Oral Motor Impairments Contribute to Weight Status of Adults with Severe Cerebral Palsy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Approval and Consent
2.2. Subjects and Study Design
2.3. Energy Intake—Weighed Dietary Recording
2.4. Energy Intake—Mealtime and Oral Motor-Function Evaluations
2.5. Energy Expenditure—Resting Metabolic Rate (RMR)
2.6. Energy Expenditure—Indirect Body Composition via Double-Labeled Water (DLW)
2.7. Metabolic Status—Blood Test
2.8. Anthropometric Measures
2.9. Analysis and Statistics
2.9.1. DLW Analysis
2.9.2. Blood Analysis
2.9.3. Statistics
3. Results
3.1. Results of the Groups
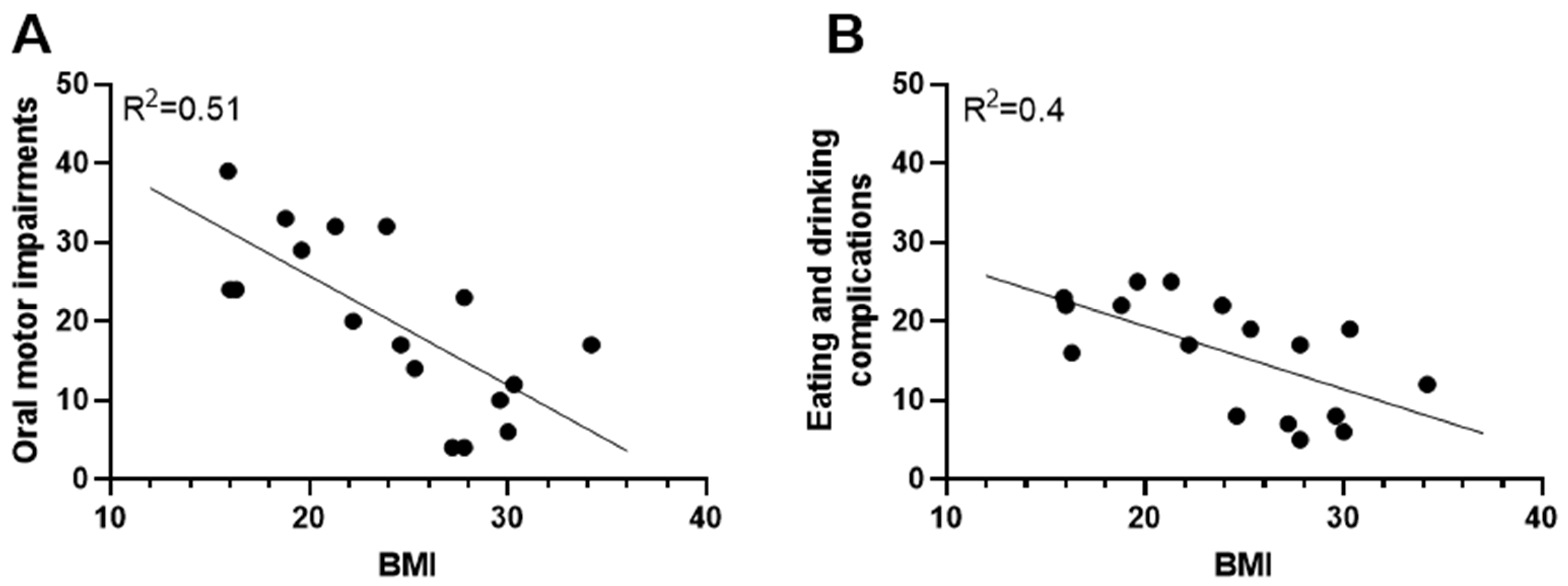
3.2. The Impact of Oral Motor Impairments on Weight Status
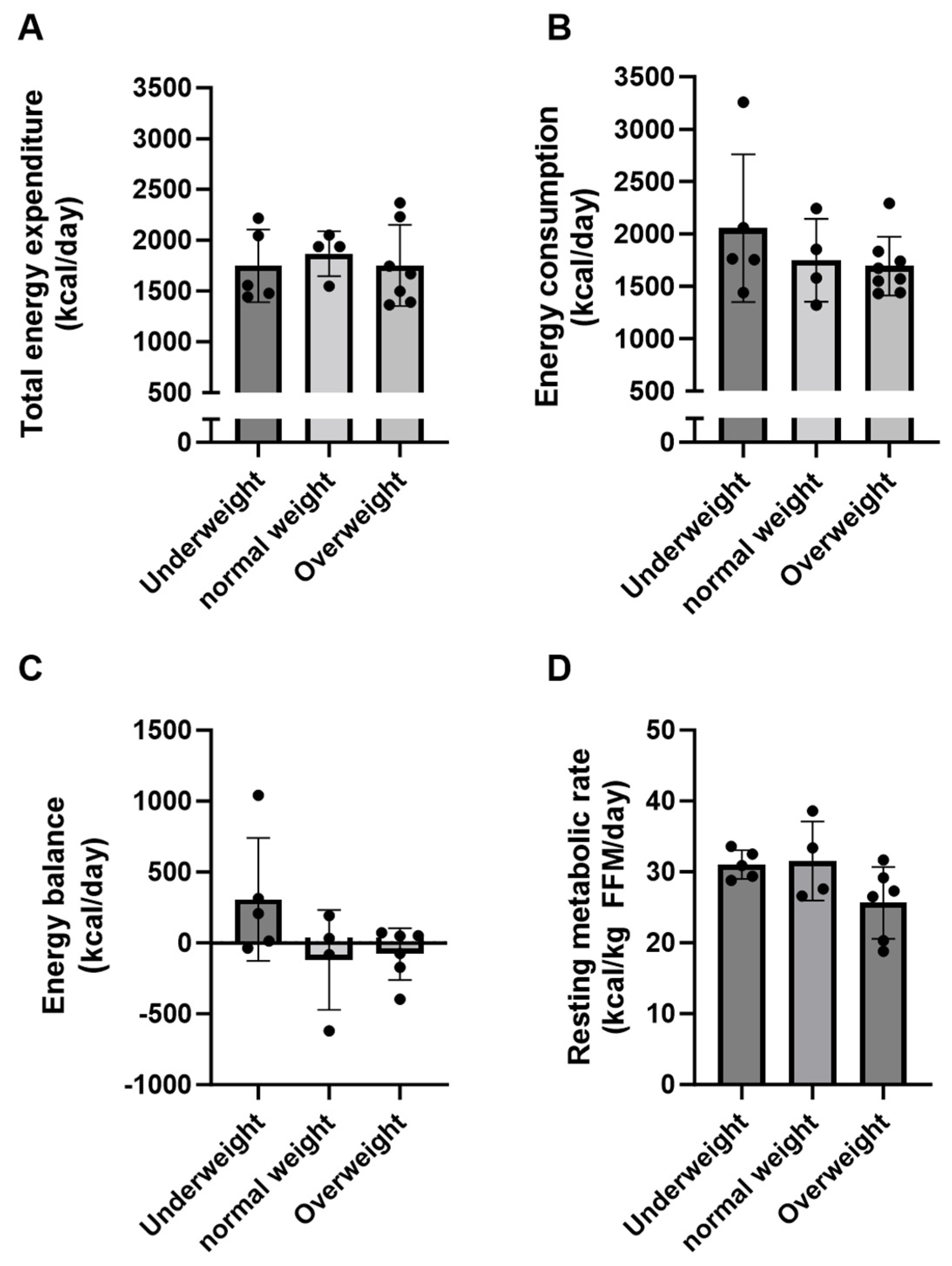
3.3. Energy Expenditure and Energy Balance in Adults with Severe CP
3.4. Diet
3.5. Metabolic Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosenbaum, P.; Paneth, N.; Leviton, A.; Goldstein, M.; Bax, M.; Damiano, D.; Dan, B.; Jacobsson, B. A report: The definition and classification of cerebral palsy April 2006. Dev. Med. Child Neurol. Suppl. 2007, 109, 8–14. [Google Scholar] [PubMed]
- Graham, H.K.; Rosenbaum, P.; Paneth, N.; Dan, B.; Lin, J.P.; Damiano, D.L.; Becher, J.G.; Gaebler-Spira, D.; Colver, A.; Reddihough, D.S.; et al. Cerebral palsy. Nat. Rev. Dis. Primers 2016, 2, 15082. [Google Scholar] [CrossRef] [PubMed]
- Aisen, M.L.; Kerkovich, D.; Mast, J.; Mulroy, S.; Wren, T.A.; Kay, R.M.; Rethlefsen, S.A. Cerebral palsy: Clinical care and neurological rehabilitation. Lancet Neurol. 2011, 10, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Ashwal, S.; Russman, B.S.; Blasco, P.A.; Miller, G.; Sandler, A.; Shevell, M.; Stevenson, R. Practice parameter: Diagnostic assessment of the child with cerebral palsy: Report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 2004, 62, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Himmelmann, K. Epidemiology of cerebral palsy. Handb. Clin. Neurol. 2013, 111, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Colver, A.; Fairhurst, C.; Pharoah, P.O. Cerebral palsy. Lancet 2014, 383, 1240–1249. [Google Scholar] [CrossRef]
- Humphries, K.; Traci, M.A.; Seekins, T. Nutrition and adults with intellectual or developmental disabilities: Systematic literature review results. Intellect. Dev. Disabil. 2009, 47, 163–185. [Google Scholar] [CrossRef]
- Strauss, D.; Ojdana, K.; Shavelle, R.; Rosenbloom, L. Decline in function and life expectancy of older persons with cerebral palsy. NeuroRehabilitation 2004, 19, 69–78. [Google Scholar] [CrossRef]
- Pettersson, K.; Rodby-Bousquet, E. Living Conditions and Social Outcomes in Adults With Cerebral Palsy. Front. Neurol. 2021, 12, 749389. [Google Scholar] [CrossRef]
- Norte, A.; Alonso, C.; Martinez-Sanz, J.M.; Gutierrez-Hervas, A.; Sospedra, I. Nutritional Status and Cardiometabolic Risk Factors in Institutionalized Adults with Cerebral Palsy. Medicina 2019, 55, 157. [Google Scholar] [CrossRef]
- Trinh, A.; Wong, P.; Fahey, M.C.; Brown, J.; Churchyard, A.; Strauss, B.J.; Ebeling, P.R.; Fuller, P.J.; Milat, F. Musculoskeletal and Endocrine Health in Adults With Cerebral Palsy: New Opportunities for Intervention. J. Clin. Endocrinol. Metab. 2016, 101, 1190–1197. [Google Scholar] [CrossRef]
- Yi, Y.G.; Oh, B.M.; Seo, H.G.; Shin, H.I.; Bang, M.S. Dysphagia-Related Quality of Life in Adults with Cerebral Palsy on Full Oral Diet Without Enteral Nutrition. Dysphagia 2019, 34, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.K.; Kim, A.R.; Kim, O.Y.; Lee, K.; Suh, Y.J.; Cho, S.R. Factors affecting bone mineral density in adults with cerebral palsy. Ann. Rehabil. Med. 2012, 36, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.L.; Lorentzen, J.; Pedersen, L.T.; Hendrich, F.L.; Jorsal, M.; Pingel, J.; Nielsen, J.B.; Kiens, B. Suboptimal Nutrition and Low Physical Activity Are Observed Together with Reduced Plasma Brain-Derived Neurotrophic Factor (BDNF) Concentration in Children with Severe Cerebral Palsy (CP). Nutrients 2019, 11, 620. [Google Scholar] [CrossRef] [PubMed]
- Balandin, S.; Hemsley, B.; Hanley, L.; Sheppard, J.J. Understanding mealtime changes for adults with cerebral palsy and the implications for support services. J. Intellect. Dev. Disabil. 2009, 34, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.; McCombie, L.; Dawes, P.; McConnell, K.N.; Dunnigan, M.G. Nutritional support for patients with intellectual disability and nutrition/dysphagia disorders in community care. J. Intellect. Disabil. Res. 1997, 41 Pt 5, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.K.; Goran, M.I.; Ferrara, M.S.; Poehlman, E.T. Athetosis increases resting metabolic rate in adults with cerebral palsy. J. Am. Diet. Assoc. 1996, 96, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Bandini, L.G.; Schoeller, D.A.; Fukagawa, N.K.; Wykes, L.J.; Dietz, W.H. Body composition and energy expenditure in adolescents with cerebral palsy or myelodysplasia. Pediatr. Res. 1991, 29, 70–77. [Google Scholar] [CrossRef]
- Stallings, V.A.; Zemel, B.S.; Davies, J.C.; Cronk, C.E.; Charney, E.B. Energy expenditure of children and adolescents with severe disabilities: A cerebral palsy model. Am. J. Clin. Nutr. 1996, 64, 627–634. [Google Scholar] [CrossRef]
- Palisano, R.; Rosenbaum, P.; Walter, S.; Russell, D.; Wood, E.; Galuppi, B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev. Med. Child Neurol. 1997, 39, 214–223. [Google Scholar] [CrossRef]
- Sellers, D.; Mandy, A.; Pennington, L.; Hankins, M.; Morris, C. Development and reliability of a system to classify the eating and drinking ability of people with cerebral palsy. Dev. Med. Child Neurol. 2014, 56, 245–251. [Google Scholar] [CrossRef]
- Pingel, J.; Andersen, C.T.; Raffalt, P.; Kowalczyk, C. Singing Therapy Improving Peak Flow, Speech and Eating Abilities in Adults with Cerebral Palsy. Open J. Ther. Rehabil. 2022, 10, 20. [Google Scholar] [CrossRef]
- Compher, C.; Frankenfield, D.; Keim, N.; Roth-Yousey, L.; Evidence Analysis Working, G. Best practice methods to apply to measurement of resting metabolic rate in adults: A systematic review. J. Am. Diet. Assoc. 2006, 106, 881–903. [Google Scholar] [CrossRef]
- Weir, J.B. New methods for calculating metabolic rate with special reference to protein metabolism. J. Physiol. 1949, 109, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Roza, A.M.; Shizgal, H.M. The Harris Benedict equation reevaluated: Resting energy requirements and the body cell mass. Am. J. Clin. Nutr. 1984, 40, 168–182. [Google Scholar] [CrossRef]
- Durnin, J.V.; Womersley, J. Body fat assessed from total body density and its estimation from skinfold thickness: Measurements on 481 men and women aged from 16 to 72 years. Br. J. Nutr. 1974, 32, 77–97. [Google Scholar] [CrossRef] [PubMed]
- Westerterp, K.R.; Wouters, L.; van Marken Lichtenbelt, W.D. The Maastricht protocol for the measurement of body composition and energy expenditure with labeled water. Obes. Res. 1995, 3 (Suppl. S1), 49–57. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J.R.; Yamada, Y.; Sagayama, H.; Berman, E.S.F.; Ainslie, P.N.; Andersen, L.F.; Anderson, L.J.; Arab, L.; Baddou, I.; Bedu-Addo, K.; et al. A standard calculation methodology for human doubly labeled water studies. Cell Rep. Med. 2021, 2, 100203. [Google Scholar] [CrossRef]
- Nordic Nutrition Recommendations. Nordic Nutrition Recommendations 2012: Integrating Nutrition and Physical Activity, 5th ed.; Nordic Council of Ministers: Copenhagen, Denmark, 2023. [Google Scholar]
- Biltoft-Jensen, A.; Ygil, K.H.; Knudsen, L.; Matthiessen, J.; Fagt, S.; Trolle, E.; Nielsen, T.H.; Hansen, D.M.; Licht, C.L.; Martens, M.; et al. Validation of the 2 × 24 h recall method and a 7-d web-based food diary against doubly labelled water in Danish adults. Br. J. Nutr. 2023, 130, 1–14. [Google Scholar] [CrossRef]
- Gisel, E. Does food and fluid texture consumption relate to dietary intake in preschool children with cerebral palsy? Dev. Med. Child Neurol. 2015, 57, 989–990. [Google Scholar] [CrossRef]
- Johnson, R.K.; Hildreth, H.G.; Contompasis, S.H.; Goran, M.I. Total energy expenditure in adults with cerebral palsy as assessed by doubly labeled water. J. Am. Diet. Assoc. 1997, 97, 966–970. [Google Scholar] [CrossRef] [PubMed]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuniga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- Colucci-D’Amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic Factor BDNF, Physiological Functions and Therapeutic Potential in Depression, Neurodegeneration and Brain Cancer. Int. J. Mol. Sci. 2020, 21, 7777. [Google Scholar] [CrossRef] [PubMed]

| Underweight | Normal Weight | Overweight | |
|---|---|---|---|
| Number of participants | 5 | 4 | 8 |
| Gender (M/F) | 5/0 | 3/1 | 5/3 |
| Age (years) | 46 ± 9.6 (34–56) | 47 ± 7.7 (39–56) | 52 ± 21.5 (22–78) |
| Height (cm) | 165 ± 7.6 (155–172) | 166 ± 6.0 (157–170) | 161 ± 8.7 (147–172) |
| Body weight (kg) | 46.9 ± 5.3 (39.9–54.4) | 63.4 ± 7.5 * (54.8–70.3) | 75.7 ± 9.6 **,(#) (60.1–89.7) |
| BMI | 17.3 ± 1.7 (15.9–19.6) | 23.0 ± 1.5 (21.3–24.6) | 29.0 ± 2.7 (25.3–34.2) |
| GMFCS (I–V) | |||
| III (n) | 3 | 0 | 2 |
| IV (n) | 1 | 2 | 5 |
| V (n) | 1 | 2 | 1 |
| Type of CP | |||
| Spastic (n) | 2 | 2 | 8 |
| Dyskinetic (n) | 3 | 2 | 0 |
| Topographic classification | |||
| Hemiplegia (n) | 0 | 0 | 1 |
| Diplegia (n) | 1 | 1 | 3 |
| Quadriplegia (n) | 4 | 3 | 4 |
| Underweight | Normal Weight | Overweight | |
|---|---|---|---|
| EDACS (I–V) | |||
| II n, (%) | 1 (20) | 1 (25) | 7 (87.5) |
| III n, (%) | 0 (0) | 1 (25) | 0 (0) |
| IV n, (%) | 4 (80) | 2 (50) | 1 (12.5) |
| Level of assistance required during eating | |||
| IND n, (%) | 0 (0) | 1 (25) | 4 (50) |
| RA n, (%) | 1 (20) | 0 | 1 (12.5) |
| TD n, (%) | 4 (80) | 3 (75) | 3 (37.5) |
| Modified food texture n, (%) | 4 (80) | 3 (75) | 1 (12.5) |
| Modified drink consistency n, (%) | 1 (20) | 2 (50) | 1 (12.5) |
| Meal time (min) | 27.4 ± 6.1 (12–45) | 23.8 ± 6.5 (9–39) | 16.3 ± 2.8 (8–28) |
| Underweight | Normal Weight | Overweight | RI | |
|---|---|---|---|---|
| Energy intake (kcal) | 2055 ± 316 | 1750 ± 198 | 1692 ± 99 | |
| Macronutrients | ||||
| Protein (E%) | 15.4 ± 2.1 | 15.0 ± 1.4 | 17.4 ± 0.9 | 10–20 |
| Protein (g/day) | 74 ± 8.8 | 64 ± 11.7 | 70 ± 4.8 | - |
| Protein (g/kg/day) | 1.58 ± 0.2 | 1.03 ± 0.2 (*) | 0.95 ± 0.1 * | ≥0.83 |
| Protein (g/kg FFM/day) | 1.94 ± 0.2 | 1.45 ± 0.3 | 1.63 ± 0.1 | - |
| Carbohydrates (E%) | 38.1 ± 4.0 | 50.0 ± 2.6 * | 47.2± 1.9 | 45–60 |
| Carbohydrates (g/day) | 175 ± 8.8 | 208 ± 19.6 | 188 ± 15.9 | - |
| Dietary fibers (g/MJ) | 1.5 ± 0.2 | 1.5 ± 0.1 | 2.7 ± 0.1 ***,### | >3 |
| Fat (E%) | 46.9 ± 5.6 | 35.7 ± 1.7 | 34.9 ± 1.8 * | 25–40 |
| N-3 fatty acids (E%) | 1.1 ± 0.2 | 1.3 ± 0.1 | 1.2 ± 0.2 | >1 |
| Added sugar (E%) | 11.2 ± 2.3 | 22.2 ± 2.7 | 10.3 ± 2.9 | <10 |
| Underweight | Normal Weight | Overweight | |
|---|---|---|---|
| Glucose (mmol/L) | 5.3 ± 0.2 | 4.8 ± 0.1 | 5.6 ± 0.3 |
| (4.9–5.8) | (4.6–5.2) | (4.8–6.4) | |
| Insulin (µU/mL) | 6.4 ± 1.5 | 6.9 ± 1.2 | 12.4 ± 2.3 |
| (3.1–11.9) | (4.4–9.2) | (4.6–22.7) | |
| HOMA-IR | 1.5 ± 0.3 | 1.5 ± 0.3 | 3.1 ± 0.7 |
| (0.7–2.6) | (0.9–1.9) | (1.2–6.5) | |
| Triacylglycerol (mmol/L) | 0.8 ± 0.1 | 2.1 ± 1.2 | 1.5 ± 0.2 |
| (0.6–1.2) | (0.8–5.7) | (0.6–2.5) | |
| Total cholesterol (mmol/L) | 4.5 ± 0.1 | 4.6 ± 0.3 | 4.8 ± 0.3 |
| (4.2–4.8) | (4.0–5.3) | (3.3–5.8) | |
| HDL cholesterol (mmol/L) | 1.4 ± 0.1 | 1.4 ± 0.3 | 1.2 ± 0.1 |
| (1.1–1.7) | (0.7–2.0) | (0.9–1.8) | |
| LDL cholesterol (mmol/L) | 3.0 ± 0.1 | 2.5 ± 0.2 | 3.2 ± 0.4 |
| (2.7–3.4) | (2.1–3.0) | (2.1–4.8) | |
| 25 (OH) vitamin D (nmol/L) | 69 ± 14.4 | 77 ± 12.6 | 44 ± 5.3 |
| (35–121) | (45–106) | (22–56) | |
| BDNF (pg/mL) | 11,031 ± 2360 (4301–16703) | 10,760 ± 1885 (*) (6723–14591) | 4407 ± 1303 * (522–9733) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyster, A.E.; Hansen, S.L.; Andersen, C.T.; Nielsen, J.B.; Westerterp, K.; Wouters, L.; Kiens, B.; Ritterband-Rosenbaum, A. Oral Motor Impairments Contribute to Weight Status of Adults with Severe Cerebral Palsy. Nutrients 2023, 15, 5042. https://doi.org/10.3390/nu15245042
Lyster AE, Hansen SL, Andersen CT, Nielsen JB, Westerterp K, Wouters L, Kiens B, Ritterband-Rosenbaum A. Oral Motor Impairments Contribute to Weight Status of Adults with Severe Cerebral Palsy. Nutrients. 2023; 15(24):5042. https://doi.org/10.3390/nu15245042
Chicago/Turabian StyleLyster, Aslak Emil, Solvejg Lis Hansen, Christina Therese Andersen, Jens Bo Nielsen, Klaas Westerterp, Loek Wouters, Bente Kiens, and Anina Ritterband-Rosenbaum. 2023. "Oral Motor Impairments Contribute to Weight Status of Adults with Severe Cerebral Palsy" Nutrients 15, no. 24: 5042. https://doi.org/10.3390/nu15245042
APA StyleLyster, A. E., Hansen, S. L., Andersen, C. T., Nielsen, J. B., Westerterp, K., Wouters, L., Kiens, B., & Ritterband-Rosenbaum, A. (2023). Oral Motor Impairments Contribute to Weight Status of Adults with Severe Cerebral Palsy. Nutrients, 15(24), 5042. https://doi.org/10.3390/nu15245042






