular Dementia. Acta Neurol. Scand. 2001, 103, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Griffin, W.S.T.; Nicoll, J.A.R.; Grimaldi, L.M.E.; Sheng, J.G.; Mrak, R.E. The Pervasiveness of Interleukin-1 in Alzheimer Pathogenesis: A Role for Specific Polymorphisms in Disease Risk. Exp. Gerontol. 2000, 35, 481. [Google Scholar] [CrossRef]
- Doroszkiewicz, J.; Kulczynska-Przybik, A.; Dulewicz, M.; Borawska, R.; Krawiec, A.; Slowik, A.; Mroczko, B. The Cerebrospinal Fluid Interleukin 8 (IL-8) Concentration in Alzheimer’s Disease (AD). Alzheimer’s Dement. 2021, 17, e051317. [Google Scholar] [CrossRef]
- Tripathy, D.; Thirumangalakudi, L.; Grammas, P. Expression of Macrophage Inflammatory Protein 1-Alpha Is Elevated in Alzheimer’s Vessels and Is Regulated by Oxidative Stress. J. Alzheimers Dis. 2007, 11, 447–455. [Google Scholar] [CrossRef]
- Gaedicke, S.; Zhang, X.; Schmelzer, C.; Lou, Y.; Doering, F.; Frank, J.; Rimbach, G. Vitamin E Dependent MicroRNA Regulation in Rat Liver. FEBS Lett. 2008, 582, 3542–3546. [Google Scholar] [CrossRef]
- Tahamtan, A.; Teymoori-Rad, M.; Nakstad, B.; Salimi, V. Anti-Inflammatory MicroRNAs and Their Potential for Inflammatory Diseases Treatment. Front. Immunol. 2018, 9, 1377. [Google Scholar] [CrossRef]
- Esau, C.; Davis, S.; Murray, S.F.; Yu, X.X.; Pandey, S.K.; Pear, M.; Watts, L.; Booten, S.L.; Graham, M.; McKay, R.; et al. MiR-122 Regulation of Lipid Metabolism Revealed by in Vivo Antisense Targeting. Cell Metab. 2006, 3, 87–98. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Żebrowska, E.; Chabowski, A. Insulin Resistance and Oxidative Stress in the Brain: What’s New? Int. J. Mol. Sci. 2019, 20, 874. [Google Scholar] [CrossRef]
| Total | HC | AD | p | |
|---|---|---|---|---|
| N | 80 | 40 | 40 | |
| F/M (n) | 45/35 | 18/22 | 27/13 | 0.043 * |
| Age (years) | 77.58 ± 3.86 | 76.33 ± 3.61 | 78.83 ± 3.74 | 0.003 |
| MMSE | 21.33 ± 8.32 | 28.83 ± 1.50 | 13.43 ± 4.13 | <0.0001 |
| GDS | 5.03 ± 3.23 | 4.92 ± 2.86 | 5.16 ± 3.67 | 0.765 |
| ADL | 4.50 ± 1.56 | 5.53 ± 0.78 | 3.48 ± 1.48 | <0.0001 |
| IADL | 3.83 ± 2.96 | 6.35 ± 1.79 | 1.30 ± 1.24 | <0.0001 |
| Glycemia (mg/dL) | 100.5 ± 23.3 | 101.8 ± 24.6 | 99.1 ± 22.2 | 0.630 |
| Creatinine (mg/dL) | 1.03 ± 0.17 | 1.06 ± 0.23 | 1.0 ± 0.11 | 0.196 |
| TC (mg/dL) | 197.4 ± 39.9 | 192.8 ± 35.7 | 202.3 ± 43.8 | 0.294 |
| HDL-C(mg/dL) | 57.2 ± 15.9 | 57.2 ± 16.9 | 57.1 ± 15.0 | 0.986 |
| LDL-C (mg/dL) | 118.4 ± 35.2 | 113.3 ± 33.5 | 123.8 ± 36.6 | 0.218 |
| Triglycerides (mg/dL) | 112.1 ± 49.5 | 118.8 ± 48.5 | 125.5 ± 50.9 | 0.546 |
| Partial Correlations | ||
|---|---|---|
| Correlation Coefficient (r) | p-Value | |
| miR-122/GM-CSF | 0.389 | 0.019 |
| miR-122/INF-α2 | 0.375 | 0.024 |
| miR-122/IL-1α | 0.360 | 0.031 |
| miR-122/IL-8 | 0.375 | 0.024 |
| miR-122/MIP-1β | 0.354 | 0.034 |
| Model 1 | ||||
| B | OR | IC 95% | p | |
| Age | 0.182 | 1.199 | 1.029–1.398 | 0.020 |
| Gender | 0.493 | 1.637 | 0.578–4.634 | 0.353 |
| α-tocopherol | −0.329 | 0.720 | 0.579–0.895 | 0.003 |
| Model 2 | ||||
| B | OR | IC 95% | p | |
| Age | 0.175 | 1.191 | 0.989–1.434 | 0.065 |
| Gender | 0.047 | 1.048 | 0.326–3.363 | 0.938 |
| α-tocopherol | −0.252 | 0.777 | 0.617–0.980 | 0.033 |
| MiR-122 | −0.215 | 0.806 | 0.654–0.995 | 0.045 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boccardi, V.; Poli, G.; Cecchetti, R.; Bastiani, P.; Scamosci, M.; Febo, M.; Mazzon, E.; Bruscoli, S.; Brancorsini, S.; Mecocci, P. miRNAs and Alzheimer’s Disease: Exploring the Role of Inflammation and Vitamin E in an Old-Age Population. Nutrients 2023, 15, 634. https://doi.org/10.3390/nu15030634
Boccardi V, Poli G, Cecchetti R, Bastiani P, Scamosci M, Febo M, Mazzon E, Bruscoli S, Brancorsini S, Mecocci P. miRNAs and Alzheimer’s Disease: Exploring the Role of Inflammation and Vitamin E in an Old-Age Population. Nutrients. 2023; 15(3):634. https://doi.org/10.3390/nu15030634
Chicago/Turabian StyleBoccardi, Virginia, Giulia Poli, Roberta Cecchetti, Patrizia Bastiani, Michela Scamosci, Marta Febo, Emanuela Mazzon, Stefano Bruscoli, Stefano Brancorsini, and Patrizia Mecocci. 2023. "miRNAs and Alzheimer’s Disease: Exploring the Role of Inflammation and Vitamin E in an Old-Age Population" Nutrients 15, no. 3: 634. https://doi.org/10.3390/nu15030634
APA StyleBoccardi, V., Poli, G., Cecchetti, R., Bastiani, P., Scamosci, M., Febo, M., Mazzon, E., Bruscoli, S., Brancorsini, S., & Mecocci, P. (2023). miRNAs and Alzheimer’s Disease: Exploring the Role of Inflammation and Vitamin E in an Old-Age Population. Nutrients, 15(3), 634. https://doi.org/10.3390/nu15030634









