Protocatechuic Acid Prevents Some of the Memory-Related Behavioural and Neurotransmitter Changes in a Pyrithiamine-Induced Thiamine Deficiency Model of Wernicke–Korsakoff Syndrome in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs, Treatment, and Early Neurological Assessment
2.3. Assessment of Motor Function
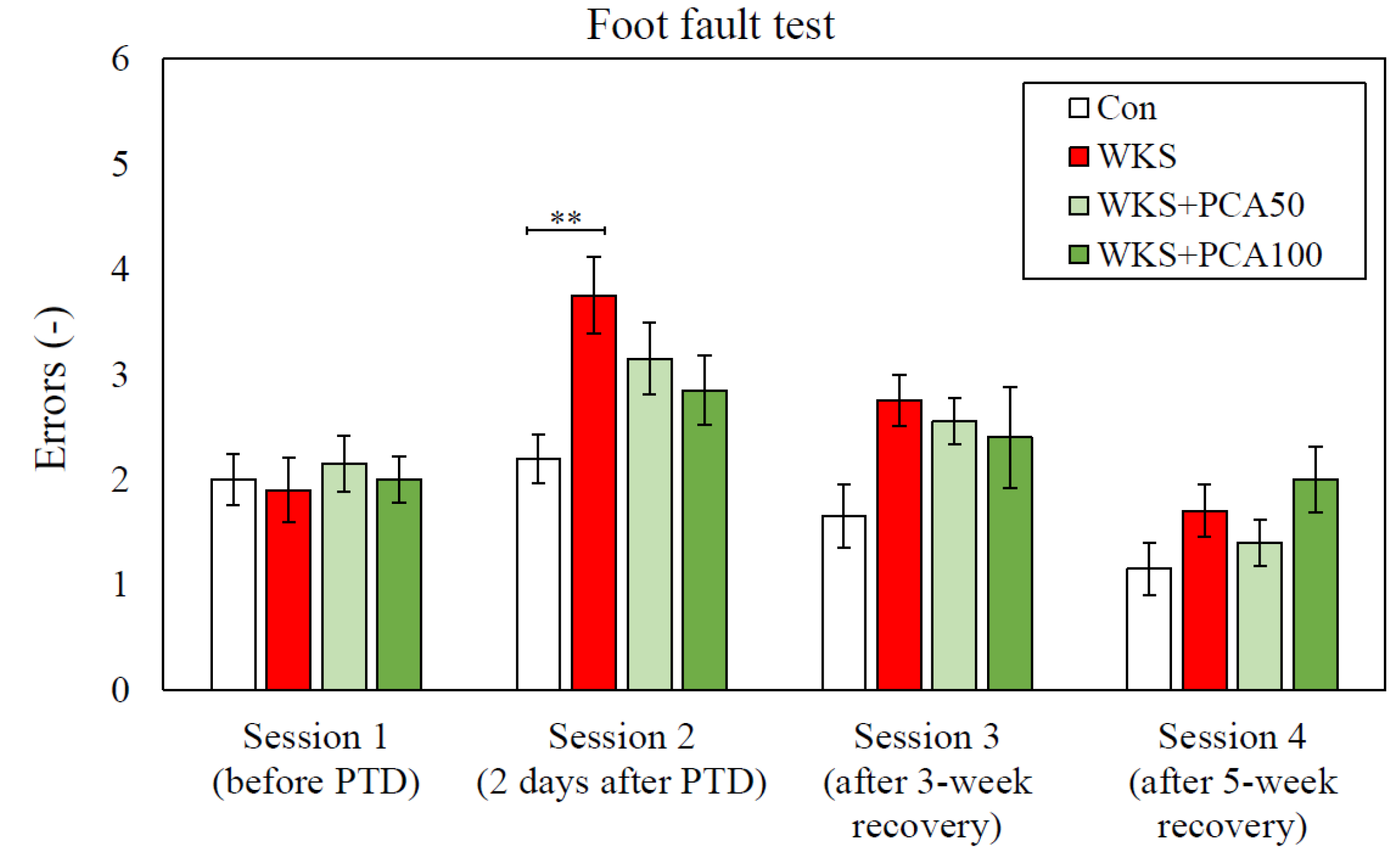
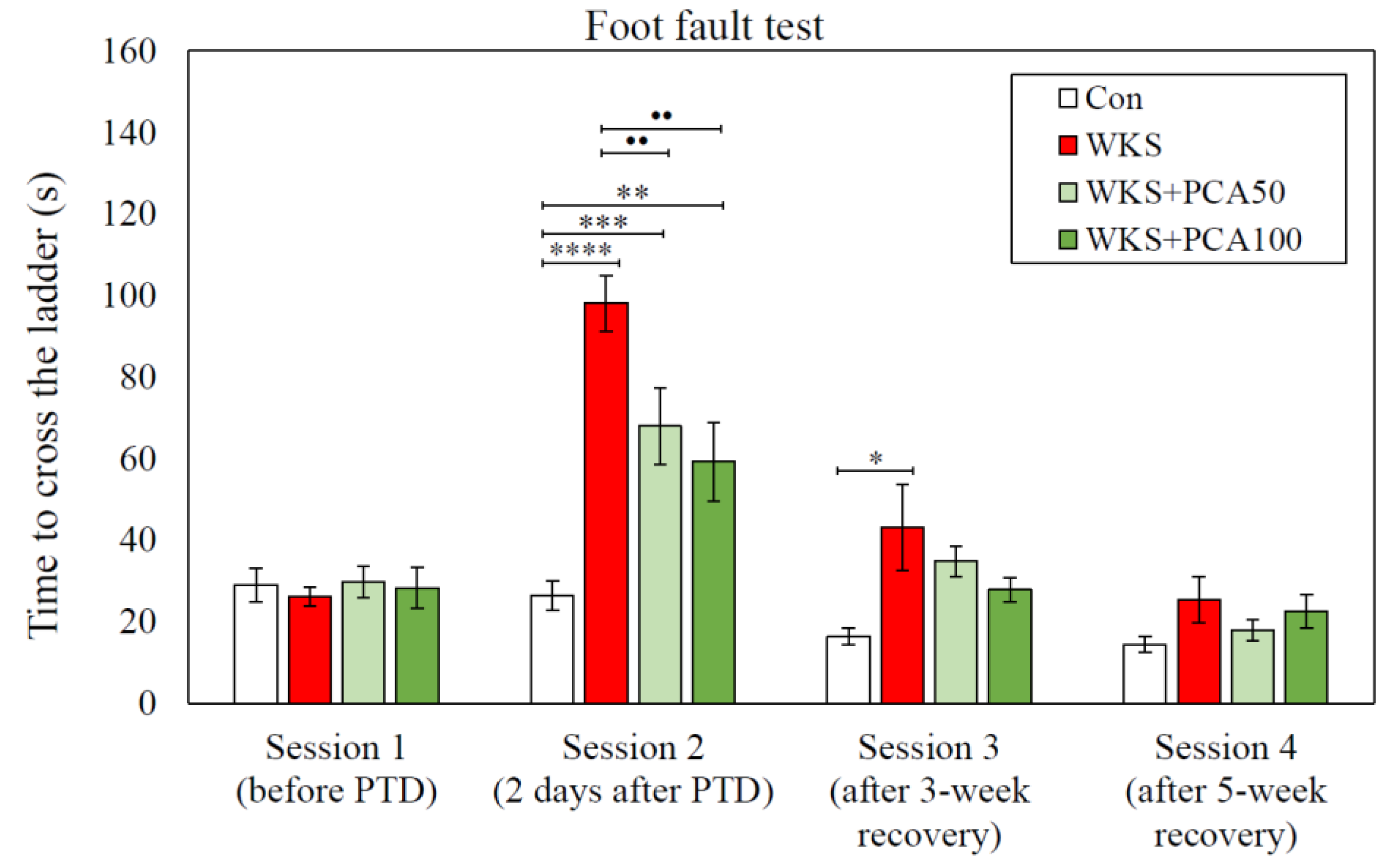
Foot Fault Test
2.4. Assessment of Cataleptic Behaviour
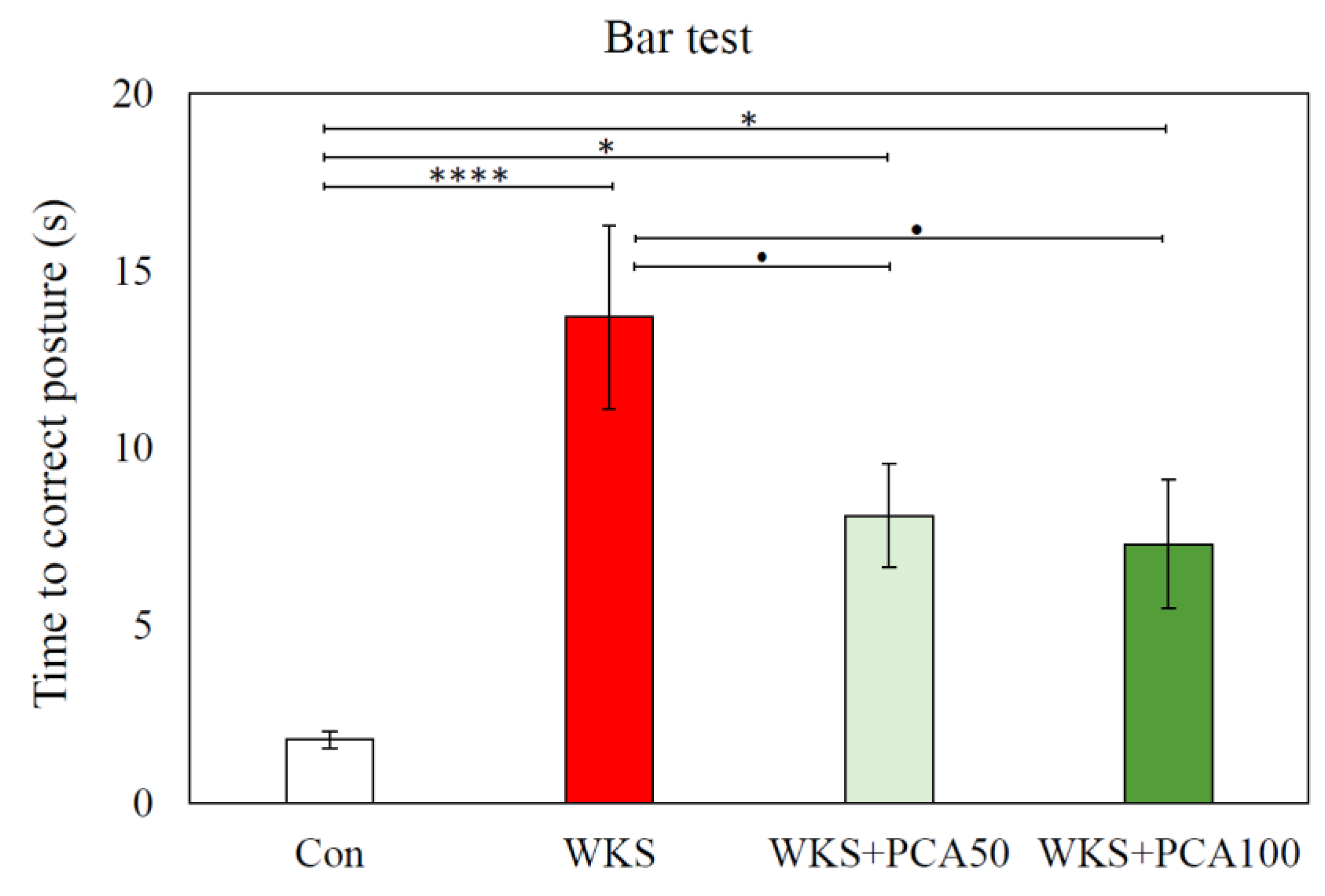
Bar Test
2.5. Cognitive Behaviour Evaluation
2.5.1. Open Field Test (OF)
2.5.2. Hole–Board Test (HB)
2.5.3. Novel Object Recognition Test (NOR)
2.5.4. Morris Water Maze Test (MWM)
2.6. Sample Preparation
2.7. Biochemical Evaluation
2.7.1. Quantification of Monoamines
2.7.2. Quantification of Amino Acids
2.8. Statistical Analysis
3. Results
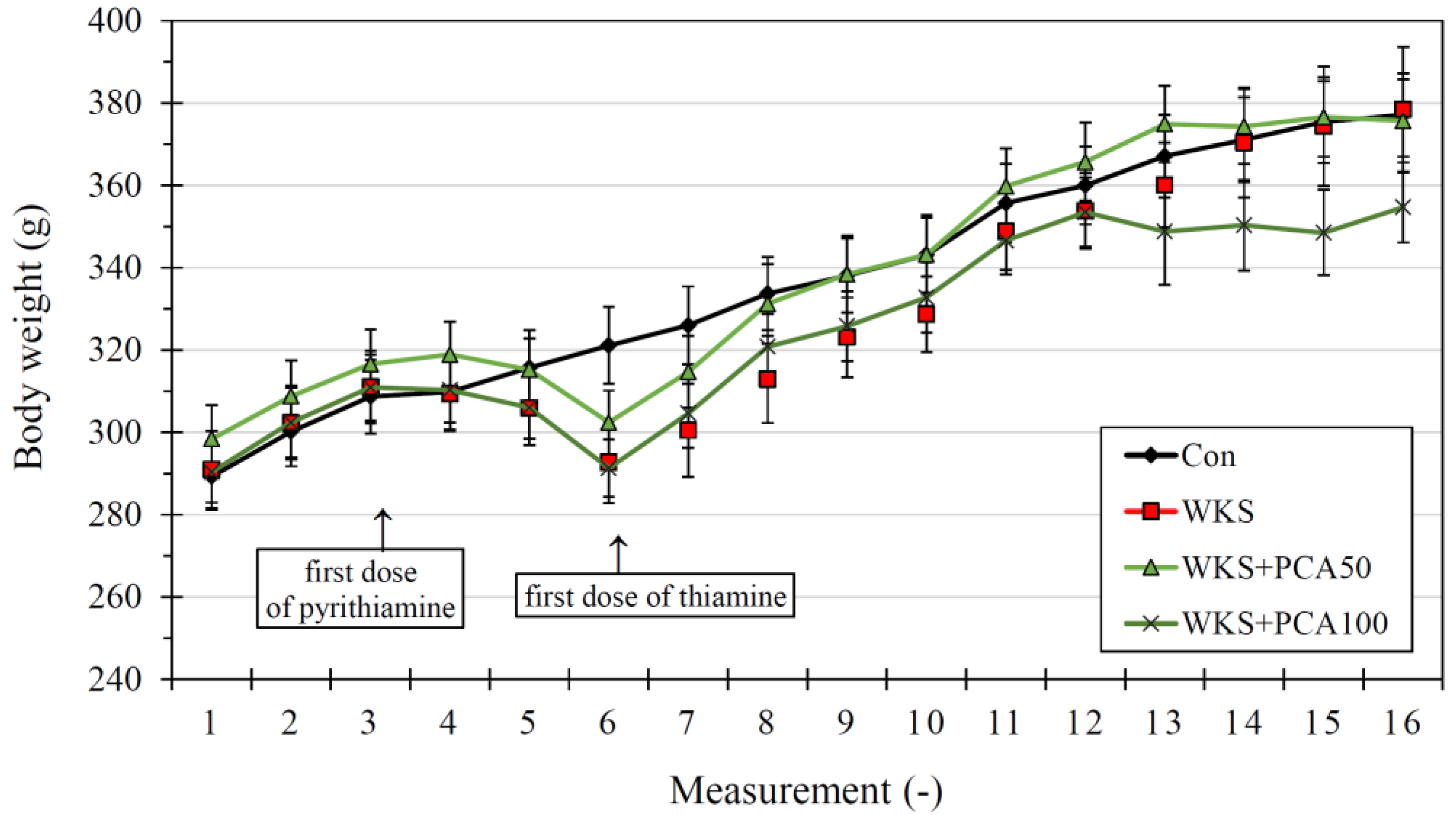
3.1. Neurological Assessment and Body Weight
3.2. Foot Fault Test
3.2.1. Accuracy of Foot Placement
3.2.2. Time Measurements
3.2.3. Bar Test
3.3. Open Field Test
3.4. Hole–Board Test
3.5. Novel Object Recognition Test
3.5.1. Familiarisation
3.5.2. Recognition
3.6. Morris Water Maze Test
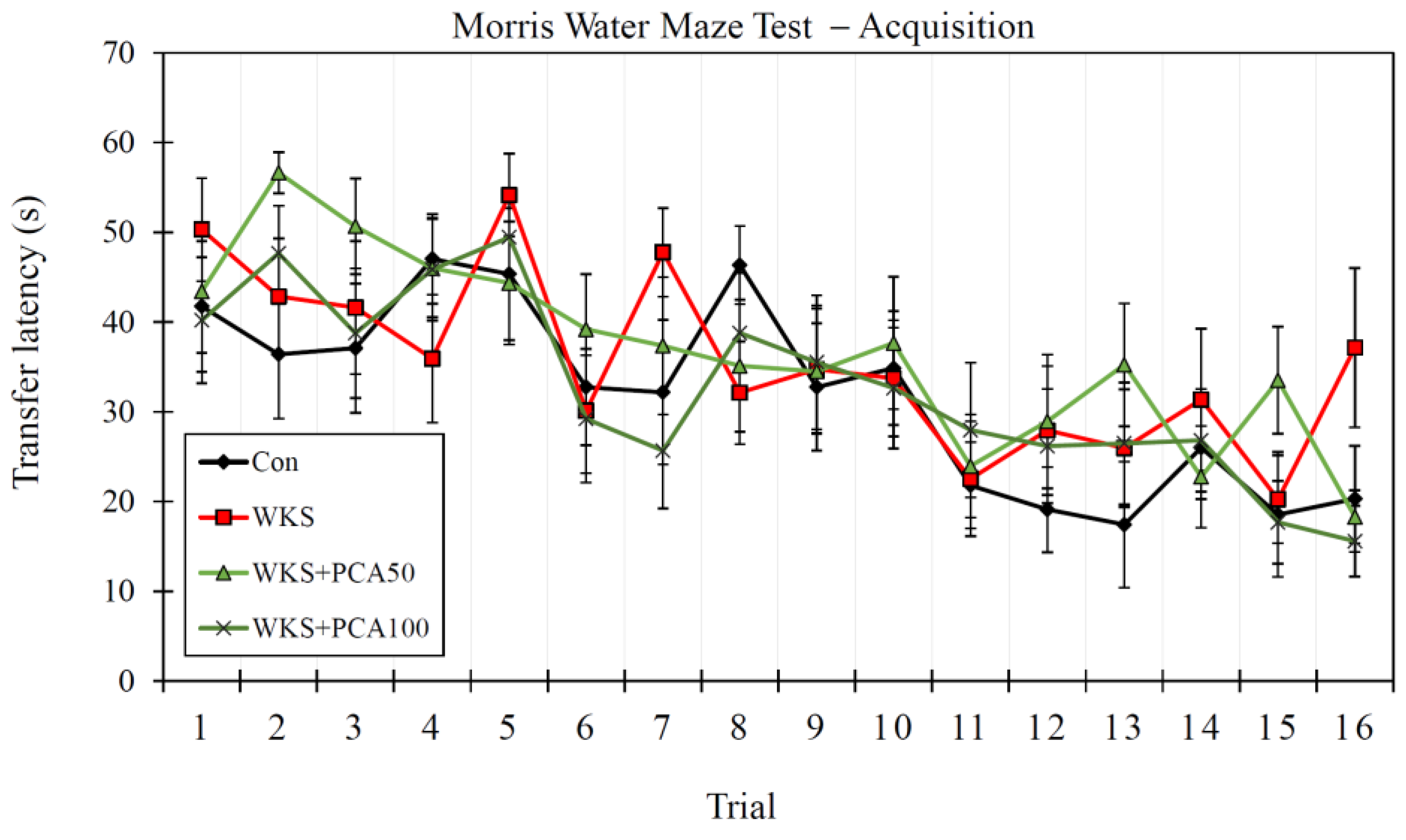
3.6.1. Acquisition
3.6.2. Recall of Memory
3.6.3. Working Memory in Reversal Training
3.6.4. Consolidation
3.6.5. Control Protocol with Visible Platform
3.7. Brain Monoamines
3.8. Brain Amino Acids
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-HIAA | 5-hydroxyindoleacetic acid |
| 5-HT | 5-hydroxytryptamine (serotonin) |
| AGEs | Advanced glycation end products |
| ALA | Alanine |
| ANOVA | Analysis of variance |
| ASP | Aspartic acid |
| b.w. | Body weight |
| Con | Healthy control group |
| CNS | Central nervous system |
| DA | Dopamine |
| DOPAC | 3,4-dihydroxyphenylacetic acid |
| EAAT1, GLAST | Astrocytic glutamate transporters 1 |
| EAAT2, GLT-1 | Astrocytic glutamate transporter 2 |
| FF | Foot Fault test |
| GABA | γ-aminobutyric acid |
| GH | Post-hoc Games–Howell test |
| GLU | Glutamate |
| HB | Hole–Board test |
| HIS | Histidine |
| HPLC-ED | High-performance liquid chromatography with electrochemical detection |
| HVA | Homovanillic acid |
| LRR | Loss of righting reflex |
| MHPG | 3-methoxy-4-hydroxyphenylglycol |
| MWM | Morris Water Maze test |
| NA | Noradrenaline |
| NK | Post-hoc Newman–Keuls test |
| NOR | Novel Object Recognition test |
| OF | Open Field test |
| PCA | Protocatechuic acid |
| PCA50 | PCA administered at dose 50 mg/kg b.w. |
| PCA100 | PCA administered at dose 100 mg/kg b.w. |
| PTD | Pyrithiamine-induced thiamine deficiency |
| SER | Serine |
| TAU | Taurine |
| TD | Thiamine deficiency |
| THR | Threonine |
| WKS | Wernicke–Korsakoff syndrome |
References
- Kimura, A.; Sugimoto, T.; Kitamori, K.; Saji, N.; Niida, S.; Toba, K.; Sakurai, T. Malnutrition is associated with behavioral and psychiatric symptoms of dementia in older women with mild cognitive impairment and early-stage Alzheimer’s disease. Nutrients 2019, 11, 1951. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Yu, W.; Liu, X.; Wan, T.; Chen, C.; Xiong, L.; Zhang, W.; Lü, Y. Associations between malnutrition and cognitive impairment in an elderly Chinese population: An analysis based on a 7-year database. Psychogeriatrics 2021, 1, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; He, Y.; Luo, C.; Feng, B.; Ran, F.; Xu, H.; Ci, Z.; Xu, R.; Han, L.; Zhang, D. New progress in the pharmacology of protocatechuic acid: A compound ingested in daily foods and herbs frequently and heavily. Pharmacol. Res. 2020, 161, 105109. [Google Scholar] [CrossRef]
- Widy-Tyszkiewicz, E. Current Evidence for Disease Prevention and Treatment by Protocatechuic Acid (PCA) and Its Precursor Protocatechuic Aldehyde (PCAL) in Animals and Humans. In Plant Antioxidants and Health; Reference Series in Phytochemistry; Ekiert, H.M., Ramawat, K.G., Arora, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar] [CrossRef]
- Krzysztoforska, K.; Mirowska-Guzel, D.; Widy-Tyszkiewicz, E. Pharmacological effects of protocatechuic acid and its therapeutic potential in neurodegenerative diseases: Review on the basis of in vitro and in vivo studies in rodents and humans. Nutr. Neurosci. 2017, 22, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Zhang, X.; Lv, C.; Li, C.; Yu, Y.; Wang, X.; Han, F. Protocatechuic acid ameliorates neurocognitive functions impairment induced by chronic intermittent hypoxia. Sci. Rep. 2015, 5, 14507. [Google Scholar] [CrossRef]
- Shi, S.H.; Zhao, X.; Liu, A.J.; Liu, B.; Li, H.; Wu, B.; Bi, K.S.; Jia, Y. Protective effect of n-butanol extract from Alpinia oxyphylla on learning and memory impairments. Physiol. Behav. 2015, 139, 13–20. [Google Scholar] [CrossRef]
- Song, Y.; Cui, T.; Xie, N.; Zhang, X.; Qian, Z.; Liu, J. Protocatechuic acid improves cognitive deficits and attenuates amyloid deposits, inflammatory response in aged AβPP/PS1 double transgenic mice. Int. Immunopharmacol. 2014, 20, 276–281. [Google Scholar] [CrossRef]
- Guan, S.; Zhang, X.L.; Ge, D.; Liu, T.Q.; Ma, X.H.; Cui, Z.F. Protocatechuic acid promotes the neuronal differentiation and facilitates survival of phenotypes differentiated from cultured neural stem and progenitor cells. Eur. J. Pharmacol. 2011, 670, 471–478. [Google Scholar] [CrossRef]
- Krzysztoforska, K.; Piechal, A.; Blecharz-Klin, K.; Pyrzanowska, J.; Joniec-Maciejak, I.; Mirowska-Guzel, D.; Widy-Tyszkiewicz, E. Administration of protocatechuic acid affects memory and restores hippocampal and cortical serotonin turnover in rat model of oral D-galactose-induced memory impairment. Behav. Brain Res. 2019, 368, 111896. [Google Scholar] [CrossRef]
- Adefegha, S.A.; Oboh, G.; Omojokun, O.S.; Adefegha, O.M. Alterations of Na+/K+-ATPase, cholinergic and antioxidant enzymes activity by protocatechuic acid in cadmium-induced neurotoxicity and oxidative stress in Wistar rats. Biomed. Pharmacother. 2016, 83, 559–568. [Google Scholar] [CrossRef]
- Kho, A.R.; Choi, B.Y.; Lee, S.H.; Hong, D.K.; Lee, S.H.; Jeong, J.H.; Park, K.H.; Song, H.K.; Choi, H.C.; Suh, S.W. Effects of protocatechuic acid (PCA) on global cerebral ischemia-induced hippocampal neuronal death. Int. J. Mol. Sci. 2018, 19, 1420. [Google Scholar] [CrossRef]
- Martin, P.R.; Singleton, C.K.; Hiller-Sturmhöfel, S. The role of thiamine deficiency in alcoholic brain disease. Alcohol Res. Health. 2003, 27, 134. [Google Scholar] [PubMed]
- Oudman, E.; Wijnia, J.W.; Oey, M.J.; van Dam, M.; Postma, A. Wernicke-Korsakoff syndrome despite no alcohol abuse: A summary of systematic reports. J. Neurol. Sci. 2021, 426, 117482. [Google Scholar] [CrossRef] [PubMed]
- Isenberg-Grzeda, E.; Kutner, H.E.; Nicolson, S.E. Wernicke-Korsakoff-syndrome: Under-recognized and under-treated. Psychosomatics 2012, 53, 507–516. [Google Scholar] [CrossRef]
- Carvalho, F.M.; Pereira, S.R.; Pires, R.G.; Ferraz, V.P.; Romano-Silva, M.A.; Oliveira-Silva, I.F.; Ribeiro, A.M. Thiamine deficiency decreases glutamate uptake in the prefrontal cortex and impairs spatial memory performance in a water maze test. Pharmacol. Biochem. Behav. 2006, 83, 481–489. [Google Scholar] [CrossRef]
- Kessels, R.P.; Kopelman, M.D. Context memory in Korsakoff’s syndrome. Neuropsychol. Rev. 2012, 22, 117–131. [Google Scholar] [CrossRef]
- Eliash, S.; Dror, V.; Cohen, S.; Rehavi, M. Neuroprotection by rasagiline in thiamine deficient rats. Brain Res. 2009, 1256, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Vetreno, R.P.; Ramos, R.L.; Anzalone, S.; Savage, L.M. Brain and behavioral pathology in an animal model of Wernicke’s encephalopathy and Wernicke–Korsakoff Syndrome. Brain Res. 2012, 1436, 178–192. [Google Scholar] [CrossRef]
- Savage, L.M.; Hall, J.M.; Resende, L.S. Translational rodent models of Korsakoff syndrome reveal the critical neuroanatomical substrates of memory dysfunction and recovery. Neuropsychol. Rev. 2012, 22, 195–209. [Google Scholar] [CrossRef]
- Hazell, A.S.; Sheedy, D.; Oanea, R.; Aghourian, M.; Sun, S.; Jung, J.Y.; Wang, D.; Wang, C. Loss of astrocytic glutamate transporters in Wernicke encephalopathy. Glia 2010, 58, 148–156. [Google Scholar] [CrossRef]
- Langlais, P.J.; Mair, R.G. Protective effects of the glutamate antagonist MK-801 on pyrithiamine-induced lesions and amino acid changes in rat brain. J. Neurosci. 1990, 10, 1664–1674. [Google Scholar] [CrossRef] [PubMed]
- Metz, G.A.; Whishaw, I.Q. Cortical and subcortical lesions impair skilled walking in the ladder rung walking test: A new task to evaluate fore-and hindlimb stepping, placing, and co-ordination. J. Neurosci. Methods 2002, 115, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.S. Emotional behavior in the rat. I. Defecation and urination as measures of individual differences in emotionality. J. Comp. Psychol. 1934, 18, 385. [Google Scholar] [CrossRef]
- Boissier, J.R. The exploration reaction in mouse. Therapie 1962, 17, 1225–1232. [Google Scholar] [PubMed]
- Ennaceur, A.; Delacour, J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav. Brain Res. 1988, 31, 47–59. [Google Scholar] [CrossRef]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Widy-Tyszkiewicz, E.; Piechal, A.; Joniec, I.; Blecharz-Klin, K. Long term administration of Hypericum perforatum improves spatial learning and memory in the water maze. Biol. Pharm. Bull. 2002, 25, 1289–1294. [Google Scholar] [CrossRef]
- Glowinski, J.; Iversen, L.L. Regional studies of catecholamines in the rat brain-I: The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain. J. Neurochem. 1966, 13, 655–669. [Google Scholar] [CrossRef]
- Rowley, H.L.; Martin, K.F.; Marsden, C.A. Determination of in vivo amino acid neurotransmitters by high-performance liquid chromatography with o-phthalaldehyde-sulphite derivatisation. J. Neurosci. Methods 1995, 57, 93–99. [Google Scholar] [CrossRef]
- Moya, M.; López-Valencia, L.; García-Bueno, B.; Orio, L. Disinhibition-Like Behavior Correlates with Frontal Cortex Damage in an Animal Model of Chronic Alcohol Consumption and Thiamine Deficiency. Biomedicines 2022, 10, 260. [Google Scholar] [CrossRef]
- Alvarez-Cervera, F.J.; Villanueva-Toledo, J.; Moo-Puc, R.E.; Heredia-López, F.J.; Alvarez-Cervera, M.; Pineda, J.C.; Góngora-Alfaro, J.L. A novel automated rat catalepsy bar test system based on a RISC microcontroller. J. Neurosci. Methods 2005, 146, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Kleim, J.A.; Boychuk, J.A.; Adkins, D.L. Rat models of upper extremity impairment in stroke. Ilar J. 2007, 48, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Ha, N.D.; Weon, Y.C.; Jang, J.C.; Kang, B.S.; Choi, S.H. Spectrum of MR imaging findings in Wernicke encephalopathy: Are atypical areas of involvement only present in nonalcoholic patients? Am. J. Neuroradiol. 2012, 33, 1398–1402. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.; Ando, K.; Katakami, T.; Kawamoto, M. Cervical cord lesions in Wernicke’s encephalopathy. Radiol. Case Rep. 2022, 17, 2424–2427. [Google Scholar] [CrossRef]
- Riedel, W.J.; Klaassen, T.; Schmitt, J.A. Tryptophan, mood, and cognitive function. Brain Behav. Immun. 2002, 16, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Harrison, B.J.; Olver, J.S.; Norman, T.R.; Burrows, G.D.; Wesnes, K.A.; Nathan, P.J. Selective effects of acute serotonin and catecholamine depletion on memory in healthy women. J. Psychopharmacol. 2004, 1, 32–40. [Google Scholar] [CrossRef]
- Haider, S.A.; Shameem, S.A.; Ahmed, S.P.; Perveen, T.A.; Haleem, D.J. Repeated administration of lead decreases brain 5-HT metabolism and produces memory deficits in rats. Cell. Mol. Biol. Lett. 2005, 10, 669. [Google Scholar]
- Flood, J.F.; Cherkin, A. Fluoxetine enhances memory processing in mice. Psychopharmacology 1987, 1, 36–43. [Google Scholar] [CrossRef]
- Cassano, G.B.; Puca, F.; Trabucchi, M.; Scapicchio, P.L. Paroxetine and fluoxetine effects on mood and cognitive functions in depressed nondemented elderly patients. J. Clin. Psychiatry 2002, 63, 13831. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Steardo, L.; Peng, L.; Parpura, V. Astroglia, glutamatergic transmission and psychiatric diseases. Adv. Neurobiol. 2016, 13, 307–326. [Google Scholar] [CrossRef]
- Langlais, P.J.; Zhang, S.X. Extracellular glutamate is increased in thalamus during thiamine deficiency-induced lesions and is blocked by MK-801. J. Neurochem. 1993, 61, 2175–2182. [Google Scholar] [CrossRef] [PubMed]
- Malar, D.S.; Prasanth, M.I.; Brimson, J.M.; Verma, K.; Prasansuklab, A.; Tencomnao, T. Hibiscus sabdariffa extract protects HT-22 cells from glutamate-induced neurodegeneration by upregulating glutamate transporters and exerts lifespan extension in C. elegans via DAF-16 mediated pathway. Nutr. Healthy Aging 2021, 6, 229–247. [Google Scholar] [CrossRef]
- Tanay, V.A.; Parent, M.B.; Wong, J.T.; Paslawski, T.; Martin, I.L.; Baker, G.B. Effects of the antidepressant/antipanic drug phenelzine on alanine and alanine transaminase in rat brain. Cell. Moll. Neurobiol. 2001, 4, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Krzysztoforska, K.; Piechal, A.; Blecharz-Klin, K.; Pyrzanowska, J.; Joniec-Maciejak, I.; Mirowska-Guzel, D.; Widy-Tyszkiewicz, E. Effect of protocatechuic acid on cognitive processes and central nervous system neuromodulators in the hippocampus, prefrontal cortex, and striatum of healthy rats. Nutr. Neurosci. 2020, 18, 1362–1373. [Google Scholar] [CrossRef] [PubMed]





| Group | Con | WKS | WKS + PCA50 | WKS + PCA100 |
|---|---|---|---|---|
| Total distance travelled (m) | 18.39 ± 0.84 | 15.79 ± 1.61 | 14.71 ± 0.99 | 13.25 ± 1.37 * |
| Average walking speed (m/s) | 0.10 ± 0.00 | 0.09 ± 0.01 | 0.08 ± 0.01 | 0.08 ± 0.01 * |
| Total time spent in the central zone (s) | 42.27 ± 5.18 | 44.99 ± 4.53 | 52.20 ± 5.19 | 62.29 ± 13.58 |
| Number of entries to the central zone (-) | 10.10 ± 0.77 | 9.70 ± 1.47 | 10.90 ± 2.47 | 8.20 ± 1.69 |
| Total time spent in the peripheral zone (s) | 137.73 ± 5.18 | 135.01 ± 4.53 | 127.80 ± 5.19 | 117.71 ± 13.58 |
| Number of rearing episodes (-) | 7.50 ± 0.67 | 5.80 ± 1.10 | 5.10 ± 0.95 | 4.90 ± 0.75 |
| Number of faecal boli produced (-) | 0.50 ± 0.34 | 1.50 ± 0.54 | 0.20 ± 0.20 | 0.60 ± 0.40 |
| Time spent in motion (%) | 78.39 ± 1.29 | 73.04 ± 2.96 | 72.88 ± 2.30 | 66.01 ± 5.50 |
| Number of grooming episodes (-) | 0.50 ± 0.22 | 0.00 ± 0.00 | 0.10 ± 0.10 | 0.30 ± 0.15 |
| Number of micturitions (-) | 0.30 ± 0.21 | 0.10 ± 0.10 | 0.10 ± 0.10 | 0.10 ± 0.10 |
| DAY 1 | ||||
| Group | Con | WKS | WKS + PCA50 | WKS + PCA100 |
| Number of head-dippings (-) | 9.60 ± 1.22 | 9.90 ± 1.73 | 10.60 ± 0.95 | 9.10 ± 1.18 |
| Number of rearing episodes (-) | 3.50 ± 0.60 | 3.10 ± 0.87 | 3.60 ± 1.01 | 2.00 ± 0.49 |
| Number of micturitions (-) | 0.40 ± 0.27 | 0.30 ± 0.21 | 0.20 ± 0.13 | 0.00 ± 0.00 |
| Number of faecal boli produced (-) | 0.40 ± 0.40 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Number of grooming episodes (-) | 0.10 ± 0.10 | 0.20 ± 0.13 | 0.10 ± 0.10 | 0.40 ± 0.16 |
| Total distance travelled (m) | 16.86 ± 0.95 | 15.92 ± 1.55 | 16.48 ± 1.21 | 26.20 ± 4.19 |
| Average walking speed (m/s) | 0.10 ± 0.01 | 0.09 ± 0.01 | 0.09 ± 0.01 | 0.15 ± 0.02 |
| Time spent in motion (%) | 72.73 ± 1.12 | 70.00 ± 3.02 | 67.95 ± 3.11 | 64.29 ± 2.33 |
| Rotation clockwise (-) | 2.50 ± 0.43 | 2.50 ± 0.43 | 2.30 ± 0.56 | 4.10 ± 1.27 |
| DAY 2 | ||||
| Group | Con | WKS | WKS + PCA50 | WKS + PCA100 |
| Number of head-dippings (-) | 7.50 ± 1.88 | 11.80 ± 2.15 | 10.70 ± 1.39 | 10.40 ± 1.17 |
| Number of rearing episodes (-) | 3.30 ± 0.68 | 1.40 ± 0.54 | 1.70 ± 0.63 | 1.10 ± 0.31 * |
| Number of micturitions (-) | 0.40 ± 0.22 | 0.10 ± 0.10 | 0.00 ± 0.00 | 0.10 ± 0.10 |
| Number of faecal boli produced (-) | 0.30 ± 0.30 | 0.40 ± 0.40 | 0.50 ± 0.40 | 0.00 ± 0.00 |
| Number of grooming episodes (-) | 0.60 ± 0.27 | 0.30 ± 0.15 | 0.40 ± 0.16 | 0.30 ± 0.15 |
| Total distance travelled (m) | 14.25 ± 1.68 | 14.10 ± 1.20 | 12.56 ± 1.32 | 14.19 ± 0.64 |
| Average walking speed (m/s) | 0.08 ± 0.01 | 0.08 ± 0.01 | 0.07 ± 0.01 | 0.08 ± 0.00 |
| Time spent in motion (%) | 61.81 ± 3.36 | 64.33 ± 2.79 | 57.92 ± 4.48 | 64.53 ± 1.86 |
| Rotation clockwise (-) | 2.10 ± 0.43 | 3.10 ± 0.80 | 1.80 ± 0.36 | 1.60 ± 0.37 |
| Monoamine | Group | Hypothalamus | Hippocampus | Prefrontal Cortex | Medulla Oblongata | Spinal Cord |
|---|---|---|---|---|---|---|
| 5-HT | Con | 815.47 ± 66.05 | 461.47 ± 48.13 | 477.33 ± 28.64 | 687.08 ± 14.99 | 455.76 ± 9.54 |
| WKS | 708 ± 47.94 | 434.49 ± 32.5 | 414.87 ± 19.11 * | 724.00 ± 14.27 | 481.16 ± 61.39 | |
| WKS + PCA50 | 578.1 ± 36.33 * | 574.03 ± 66.07 | 355.49 ± 15.19 **** | 713.67 ± 10.89 | 425.77 ± 12.26 | |
| WKS + PCA100 | 644.03 ± 74.56 | 475.7 ± 56.04 | 383.09 ± 15.78 ** | 693.17 ± 15.12 | 445.77 ± 7.9 | |
| 5-HIAA | Con | 859.69 ± 76.28 | 430.51 ± 20.11 | 367.38 ± 21.04 | 437.71 ± 10.23 | 257.94 ± 9.6 |
| WKS | 750.95 ± 28.68 | 360.24 ± 18.17 | 403.68 ± 24.47 | 429.55 ± 14.47 | 270.29 ± 23.81 | |
| WKS + PCA50 | 897.56 ± 30.52 | 412.45 ± 24.73 | 436.32 ± 20.46 | 433.52 ± 13.31 | 246.9 ± 9.35 | |
| WKS + PCA100 | 753.3 ± 87.59 | 373.23 ± 30.41 | 364.44 ± 18.24 | 408.70 ± 15.89 | 253.95 ± 9.77 | |
| 5-HIAA/ | Con | 1.15 ± 0.17 | 1.03 ± 0.11 | 0.8 ± 0.07 | 0.64 ± 0.02 | 0.57 ± 0.02 |
| 5-HT | WKS | 1.12 ± 0.1 | 0.85 ± 0.04 | 1.01 ± 0.1 | 0.59 ± 0.01 | 0.58 ± 0.02 |
| WKS + PCA50 | 1.6 ± 0.09 *• | 0.78 ± 0.07 | 1.24 ± 0.05 •**** | 0.61 ± 0.01 | 0.58 ± 0.02 | |
| WKS + PCA100 | 1.19 ± 0.07 ^ | 0.84 ± 0.07 | 0.96 ± 0.05 ^ | 0.59 ± 0.03 | 0.57 ± 0.02 | |
| DA | Con | 174.28 ± 35.21 | 36.45 ± 9.04 | 20.18 ± 2.55 | 54.60 ± 1.33 | 46.47 ± 2.24 |
| WKS | 195.77 ± 40.41 | 41.47 ± 9.52 | 18.09 ± 3.71 | 57.55 ± 2.55 | 41.32 ± 1.93 | |
| WKS + PCA50 | 147.48 ± 24.83 | 72.65 ± 29.57 | 16.75 ± 2.63 | 54.89 ± 1.11 | 41.58 ± 1.38 | |
| WKS + PCA100 | 121.12 ± 37.38 | 79.25 ± 48.13 | 12.74 ± 1.73 | 54.78 ± 0.99 | 41.68 ± 1.26 | |
| DOPAC | Con | 66.22 ± 9.66 | 21.15 ± 5.64 | 27.56 ± 3.94 | 21.79 ± 1.48 | 8.03 ± 0.67 |
| WKS | 107.95 ± 15.34 | 17.71 ± 3.7 | 32.25 ± 3.35 | 20.8 ± 0.74 | 8.10 ± 0.84 | |
| WKS + PCA50 | 119.39 ± 15.58 | 63.62 ± 36.09 | 36.92 ± 2.85 | 20.75 ± 1.41 | 7.14 ± 0.32 | |
| WKS + PCA100 | 70.88 ± 15.18 | 21.13 ± 8.27 | 25.37 ± 2.32 | 19.76 ± 0.62 | 6.08 ± 0.4 | |
| DOPAC/ | Con | 0.53 ± 0.12 | 0.58 ± 0.04 | 1.42 ± 0.18 | 0.40 ± 0.02 | 0.18 ± 0.02 |
| DA | WKS | 0.74 ± 0.12 | 0.44 ± 0.04 | 2.31 ± 0.32 | 0.36 ± 0.01 | 0.20 ± 0.02 |
| WKS + PCA50 | 0.91 ± 0.08 | 0.52 ± 0.11 | 2.62 ± 0.37 * | 0.38 ± 0.02 | 0.17 ± 0.01 | |
| WKS + PCA100 | 0.77 ± 0.1 | 0.44 ± 0.06 | 2.21 ± 0.23 | 0.36 ± 0.01 | 0.14 ± 0.01 | |
| HVA | Con | 222.91 ± 16.55 | 81.12 ± 2.4 | 196.17 ± 7.1 | 80.36 ± 2.19 | 86.91 ± 2.92 |
| WKS | 196.76 ± 7.1 | 76.02 ± 1.99 | 198.31 ± 7.62 | 72.97 ± 2.06 * | 72.78 ± 2.88 *** | |
| WKS + PCA50 | 183.87 ± 7.43 | 98.63 ± 13.03 | 181.56 ± 7.37 | 70.48 ± 2.36 * | 70.46 ± 3.13 *** | |
| WKS + PCA100 | 173.24 ± 20.32 | 83.39 ± 3.02 | 178.62 ± 8.24 | 71.05 ± 3.51* | 73.24 ± 2.88 *** | |
| HVA/ | Con | 2.07 ± 0.46 | 2.99 ± 0.4 | 11.22 ± 1.4 | 1.48 ± 0.04 | 1.90 ± 0.09 |
| DA | WKS | 1.77 ± 0.46 | 2.44 ± 0.31 | 16.38 ± 3.61 | 1.29 ± 0.06 * | 1.78 ± 0.07 |
| WKS + PCA50 | 1.77 ± 0.37 | 2.18 ± 0.37 | 13.97 ± 2.53 | 1.28 ± 0.03 * | 1.69 ± 0.04 | |
| WKS + PCA100 | 2.43 ± 0.4 | 2.97 ± 0.52 | 16.6 ± 2.44 | 1.30 ± 0.06 * | 1.76 ± 0.07 | |
| NA | Con | 1271.21 ± 231.25 | 552.44 ± 28.45 | 433.32 ± 12.78 | 572.54 ± 11.01 | 292.85 ± 6.07 |
| WKS | 1353.06 ± 184.68 | 509.64 ± 30.33 | 402.78 ± 20.17 | 595.31 ± 12.15 | 299.63 ± 15.28 | |
| WKS + PCA50 | 1116.44 ± 159.15 | 552.42 ± 36.8 | 373.00 ± 13.93 | 586.34 ± 10.01 | 296.35 ± 8.71 | |
| WKS + PCA100 | 865.37 ± 187.56 | 537.88 ± 49.22 | 395.95 ± 13.66 | 569.62 ± 14.17 | 280.16 ± 6.53 | |
| MHPG | Con | 3.84 ± 0.62 | 2.32 ± 0.26 | 1.67 ± 0.31 | 7.19 ± 0.32 | 2.60 ± 0.25 |
| WKS | 3.08 ± 0.57 | 1.76 ± 0.14 | 3.50 ± 0.61 *** | 6.03 ± 0.36 ** | 2.68 ± 0.25 | |
| WKS + PCA50 | 4.02 ± 0.66 | 1.56 ± 0.22 | 5.15 ± 0.29 ****• | 5.96 ± 0.22 * | 2.28 ± 0.22 | |
| WKS + PCA100 | 1.91 ± 0.42 | 2.00 ± 0.35 | 3.82 ± 0.34 ***^ | 5.86 ± 0.19* | 1.99 ± 0.21 |
| Amino Acid | Group | Hypothalamus | Hippocampus | Prefrontal Cortex | Medulla Oblongata | Spinal Cord |
|---|---|---|---|---|---|---|
| TAU | Con | 487.13 ± 24.6 | 858.97 ± 15.67 | 1067.58 ± 36.00 | 231.51 ± 2.23 | 183.57 ± 8.1 |
| WKS | 479.81 ± 23.34 | 851.18 ± 19.63 | 1100.47 ± 34.5 | 225.28 ± 2.12 | 167.17 ± 5.96 | |
| WKS + PCA50 | 457.31 ± 27.9 | 879.43 ± 26.56 | 1127.26 ± 38.44 | 237.53 ± 3.17 | 161.42 ± 5.45 | |
| WKS + PCA100 | 437.5 ± 48.5 | 842.73 ± 23.02 | 1043.9 ± 26.86 | 230.78 ± 3.97 | 161.07 ± 5.45 | |
| HIS | Con | 19.64 ± 4.03 | 13.22 ± 1.1 | 11.88 ± 0.31 | 5.04 ± 0.17 | 7.43 ± 0.5 |
| WKS | 12.23 ± 0.66 | 12.59 ± 0.43 | 12.48 ± 0.92 | 5.00 ± 0.20 | 11.39 ± 5.31 | |
| WKS + PCA50 | 38.42 ± 25.63 | 17.42 ± 2.15 | 13.24 ± 0.66 | 5.43 ± 0.11 | 7.68 ± 1.32 | |
| WKS + PCA100 | 13.64 ± 2.61 | 14.15 ± 0.8 | 12.78 ± 1.06 | 5.19 ± 0.13 | 7.12 ± 0.54 | |
| SER | Con | 88.35 ± 4.34 | 123.09 ± 5.22 | 136.59 ± 4.44 | 48.82 ± 0.70 | 65.84 ± 3.18 |
| WKS | 83.53 ± 4.26 | 119.68 ± 5.02 | 143.59 ± 4.22 | 47.89 ± 0.87 | 57.66 ± 1.98 * | |
| WKS + PCA50 | 80.72 ± 4.04 | 129.54 ± 6.36 | 145.53 ± 4.31 | 49.08 ± 1.07 | 55.02 ± 1.92 *** | |
| WKS + PCA100 | 73.79 ± 7.79 | 127.63 ± 4.48 | 140.97 ± 3.20 | 48.05 ± 0.81 | 53.92 ± 1.56 *** | |
| ASP | Con | 414.65 ± 16.28 | 283.09 ± 16.2 | 455.5 ± 17.45 | 412.75 ± 5.19 | 359.24 ± 7.57 |
| WKS | 378.88 ± 15.73 | 242.02 ± 14.5 | 474.05 ± 16.04 | 391.57 ± 6.57 | 340.95 ± 12.66 | |
| WKS + PCA50 | 392.77 ± 15.25 | 262.51 ± 12.98 | 438.55 ± 14.77 | 396.67 ± 6.28 | 357.07 ± 7.44 | |
| WKS + PCA100 | 356.05 ± 37.97 | 272.01 ± 19.09 | 476.04 ± 7.69 | 409.31 ± 7.52 | 364.95 ± 10.45 | |
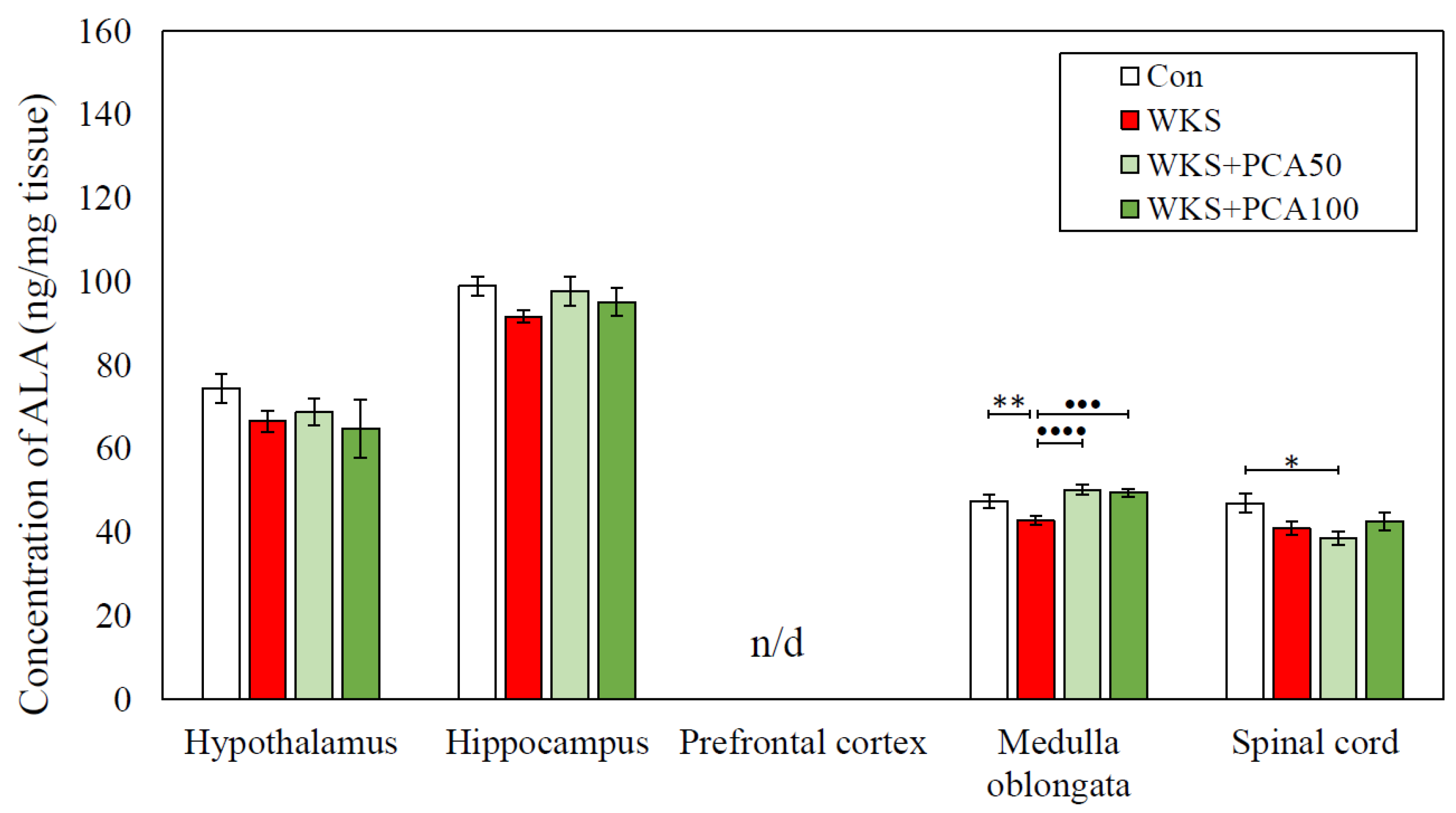
| ALA | Con | 74.49 ± 3.39 | 99.01 ± 2.28 | n/d | 47.51 ± 1.62 | 47.06 ± 2.27 |
| WKS | 66.63 ± 2.52 | 91.69 ± 1.55 | 42.79 ± 1.04 ** | 40.99 ± 1.55 | ||
| WKS + PCA50 | 68.82 ± 3.12 | 97.83 ± 3.52 | 50.2 ± 1.14 •••• | 38.72 ± 1.59 * | ||
| WKS + PCA100 | 64.85 ± 7.00 | 95.19 ± 3.23 | 49.58 ± 0.96 ••• | 42.57 ± 2.21 | ||
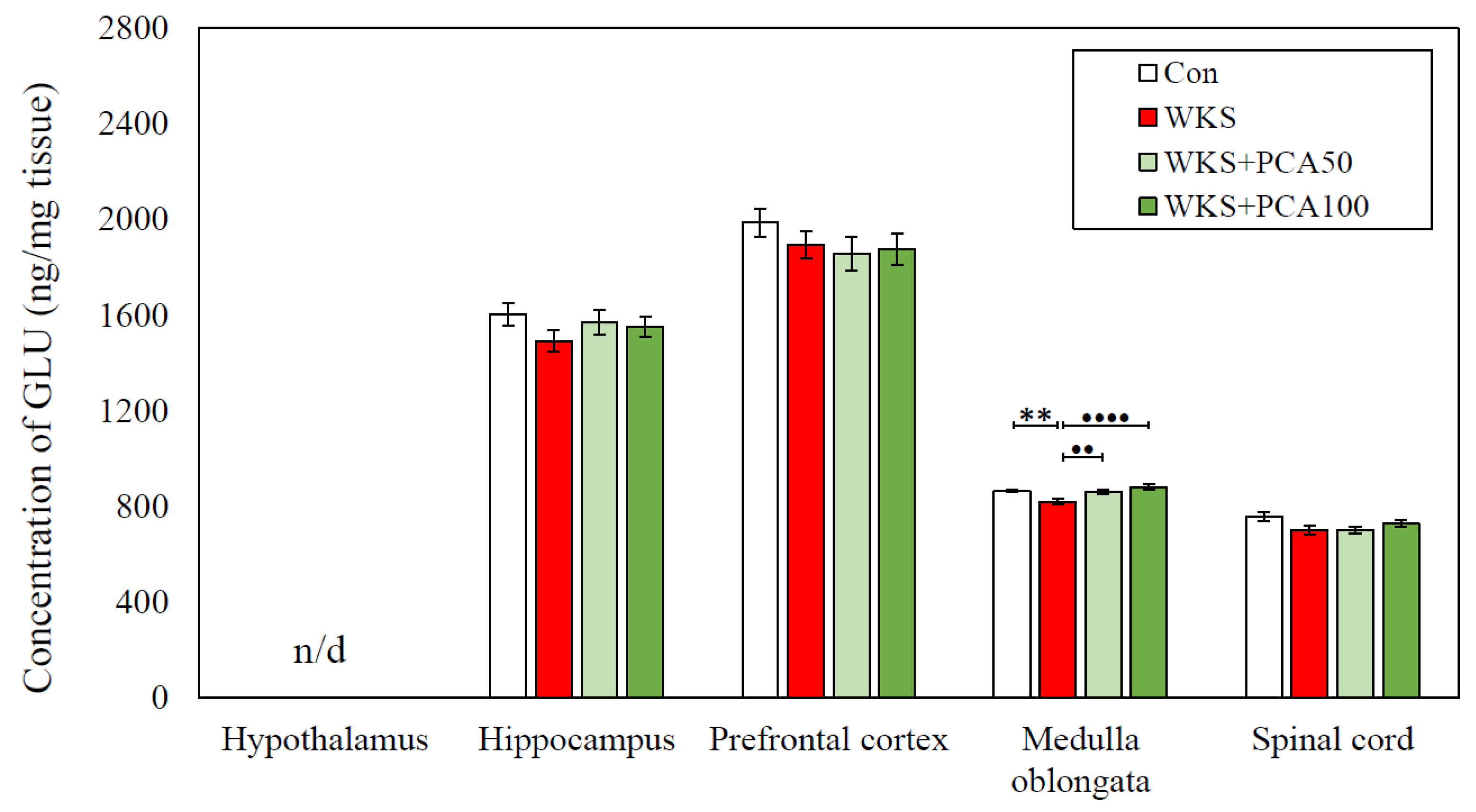
| GLU | Con | n/d | 1601.9 ± 45.64 | 1985.52 ± 60.30 | 864.12 ± 6.49 | 756.23 ± 20.51 |
| WKS | 1491.4 ± 43.13 | 1893.71 ± 54.31 | 819.8 ± 11.51 ** | 700.31 ± 20.3 | ||
| WKS + PCA50 | 1571.26 ± 51.97 | 1856.07 ± 70.24 | 858.36 ± 8.87 •• | 699.9 ± 15.59 | ||
| WKS + PCA100 | 1551.25 ± 41.31 | 1875.73 ± 65.19 | 880.98 ± 9.89 •••• | 726.5 ± 14.67 | ||
| GABA | Con | 685.22 ± 73.43 | 265.56 ± 10.67 | 304.09 ± 8.75 | 187.72 ± 3.17 | 116.91 ± 5.58 |
| WKS | 623.73 ± 71.04 | 253.99 ± 9.28 | 371.77 ± 45.38 | 178.84 ± 3.64 | 108.08 ± 3.98 | |
| WKS + PCA50 | 693.21 ± 64.03 | 293.62 ± 20.24 | 343.6 ± 12.59 | 189.95 ± 3.78 | 104.71 ± 4.38 | |
| WKS + PCA100 | 523.66 ± 76.69 | 252.95 ± 12.52 | 318.41 ± 6.83 | 189.12 ± 4.02 | 108.31 ± 5.75 | |
| THR | Con | 112.39 ± 3.82 | 96.98 ± 4.37 | 96.40 ± 2.15 | 122.83 ± 2.72 | 114.6 ± 2.97 |
| WKS | 104.35 ± 4.96 | 84.52 ± 1.36 * | 94.86 ± 2.51 | 114.23 ± 2.31 * | 101.96 ± 2.71 * | |
| WKS + PCA50 | 111.91 ± 2.98 | 92.53 ± 1.97 | 97.96 ± 2.42 | 114.87 ± 1.91 * | 106.03 ± 3.06 * | |
| WKS + PCA100 | 93.57 ± 11.23 | 85.23 ± 2.33 * | 94.02 ± 2.28 | 108.74 ± 2.08 **** | 103.32 ± 2.76 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzysztoforska, K.; Piechal, A.; Wojnar, E.; Blecharz-Klin, K.; Pyrzanowska, J.; Joniec-Maciejak, I.; Krzysztoforski, J.; Widy-Tyszkiewicz, E. Protocatechuic Acid Prevents Some of the Memory-Related Behavioural and Neurotransmitter Changes in a Pyrithiamine-Induced Thiamine Deficiency Model of Wernicke–Korsakoff Syndrome in Rats. Nutrients 2023, 15, 625. https://doi.org/10.3390/nu15030625
Krzysztoforska K, Piechal A, Wojnar E, Blecharz-Klin K, Pyrzanowska J, Joniec-Maciejak I, Krzysztoforski J, Widy-Tyszkiewicz E. Protocatechuic Acid Prevents Some of the Memory-Related Behavioural and Neurotransmitter Changes in a Pyrithiamine-Induced Thiamine Deficiency Model of Wernicke–Korsakoff Syndrome in Rats. Nutrients. 2023; 15(3):625. https://doi.org/10.3390/nu15030625
Chicago/Turabian StyleKrzysztoforska, Kinga, Agnieszka Piechal, Ewa Wojnar, Kamilla Blecharz-Klin, Justyna Pyrzanowska, Ilona Joniec-Maciejak, Jan Krzysztoforski, and Ewa Widy-Tyszkiewicz. 2023. "Protocatechuic Acid Prevents Some of the Memory-Related Behavioural and Neurotransmitter Changes in a Pyrithiamine-Induced Thiamine Deficiency Model of Wernicke–Korsakoff Syndrome in Rats" Nutrients 15, no. 3: 625. https://doi.org/10.3390/nu15030625
APA StyleKrzysztoforska, K., Piechal, A., Wojnar, E., Blecharz-Klin, K., Pyrzanowska, J., Joniec-Maciejak, I., Krzysztoforski, J., & Widy-Tyszkiewicz, E. (2023). Protocatechuic Acid Prevents Some of the Memory-Related Behavioural and Neurotransmitter Changes in a Pyrithiamine-Induced Thiamine Deficiency Model of Wernicke–Korsakoff Syndrome in Rats. Nutrients, 15(3), 625. https://doi.org/10.3390/nu15030625





