Health Economic Evaluation of a Controlled Lifestyle Intervention: The Healthy Lifestyle Community Program (Cohort 2; HLCP-2)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Measures
2.4. Intervention
2.5. Analysis
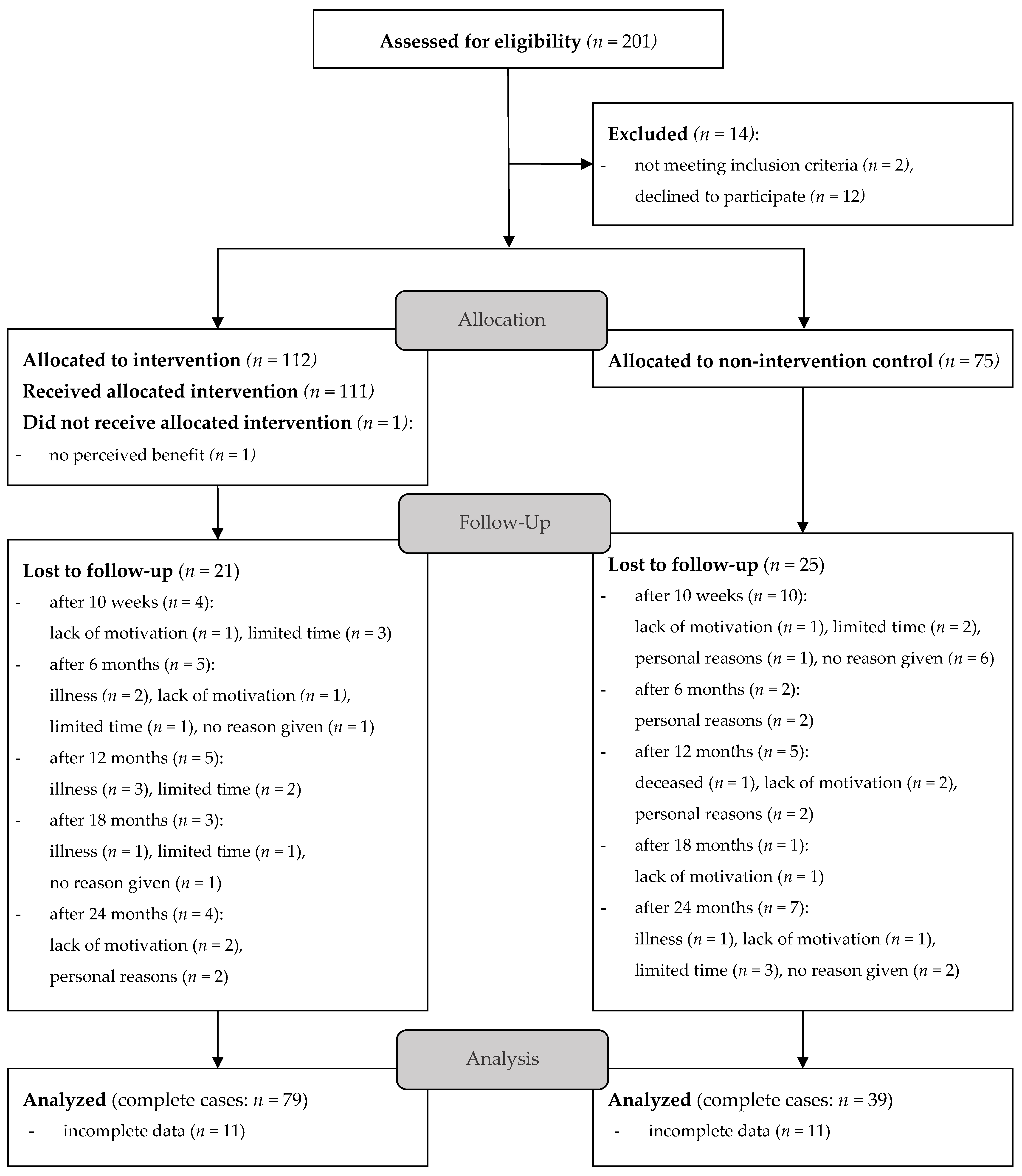
3. Results
3.1. Baseline Characteristics
3.2. Calculation of Costs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vuik, S.; Lerouge, A.; Guillemette, Y.; Feigl, A.; Aldea, A. The Heavy Burden of Obesity; OECD: Paris, France, 2019; ISBN 9789264330047. [Google Scholar]
- Vos, T.; Abajobir, A.A.; Abate, K.H.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abdulkader, R.S.; Abdulle, A.M.; Abebo, T.A.; Abera, S.F.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef] [PubMed]
- Goryakin, Y.; Thiébaut, S.P.; Cortaredona, S.; Lerouge, M.A.; Cecchini, M.; Feigl, A.B.; Ventelou, B. Assessing the future medical cost burden for the European health systems under alternative exposure-to-risks scenarios. PLoS ONE 2020, 15, e0238565. [Google Scholar] [CrossRef] [PubMed]
- Cortaredona, S.; Ventelou, B. The extra cost of comorbidity: Multiple illnesses and the economic burden of non-communicable diseases. BMC Med. 2017, 15, 216. [Google Scholar] [CrossRef] [PubMed]
- Robert Koch-Institut. Gesundheit in Deutschland. Gesundheitsberichterstattung des Bundes; Robert Koch-Institut: Berlin, Germany, 2015. [Google Scholar]
- RKI. Surveillance Nichtübertragbarer Krankheiten. Available online: https://www.rki.de/DE/Content/Gesundheitsmonitoring/Studien/NCD-Surveillance/NCD-Surveillance_inhalt.html (accessed on 27 January 2023).
- Ding, D.; Lawson, K.D.; Kolbe-Alexander, T.L.; Finkelstein, E.A.; Katzmarzyk, P.T.; van Mechelen, W.; Pratt, M. The economic burden of physical inactivity: A global analysis of major non-communicable diseases. Lancet 2016, 388, 1311–1324. [Google Scholar] [CrossRef] [PubMed]
- Puska, P.; Laatikainen, T.; Korpelainen, V.; Vartiainen, E. Contribution of the North Karelia Project to International Work in CVD and NCD Prevention and Health Promotion. Glob. Heart 2016, 11, 243–246. [Google Scholar] [CrossRef]
- Probst-Hensch, N.; Tanner, M.; Kessler, C.; Burri, C.; Künzli, N. Prevention—A cost-effective way to fight the non-communicable disease epidemic: An academic perspective of the United Nations High-level NCD Meeting. Swiss Med. Wkly. 2011, 141, w13266. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Urbano, R.E.; Paredes, A.; Vargas Chambi, F.R.; Guedes Ruela, P.; Olivares, D.E.V.; Souza Pereira, B.T.; Pacheco, S.O.S.; Pacheco, F.J. Reception of Dietary and Other Health-Related Lifestyle Advice to Address Non-communicable Diseases in a Primary Care Context: A Mixed-Method Study in Central Argentina. Front. Nutr. 2021, 8, 622543. [Google Scholar] [CrossRef]
- O’Donnell, M.P.; Schultz, A.B.; Yen, L. The Portion of Health Care Costs Associated With Lifestyle-Related Modifiable Health Risks Based on a Sample of 223,461 Employees in Seven Industries: The UM-HMRC Study. J. Occup. Environ. Med. 2015, 57, 1284–1290. [Google Scholar] [CrossRef]
- Phillips, E.M.; Frates, E.P.; Park, D.J. Lifestyle Medicine. Phys. Med. Rehabil. Clin. N. Am. 2020, 31, 515–526. [Google Scholar] [CrossRef]
- Bosy-Westphal, A.; Müller, M.J. Prävention nicht übertragbarer chronischer Erkrankungen durch “gesunde” Ernährung. Dtsch. Med. Wochenschr. 2021, 146, 389–397. [Google Scholar] [CrossRef]
- Koeder, C.; Alzughayyar, D.; Anand, C.; Kranz, R.-M.; Husain, S.; Schoch, N.; Hahn, A.; Englert, H. The healthful plant-based diet index as a tool for obesity prevention—The healthy lifestyle community program cohort 3 study. Obes. Sci. Pract. 2022, 9, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Koeder, C.; Hahn, A.; Englert, H. Effect of a 6-Month Controlled Lifestyle Intervention on Common Carotid Intima-Media Thickness. J. Nutr. Health Aging 2021, 25, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Anand, C.; Kranz, R.-M.; Husain, S.; Koeder, C.; Schoch, N.; Alzughayyar, D.-K.; Gellner, R.; Hengst, K.; Englert, H. Bridging the gap between science and society: Long-term effects of the Healthy Lifestyle Community Programme (HLCP, cohort 1) on weight and the metabolic risk profile: A controlled study. BMJ Nutr. Prev. Health 2022, 5, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Koeder, C.; Kranz, R.-M.; Anand, C.; Husain, S.; Alzughayyar, D.; Schoch, N.; Hahn, A.; Englert, H. Effect of a 1-Year Controlled Lifestyle Intervention on Body Weight and Other Risk Markers (the Healthy Lifestyle Community Programme, Cohort 2). Obes. Facts 2022, 15, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Fleßa, S.; Greiner, W. Grundlagen der Gesundheitsökonomie; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 978-3-642-30918-2. [Google Scholar]
- Willems, R.; Pil, L.; Lambrinou, C.-P.; Kivelä, J.; Wikström, K.; Gonzalez-Gil, E.M.; de Miguel-Etayo, P.; Nánási, A.; Semánová, C.; van Stappen, V.; et al. Methodology of the health economic evaluation of the Feel4Diabetes-study. BMC Endocr. Disord. 2020, 20, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Siegel, K.R.; Ng, B.P.; Jawanda, S.; Proia, K.K.; Zhang, X.; Albright, A.L.; Zhang, P. Cost-effectiveness of Diabetes Prevention Interventions Targeting High-risk Individuals and Whole Populations: A Systematic Review. Diabetes Care 2020, 43, 1593–1616. [Google Scholar] [CrossRef] [PubMed]
- Hewage, S.S.; Wu, S.; Neelakantan, N.; Yoong, J. Systematic review of effectiveness and cost-effectiveness of lifestyle interventions to improve clinical diabetes outcome measures in women with a history of GDM. Clin. Nutr. ESPEN 2020, 35, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Dorhout, B.G.; Haveman-Nies, A.; van Dongen, E.J.I.; Wezenbeek, N.L.W.; Doets, E.L.; Bulten, A.; de Wit, G.A.; de Groot, L.C. Cost-effectiveness of a Diet and Resistance Exercise Intervention in Community-Dwelling Older Adults: ProMuscle in Practice. J. Am. Med. Dir. Assoc. 2021, 22, 792–802.e2. [Google Scholar] [CrossRef]
- Wolf, A.M.; Siadaty, M.; Yaeger, B.; Conaway, M.R.; Crowther, J.Q.; Nadler, J.L.; Bovbjerg, V.E. Effects of lifestyle intervention on health care costs: Improving Control with Activity and Nutrition (ICAN). J. Am. Diet. Assoc. 2007, 107, 1365–1373. [Google Scholar] [CrossRef]
- Li, R.; Qu, S.; Zhang, P.; Chattopadhyay, S.; Gregg, E.W.; Albright, A.; Hopkins, D.; Pronk, N.P. Economic Evaluation of Combined Diet and Physical Activity Promotion Programs to Prevent Type 2 Diabetes Among Persons at Increased Risk: A Systematic Review for the Community Preventive Services Task Force. Ann. Intern. Med. 2015, 163, 452–460. [Google Scholar] [CrossRef]
- Alouki, K.; Delisle, H.; Bermúdez-Tamayo, C.; Johri, M. Lifestyle Interventions to Prevent Type 2 Diabetes: A Systematic Review of Economic Evaluation Studies. J. Diabetes Res. 2016, 2016, 2159890. [Google Scholar] [CrossRef]
- Müller-Nordhorn, J.; Englert, H.; Wegscheider, K.; Berger, H.; Sonntag, F.; Völler, H.; Meyer-Sabellek, W.; Reinhold, T.; Windler, E.; Katus, H.A.; et al. Productivity loss as a major component of disease-related costs in patients with hypercholesterolemia in Germany. Clin. Res. Cardiol. 2008, 97, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Sanson-Fisher, R.W.; D’Este, C.A.; Carey, M.L.; Noble, N.; Paul, C.L. Evaluation of systems-oriented public health interventions: Alternative research designs. Annu. Rev. Public Health 2014, 35, 9–27. [Google Scholar] [CrossRef]
- Scholz, S.; Biermann-Stallwitz, J.; Brettschneider, C.; Damm, O.; Freytag, A.; Greiner, W.; Icks, A.; König, H.-H.; Krauth, C.; Kuhlmann, A.; et al. Standardisierte Kostenberechnungen im deutschen Gesundheitswesen: Bericht der Arbeitsgruppe “Standardkosten” des Ausschusses “ökonomische Evaluation” der dggö. Gesundh Ökon Qual Manag. 2020, 25, 52–59. [Google Scholar] [CrossRef]
- Burchardi, H. Einführung des Fallpauschalensystems in Deutschland: Ein geschichtlicher Rückblick. Med. Klin. Intensivmed. Notfmed. 2018, 113, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Köder, C. Effect of the Community-Based Intervention Healthy Lifestyle Community Programme on Common Carotid Intima-Media Thickness and Other Cardiovascular Markers; Institutionelles Repositorium der Leibniz Universität Hannover: Hannover, Germany, 2022. [Google Scholar]
- Berra, K.; Franklin, B.; Jennings, C. Community-Based Healthy Living Interventions. Prog. Cardiovasc. Dis. 2017, 59, 430–439. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, D.B.; Thayer, J.F.; Vedhara, K. Stress and Health: A Review of Psychobiological Processes. Annu. Rev. Psychol. 2021, 72, 663–688. [Google Scholar] [CrossRef] [PubMed]
- Posadzki, P.; Pieper, D.; Bajpai, R.; Makaruk, H.; Könsgen, N.; Neuhaus, A.L.; Semwal, M. Exercise/physical activity and health outcomes: An overview of Cochrane systematic reviews. BMC Public Health 2020, 20, 1724. [Google Scholar] [CrossRef] [PubMed]
- Jayedi, A.; Soltani, S.; Abdolshahi, A.; Shab-Bidar, S. Healthy and unhealthy dietary patterns and the risk of chronic disease: An umbrella review of meta-analyses of prospective cohort studies. Br. J. Nutr. 2020, 124, 1133–1144. [Google Scholar] [CrossRef]
- Koeder, C.; Husain, S.; Kranz, R.-M.; Anand, C.; Alzughayyar, D.; Schoch, N.; Hahn, A.; Englert, H. Healthy lifestyle changes favourably affect common carotid intima-media thickness: The Healthy Lifestyle Community Programme (cohort 2). J. Nutr. Sci. 2022, 11, e47. [Google Scholar] [CrossRef]
- Kapsner, L.A.; Kampf, M.O.; Seuchter, S.A.; Gruendner, J.; Gulden, C.; Mate, S.; Mang, J.M.; Schüttler, C.; Deppenwiese, N.; Krause, L.; et al. Reduced Rate of Inpatient Hospital Admissions in 18 German University Hospitals During the COVID-19 Lockdown. Front. Public Health 2020, 8, 594117. [Google Scholar] [CrossRef] [PubMed]
- Sarria-Santamera, A.; Alexeyeva, Z.; Yen Chan, M.; Ortega, M.A.; Asunsolo-Del-Barco, A.; Navarro-García, C. Direct and Indirect Costs Related to Physical Activity Levels in Patients with Diabetes Mellitus in Spain: A Cross-Sectional Study. Healthcare 2022, 10, 752. [Google Scholar] [CrossRef] [PubMed]
- Kranz, R.-M.; Kettler, C.; Anand, C.; Koeder, C.; Husain, S.; Schoch, N.; Buyken, A.; Englert, H. Effect of a controlled lifestyle intervention on medication use and costs: The Healthy Lifestyle Community Program (cohort 2). Nutr. Health 2023, 2601060231164665. [Google Scholar] [CrossRef]
- Kang, Y.J.; Kang, M.-Y. Chronic Diseases, Health Behaviors, and Demographic Characteristics as Predictors of Ill Health Retirement: Findings from the Korea Health Panel Survey (2008–2012). PLoS ONE 2016, 11, e0166921. [Google Scholar] [CrossRef] [PubMed]
- Robroek, S.J.W.; Schuring, M.; Croezen, S.; Stattin, M.; Burdorf, A. Poor health, unhealthy behaviors, and unfavorable work characteristics influence pathways of exit from paid employment among older workers in Europe: A four year follow-up study. Scand. J. Work Environ. Health 2013, 39, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Bakaloudi, D.R.; Barazzoni, R.; Bischoff, S.C.; Breda, J.; Wickramasinghe, K.; Chourdakis, M. Impact of the first COVID-19 lockdown on body weight: A combined systematic review and a meta-analysis. Clin. Nutr. 2022, 41, 3046–3054. [Google Scholar] [CrossRef] [PubMed]
- Tremmel, M.; Gerdtham, U.-G.; Nilsson, P.M.; Saha, S. Economic Burden of Obesity: A Systematic Literature Review. Int. J. Environ. Res. Public Health 2017, 14, 435. [Google Scholar] [CrossRef]
| Segment | Information Base | |
|---|---|---|
| Direct costs | Medications | Lauer-Taxe® |
| Ambulatory physician sector | Report on the results of the 2019 fee distribution | |
| Treatments | Statutory health insurance: EBM; private health insurance: GoÄ | |
| Therapeutic appliances | Reference amounts of the National Association of Statutory Health Insurance Funds; average prices of an online medical supply store | |
| Remedies | Remedies prices of the National Association of Statutory Health Insurance Funds | |
| Inpatient visit | Report on the results of the 2019 fee distribution | |
| Outpatient visits | Hospital: hospital statistics based on flat rates per case (DRG-statistics); rehabilitation clinic, when employed: rehabilitation report 2019 of the German pension insurance; rehabilitation clinic, when unemployed: statistics of the Federal Ministry of Health | |
| Ambulance service | Local statutes of the municipality | |
| Care service | German Code of Social Law | |
| Indirect costs | Time of incapacity to work | Statistical yearbook 2019 |
| Early retirement | Statistical yearbook 2019 |
| Variable | Intervention Group (n = 79) | Control Group (n = 39) | p-Value |
|---|---|---|---|
| Age, years: mean ± SD | 59.2 ± 7.8 | 56.4 ± 9.2 | 0.031 c |
| Sex, n (%) | 0.402 a | ||
| Male | 23 (29.1) | 15 (38.5) | |
| Female | 56 (70.9) | 24 (61.5) | |
| Body weight, kg: mean ± SD | 80.7 ± 19.0 | 83.9 ± 18.1 | 0.260 c |
| BMI, kg/m2: mean ± SD | 27.4 ± 5.3 | 28.1 ± 5.7 | 0.542 c |
| Waist circumference, cm: mean ± SD | 97.5 ± 15.1 | 96.8 ± 14.8 | 0.975 c |
| Education level, n (%) | 0.019 a | ||
| Lower secondary school | 14 (17.7) | 14 (35.9) | |
| Secondary school | 36 (45.6) | 12 (30.8) | |
| University entrance qualification | 14 (17.7) | 11 (28.2) | |
| University degree | 15 (19) | 2 (5.1) | |
| Marital status, n (%) | 0.547 a | ||
| Married | 63 (79.7) | 35 (89.7) | |
| Partner, unmarried | 5 (6.3) | 1 (2.6) | |
| Single (not widowed) | 7 (8.9) | 1 (2.6) | |
| Single (widowed) | 4 (5.1) | 2 (5.1) | |
| Blood pressure: mean ± SD | |||
| Systolic blood pressure, mm Hg | 133.3 ± 15.0 | 134.9 ± 14.5 | 0.585 b |
| Diastolic blood pressure, mm Hg | 81.3 ± 9.0 | 80.7 ± 9.3 | 0.636 c |
| Blood parameters: mean ± SD | |||
| Total cholesterol, mg/dL | 209.2 ± 38.0 | 205.9 ± 44.8 | 0.677 b |
| HDL cholesterol, mg/dL | 66.6 ± 18.9 | 62.1 ± 17.9 | 0.226 c |
| LDL cholesterol, mg/dL | 134.1 ± 36.2 | 138.5 ± 41.5 | 0.555 b |
| Triglycerides, mg/dL | 104.6 ± 52.7 | 112.6 ± 49.3 | 0.292 c |
| Fasting glucose, mg/dL | 98.3 ± 11.5 | 102.0 ± 12.5 | 0.352 c |
| HbA1c, % | 5.4 ± 0.5 | 5.5 ± 0.4 | 0.691 c |
| Health insurance, n (%) | 0.092 a | ||
| Statutory health insurance | 65 (82.3) | 35 (89.7) | |
| Private health insurance | 13 (16.5) | 2 (5.1) | |
| Other | 1 (1.3) | 2 (5.1) | |
| Diagnosis of diseases, n (%) | 64 (81.0) | 29 (74.4) | 0.474 a |
| Medication use (regularly), n (%) | 57 (72.2) | 23 (59.0) | 0.208 a |
| Employment, n (%) | 0.129 a | ||
| Employed | 50 (63.3) | 29 (74.4) | |
| Pensioner | 25 (31.6) | 6 (15.4) | |
| Unemployed | 0 (0.0) | 0 (0.0) | |
| Housewife/househusband | 4 (5.1) | 4 (10.3) |
| Change after | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Costs at Baseline | 10 Weeks | p-Value # | 6 Months | p- Value # | 12 Months | p- Value # | 18 Months | p- Value # | 24 Months | p- Value # | |||
| Total costs 1 | IG | mean ± SD | 67.80 ± 69.17 | −49.73 *** ± 65.59 | 0.012 | −40.91 *** ± 60.00 | 0.004 | −28.83 *** ± 60.57 | 0.259 | −39.71 *** ± 62.50 | 0.240 | −22.92 ** ± 85.67 | 0.305 |
| CG | mean ± SD | 48.73 ± 54.41 | −18.40 ± 53.77 | 0.39 ± 70.57 | −23.73 ± 54.60 | −22.43 * ± 46.82 | −18.25 ± 53.48 | ||||||
| Direct costs 2 | IG | mean ± SD | 47.84 ± 42.80 | −24.92 *** ± 34.90 | 0.017 | −20.44 *** ± 33.83 | 0.041 | −21.83 *** ± 33.64 | 0.012 | −21.70 *** ± 36.06 | 0.070 | −18.66 *** ± 43.19 | 0.243 |
| CG | mean ± SD | 33.21 ± 33.65 | −5.97 ± 23.76 | −4.98 ± 26.09 | −5.66 ± 31.11 | −7.39 ± 32.05 | −8.91 ± 30.99 | ||||||
| Indirect costs 3 | IG | mean ± SD | 11.39 ± 29.13 | −11.06 ** ± 29.28 | 0.021 | −10.38 ** ± 28.85 | 0.044 | −6.23 * ± 19.80 | 0.057 | −6.96 * ± 22.68 | 0.059 | −7.33 * ± 24.00 | 0.058 |
| CG | mean ± SD | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | ||||||
| ß | SE | p-Value | |
|---|---|---|---|
| 10 weeks 1 | |||
| constant (ß0) | 9.400 | 3.566 | 0.010 |
| group (ref. intervention) | 13.697 | 4.733 | 0.005 |
| total costs at baseline | −0.875 | 0.034 | <0.001 |
| 6 months 2 | |||
| constant (ß0) | 15.275 | 5.710 | 0.009 |
| group (ref. intervention) | 28.236 | 7.282 | <0.001 |
| total costs at baseline | −0.896 | 0.058 | <0.001 |
| 12 months 3 | |||
| constant (ß0) | 20.189 | 5.153 | <0.001 |
| group (ref. intervention) | −3.489 | 6.691 | 0.603 |
| total costs at baseline | −0.847 | 0.056 | <0.001 |
| 18 months 4 | |||
| constant (ß0) | −33.271 | 21.496 | 0.125 |
| group (ref. intervention) | 4.827 | 5.121 | 0.348 |
| diastolic blood pressure | 0.587 | 0.265 | 0.029 |
| total costs at baseline | −0.873 | 0.040 | <0.001 |
| 24 months 5 | |||
| constant (ß0) | 33.598 | 8.566 | <0.001 |
| group (ref. intervention) | −9.742 | 11.220 | 0.387 |
| total costs at baseline | −0.880 | 0.085 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kranz, R.-M.; Kettler, C.; Koeder, C.; Husain, S.; Anand, C.; Schoch, N.; Englert, H. Health Economic Evaluation of a Controlled Lifestyle Intervention: The Healthy Lifestyle Community Program (Cohort 2; HLCP-2). Nutrients 2023, 15, 5045. https://doi.org/10.3390/nu15245045
Kranz R-M, Kettler C, Koeder C, Husain S, Anand C, Schoch N, Englert H. Health Economic Evaluation of a Controlled Lifestyle Intervention: The Healthy Lifestyle Community Program (Cohort 2; HLCP-2). Nutrients. 2023; 15(24):5045. https://doi.org/10.3390/nu15245045
Chicago/Turabian StyleKranz, Ragna-Marie, Carmen Kettler, Christian Koeder, Sarah Husain, Corinna Anand, Nora Schoch, and Heike Englert. 2023. "Health Economic Evaluation of a Controlled Lifestyle Intervention: The Healthy Lifestyle Community Program (Cohort 2; HLCP-2)" Nutrients 15, no. 24: 5045. https://doi.org/10.3390/nu15245045
APA StyleKranz, R.-M., Kettler, C., Koeder, C., Husain, S., Anand, C., Schoch, N., & Englert, H. (2023). Health Economic Evaluation of a Controlled Lifestyle Intervention: The Healthy Lifestyle Community Program (Cohort 2; HLCP-2). Nutrients, 15(24), 5045. https://doi.org/10.3390/nu15245045






