Barriers to and Facilitators of the Implementation of a Micronutrient Powder Program for Children: A Systematic Review Based on the Consolidated Framework for Implementation Research
Highlights
- As one of the most cost-effective investments for improving child nutrition, micronutrient powder (MNP) has been widely used in many countries to underpin the Sustainable Development Goals, yet challenges remain regarding implementation at scale.
- This article firstly provided a comprehensive analysis of the barriers and facilitators of implementing MNP programmes for children. The findings identified the main challenges in terms of organization structure, available resources, and knowledge levels.
- This research suggested the internal relationships between commonly reported barriers and facilitators and underlying factors influencing implementation, providing a transparent approach for decision-makers at the policy level.
- This evidence provides priorities for implementers to directly assess challenges when developing MNP programmes and similar interventions.
Abstract
:1. Background
2. Methods
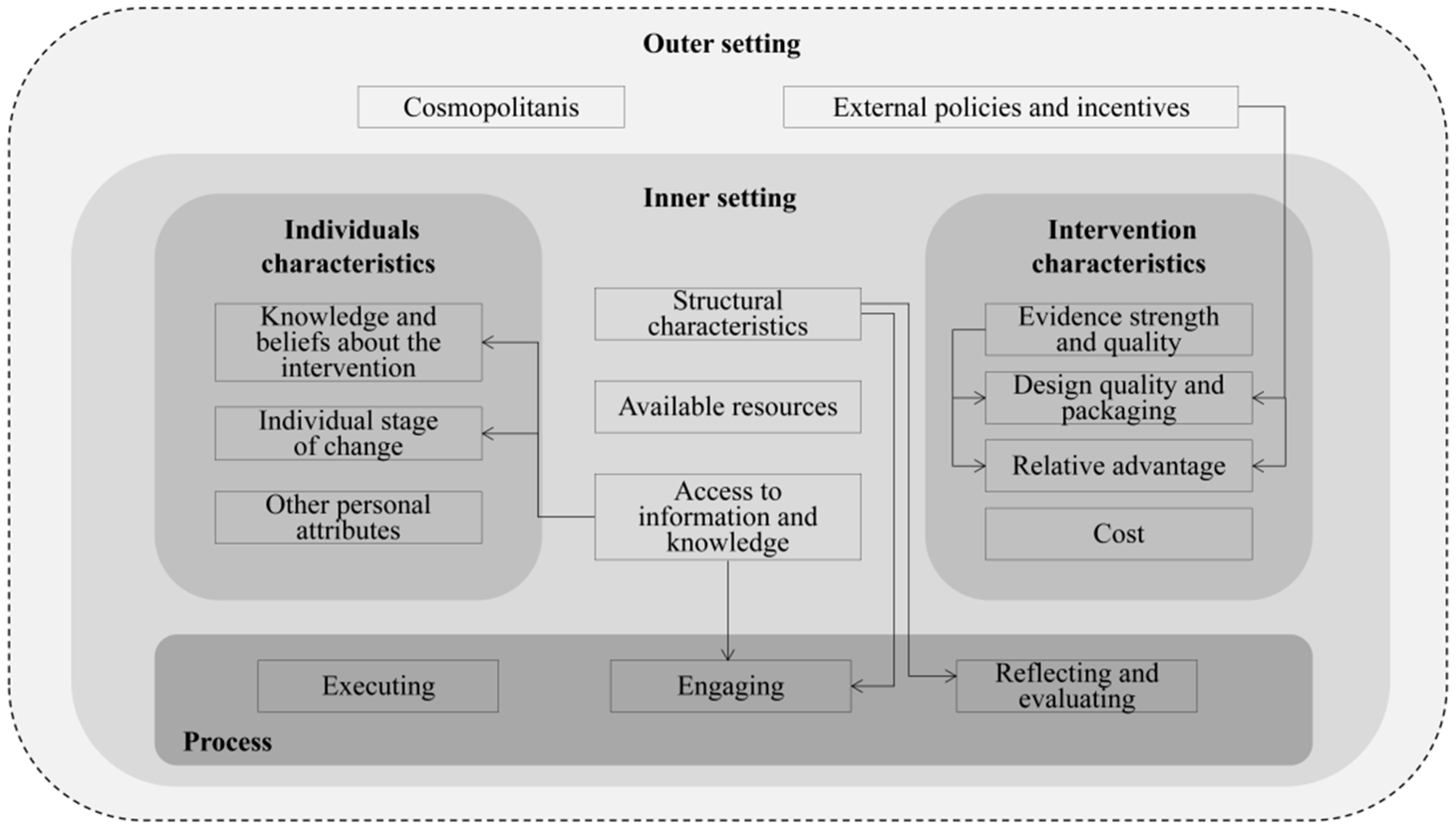
2.1. Conceptual Framework
2.2. Search Strategy
2.3. Study Screening and Eligibility
2.4. Data Extraction
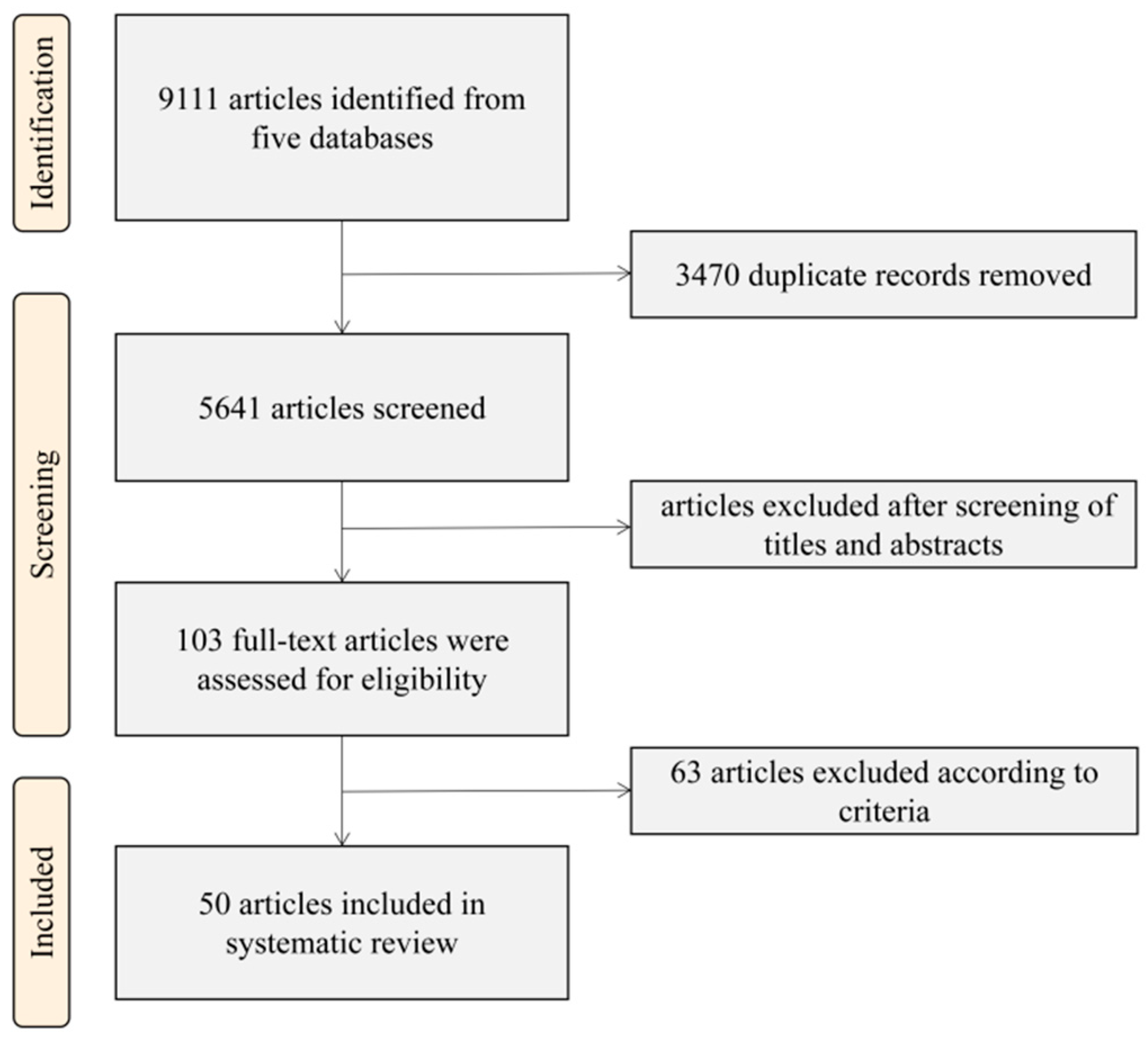
2.5. Search Results and Included Studies
3. Results
3.1. Study Characteristics
3.2. Barriers and Facilitators
3.3. Domain 1: Intervention Characteristics
3.4. Domain 2: Outer Setting
3.5. Domain 3: Inner Setting
3.6. Domain 4: Characteristics of the Individuals Involved
3.7. Domain 5: The Process of Implementation
3.8. Relationship between Constructs
4. Discussion
4.1. Implications and Recommendations for Policy and Practice
4.2. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Luo, R.; Shi, Y.; Zhou, H.; Yue, A.; Zhang, L.; Sylvia, S.; Medina, A.; Rozelle, S. Micronutrient deficiencies and developmental delays among infants: Evidence from a cross-sectional survey in rural China. BMJ Open 2015, 5, e008400. [Google Scholar] [CrossRef]
- Dicker, D.; Nguyen, G.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; et al. Global, regional, and national age-sex-specific mortality and life expectancy, 1950–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1684–1735. [Google Scholar] [CrossRef]
- Kyei-Arthur, F.; Situma, R.; Aballo, J.; Mahama, A.B.; Selenje, L.; Amoaful, E.; Adu-Afarwuah, S. Lessons learned from implementing the pilot Micronutrient Powder Initiative in four districts in Ghana. BMC Nutr. 2020, 6, 50. [Google Scholar] [CrossRef]
- Shekar, M.; Condo, J.; Pate, M.A.; Nishtar, S. Maternal and child undernutrition: Progress hinges on supporting women and more implementation research. Lancet 2021, 397, 1329–1331. [Google Scholar] [CrossRef]
- WHO. UNICEF/WHO/WB Joint Child Malnutrition Estimates. 6 May 2021. Available online: https://www.who.int/news/item/06-05-2021-the-unicef-who-wb-joint-child-malnutrition-estimates-group-released-new-data-for-2021 (accessed on 2 May 2023).
- Stevens, G.A.; Bennett, J.E.; Hennocq, Q.; Lu, Y.; De-Regil, L.M.; Rogers, L.; Danaei, G.; Li, G.; White, R.A.; Flaxman, S.R.; et al. Trends and mortality effects of vitamin A deficiency in children in 138 low-income and middle-income countries between 1991 and 2013: A pooled analysis of population-based surveys. Lancet Glob. Health 2015, 3, e528–e536. [Google Scholar] [CrossRef]
- Kapil, U.S. Technical consultation on Strategies for Prevention and Control of Iron Deficiency Anemia amongst under three children in India. Indian Pediatr. 2002, 39, 640–647. [Google Scholar]
- WHO. WHO Guideline: Use of Multiple Micronutrient Powders for Home Fortification of Foods Consumed by Infants and Children 6–23 Months of Age; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- WHO. Multiple Micronutrient Powders for Point-of-Use Fortification of Foods Consumed by Children 2–12 Years of Age. 19 August 2023. Available online: https://www.who.int/tools/elena/interventions/micronutrientpowder-children (accessed on 2 May 2023).
- Tam, E.; Keats, E.C.; Rind, F.; Das, J.K.; Bhutta, Z.A. Micronutrient supplementation and fortification interventions on health and development outcomes among children under-five in low- and middle-income countries: A systematic review and me-ta-analysis. Nutrients 2020, 12, 289. [Google Scholar] [CrossRef]
- UNICEF. Global Nutrition Programme Monitoring; NutriDash 2020 Key Findings; UNICEF: New York, NY, USA, 2021. [Google Scholar]
- Reerink, I.; Namaste, S.M.; Poonawala, A.; Dhillon, C.N.; Aburto, N.; Chaudhery, D.; Kroeun, H.; Griffiths, M.; Haque, M.R.; Bonvecchio, A.; et al. Experiences and lessons learned for delivery of micronutrient powders interventions. Matern. Child Nutr. 2017, 13, e12495. [Google Scholar] [CrossRef]
- Habicht, J.-P.; Pelto, G.H. From biological to program efficacy: Promoting dialogue among the research, policy, and program communities. Adv. Nutr. Int. Rev. J. 2014, 5, 27–34. [Google Scholar] [CrossRef]
- Bhutta, Z.A.; Das, J.K.; Rizvi, A.; Gaffey, M.F.; Walker, N.; Horton, S.; Webb, P.; Lartey, A.; Black, R.E.; The Lancet Nutrition Interventions Review Group; et al. Evidence-based interventions for improvement of maternal and child nutrition: What can be done and at what cost? Lancet 2013, 382, 452–477. [Google Scholar] [CrossRef]
- Damschroder, L.J.; Aron, D.C.; Keith, R.E.; Kirsh, S.R.; Alexander, J.A.; Lowery, J.C. Fostering implementation of health services research findings into practice: A consolidated framework for advancing implementation science. Implement. Sci. 2009, 4, 50. [Google Scholar] [CrossRef]
- Damschroder, L.J.; Reardon, C.M.; Widerquist, M.A.O.; Lowery, J. The updated Consolidated Framework for Implementation Research based on user feedback. Implement. Sci. 2022, 17, 75. [Google Scholar] [CrossRef]
- Cruess, S.R.; Cruess, R.L.; Steinert, Y. Role modelling—Making the most of a powerful teaching strategy. BMJ 2008, 336, 718–721. [Google Scholar] [CrossRef]
- El-Awaisi, A.; Joseph, S.; El Hajj, M.S.; Diack, L. A comprehensive systematic review of pharmacy perspectives on interprofessional education and collaborative practice. Res. Soc. Adm. Pharm. 2018, 14, 863–882. [Google Scholar] [CrossRef]
- Nyhus Dhillon, C.; Sarkar, D.; Klemm, R.D.; Neufeld, L.M.; Rawat, R.; Tumilowicz, A.; Namaste, S.M. Executive summary for the Micronutrient Powders Consultation: Lessons Learned for Operational Guidance. Matern. Child Nutr. 2017, 13 (Suppl. S1), e12493. [Google Scholar] [CrossRef]
- Suchdev, P.S.; Ruth, L.; Obure, A.; Were, V.; Ochieng, C.; Ogange, L.; Owuor, M.; Ngure, F.; Quick, R.; Juliao, P.; et al. Monitoring the marketing, distribution, and use of sprinkles micronutrient powders in rural western Kenya. Food Nutr. Bull. 2010, 31 (Suppl. S2), S168–S178. [Google Scholar] [CrossRef]
- Kodish, S.; Rah, J.H.; Kraemer, K.; de Pee, S.; Gittelsohn, J. Understanding low usage of micronutrient powder in the kakuma refugee camp, Kenya: Findings from a qualitative study. Food Nutr. Bull. 2011, 32, 292–303. [Google Scholar] [CrossRef]
- Michaux, K.; Anema, A.; Green, T.; Smith, L.; McLean, J.; Omwega, A.; Ahimbisibwei, M. Home Fortification with Micronutrient Powders: Lessons learned from formative research across six countries. Sight Life 2014, 28, 26–35. [Google Scholar]
- Korenromp, E.L.; Adeosun, O.; Adegoke, F.; Akerele, A.; Anger, C.; Ohajinwa, C.; Hotz, C.; Umunna, L.; Aminu, F. Micronutrient powder distribution through Maternal, Neonatal and Child Health Weeks in Nigeria: Process evaluation of feasibility and use. Public Health Nutr. 2016, 19, 1882–1892. [Google Scholar] [CrossRef]
- Lundeen, E.; Schueth, T.; Toktobaev, N.; Zlotkin, S.; Hyder, S.Z.; Houser, R. Daily use of sprinkles micronutrient powder for 2 months reduces anemia among children 6 to 36 months of age in the Kyrgyz republic: A cluster-randomized trial. Food Nutr. Bull. 2010, 31, 446–460. [Google Scholar] [CrossRef]
- Locks, L.M.; Reerink, I.; Brown, A.T.; Gnegne, S.; Ramalanjaona, N.; Nanama, S.; Duggan, C.P.; Garg, A. The Impact of Integrated Infant and Young Child Feeding and Micronutrient Powder Intervention on Feeding Practices and Anemia in Children Aged 6–23 Months in Madagascar. Nutrients 2017, 9, 581. [Google Scholar] [CrossRef] [PubMed]
- Adu-Afarwuah, S.; Lartey, A.; Brown, K.H.; Zlotkin, S.; Briend, A.; Dewey, K.G. Home fortification of complementary foods with micronutrient supplements is well accepted and has positive effects on infant iron status in Ghana. Am. J. Clin. Nutr. 2008, 87, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Jefferds, M.E.; Irizarry, L.; Timmer, A.; Tripp, K. UNICEF-CDC global assessment of home fortification interventions 2011: Current status, new directions, and implications for policy and programmatic guidance. Food Nutr. Bull. 2013, 34, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Hyder, S.Z.; Haseen, F.; Rahman, M.; Tondeur, M.C.; Zlotkin, S.H. Effect of daily versus once-weekly home fortification with micronutrient sprinkles on hemoglobin and iron status among young children in rural Bangladesh. Food Nutr. Bull. 2007, 28, 156–164. [Google Scholar] [CrossRef]
- Brewer, J.D.; Santos, M.P.; Román, K.; Riley-Powell, A.R.; Oberhelman, R.A.; Paz-Soldan, V.A. Micronutrient powder use in Arequipa, Peru: Barriers and enablers across multiple levels. Matern. Child Nutr. 2020, 16, e12915. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Tariqujjaman; Mahfuz, M.; Ahmed, T.; Sarma, H. Caregiver perceived barriers to the use of micronutrient powder for children aged 6–59 months in Bangladesh. PLoS ONE 2021, 16, e0260773. [Google Scholar] [CrossRef]
- Vossenaar, M.; Tumilowicz, A.; D’Agostino, A.; Bonvecchio, A.; Grajeda, R.; Imanalieva, C.; Irizarry, L.; Mulokozi, G.; Sudardjo, M.N.; Tsevegsuren, N.; et al. Experiences and lessons learned for programme improvement of micronutrient powders interventions. Matern. Child Nutr. 2017, 13 (Suppl. S1), e12496. [Google Scholar] [CrossRef]
- Sarma, H.; Uddin, F.; Harbour, C.; Ahmed, T. Factors Influencing Child Feeding Practices Related to Home Fortification with Micronutrient Powder Among Caregivers of Under-5 Children in Bangladesh. Food Nutr. Bull. 2016, 37, 340–352. [Google Scholar] [CrossRef]
- Nguyen, M.; Poonawala, A.; Leyvraz, M.; Berger, J.; Schofield, D.; Nga, T.T.; Van, T.K.; Hoa, D.T.B.; Wieringa, F.T. A Delivery Model for Home Fortification of Complementary Foods with Micronutrient Powders: In-novation in the Context of Vietnamese Health System Strengthening. Nutrients 2016, 8, 259. [Google Scholar] [CrossRef]
- Creed-Kanashiro, H.; Bartolini, R.; Abad, M.; Arevalo, V. Promoting multi-micronutrient powders (MNP) in Peru: Acceptance by caregivers and role of health personnel. Matern. Child Nutr. 2016, 12, 152–163. [Google Scholar] [CrossRef]
- Inayati, D.A.; Scherbaum, V.; Purwestri, R.C.; Wirawan, N.N.; Suryantan, J.; Hartono, S.; Bloem, M.A.; Pangaribuan, R.V.; Biesalski, H.K.; Hoffmann, V.; et al. Combined intensive nutrition education and micronutrient powder supplementation improved nutri-tional status of mildly wasted children on Nias Island, Indonesia. Asia Pac. J. Clin. Nutr. 2012, 21, 361–373. [Google Scholar] [PubMed]
- Jefferds, M.E.D.; Mirkovic, K.R.; Subedi, G.R.; Mebrahtu, S.; Dahal, P.; Perrine, C.G. Predictors of micronutrient powder sachet coverage in Nepal. Matern. Child Nutr. 2015, 11 (Suppl. S4), 77–89. [Google Scholar] [CrossRef] [PubMed]
- Ford, N.D.; Ruth, L.J.; Ngalombi, S.; Lubowa, A.; Halati, S.; Ahimbisibwe, M.; Mapango, C.; Whitehead, R.D., Jr.; Jefferds, M.E. Predictors of micronutrient powder sachet coverage and recent intake among children 12–23 months in Eastern Uganda. Matern. Child Nutr. 2019, 15 (Suppl. S5), e12792. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Fang, J.Q.; Luo, J.Y.; Wang, H.; Du, Q.Y.; Huang, G.W.; Feng, B.B. Factors related to effective use of nutrition kits for infants aged 6 to 24 months in poor rural areas of Hunan Province. Health Res. 2017, 46, 256–261. (In Chinese) [Google Scholar]
- Shen, H.; Li, G.Z. Comprehensive nutrition intervention of infants and young children in rural Shaanxi Province. Chin. Clin. Study 2011, 24, 857–858. (In Chinese) [Google Scholar]
- Suchdev, P.S.; Shah, A.; Jefferds, M.E.D.; Eleveld, A.; Patel, M.; Stein, A.D.; Macdonald, B.; Ruth, L. Sustainability of market-based community distribution of Sprinkles in western Kenya. Matern. Child Nutr. 2013, 9 (Suppl. S1), 78–88. [Google Scholar] [CrossRef]
- Zhang, W.J.; Yang, H.; Peng, M.; Li, P. Analysis on the influencing factors of nutrition package administration in the nutrition improvement project for children in poor areas of Gansu Province. China Matern. Child Health Care 2019, 34, 4851–4855. [Google Scholar]
- Zlotkin, S.H.; Schauer, C.; Christofides, A.; Sharieff, W.; Tondeur, M.C.; Hyder, S.M.Z. Micronutrient Sprinkles to Control Childhood Anaemia. PLoS Med. 2005, 2, e1. [Google Scholar] [CrossRef]
- Samuel, A.; Brouwer, I.D.; Pamungkas, N.P.; Terra, T.; Lelisa, A.; Kebede, A.; Osendarp, S.J.M. Determinants of adherence to micronutrient powder use among young children in Ethiopia. Matern. Child Nutr. 2021, 17, e13111. [Google Scholar] [CrossRef]
- Roschnik, N.; Diarra, H.; Dicko, Y.; Diarra, S.; Stanley, I.; Moestue, H.; McClean, J.; Verhoef, H.; Clarke, S.E. Adherence and acceptability of community-based distribution of micronutrient powders in Southern Mali. Matern. Child Nutr. 2019, 15 (Suppl. S5), e12831. [Google Scholar] [CrossRef]
- Jefferds, M.E.D.; Ogange, L.; Owuor, M.; Cruz, K.; Person, B.; Obure, A.; Suchdev, P.S.; Ruth, L.J. Formative research exploring acceptability, utilization, and promotion in order to develop a micro-nutrient powder (Sprinkles) intervention among Luo families in western Kenya. Food Nutr. Bull. 2010, 31 (Suppl. S2), S179–S185. [Google Scholar] [CrossRef]
- D’Agostino, A.; Ssebiryo, F.; Murphy, H.; Cristello, A.; Nakiwala, R.; Otim, K.; Sarkar, D.; Ngalombi, S.; Schott, W.; Katuntu, D.; et al. Facility- and community-based delivery of micronutrient powders in Uganda: Opening the black box of implementation using mixed methods. Matern. Child Nutr. 2019, 15 (Suppl. S5), e12798. [Google Scholar] [CrossRef]
- Locks, L.M.; Dahal, P.; Pokharel, R.; Joshi, N.; Paudyal, N.; Whitehead, R.D., Jr.; Chitekwe, S.; Mei, L.M.; Lamichhane, B.; Garg, A.; et al. Predictors of micronutrient powder (MNP) knowledge, coverage, and consumption during the scale-up of an integrated infant and young child feeding (IYCF-MNP) programme in Nepal. Matern. Child Nutr. 2019, 15 (Suppl. S5), e12712. [Google Scholar] [CrossRef]
- Sun, J.; Dai, Y.; Zhang, S.; Huang, J.; Yang, Z.; Huo, J.; Chen, C. Implementation of a programme to market a complementary food supplement (Ying Yang Bao) and impacts on anaemia and feeding practices in Shanxi, China. Matern. Child Nutr. 2011, 7 (Suppl. S3), 96–111. [Google Scholar] [CrossRef]
- Sutrisna, A.; Vossenaar, M.; Poonawala, A.; Mallipu, A.; Izwardy, D.; Menon, R.; Tumilowicz, A. Improved Information and Educational Messages on Outer Packaging of Micronutrient Powders Dis-tributed in Indonesia Increase Caregiver Knowledge and Adherence to Recommended Use. Nutrients 2018, 10, 747. [Google Scholar] [CrossRef]
- Pelto, G.H.; Tumilowicz, A.; Schnefke, C.H.; Gebreyesus, S.H.; Hrabar, M.; Gonzalez, W.; Wodajo, H.Y.; Neufeld, L.M. Ethiopian mothers’ experiences with micronutrient powders: Perspectives from continuing and non-continuing users. Matern. Child Nutr. 2019, 15 (Suppl. S5), e12708. [Google Scholar] [CrossRef]
- Sutrisna, A.; Vossenaar, M.; Izwardy, D.; Tumilowicz, A. Sensory Evaluation of Foods with Added Micronutrient Powder (MNP) “Taburia” to Assess Acceptability among Children Aged 6–24 Months and Their Caregivers in Indonesia. Nutrients 2017, 9, 979. [Google Scholar] [CrossRef]
- Dusingizimana, T.; Weber, J.L.; Ramilan, T.; Iversen, P.O.; Brough, L. A Mixed-Methods Study of Factors Influencing Access to and Use of Micronutrient Powders in Rwanda. Glob. Health Sci. Pract. 2021, 9, 274–285. [Google Scholar] [CrossRef]
- Christofides, A.; Schauer, C.; Sharieff, W.; Zlotkin, S.H. Acceptability of micronutrient sprinkles: A new food-based approach for delivering iron to First Nations and Inuit children in Northern Canada. Chronic Dis. Can. 2005, 26, 114–120. [Google Scholar]
- Mirkovic, K.R.; Perrine, C.G.; Subedi, G.R.; Mebrahtu, S.; Dahal, P.; Staatz, C.; Jefferds, M.E.D. Predictors of micronutrient powder intake adherence in a pilot programme in Nepal. Public Health Nutr. 2016, 19, 1768–1776. [Google Scholar] [CrossRef]
- Niu, H.; Wang, Y.; Tang, H.; Gong, L. Dietary compliance of poor rural children in Guizhou, Yunnan and Shanxi provinces and its influencing factors. Health Res. 2017, 46, 262–265+271. (In Chinese) [Google Scholar]
- Loechl, C.U.; Menon, P.; Arimond, M.; Ruel, M.T.; Pelto, G.; Habicht, J.-P.; Michaud, L. Using programme theory to assess the feasibility of delivering micronutrient Sprinkles through a food-assisted maternal and child health and nutrition programme in rural Haiti. Matern. Child Nutr. 2009, 5, 33–48. [Google Scholar] [CrossRef]
- Yao, C.; Sun, C.; Ye, R.; Wu, Y.; Zheng, L.; Zhou, H. Influence of information dissemination on YYB feeding behavior of infant and young child caregivers in different generations in rural areas. J. Sichuan Univ. (Med. Ed.) 2022, 53, 1061–1067. (In Chinese) [Google Scholar]
- Zhang, W.J.; Yang, H.X.; Li, P.X.; Peng, M.Q. Analysis of influencing factors of YYB administration of a county. China J. Matern. Child Health 2015, 6, 26–29. (In Chinese) [Google Scholar]
- Angdembe, M.R.; Choudhury, N.; Haque, M.R.; Ahmed, T. Adherence to multiple micronutrient powder among young children in rural Bangladesh: A cross-sectional study. BMC Public Health 2015, 15, 440. [Google Scholar] [CrossRef]
- Ouedraogo, O.; Doudou, M.H.; Drabo, K.M.; Kiburente, M.; Cissé, D.; Mésenge, C.; Sanou, D.; Zagre, N.M.; Donnen, P. Facilitating factors and challenges of the implementation of multisectoral nutrition programmes at the community level to improve optimal infant and young child feeding practices: A qualitative study in Burkina Faso. Public Health Nutr. 2021, 24, 3756–3767. [Google Scholar] [CrossRef]
- Sarma, H.; Mbuya, M.N.; Tariqujjaman; Rahman, M.; Askari, S.; Khondker, R.; Sultana, S.; Shahin, S.A.; Bossert, T.J.; Banwell, C.; et al. Role of home visits by volunteer community health workers: To improve the coverage of micronutrient powders in rural Bangladesh. Public Health Nutr. 2021, 24, s48–s58. [Google Scholar] [CrossRef]
- Black, M.M.; Drennen, C.R. Nutritional and growth issues related to child neglect. Pediatr. Ann. 2014, 43, e266–e270. [Google Scholar] [CrossRef]
- Results for Development Institute. Nutrition for a Better Tomorrow: Scaling up Delivery of Micronutrient Powders for Infants and Young Children; Results for Development Institute: Washington, DC, USA, 2018. [Google Scholar]
- Yang, M.; Sun, Y.; Ma, J.; Zhang, K.; Guo, Y.; Huang, Y. Analysis on the supply path, advantages and challenges of YYB for children in China. Chin. Food Nutr. 2022, 28, 16–20. (In Chinese) [Google Scholar]
- UNICEF. Multiple Micronutrient Powder Supply and Market Update; UNICEF: New York, NY, USA, 2021. [Google Scholar]
- Ansell, L.H. 309Community-Based Health Interventions: Past, Present, and Future, in Urban Health: Combating Disparities with Local Data; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Hirve, S.; Martini, E.; Juvekar, S.K.; Agarwal, D.; Bavdekar, A.; Sari, M.; Molwane, M.; Janes, S.; Haselow, N.; Yeung, D.L.; et al. Delivering Sprinkles Plus through the Integrated Child Development Services (ICDS) to Reduce Anemia in Pre-school Children in India. Indian J. Pediatr. 2013, 80, 990–995. [Google Scholar] [CrossRef]
- Eileen, K.; Jennifer, S.; Meghan, K.; Sibhatu, B. Impact of Social and Behavior Change Communication in Nutrition Specific Interventions on Selected Indicators of Nutritional Status. J. Hum. Nutr. 2018, 2, 34–46. [Google Scholar] [CrossRef]
- Tadelis, S.; Zettelmeyer, F. Information Disclosure as a Matching Mechanism: Theory and Evidence from a Field Experiment. Am. Econ. Rev. 2015, 105, 886–905. [Google Scholar] [CrossRef]
- Hotz, C.; Gibson, R.S. Participatory nutrition education and adoption of new feeding practices are associated with im-proved adequacy of complementary diets among rural Malawian children: A pilot study. Eur. J. Clin. Nutr. 2005, 59, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Alam, K.; Oliveras, E. Retention of female volunteer community health workers in Dhaka urban slums: A prospective cohort study. Hum. Resour. Health 2014, 12, 29. [Google Scholar] [CrossRef]
- Ahmed, S.; Sarma, H.; Hasan, Z.; Rahman, M.; Ahmed, M.W.; Islam, M.A.; Djimeu, E.W.; Mbuya, M.N.; Ahmed, T.; Khan, J.A. Cost-effectiveness of a market-based home fortification of food with micronutrient powder programme in Bangladesh. Public Health Nutr. 2021, 24, s59–s70. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
| Selection Criteria | Inclusion | Exclusion |
|---|---|---|
| Language | English or Chinese | All languages except English or Chinese |
| Study design | Original studies: qualitative, quantitative, randomized controlled trials, non-randomized trials, and mixed methods studies | Editorials, commentary pieces, and systematic reviews |
| Study population | Children aged 6–23 months or 6–36 months | Children in other age group |
| Study | Studies that reported implementation facilitators and barriers from data collection after implementing MNP interventions | Studies in which the facilitators and barriers were not reported |
| Number of Studies | Percentage % | |
|---|---|---|
| Summary by study region | ||
| High income | 1 | 2.0 |
| Low income | 9 | 18.0 |
| Lower-middle income | 26 | 52.0 |
| Upper-middle income | 9 | 18.0 |
| Low and middle income | 5 | 10.0 |
| Summary by study population | ||
| Providers | / | |
| Users | 27 | 54.0 |
| Providers and Users | 23 | 46.0 |
| Summary by study design | ||
| Randomized controlled trial | 14 | 28.0 |
| Cross-sectional study | 10 | 20.0 |
| Mixed-methods study | 16 | 32.0 |
| Qualitative study | 9 | 18.0 |
| Case study | 1 | 2.0 |
| Summary by implementation settings | ||
| Free distribution | 39 | 78.0 |
| Market-based | 11 | 22.0 |
| CFIR Framework Constructs | Barriers | Facilitators | ||
|---|---|---|---|---|
| B. Cosmopolitanism | 8 | Partnerships and coordination between both private and public sectors | ||
| D. External policies and incentives | 2 | A gap exists between the guidelines for use and successful operational protocols | 15 | Global evidence-based guidelines |
| 3. Inner setting | ||||
| 3 | Added burden on struggling health systems | 10 | Community-driven, decentralized, and integrated delivery approach |
| 2 | Lack of coordination with communities and interagency coordination | ||
| 2 | Insufficient attention to cultural situations, perceptions, routines, and practices | ||
| D4. Organizational incentives and rewards | 2 | Health workers had low motivation to accept MNP distribution tasks, expressing concerns about work overload and inadequate financial compensation | 1 | |
| E2. Available resources | 14 | Interrupted or insufficient supply of MNP Insufficient staff Inadequate funding for MNP interventions | 3 | Sustainable availability and establishing various contact points for supplying MNP |
| E3. Access to information and knowledge | 10 | Absence of refresher training for frontline workers, inconsistent training on MNP counseling techniques, and lack of counseling materials or information | 18 | Positive experiences with training and supervision of primary-level workers |
| 4. Characteristics of the individuals involved | ||||
| 14 | Lack of awareness and inadequate knowledge of MNP among caregivers Perceptions of side effects of MNP | 10 | Perceived positive changes in children following MNP use |
| 1 | Health workers’ confidence in ability to explain MNP benefits | ||
| 4 | Lack of familial and peer support for MNP use | 2 | Approval from family members Positive testimonies about the effectiveness of the MNP from relatives and neighbors |
| 1 | 3 | Trust in the government and field staff | |
| 10 | Low educational level of caregivers, lack of time or knowledge of MNP use in caregivers | 1 | Mother’s age > 25 years |
| 5. The process of implementation | ||||
| A. Engaging | 2 | Inadequate communication regarding the health benefits and use of micronutrient powder for caregivers Insufficient social marketing and dissemination | 6 | Social and behavior change communication on how to use MNP and resolve side effects of MNP, home visits by community health workers Social encouragement and advocacy by private sectors |
| B1. Opinion leaders | 1 | Authority of health center staff | ||
| C. Executing | 5 | Complicated importation procedures and import taxes | ||
| D. Reflecting and evaluating | 5 | Insufficient capacity for monitoring the program | 8 | Monitoring MNP distribution, usage, and adherence; evaluate the effectiveness of MNP program |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Ma, J.; Wei, X.; Dong, J.; Wu, S.; Huang, Y. Barriers to and Facilitators of the Implementation of a Micronutrient Powder Program for Children: A Systematic Review Based on the Consolidated Framework for Implementation Research. Nutrients 2023, 15, 5073. https://doi.org/10.3390/nu15245073
Sun Y, Ma J, Wei X, Dong J, Wu S, Huang Y. Barriers to and Facilitators of the Implementation of a Micronutrient Powder Program for Children: A Systematic Review Based on the Consolidated Framework for Implementation Research. Nutrients. 2023; 15(24):5073. https://doi.org/10.3390/nu15245073
Chicago/Turabian StyleSun, Yinuo, Jiyan Ma, Xiaolin Wei, Jingya Dong, Shishi Wu, and Yangmu Huang. 2023. "Barriers to and Facilitators of the Implementation of a Micronutrient Powder Program for Children: A Systematic Review Based on the Consolidated Framework for Implementation Research" Nutrients 15, no. 24: 5073. https://doi.org/10.3390/nu15245073
APA StyleSun, Y., Ma, J., Wei, X., Dong, J., Wu, S., & Huang, Y. (2023). Barriers to and Facilitators of the Implementation of a Micronutrient Powder Program for Children: A Systematic Review Based on the Consolidated Framework for Implementation Research. Nutrients, 15(24), 5073. https://doi.org/10.3390/nu15245073







