Utilizing the Off-Target Effects of T1R3 Antagonist Lactisole to Enhance Nitric Oxide Production in Basal Airway Epithelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Live Cell Imaging
2.2. Culture of Primary Human Cells
2.3. Knockdown of T1Rs
2.4. Quantitative PCR (qPCR)
2.5. Genotyping T2R38 PAV/AVI
2.6. Data Analysis and Statistics
3. Results
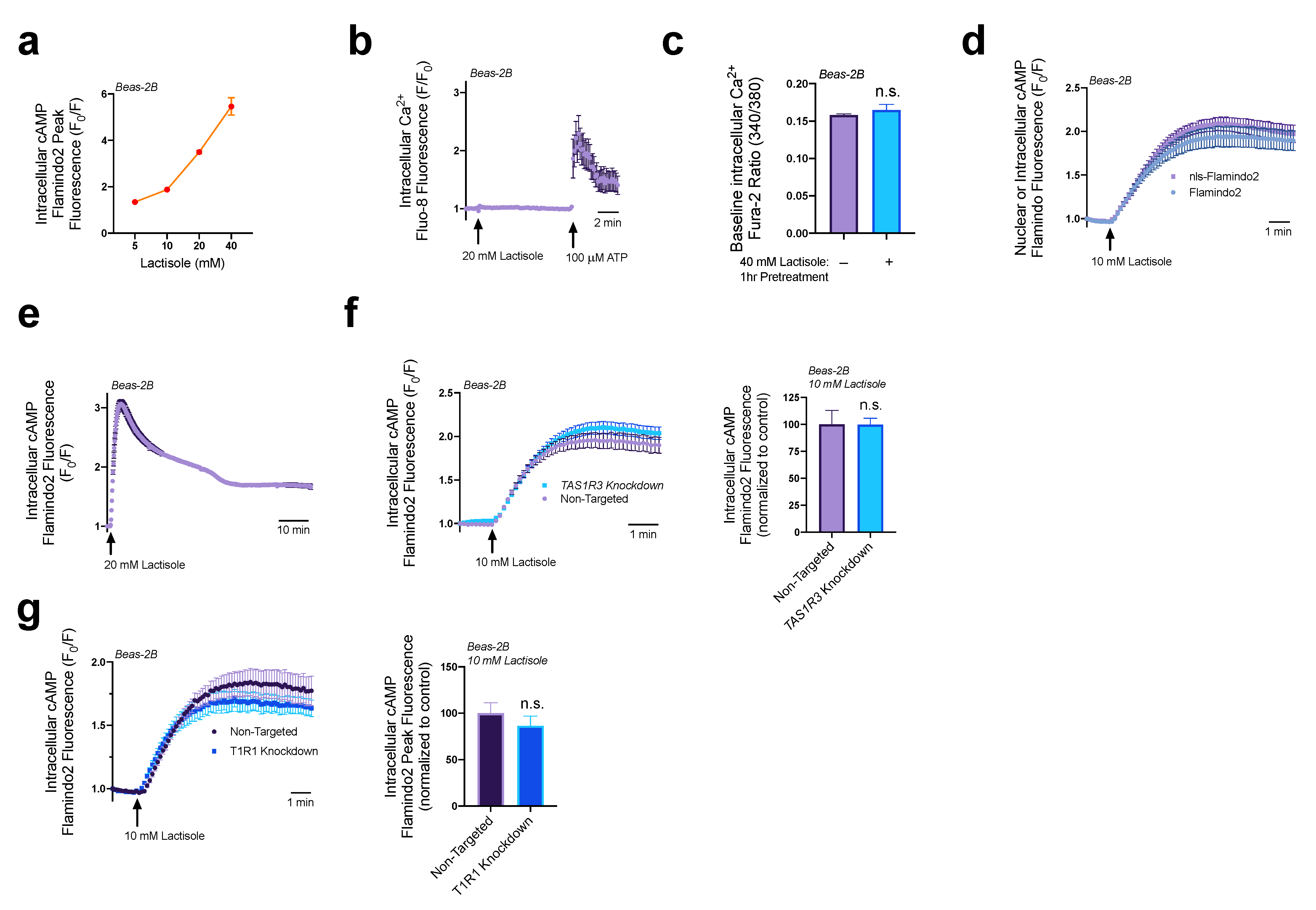
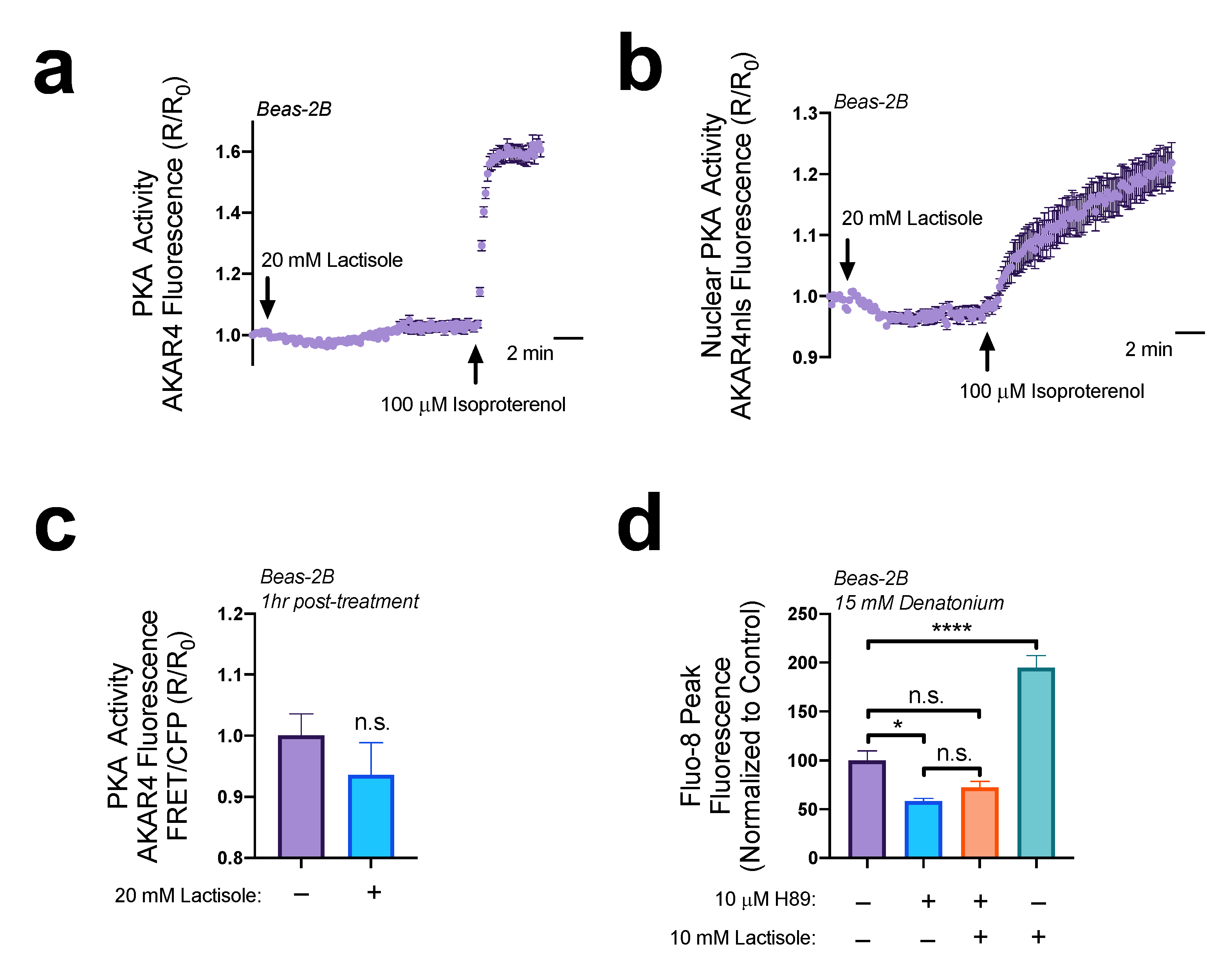
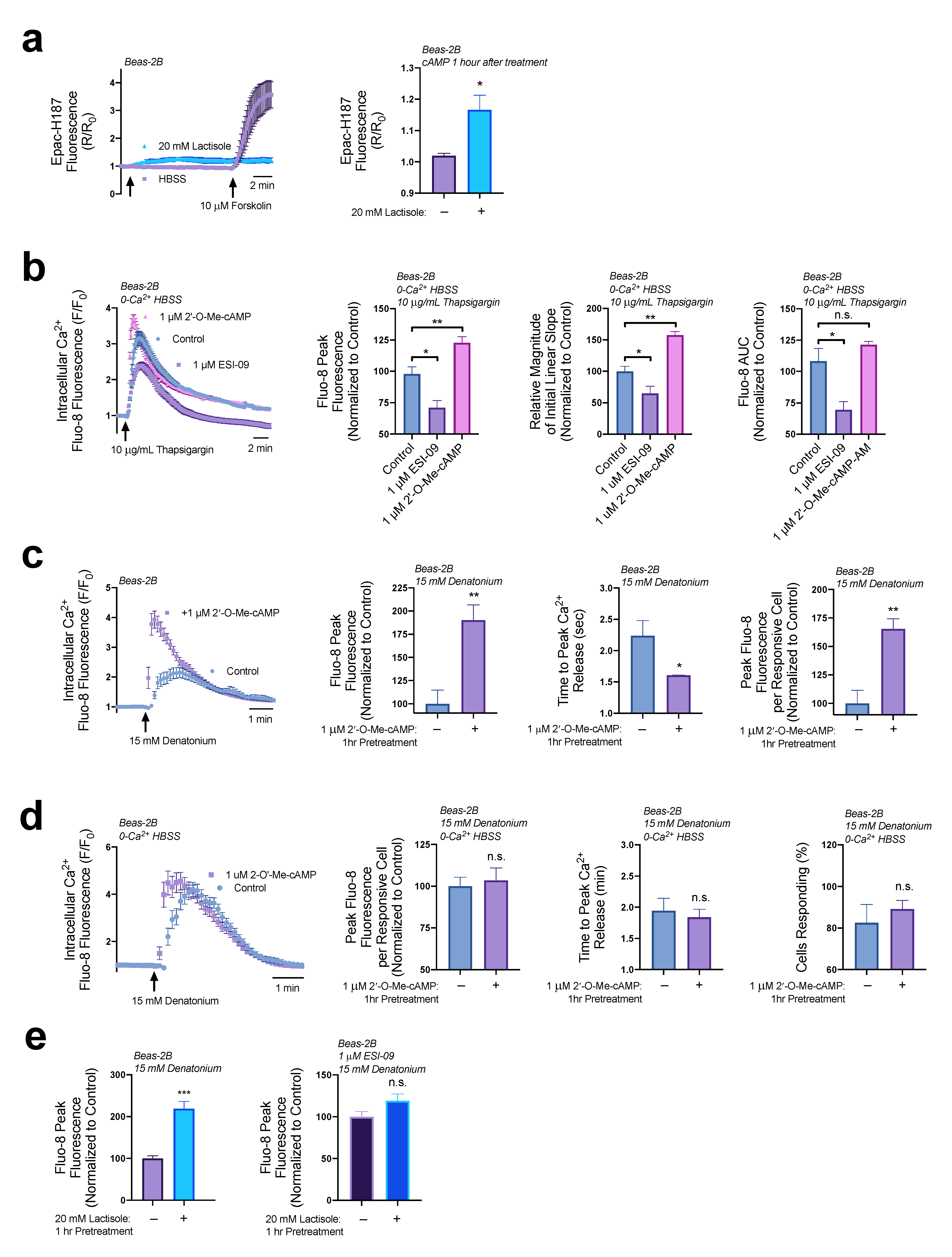
3.1. Lactisole Increases Intracellular cAMP
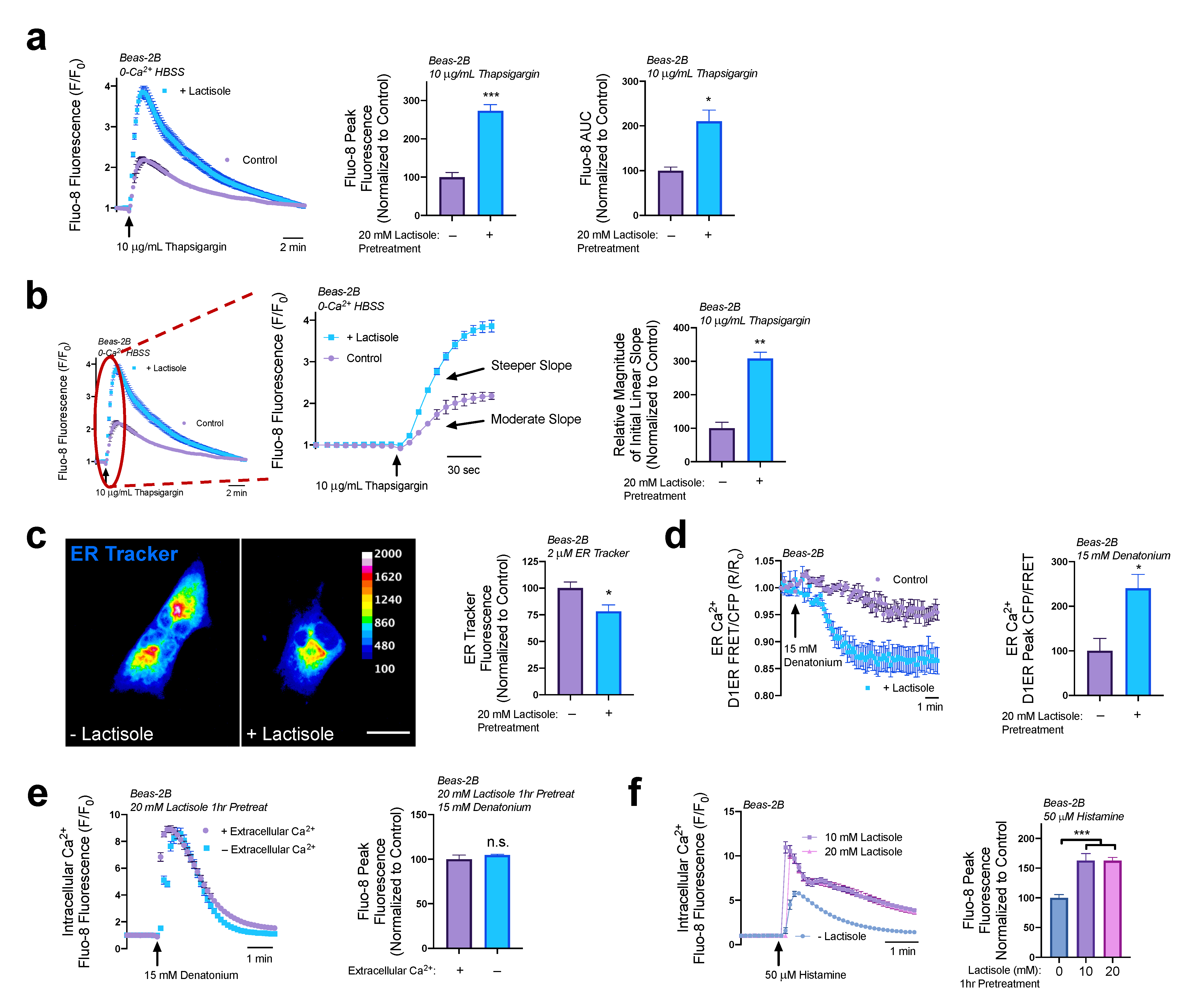
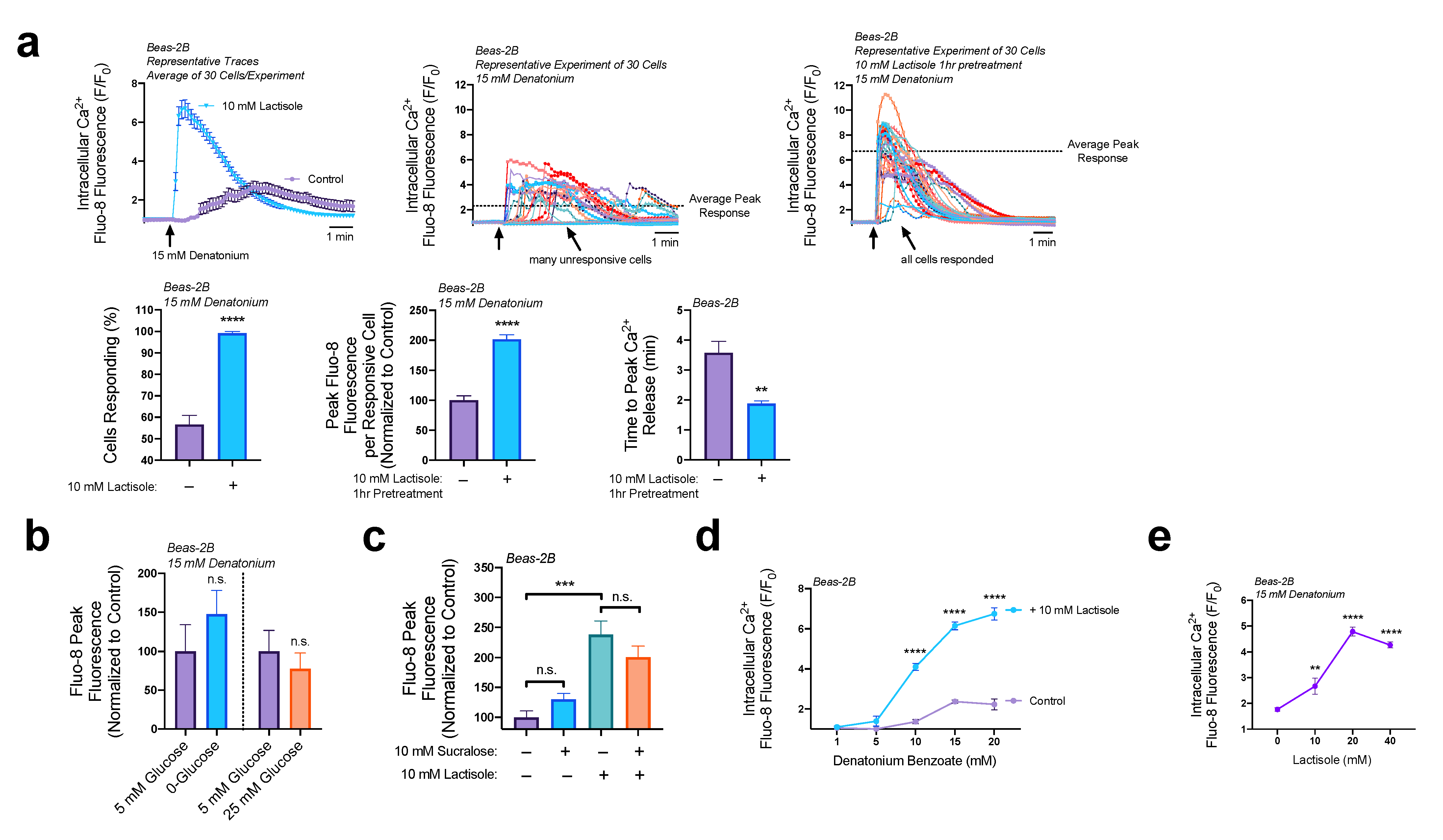
3.2. Lactisole Increases ER Ca2+ Content, ER Ca2+ Efflux, and GPCR-Modulated Ca2+ Signaling
3.3. EPAC Increases ER Ca2+ Efflux
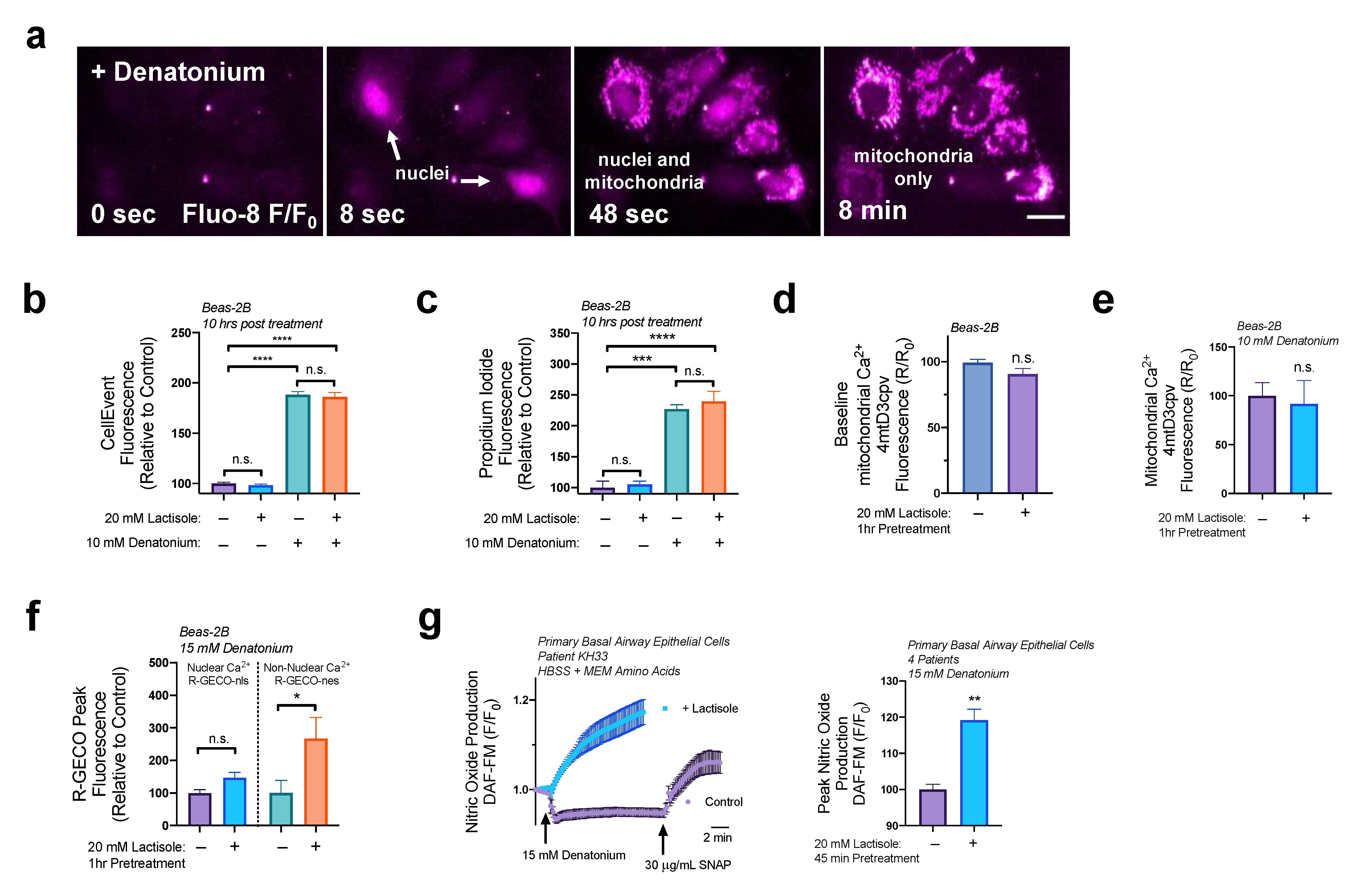
3.4. Lactisole Increases Denatonium-Induced Cytosolic Ca2+ to Activate NO Production
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nelson, G.; Hoon, M.A.; Chandrashekar, J.; Zhang, Y.; Ryba, N.J.; Zuker, C.S. Mammalian Sweet Taste Receptors. Cell 2001, 106, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Staszewski, L.; Xu, H.; Durick, K.; Zoller, M.; Adler, E. Human Receptors for Sweet and Umami Taste. Proc. Natl. Acad. Sci. USA 2002, 99, 4692–4696. [Google Scholar] [CrossRef] [PubMed]
- Nelson, G.; Chandrashekar, J.; Hoon, M.A.; Feng, L.; Zhao, G.; Ryba, N.J.P.; Zuker, C.S. An Amino-Acid Taste Receptor. Nature 2002, 416, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Masubuchi, Y.; Ma, J.; Suzuki, T.; Kojima, I.; Inagaki, T.; Shibata, H. T1R3 Homomeric Sweet Taste Receptor Negatively Regulates Insulin-Induced Glucose Transport through Gαs-Mediated Microtubules Disassembly in 3T3-L1 Adipocytes. Endocr. J. 2022, 69, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Kojima, I.; Medina, J.; Nakagawa, Y. Role of the Glucose-Sensing Receptor in Insulin Secretion. Diabetes Obes. Metab. 2017, 19 (Suppl. 1), 54–62. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Nagasawa, M.; Medina, J.; Kojima, I. Glucose Evokes Rapid Ca2+ and Cyclic AMP Signals by Activating the Cell-Surface Glucose-Sensing Receptor in Pancreatic β-Cells. PLoS ONE 2015, 10, e0144053. [Google Scholar] [CrossRef]
- Kojima, I.; Nakagawa, Y.; Ohtsu, Y.; Hamano, K.; Medina, J.; Nagasawa, M. Return of the Glucoreceptor: Glucose Activates the Glucose-Sensing Receptor T1R3 and Facilitates Metabolism in Pancreatic β-Cells. J. Diabetes Investig. 2015, 6, 256–263. [Google Scholar] [CrossRef]
- Kojima, I.; Nakagawa, Y.; Hamano, K.; Medina, J.; Li, L.; Nagasawa, M. Glucose-Sensing Receptor T1R3: A New Signaling Receptor Activated by Glucose in Pancreatic β-Cells. Biol. Pharm. Bull. 2015, 38, 674–679. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Ohtsu, Y.; Nagasawa, M.; Shibata, H.; Kojima, I. Glucose Promotes Its Own Metabolism by Acting on the Cell-Surface Glucose-Sensing Receptor T1R3. Endocr. J. 2014, 61, 119–131. [Google Scholar] [CrossRef]
- Rathbone, E.B.; Patel, G.D.; Butters, R.W.; Cookson, D.; Robinson, J.L. Occurrence of 2-(4-Methoxyphenoxy)Propanoic Acid in Roasted Coffee Beans: Analysis by Gas-Liquid Chromatography and by High-Performance Liquid Chromatography. J. Agric. Food Chem. 1989, 37, 54–58. [Google Scholar] [CrossRef]
- Jiang, P.; Cui, M.; Zhao, B.; Liu, Z.; Snyder, L.A.; Benard, L.M.J.; Osman, R.; Margolskee, R.F.; Max, M. Lactisole Interacts with the Transmembrane Domains of Human T1R3 to Inhibit Sweet Taste. J. Biol. Chem. 2005, 280, 15238–15246. [Google Scholar] [CrossRef]
- Alvarado, C.; Nachtigal, D.; Slack, J.P.; Green, B.G. Differential Modulation of the Lactisole ‘Sweet Water Taste’ by Sweeteners. PLoS ONE 2017, 12, e0180787. [Google Scholar] [CrossRef]
- Galindo-Cuspinera, V.; Breslin, P.A.S. The Liaison of Sweet and Savory. Chem. Senses 2006, 31, 221–225. [Google Scholar] [CrossRef]
- Lee, R.J.; Xiong, G.; Kofonow, J.M.; Chen, B.; Lysenko, A.; Jiang, P.; Abraham, V.; Doghramji, L.; Adappa, N.D.; Palmer, J.N.; et al. T2R38 Taste Receptor Polymorphisms Underlie Susceptibility to Upper Respiratory Infection. J. Clin. Invest. 2012, 122, 4145–4159. [Google Scholar] [CrossRef]
- Kuek, L.E.; McMahon, D.B.; Ma, R.Z.; Miller, Z.A.; Jolivert, J.F.; Adappa, N.D.; Palmer, J.N.; Lee, R.J. Cilia Stimulatory and Antibacterial Activities of T2R Bitter Taste Receptor Agonist Diphenhydramine: Insights into Repurposing Bitter Drugs for Nasal Infections. Pharmaceuticals 2022, 15, 452. [Google Scholar] [CrossRef]
- Hariri, B.M.; McMahon, D.B.; Chen, B.; Freund, J.R.; Mansfield, C.J.; Doghramji, L.J.; Adappa, N.D.; Palmer, J.N.; Kennedy, D.W.; Reed, D.R.; et al. Flavones Modulate Respiratory Epithelial Innate Immunity: Anti-Inflammatory Effects and Activation of the T2R14 Receptor. J. Biol. Chem. 2017, 292, 8484–8497. [Google Scholar] [CrossRef]
- Shah, A.S.; Ben-Shahar, Y.; Moninger, T.O.; Kline, J.N.; Welsh, M.J. Motile Cilia of Human Airway Epithelia Are Chemosensory. Science 2009, 325, 1131–1134. [Google Scholar] [CrossRef]
- Finger, T.E.; Bottger, B.; Hansen, A.; Anderson, K.T.; Alimohammadi, H.; Silver, W.L. Solitary Chemoreceptor Cells in the Nasal Cavity Serve as Sentinels of Respiration. Proc. Natl. Acad. Sci. USA 2003, 100, 8981–8986. [Google Scholar] [CrossRef]
- Lee, R.J.; Kofonow, J.M.; Rosen, P.L.; Siebert, A.P.; Chen, B.; Doghramji, L.; Xiong, G.; Adappa, N.D.; Palmer, J.N.; Kennedy, D.W.; et al. Bitter and Sweet Taste Receptors Regulate Human Upper Respiratory Innate Immunity. J. Clin. Invest. 2014, 124, 1393–1405. [Google Scholar] [CrossRef]
- Lopez-Souza, N.; Dolganov, G.; Dubin, R.; Sachs, L.A.; Sassina, L.; Sporer, H.; Yagi, S.; Schnurr, D.; Boushey, H.A.; Widdicombe, J.H. Resistance of Differentiated Human Airway Epithelium to Infection by Rhinovirus. Am. J. Physiol. Lung Cell Mol. Physiol. 2004, 286, L373–L381. [Google Scholar] [CrossRef]
- Robinot, R.; Hubert, M.; de Melo, G.D.; Lazarini, F.; Bruel, T.; Smith, N.; Levallois, S.; Larrous, F.; Fernandes, J.; Gellenoncourt, S.; et al. SARS-CoV-2 Infection Induces the Dedifferentiation of Multiciliated Cells and Impairs Mucociliary Clearance. Nat. Commun. 2021, 12, 4354. [Google Scholar] [CrossRef] [PubMed]
- Gudis, D.; Zhao, K.Q.; Cohen, N.A. Acquired Cilia Dysfunction in Chronic Rhinosinusitis. Am. J. Rhinol. Allergy 2012, 26, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Dunnill, M.S. The Pathology of Asthma, with Special Reference to Changes in the Bronchial Mucosa. J. Clin. Pathol. 1960, 13, 27–33. [Google Scholar] [CrossRef] [PubMed]
- McMahon, D.B.; Kuek, L.E.; Johnson, M.E.; Johnson, P.O.; Horn, R.L.J.; Carey, R.M.; Adappa, N.D.; Palmer, J.N.; Lee, R.J. The Bitter End: T2R Bitter Receptor Agonists Elevate Nuclear Calcium and Induce Apoptosis in Non-Ciliated Airway Epithelial Cells. Cell Calcium 2021, 101, 102499. [Google Scholar] [CrossRef] [PubMed]
- McMahon, D.B.; Jolivert, J.F.; Kuek, L.E.; Adappa, N.D.; Palmer, J.N.; Lee, R.J. Savory Signaling: T1R Umami Receptor Modulates Endoplasmic Reticulum Calcium Store Content and Release Dynamics in Airway Epithelial Cells. Nutrients 2022, 15, 496. [Google Scholar] [CrossRef]
- Wölfle, U.; Elsholz, F.A.; Kersten, A.; Haarhaus, B.; Schumacher, U.; Schempp, C.M. Expression and Functional Activity of the Human Bitter Taste Receptor TAS2R38 in Human Placental Tissues and JEG-3 Cells. Molecules 2016, 21, 306. [Google Scholar] [CrossRef]
- Winnig, M.; Bufe, B.; Meyerhof, W. Valine 738 and Lysine 735 in the Fifth Transmembrane Domain of RTas1r3 Mediate Insensitivity towards Lactisole of the Rat Sweet Taste Receptor. BMC Neurosci. 2005, 6, 22. [Google Scholar] [CrossRef]
- Xu, H.; Staszewski, L.; Tang, H.; Adler, E.; Zoller, M.; Li, X. Different Functional Roles of T1R Subunits in the Heteromeric Taste Receptors. Proc. Natl. Acad. Sci. USA 2004, 101, 14258–14263. [Google Scholar] [CrossRef]
- Hamano, K.; Nakagawa, Y.; Ohtsu, Y.; Li, L.; Medina, J.; Tanaka, Y.; Masuda, K.; Komatsu, M.; Kojima, I. Lactisole Inhibits the Glucose-Sensing Receptor T1R3 Expressed in Mouse Pancreatic β-Cells. J. Endocrinol. 2015, 226, 57–66. [Google Scholar] [CrossRef]
- Palmer, A.E.; Jin, C.; Reed, J.C.; Tsien, R.Y. Bcl-2-Mediated Alterations in Endoplasmic Reticulum Ca2+ Analyzed with an Improved Genetically Encoded Fluorescent Sensor. Proc. Natl. Acad. Sci. USA 2004, 101, 17404–17409. [Google Scholar] [CrossRef]
- Meyerhof, W.; Batram, C.; Kuhn, C.; Brockhoff, A.; Chudoba, E.; Bufe, B.; Appendino, G.; Behrens, M. The Molecular Receptive Ranges of Human TAS2R Bitter Taste Receptors. Chem. Senses 2010, 35, 157–170. [Google Scholar] [CrossRef]
- Bufe, B.; Breslin, P.A.; Kuhn, C.; Reed, D.R.; Tharp, C.D.; Slack, J.P.; Kim, U.K.; Drayna, D.; Meyerhof, W. The Molecular Basis of Individual Differences in Phenylthiocarbamide and Propylthiouracil Bitterness Perception. Curr. Biol. 2005, 15, 322–327. [Google Scholar] [CrossRef]
- Depry, C.; Allen, M.D.; Zhang, J. Visualization of PKA Activity in Plasma Membrane Microdomains. Mol. Biosyst. 2011, 7, 52–58. [Google Scholar] [CrossRef]
- Sample, V.; DiPilato, L.M.; Yang, J.H.; Ni, Q.; Saucerman, J.J.; Zhang, J. Regulation of Nuclear PKA Revealed by Spatiotemporal Manipulation of Cyclic AMP. Nat. Chem. Biol. 2012, 8, 375–382. [Google Scholar] [CrossRef]
- Klarenbeek, J.B.; Goedhart, J.; Hink, M.A.; Gadella, T.W.J.; Jalink, K. A MTurquoise-Based CAMP Sensor for Both FLIM and Ratiometric Read-out Has Improved Dynamic Range. PLoS ONE 2011, 6, e19170. [Google Scholar] [CrossRef]
- Odaka, H.; Arai, S.; Inoue, T.; Kitaguchi, T. Genetically-Encoded Yellow Fluorescent CAMP Indicator with an Expanded Dynamic Range for Dual-Color Imaging. PLoS ONE 2014, 9, e100252. [Google Scholar] [CrossRef]
- Lacabaratz-Porret, C.; Corvazier, E.; Kovàcs, T.; Bobe, R.; Bredoux, R.; Launay, S.; Papp, B.; Enouf, J. Platelet Sarco/Endoplasmic Reticulum Ca2+ATPase Isoform 3b and Rap 1b: Interrelation and Regulation in Physiopathology. Biochem. J. 1998, 332, 173–181. [Google Scholar] [CrossRef]
- Pereira, L.; Métrich, M.; Fernández-Velasco, M.; Lucas, A.; Leroy, J.; Perrier, R.; Morel, E.; Fischmeister, R.; Richard, S.; Bénitah, J.-P.; et al. The CAMP Binding Protein Epac Modulates Ca 2+ Sparks by a Ca 2+/Calmodulin Kinase Signalling Pathway in Rat Cardiac Myocytes: EPAC and Calcium Handling. J. Physiol. 2007, 583, 685–694. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Takahashi, N.; Kishimoto, T.; Nemoto, T.; Kasai, H. Two CAMP-Dependent Pathways Differentially Regulate Exocytosis of Large Dense-Core and Small Vesicles in Mouse β-Cells: Regulation of Exocytosis by Epac and PKA. J. Physiol. 2007, 582, 1087–1098. [Google Scholar] [CrossRef]
- Kang, G.; Joseph, J.W.; Chepurny, O.G.; Monaco, M.; Wheeler, M.B.; Bos, J.L.; Schwede, F.; Genieser, H.-G.; Holz, G.G. Epac-Selective CAMP Analog 8-PCPT-2′-O-Me-CAMP as a Stimulus for Ca2+-Induced Ca2+ Release and Exocytosis in Pancreatic β-Cells. J. Biol. Chem. 2003, 278, 8279–8285. [Google Scholar] [CrossRef]
- Palmer, A.E.; Giacomello, M.; Kortemme, T.; Hires, S.A.; Lev-Ram, V.; Baker, D.; Tsien, R.Y. Ca2+ Indicators Based on Computationally Redesigned Calmodulin-Peptide Pairs. Chem. Biol. 2006, 13, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Araki, S.; Wu, J.; Teramoto, T.; Chang, Y.F.; Nakano, M.; Abdelfattah, A.S.; Fujiwara, M.; Ishihara, T.; Nagai, T.; et al. An Expanded Palette of Genetically Encoded Ca2+ Indicators. Science 2011, 333, 1888–1891. [Google Scholar] [CrossRef] [PubMed]
- Garcia, V.; Sessa, W.C. Endothelial NOS: Perspective and Recent Developments. Br. J. Pharm. 2019, 176, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Carreras-Sureda, A.; Pihán, P.; Hetz, C. Calcium Signaling at the Endoplasmic Reticulum: Fine-Tuning Stress Responses. Cell Calcium 2018, 70, 24–31. [Google Scholar] [CrossRef]
- Berridge, M.J. The Endoplasmic Reticulum: A Multifunctional Signaling Organelle. Cell Calcium 2002, 32, 235–249. [Google Scholar] [CrossRef]
- Kong, H.; Jones, P.P.; Koop, A.; Zhang, L.; Duff, H.J.; Chen, S.R.W. Caffeine Induces Ca2+ Release by Reducing the Threshold for Luminal Ca2+ Activation of the Ryanodine Receptor. Biochem. J. 2008, 414, 441–452. [Google Scholar] [CrossRef]
- Dagan-Wiener, A.; Di Pizio, A.; Nissim, I.; Bahia, M.S.; Dubovski, N.; Margulis, E.; Niv, M.Y. BitterDB: Taste Ligands and Receptors Database in 2019. Nucleic Acids Res. 2019, 47, D1179–D1185. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McMahon, D.B.; Jolivert, J.F.; Kuek, L.E.; Adappa, N.D.; Palmer, J.N.; Lee, R.J. Utilizing the Off-Target Effects of T1R3 Antagonist Lactisole to Enhance Nitric Oxide Production in Basal Airway Epithelial Cells. Nutrients 2023, 15, 517. https://doi.org/10.3390/nu15030517
McMahon DB, Jolivert JF, Kuek LE, Adappa ND, Palmer JN, Lee RJ. Utilizing the Off-Target Effects of T1R3 Antagonist Lactisole to Enhance Nitric Oxide Production in Basal Airway Epithelial Cells. Nutrients. 2023; 15(3):517. https://doi.org/10.3390/nu15030517
Chicago/Turabian StyleMcMahon, Derek B., Jennifer F. Jolivert, Li Eon Kuek, Nithin D. Adappa, James N. Palmer, and Robert J. Lee. 2023. "Utilizing the Off-Target Effects of T1R3 Antagonist Lactisole to Enhance Nitric Oxide Production in Basal Airway Epithelial Cells" Nutrients 15, no. 3: 517. https://doi.org/10.3390/nu15030517
APA StyleMcMahon, D. B., Jolivert, J. F., Kuek, L. E., Adappa, N. D., Palmer, J. N., & Lee, R. J. (2023). Utilizing the Off-Target Effects of T1R3 Antagonist Lactisole to Enhance Nitric Oxide Production in Basal Airway Epithelial Cells. Nutrients, 15(3), 517. https://doi.org/10.3390/nu15030517






