24,25-Dihydroxy Vitamin D and Vitamin D Metabolite Ratio as Biomarkers of Vitamin D in Chronic Kidney Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Vitamin D Level Measurement and Calculation of VMR
2.3. Demographics and Laboratory Findings
2.4. Statistical Analysis
3. Results
3.1. Demographics and Laboratory Findings of Study Participants
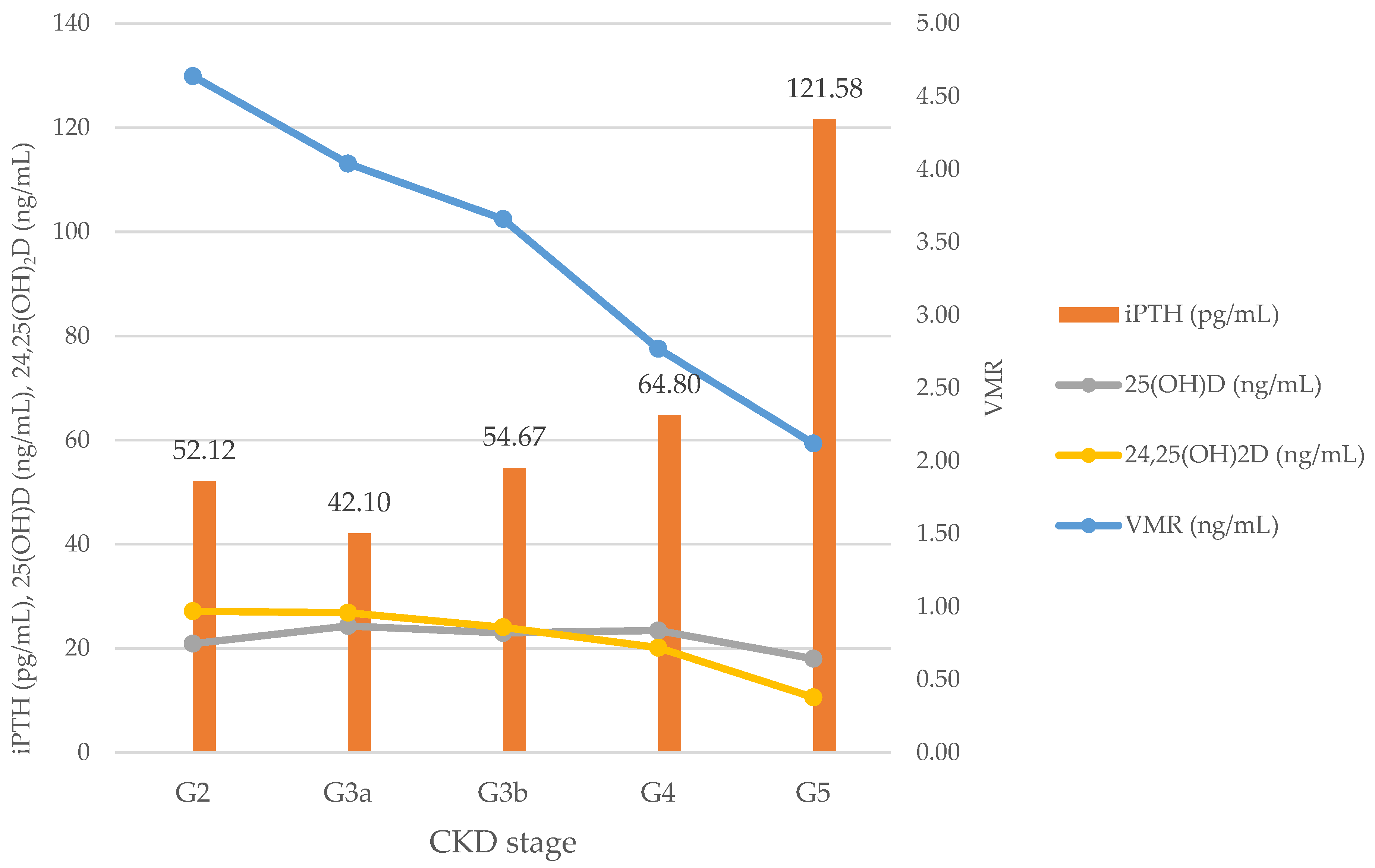
3.2. iPTH and Vitamin D Biomarkers According to CKD Stage
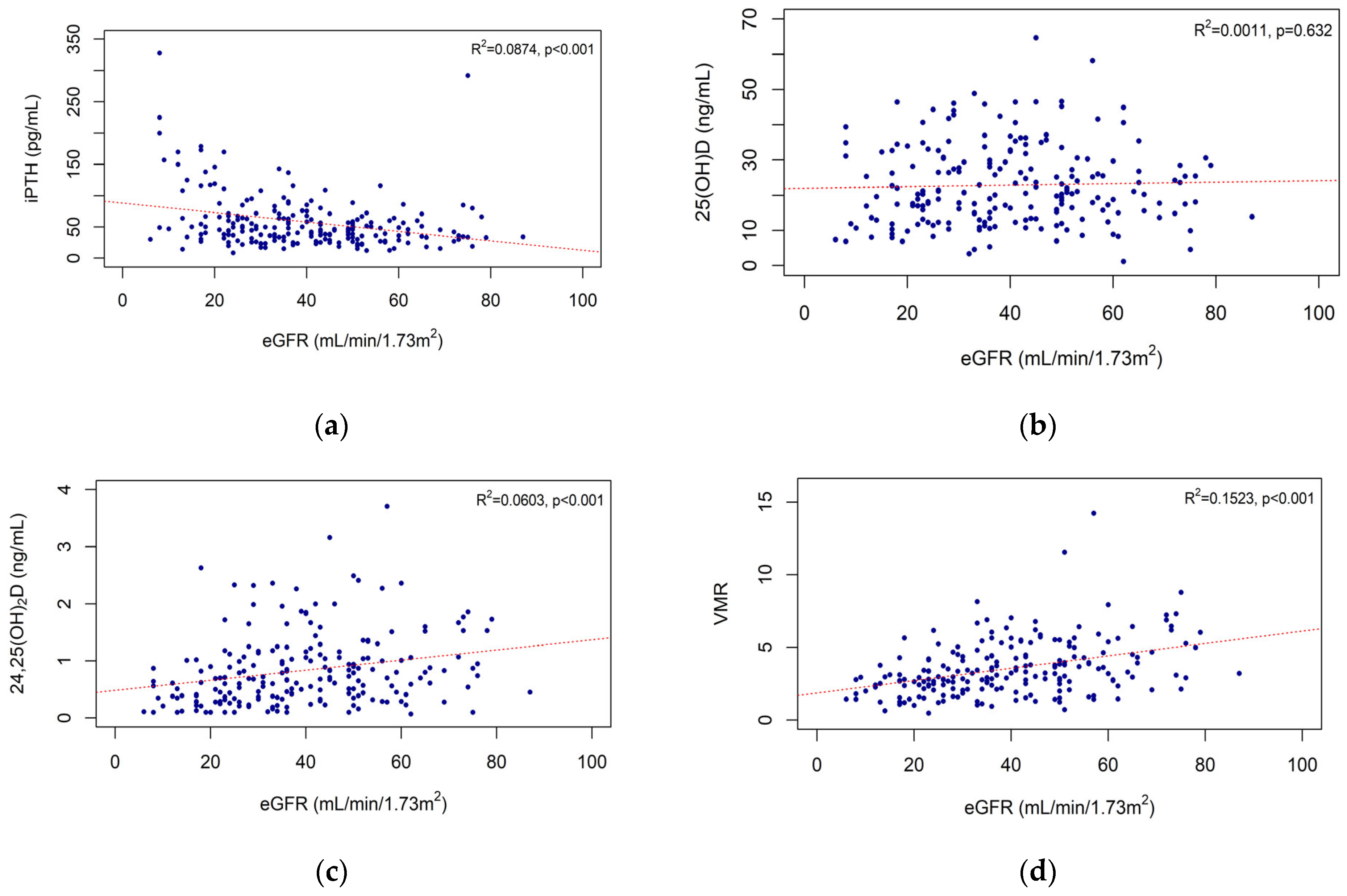
3.3. Correlations of eGFR with iPTH Level and Vitamin D Biomarkers
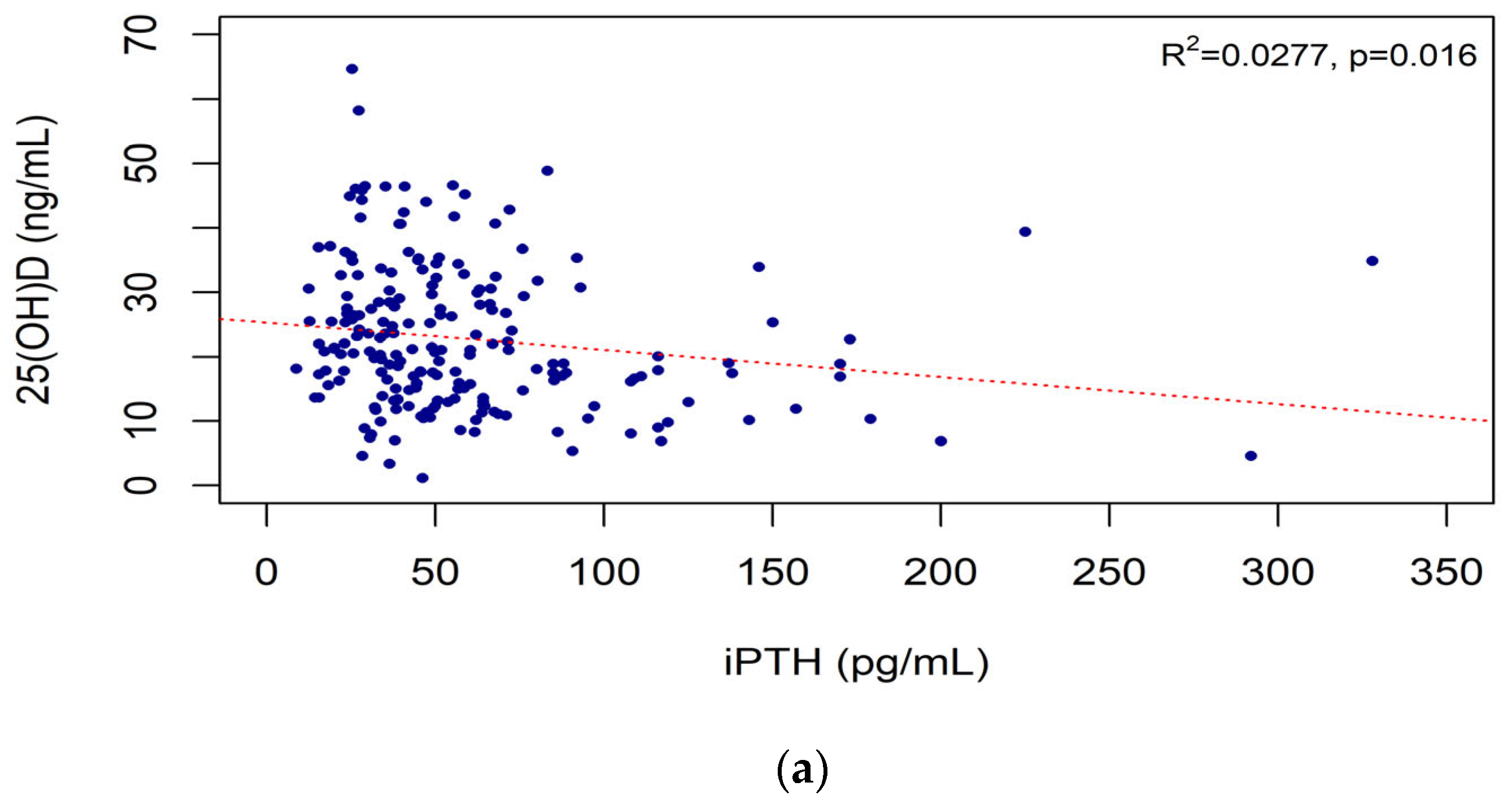
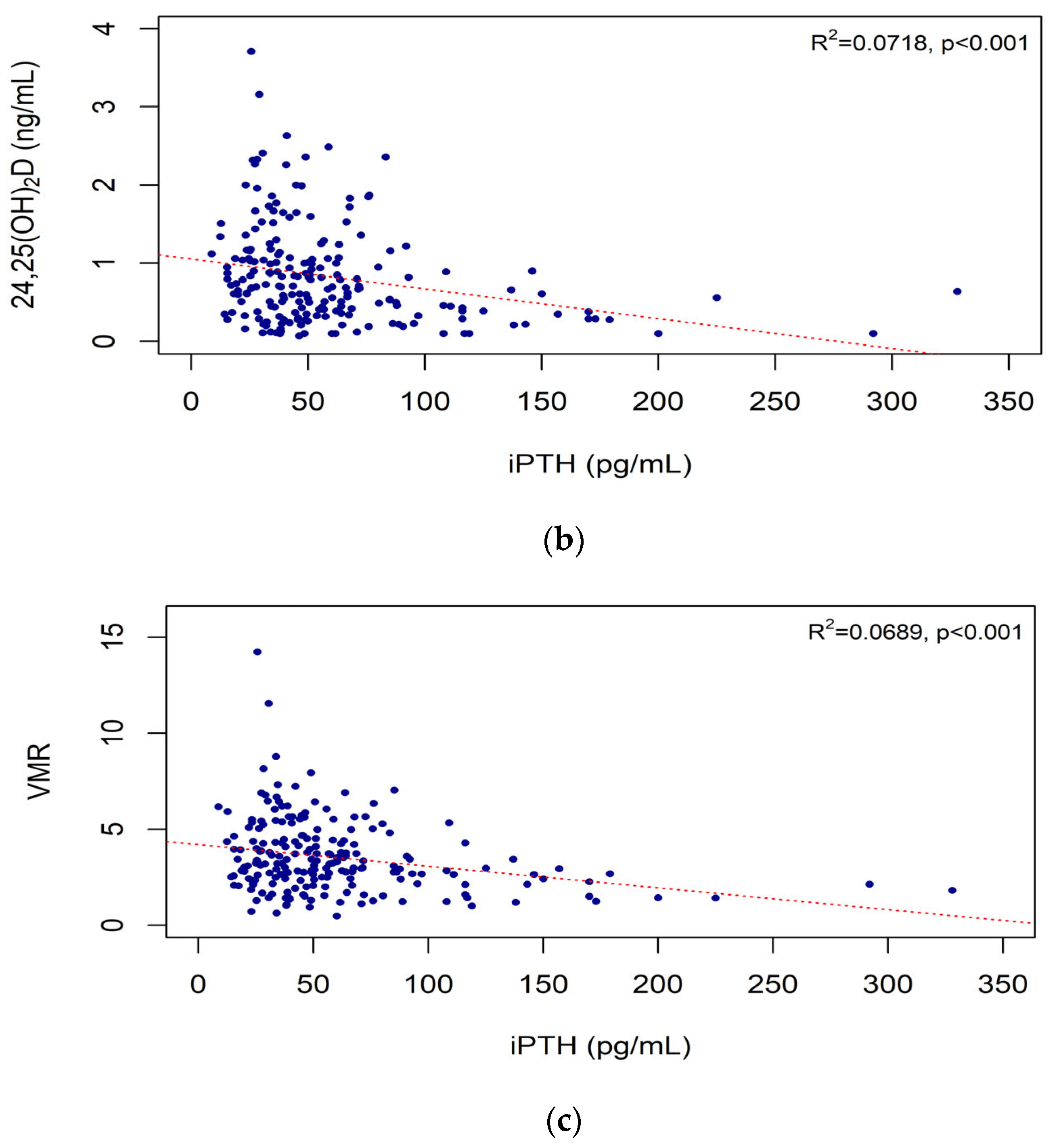
3.4. Correlations of iPTH Level with Vitamin D Biomarkers
3.5. Vitamin D Biomarkers According to eGFR and iPTH Level
3.6. Vitamin D Biomarkers and Bone Density
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| eGFR | iPTH | 25(OH)D | 24,25(OH)2D | VMR | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | |
| Calcium (mg/dL) | 0.023 | 0.739 | −0.114 | 0.101 | 0.03 | 0.671 | 0.045 | 0.519 | −0.026 | 0.714 |
| Phosphate (mg/dL) | −0.327 | <0.001 | 0.126 | 0.069 | −0.022 | 0.755 | −0.046 | 0.509 | −0.044 | 0.531 |
| Ca × P (mg2/dL2) | −0.359 | <0.001 | 0.101 | 0.147 | −0.034 | 0.624 | −0.044 | 0.527 | −0.053 | 0.444 |
References
- DeLuca, H.F. Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutr. 2004, 80, 1689S–1696S. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.M.; Shin, E.A. Exploring vitamin D metabolism and function in cancer. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef]
- Keung, L.; Perwad, F. Vitamin D and kidney disease. Bone Rep. 2018, 9, 93–100. [Google Scholar] [CrossRef] [PubMed]
- De Brito Galvao, J.F.; Nagode, L.A.; Schenck, P.A.; Chew, D.J. Calcitriol, calcidiol, parathyroid hormone, and fibroblast growth factor-23 interactions in chronic kidney disease. J. Vet. Emerg. Crit. Care 2013, 23, 134–162. [Google Scholar] [CrossRef]
- Bhan, I.; Powe, C.E.; Berg, A.H.; Ankers, E.; Wenger, J.B.; Karumanchi, S.A.; Thadhani, R.I. Bioavailable vitamin D is more tightly linked to mineral metabolism than total vitamin D in incident hemodialysis patients. Kidney Int. 2012, 82, 84–89. [Google Scholar] [CrossRef]
- Terai, K.; Nara, H.; Takakura, K.; Mizukami, K.; Sanagi, M.; Fukushima, S.; Fujimori, A.; Itoh, H.; Okada, M. Vascular calcification and secondary hyperparathyroidism of severe chronic kidney disease and its relation to serum phosphate and calcium levels. Br. J. Pharmacol. 2009, 156, 1267–1278. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.; Bouillon, R.; Thadhani, R.; Schoenmakers, I. Vitamin D metabolites in captivity? Should we measure free or total 25(OH)D to assess vitamin D status? J. Steroid Biochem. Mol. Biol. 2017, 173, 105–116. [Google Scholar] [CrossRef]
- Castillo-Peinado, L.D.L.S.; Calderón-Santiago, M.; Herrera-Martínez, A.D.; León-Idougourram, S.; Gálvez-Moreno, M.Á.; Sánchez-Cano, R.L.; Priego-Capote, F. Measuring Vitamin D3 Metabolic Status, Comparison between Vitamin D Deficient and Sufficient Individuals. Separations 2022, 9, 141. [Google Scholar] [CrossRef]
- Ginsberg, C.; Katz, R.; de Boer, I.H.; Kestenbaum, B.R.; Chonchol, M.; Shlipak, M.G.; Sarnak, M.J.; Hoofnagle, A.N.; Rifkin, D.E.; Garimella, P.S.; et al. The 24,25 to 25-hydroxyvitamin D ratio and fracture risk in older adults: The cardiovascular health study. Bone 2018, 107, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Burt, L.A.; Billington, E.O.; Rose, M.S.; Raymond, D.A.; Hanley, D.A.; Boyd, S.K. Effect of High-Dose Vitamin D Supplementation on Volumetric Bone Density and Bone Strength: A Randomized Clinical Trial. JAMA 2019, 322, 736–745. [Google Scholar] [CrossRef] [PubMed]
- LeBoff, M.S.; Chou, S.H.; Murata, E.M.; Donlon, C.M.; Cook, N.R.; Mora, S.; Lee, I.M.; Kotler, G.; Bubes, V.; Buring, J.E.; et al. Effects of Supplemental Vitamin D on Bone Health Outcomes in Women and Men in the VITamin D and OmegA-3 TriaL (VITAL). J. Bone Miner. Res. 2020, 35, 883–893. [Google Scholar] [CrossRef]
- Souberbielle, J.C.; Cavalier, E.; Delanaye, P.; Massart, C.; Brailly-Tabard, S.; Cormier, C.; Borderie, D.; Benachi, A.; Chanson, P. Serum calcitriol concentrations measured with a new direct automated assay in a large population of adult healthy subjects and in various clinical situations. Clin. Chim. Acta 2015, 451, 149–153. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine, S. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Obi, Y.; Hamano, T.; Isaka, Y. Prevalence and prognostic implications of vitamin D deficiency in chronic kidney disease. Dis. Markers 2015, 2015, 868961. [Google Scholar] [CrossRef]
- Moe, S.M.; Drüeke, T.B.; Block, G.A.; Cannata-Andía, J.B.; Elder, G.J.; Fukagawa, M.; Jorgetti, V.; Ketteler, M.; Langman, C.B.; Levin, A.; et al. Kidney Disease: Improving Global Outcomes, C.K.D.M.B.D.W.G. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. Suppl. 2009, 76, S1–S130. [Google Scholar] [CrossRef]
- Alonso, N.; Zelzer, S.; Eibinger, G.; Herrmann, M. Vitamin D Metabolites: Analytical Challenges and Clinical Relevance. Calcif. Tissue Int. 2022, 112, 158–177. [Google Scholar] [CrossRef]
- Bosworth, C.R.; Levin, G.; Robinson-Cohen, C.; Hoofnagle, A.N.; Ruzinski, J.; Young, B.; Schwartz, S.M.; Himmelfarb, J.; Kestenbaum, B.; de Boer, I.H. The serum 24,25-dihydroxyvitamin D concentration, a marker of vitamin D catabolism, is reduced in chronic kidney disease. Kidney Int. 2012, 82, 693–700. [Google Scholar] [CrossRef]
- Jones, G. Pharmacokinetics of vitamin D toxicity. Am. J. Clin. Nutr. 2008, 88, 582S–586S. [Google Scholar] [CrossRef]
- Tryfonidou, M.A.; Oosterlaken-Dijksterhuis, M.A.; Mol, J.A.; van den Ingh, T.S.; van den Brom, W.E.; Hazewinkel, H.A. 24-Hydroxylase: Potential key regulator in hypervitaminosis D3 in growing dogs. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E505–E513. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, C.; Hoofnagle, A.N.; Katz, R.; Hughes-Austin, J.; Miller, L.M.; Becker, J.O.; Kritchevsky, S.B.; Shlipak, M.G.; Sarnak, M.J.; Ix, J.H. The Vitamin D Metabolite Ratio Is Associated With Changes in Bone Density and Fracture Risk in Older Adults. J. Bone Miner. Res. 2021, 36, 2343–2350. [Google Scholar] [CrossRef] [PubMed]
- Selamet, U.; Katz, R.; Ginsberg, C.; Rifkin, D.E.; Fried, L.F.; Kritchevsky, S.B.; Hoofnagle, A.N.; Bibbins-Domingo, K.; Drew, D.; Harris, T.; et al. Serum Calcitriol Concentrations and Kidney Function Decline, Heart Failure, and Mortality in Elderly Community-Living Adults: The Health, Aging, and Body Composition Study. Am. J. Kidney Dis. 2018, 72, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Prosser, D.E.; Kaufmann, M. 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): Its important role in the degradation of vitamin D. Arch. Biochem. Biophys. 2012, 523, 9–18. [Google Scholar] [CrossRef] [PubMed]
- van den Ouweland, J.M.; Beijers, A.M.; Demacker, P.N.; van Daal, H. Measurement of 25-OH-vitamin D in human serum using liquid chromatography tandem-mass spectrometry with comparison to radioimmunoassay and automated immunoassay. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2010, 878, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.H.; Powe, C.E.; Evans, M.K.; Wenger, J.; Ortiz, G.; Zonderman, A.B.; Suntharalingam, P.; Lucchesi, K.; Powe, N.R.; Karumanchi, S.A.; et al. 24,25-Dihydroxyvitamin d3 and vitamin D status of community-dwelling black and white Americans. Clin. Chem. 2015, 61, 877–884. [Google Scholar] [CrossRef]
- Levey, A.S.; Coresh, J.; Greene, T.; Stevens, L.A.; Zhang, Y.L.; Hendriksen, S.; Kusek, J.W.; Van Lente, F. Chronic Kidney Disease Epidemiology, C. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann. Intern. Med. 2006, 145, 247–254. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Norman, M.E.; Mazur, A.T.; Borden, S.t.; Gruskin, A.; Anast, C.; Baron, R.; Rasmussen, H. Early diagnosis of juvenile renal osteodystrophy. J. Pediatr. 1980, 97, 226–232. [Google Scholar] [CrossRef]
- Haffner, D.; Leifheit-Nestler, M. Treatment of hyperphosphatemia: The dangers of aiming for normal PTH levels. Pediatr. Nephrol. 2020, 35, 485–491. [Google Scholar] [CrossRef]
- Levin, A.; Bakris, G.L.; Molitch, M.; Smulders, M.; Tian, J.; Williams, L.A.; Andress, D.L. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int. 2007, 71, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Toribio, M.J.; Priego-Capote, F.; Perez-Gomez, B.; Fernandez de Larrea-Baz, N.; Ruiz-Moreno, E.; Castello, A.; Lucas, P.; Sierra, M.A.; Pino, M.N.; Martinez-Cortes, M.; et al. Factors Associated with Serum Vitamin D Metabolites and Vitamin D Metabolite Ratios in Premenopausal Women. Nutrients 2021, 13, 3747. [Google Scholar] [CrossRef]
- Fabregat-Cabello, N.; Farre-Segura, J.; Huyghebaert, L.; Peeters, S.; Le Goff, C.; Souberbielle, J.C.; Cavalier, E. A fast and simple method for simultaneous measurements of 25(OH)D, 24,25(OH)(2)D and the Vitamin D Metabolite Ratio (VMR) in serum samples by LC-MS/MS. Clin. Chim. Acta 2017, 473, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Shah, A.; Duong, U.; Hechter, R.C.; Dukkipati, R.; Kovesdy, C.P. Kidney bone disease and mortality in CKD: Revisiting the role of vitamin D, calcimimetics, alkaline phosphatase, and minerals. Kidney Int. Suppl. 2010, 59, S10–S21. [Google Scholar] [CrossRef]
- Fraser, D.R.; Kodicek, E. Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature 1970, 228, 764–766. [Google Scholar] [CrossRef] [PubMed]
- Shinki, T.; Jin, C.H.; Nishimura, A.; Nagai, Y.; Ohyama, Y.; Noshiro, M.; Okuda, K.; Suda, T. Parathyroid hormone inhibits 25-hydroxyvitamin D3-24-hydroxylase mRNA expression stimulated by 1 alpha,25-dihydroxyvitamin D3 in rat kidney but not in intestine. J. Biol. Chem. 1992, 267, 13757–13762. [Google Scholar] [CrossRef] [PubMed]
- Akeno, N.; Saikatsu, S.; Kawane, T.; Horiuchi, N. Mouse vitamin D-24-hydroxylase: Molecular cloning, tissue distribution, and transcriptional regulation by 1alpha,25-dihydroxyvitamin D3. Endocrinology 1997, 138, 2233–2240. [Google Scholar] [CrossRef]
- Shimada, T.; Hasegawa, H.; Yamazaki, Y.; Muto, T.; Hino, R.; Takeuchi, Y.; Fujita, T.; Nakahara, K.; Fukumoto, S.; Yamashita, T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J. Bone Miner. Res. 2004, 19, 429–435. [Google Scholar] [CrossRef]
- Leeuwenkamp, O.R.; van der Wiel, H.E.; Lips, P.; van der Vijgh, W.J.; Barto, R.; Greuter, H.; Netelenbos, J.C. Human pharmacokinetics of orally administered (24 R)-hydroxycalcidiol. Eur. J. Clin. Chem. Clin. Biochem. 1993, 31, 419–426. [Google Scholar] [CrossRef]
- Sunshine & Daylight Hours in Seoul, South Korea. Available online: http://www.seoul.climatemps.com/sunlight.php (accessed on 3 January 2023).
| N = 208 | |
|---|---|
| Age (year) | 64.3 ± 12.7 |
| Sex, male, (n, %) | 133 (63.9%) |
| BMI (kg/m2) | 27.2 ± 21.5 |
| Comorbidities | |
| DM | 98 (47.1%) |
| HTN | 167 (80.3%) |
| Cause of CKD | |
| Glomerular disease | |
| DN | 45 (21.6%) |
| CGN | 61 (33.8%) |
| CTIN | 57 (27.4%) |
| Vascular disease | 37 (17.8%) |
| PKD and other | 8 (3.8%) |
| Serum Cr (mg/dL) | 2.00 ± 1.07 |
| CKD stage (eGFR, mL/min/1.73 m2) | |
| G2 (>60) | 29 (13.9%) |
| G3a (45–60) | 49 (23.6%) |
| G3b (30–44) | 62 (29.8%) |
| G4 (15–29) | 54 (26.0%) |
| G5 (<15) | 14 (6.7%) |
| Albumin (g/dL) | 4.4 ± 0.4 |
| Ca (mg/dL) | 9.4 ± 0.6 |
| P (mg/dL) | 3.8 ± 0.8 |
| Ca × P (mg2/dL2) | 35.1 ± 6.5 |
| iPTH (pg/mL) | 58.5 ± 44.4 |
| Vitamin D Biomarkers | Total | CKD Stage | p | ||||
|---|---|---|---|---|---|---|---|
| G2 | G3a | G3b | G4 | G5 | |||
| iPTH (pg/mL) | 58.49 ± 44.42 | 52.12 ± 50.00 | 42.10 ± 20.43 | 54.67 ± 29.63 | 64.80 ± 40.29 | 121.58 ± 90.14 | <0.001 |
| 25(OH)D (ng/mL) | 22.81 ± 11.24 | 20.93 ± 10.13 | 24.35 ± 12.37 | 22.99 ± 10.89 | 23.45 ± 11.24 | 18.05 ± 10.59 | 0.353 |
| 24,25(OH)2D (ng/mL) | 0.83 ± 0.63 | 0.97 ± 0.60 | 0.96 ± 0.75 | 0.86 ± 0.58 | 0.72 ± 0.60 | 0.38 ± 0.24 | 0.011 |
| VMR | 3.55 ± 1.91 | 4.64 ± 1.98 | 4.04 ± 2.42 | 3.66 ± 1.64 | 2.77 ± 1.23 | 2.12 ± 0.85 | <0.001 |
| Model | Explan Variables | Adjusted R2 | AIC | BIC |
|---|---|---|---|---|
| Model 1: 25(OH)D = α + β1(eGFR) + β2(iPTH) + ε | eGFR, iPTH | 0.022 | 1517.1 | 1530.2 |
| Model 2: 24,25(OH)2D = α + β1(eGFR) + β2(iPTH) + ε | eGFR, iPTH | 0.087 | 368.5 | 381.6 |
| Model 3: VMR = α + β1(eGFR) + β2(iPTH) + ε | eGFR, iPTH | 0.145 | 788.4 | 801.6 |
| Model 4: eGFR= α + β1(25(OH)D) + ε | 25(OH)D, iPTH | 0.079 | 1644.2 | 1657.3 |
| Model 5: eGFR = α + β1(24,25(OH)2D) + ε | 24,25(OH)2D, iPTH | 0.105 | 1638.5 | 1651.6 |
| Model 6: eGFR = α + β1(VMR) + ε | VMR, iPTH | 0.167 | 1624.4 | 1637.6 |
| Model 7: iPTH= α + β1(25(OH)D) + ε | 25(OH)D, eGFR | 0.105 | 2037.9 | 2051.0 |
| Model 8: iPTH= α + β1(24,25(OH)2D) + ε | 24,25(OH)2D, eGFR | 0.119 | 2034.7 | 2047.8 |
| Model 9: iPTH= α + β1(VMR) + ε | VMR, eGFR | 0.104 | 2038.2 | 2051.3 |
| Normal (n = 25) | Osteopenia (n = 14) | Osteoporosis (n = 4) | p | |
|---|---|---|---|---|
| eGFR (mL/min/1.73 m2) | 35.40 ± 19.46 | 33.86 ± 20.49 | 28.50 ± 20.86 | 0.552 |
| 25(OH)D (ng/mL) | 23.92 ± 14.47 | 23.32 ± 9.41 | 15.88 ± 9.36 | 0.349 |
| 24,25(OH)2D (ng/mL) | 0.84 ± 0.62 | 0.88 ± 0.54 | 0.35 ± 0.39 | 0.304 |
| VMR | 3.45 ± 1.60 | 3.55 ± 1.50 | 1.81 ± 1.05 | 0.177 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Chung, H.J.; Jung, S.; Jang, H.N.; Chang, S.-H.; Kim, H.-J.; Cho, M.-C. 24,25-Dihydroxy Vitamin D and Vitamin D Metabolite Ratio as Biomarkers of Vitamin D in Chronic Kidney Disease. Nutrients 2023, 15, 578. https://doi.org/10.3390/nu15030578
Lee S, Chung HJ, Jung S, Jang HN, Chang S-H, Kim H-J, Cho M-C. 24,25-Dihydroxy Vitamin D and Vitamin D Metabolite Ratio as Biomarkers of Vitamin D in Chronic Kidney Disease. Nutrients. 2023; 15(3):578. https://doi.org/10.3390/nu15030578
Chicago/Turabian StyleLee, Seunghye, Hye Jin Chung, Sehyun Jung, Ha Nee Jang, Se-Ho Chang, Hyun-Jung Kim, and Min-Chul Cho. 2023. "24,25-Dihydroxy Vitamin D and Vitamin D Metabolite Ratio as Biomarkers of Vitamin D in Chronic Kidney Disease" Nutrients 15, no. 3: 578. https://doi.org/10.3390/nu15030578
APA StyleLee, S., Chung, H. J., Jung, S., Jang, H. N., Chang, S.-H., Kim, H.-J., & Cho, M.-C. (2023). 24,25-Dihydroxy Vitamin D and Vitamin D Metabolite Ratio as Biomarkers of Vitamin D in Chronic Kidney Disease. Nutrients, 15(3), 578. https://doi.org/10.3390/nu15030578





